Abstract
Background: Although KCNH2 (HERG) K897T polymorphism has been shown to be associated with the QT interval measured from 12‐lead electrocardiogram (ECG), the functional significance of K897T polymorphism has been debated. The aim of this study was to test whether the K897T polymorphism of the KCNH2 (HERG) gene coding for the rapidly activating delayed rectifier K+ channel influences cardiac repolarization assessed by principal component analysis (PCA) of T‐wave morphology.
Methods: Twelve‐lead ECGs were digitized and T‐wave morphology was analyzed with a PCA method in a population consisting of 228 healthy middle‐aged subjects (121 women and 107 men). DNA samples were genotyped for the nucleotide 2690 A>C variation of the KCNH2 gene, corresponding to the KCNH2 K(lysine)897T(threonine) amino acid polymorphism.
Results: The allele frequencies were 0.86 (K) and 0.14 (T). The KCNH2 K897T polymorphism was associated with the total cosine R‐to‐T (TCRT), which reflects the wave front direction between depolarization and repolarization. TCRT was 0.421 in the genotype KK and 0.300 in the genotypes KT and TT (P = 0.04). The difference of TCRT was more marked between the KCNH2 K897T genotypes in women (P = 0.03) than in men (P = 0.52).
Conclusions: The common K897T polymorphism of the cardiac potassium channel KCNH2 has functional significance for cardiac electrical properties. Subjects with a less common genotype, KT or TT, have smaller TCRT, which reflects dyssynchrony between depolarization and repolarization and is associated with an increased risk of cardiac mortality.
Keywords: ventricular repolarization, ion channels, genes
The common polymorphism K897T of the KCNH2 (HERG) gene coding for the major subunit of the rapidly activating delayed rectifier K+ channel (Ikr) has been shown to be associated with the QT interval measured from the 12‐lead surface electrocardiogram (ECG). 1 , 2 , 3 , 4 In vitro studies have also shown that this polymorphism alters the function of the ion channel. 3 , 4 , 5 However, there has been some disparity between the results, with others suggesting hastened 4 and others delayed repolarization. 3
Novel methods of analyzing the cardiac repolarization process from surface ECG have been recently proposed to overcome the problems of manual ECG measurements and subjective estimates of the end of the T wave. 6 These new variables describe the morphology of the T wave patterns and are calculated using digital signals from the 12‐lead surface ECG. These variables have been shown to provide novel information on cardiac repolarization that cannot be obtained by measuring the duration of QT intervals from different ECG leads. 7 , 8 Some of these parameters, such as the total cosine R‐to‐T (TCRT), that corresponds the cosine of the angle between the main vectors of depolarization and repolarization fronts, have also been shown to predict survival and arrhythmic events. 7 , 9 , 10 , 11 , 12
The purpose of the present study was to test whether the KCNH2 K897T polymorphism is associated with the novel measures of repolarization and T‐wave morphology derived from digitized 12‐lead surface ECG by principal component analysis (PCA).
METHODS
Population
The study population was randomly selected from the Social Insurance Register covering all the inhabitants of the city of Oulu in northern Finland, which has been used for a population‐based epidemiological study of cardiovascular risk factors, Oulu project elucidating risk of atherosclerosis (OPERA). Three hundred women and 300 men between 40 and 60 years of age were invited to take part, and 88% participated (267 women and 259 men). The details of this population have been previously described. 13 , 14 , 15 Past and current medical history, smoking habits, alcohol consumption, and physical activity were assessed by a standardized health questionnaire. A standard 12‐lead ECG and a peripheral blood sample were obtained from each subject.
Routine blood tests and DNA extraction were performed on each blood sample. M mode and two‐dimensional echocardiographic examinations were also performed. Subjects on any medication affecting cardiac repolarization were excluded from the study. The study was approved by the Ethics Committee of the University of Oulu, and the subjects gave an informed consent.
The KCNH2 K897T polymorphism was genotyped from each subject, and the 12‐lead ECG was analyzed from each measurable lead. ECGs of poor quality were excluded from the analyses, and the remaining ECGs were chosen for further digital analyses. Complete manual and digital electrocardiographic data and the KCNH2 K897T genotype were obtained from 107 men and 121 women.
Electrocardiograms
A standard 12‐lead ECG was obtained from each study subject at a paper speed of 50 mm/s. All subjects were in sinus rhythm. The methods for manual QT interval analyses were presented in detail in our previous report. 2 The ECGs were scanned and digitized by using UN‐SCAN‐IT Graph Digitizing System Version 6.0 (Silk Scientific, Orem, Utah, USA). Several descriptors of T‐wave morphology were automatically calculated from the 12‐lead ECGs, using a custom‐made software application.
T‐Wave Descriptors
The software automatically creates T wave and QRS loops in Frank's three‐dimensional space using matrix modification and the singular value decomposition technique. The software calculates a plane where the loop has the maximum first and second dimensions. The loop is rotated until its longest axis is parallel to the x‐axis. The longest axis of the loop is defined as the width of the loop (W), and the second longest axis perpendicular to the longest axis as the height of the loop (H). The ratio of H to W (H/W), which indicates the shape of the loop, is then calculated. Next, a rectangle is adjusted around the loop. The rectangle is divided into 100 (10 × 10) subrectangles. The T‐wave loop dispersion (TWLD) is defined as the number of subrectangles traversed by the borderline of the corresponding loop. The cosine of the angle between the main vectors of the T‐wave loop and the QRS loop in the three‐dimensional space (TCRT) is determined. It represents the relationship between the orientations of the repolarization and depolarization fronts. 7
Genetic Analysis
DNA was extracted from peripheral blood nucleated cells by standard methods. 16 The analysis of the KCNH2 (HERG) K897T polymorphism was performed by primer‐induced restriction analysis as previously described. 1 , 17 The site of this polymorphism is in the exon 11 of the KCNH2 gene. A nucleotide transition (2690A > C) is predicted to substitute threonine (T) for lysine (K) at codon 897.
Statistical Analysis
The statistical analyses were performed with the SPSS for Windows software package (SPSS 10.1., SPSS Inc., Chicago, IL). The standard t‐test was used to assess the statistical significances of the differences of the variables between the groups in cases of normal distribution of the data according to the Kolmogorov‐Smirnov test (z‐value < 1.0). In other cases, the non‐parametric Mann‐Whitney test was used. The Spearman correlation coefficients were determined for estimating the associations between the variables. Because only 4 women and 3 men belonged to the genotype TT, the genotypes KT and TT were combined for the statistical analyses. Multivariate analysis was performed with Kruskal–Wallis analysis. P < 0.05 was considered to be statistically significant.
RESULTS
Effects of the KCNH2 K897T Polymorphism on Cardiac Repolarization
The allele frequencies of the KCNH2 K897T polymorphism were 0.86(K) and 0.14(T). Similar allele frequencies have been reported recently for other Finnish populations. 1 , 2 , 3
The effect of the KCNH2 K897T polymorphism on the manually measured QT interval variables was described in detail in our previous article. 2 In the present study, the KCNH2 K897T polymorphism was associated with TCRT in the total study population. TCRT was significantly smaller with the genotypes KT and TT than with the genotype KK. There were no significant differences in the other T‐wave loop parameters between the different genotypes. The results are shown in Table 1. When TCRT was categorized at quartiles, the association between the KCNH2 polymorphism and TCRT remained significant (P = 0.03) (Table 2).
Table 1.
Electrocardiographic Variables in Different KCNH2 Genotypes
| KK (n = 167) | KT or TT (n = 61) | P Value | |
|---|---|---|---|
| All | |||
| T‐W | 0.404 + 0.175 | 0.403 + 0.157 | 0.70 |
| T‐H | 0.138 + 0.066 | 0.134 + 0.052 | 0.49 |
| T‐H/T‐W | 0.371 + 0.152 | 0.359 + 0.145 | 0.87 |
| TWLD | 38.50 + 4.84 | 37.66 + 4.60 | 0.23 |
| TCRT | 0.421 + 0.502 | 0.300 + 0.500 | 0.04* |
| Men | n = 77 | n = 30 | |
| T‐W | 0.499 ± 0.177 | 0.463 ± 0.155 | 0.58 |
| T‐H | 0.164 ± 0.076 | 0.157 ± 0.052 | 0.34 |
| T‐H/T‐W | 0.343 ± 0.145 | 0.366 ± 0.142 | 0.29 |
| TWLD | 38.21 ± 5.32 | 37.00 ± 3.73 | 0.27 |
| TCRT | 0.291 ± 0.556 | 0.234 ± 0.558 | 0.52 |
| Women | n = 90 | n = 31 | |
| T‐W | 0.322 ± 0.126 | 0.346 ± 0.139 | 0.56 |
| T‐H | 0.117 ± 0.046 | 0.112 ± 0.043 | 0.99 |
| T‐H/T‐W | 0.394 ± 0.154 | 0.352 ± 0.150 | 0.16 |
| TWLD | 38.76 ± 4.41 | 38.29 ± 5.29 | 0.99 |
| TCRT | 0.531 ± 0.419 | 0.364 ± 0.437 | 0.03* |
The values are means ± SD.
TCRT = total cosine R‐to‐T; T‐H = the height of the T‐wave loop; T‐H/T‐W = the ratio of H and W of the T‐wave loop; T‐W = the width of the T‐wave loop; TWLD = T‐wave loop dispersion.
*P < 0.05.
Table 2.
Association Between KCNH2 Genotypes and Total Cosine R‐to‐T
| TCRT | KK (n = 167) | KT or TT (n = 61) |
|---|---|---|
| 1st quartile (−0.988 to 0.132) | 38 (68%) | 18 (32%) |
| 2nd quartile (0.152–0.501) | 36 (62%) | 22 (38%) |
| 3rd quartile (0.505–0.767) | 45 (79%) | 12 (21%) |
| 4th quartile (0.775–0.998) | 48 (84%) | 9 (16%)* |
TCRT=total cosine R‐to‐T.
*P = 0.03 between the groups.
Gender Differences
The electrocardiographic data of the study population are presented in Table 3. TCRT was significantly lower in men than in women (0.273 ± 0.560 in men and 0.492 ± 0.428 in women, P = 0.001). When the repolarization parameters were compared in men and women, the values of TCRT were lower in the less common genotypes KT and TT in both males and females. However, the difference in TCRT between the genotypes reached statistical significance only in women (P = 0.03) but not in men (P = 0.52). TCRT was higher in females with the less common genotype, KT or TT (0.364 ± 0.437), than in males even with the more common genotype, KK (0.291 ± 0.556) (Table 1).
Table 3.
Electrocardiographic Data of the Study Population
| Men (n = 107) | Women (n = 121) | P Value | |
|---|---|---|---|
| QTcmax (ms) | 462 ± 93 | 453 ± 79 | NS |
| Tpeak‐Tend (ms) | 146 ± 94 | 126 ± 74 | NS |
| Heart rate (beats/min) | 64 ± 11 | 65 ± 9 | NS |
| T‐W | 0.487 ± 0.178 | 0.330 ± 0.129 | <0.001 |
| T‐H | 0.161 ± 0.071 | 0.116 ± 0.045 | <0.001 |
| T‐H/T‐W | 0.351 ± 0.150 | 0.382 ± 0.154 | NS |
| TWLD | 38.09 ± 5.42 | 38.63 ± 4.61 | NS |
| TCRT | 0.273 ± 0.560 | 0.492 ± 0.428 | 0.001 |
The values are means±SD.
TCRT = total cosine R‐to‐T; T‐H = the height of the T‐wave loop; T‐H/T‐W = the ratio of H and W of the T‐wave loop; T‐W = the width of the T‐wave loop; TWLD = T‐wave loop dispersion.
Relationship Between the Repolarization Variables and Other Baseline Parameters
TCRT was not associated with any of the conventional QT interval variables in the total study population. In the multivariate analysis adjusting with age, body mass index, and cholesterol, TCRT remained significantly different between the HERG genotypes in the total population (P = 0.04) and in women (P = 0.03) but not in men (P = 0.6).
DISCUSSION
The present study confirms the assumption that the K897T polymorphism of the KCNH2 gene, coding for a major subunit of the cardiac potassium channel Ikr, has an influence on cardiac electrical properties in humans. The novel repolarization variable TCRT, reflecting the heterogeneity of global repolarization measured from digitized 12‐lead surface ECG, was lower in subjects carrying the less common polymorphic allele T (Fig. 1).
Figure 1.
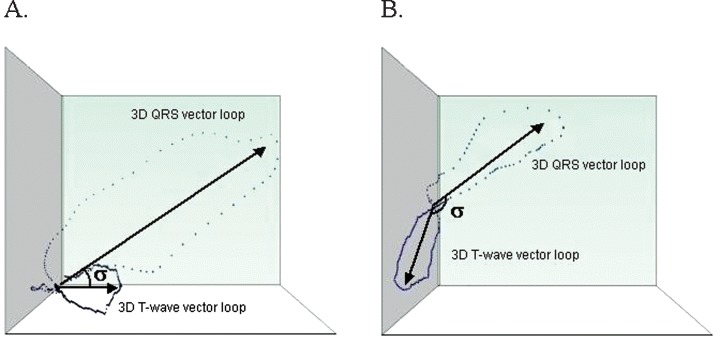
Examples of TCRT in subjects with (A) high TCRT (small θ) and (B) low TCRT (large θ).
Effects of the KCNH2 Gene on Repolarization
Mutations in the KCNH2 (HERG) gene cause the long QT syndrome type 2 (LQT2). 18 Due to mutations causing LQT2, the Ikr current is decreased, delaying the repolarization of the myocardium and resulting in a prolonged QT interval. Because of the delayed repolarization, LQT2 patients are more vulnerable to Torsade de Pointes type of ventricular arrhythmia, which may cause fainting or even sudden cardiac death. Several research groups have attempted to clarify whether the common K897T polymorphism of the KCNH2 gene has an influence on the function of the channel. However, the results have been ambiguous. Scherer et al. 19 found no differences between the polymorphic and wild‐type (WT) current or other properties of the channel, including the sensitivity to drug block. Bezzina et al. 4 found no differences in current amplitude or channel inactivation, but reported that polymorphic channels express quickened activation and deactivation kinetics at specific voltages and have a hyperpolarized shift in channel activation. Paavonen et al. 3 found no differences in channel activation, but reported decreased 897T protein levels, slower channel deactivation and inactivation kinetics, and a hyperpolarized shift in steady‐state inactivation. The most recent study by Anson et al. 5 described lower current density, activation at more negative potentials, faster inactivation and recovery, and subtle differences in the polymorphic K897T channel compared with other polymorphic channels and the WT channel.
The association between the K897T polymorphism and the QT interval has also been studied in several different populations. We recently reported the association between the polymorphism and the QT and Tpeak‐Tend intervals, which were longer in middle‐aged Finnish females with the genotypes KT or TT. 2 Similar results were obtained in Finnish LQT1 patients by Paavonen et al., who found that the QT intervals of LQT1 patients were prolonged during an exercise test in the genotypes KT and TT, whereas exercise had no effect on the QT intervals of the patients with the genotype KK. 3
Novel ECG Variables
The duration of the QT interval, measured from standard 12‐lead ECG, is only a gross estimate of repolarization, and it is biased by many methodological problems, such as inaccuracy of the determination of the onset of the QRS complex and offset of the T wave, poor reproducibility, and the laborious procedure of manual measurement. Responding to the need for more accurate measures, Acar et al. 6 introduced novel ECG variables indicative of the T‐wave morphology, which are derived from a single beat of the digitized 12‐lead surface ECG with high reproducibility and are easily calculated by a computer program. These values require no time domain measurements and hence avoid the inaccuracies associated with the conventional QT interval–related parameters. Several studies have recently evaluated these novel ECG variables. 7 , 9 , 10 , 11 , 12 . Of these, the TCRT, reflecting the wave front direction between depolarization and repolarization, has been shown to serve as a strong and independent predictor of all‐cause mortality, 7 cardiovascular risk and sudden death, 10 cardiac and arrhythmic death, 11 and cardiovascular mortality 12 in different clinical settings.
In the present study, the KCNH2 K897T polymorphism was associated with TCRT in healthy subjects, particularly in women. The subjects with the less common genotypes, KT and TT had smaller values of TCRT. Reduced TCRT is due to a larger difference in the wave front direction between depolarization and repolarization, and it thereby reflects dyssynchrony between the global depolarizing and repolarizing currents. Concurrently with a previous study, men had smaller TCRT than women. 8 In fact, women with the genotypes KT or TT had larger TCRT than men with any genotype.TCRT had no correlation with the standard QT interval measurements, confirming that this index reflects different aspects of repolarization than those obtained by measurement of QT intervals from the standard ECG.
CONCLUSION
In the present study, reduced TCRT was associated with the less common genotypes of the KCNH2 K897T polymorphism. It has been shown earlier that TCRT serves as an independent predictor of survival and arrhythmias. 7 , 10 , 11 , 12 Our preliminary data from an ongoing study suggest that women with the genotype KT or TT are at an increased risk of sudden death during an ischemic event, 20 and another study group found a similar association in patients with ischemic cardiomyopathy. 21 These observations support the view that the KCNH2 K897T polymorphism may also have some clinical importance, which may be explained by its effects on cardiac repolarization. Subjects with the less common genotype KT or TT seem to have reduced synchrony between depolarization and repolarization, reflected as reduced TCRT, and that may increase their vulnerability to arrhythmic events. Further studies are needed to confirm this finding.
This study was supported by the Medical Council of the Finnish Academy of Science, Helsinki, Finland; and Sigrid Juselius Foundation, Helsinki, Finland.
REFERENCES
- 1. Laitinen P, Fodstad H, Pippo K, et al Survey of the coding region of the HERG gene in long QT syndrome reveals six novel mutations and an amino acid polymorphism with possible phenotypic effects. Hum Mutat 2000;15: 580–581. [DOI] [PubMed] [Google Scholar]
- 2. Pietilä E, Fodstad H, Niskasaari E, et al Association between HERG K897T polymorphism and QT interval in middle‐aged Finnish women. J Am Coll Cardiol 2002;40: 511–514. [DOI] [PubMed] [Google Scholar]
- 3. Paavonen KJ, Chapman H, Laitinen PJ, et al Functional characterization of the common amino acid 897 polymorphism of the cardiac potassium channel KCNH2 (HERG). Cardiovasc Res 2003;59: 603–611. [DOI] [PubMed] [Google Scholar]
- 4. Bezzina CR, Verkerk AO, Busjahn A, et al A common polymorphism in KCNH2 (HERG) hastens cardiac repolarization. Cardiovasc Res 2003;59: 27–36. [DOI] [PubMed] [Google Scholar]
- 5. Anson BD, Ackerman MJ, Tester DJ, et al Molecular and functional characterization of common polymorphisms in HERG (KCNH2) potassium channels. Am J Physiol Heart Circ Physiol 2004;286: H2434–H2441. [DOI] [PubMed] [Google Scholar]
- 6. Acar B, Yi G, Hnatkova K, et al Spatial, temporal and wavefront direction characteristics of 12‐lead T‐wave morphology. Med Biol Eng Comput 1999;37: 574–584. [DOI] [PubMed] [Google Scholar]
- 7. Zabel M, Acar B, Klingenheben T, et al Analysis of 12‐lead T‐wave morphology for risk stratification after myocardial infarction. Circulation 2000;102: 1252–1257. [DOI] [PubMed] [Google Scholar]
- 8. Smetana P, Batchvarov VN, Hnatkova K, et al Sex differences in repolarization homogeneity and its circadian pattern. Am J Physiol Heart Circ Physiol 2002;282: H1889—H1897. [DOI] [PubMed] [Google Scholar]
- 9. Zabel M, Malik M, Hnatkova K, et al Analysis of T‐wave morphology from the 12‐lead electrocardiogram for prediction of long‐term prognosis in male US veterans. Circulation 2002;105: 1066–1070. [DOI] [PubMed] [Google Scholar]
- 10. Kardys I, Kors JA, Van Der Meer IM, et al Spatial QRS‐T angle predicts cardiac death in a general population. Eur Heart J 2003;24: 1357–1364. [DOI] [PubMed] [Google Scholar]
- 11. Batcvarov VN, Hnatkova K, Poloniecki J, et al Prognostic value of heterogeneity of ventricular repolarization in survivors of acute myocardial infarction. Clin Cardiol 2004;27: 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamazaki T, Froelicher VF, Myers J, et al Spatial QRS‐T angle predicts cardiac death in a clinical population. Heart Rhythm 2005;2: 73–78. [DOI] [PubMed] [Google Scholar]
- 13. Huikuri HV, Pikkujämsä SV, Airaksinen KE, et al Sex‐related differences in autonomic modulation of heart rate in middle‐aged subjects. Circulation 1996;94: 122–125. [DOI] [PubMed] [Google Scholar]
- 14. Rantala AO, Kauma H, Lilja M, et al Prevalence of the metabolic syndrome in drug‐treated hypertensive patients and control subjects. J Intern Med 1999;245: 163–174. [DOI] [PubMed] [Google Scholar]
- 15. Ylitalo A, Airaksinen KE, Hautanen A, et al Baroreflex sensitivity and variants of the renin angiotensin system genes. J Am Coll Cardiol 2000;35: 194–200. [DOI] [PubMed] [Google Scholar]
- 16. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 1988;16: 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saarinen K, Swan H, Kainulainen K, et al Molecular genetics of the long QT syndrome: Two novel mutations of the KVLQT1 gene and phenotypic expression of the mutant gene in a large kindred. Hum Mutat 1998;11: 158–165. [DOI] [PubMed] [Google Scholar]
- 18. Curran ME, Splawski I, Timothy KW, et al A molecular basis for cardiac arrhythmia. HERG mutations cause long QT syndrome. Cell 1995;80: 795–803. [DOI] [PubMed] [Google Scholar]
- 19. Scherer CR, Lerche C, Decher N, et al The antihistamine fexofenadine does not affect I(Kr) currents in a case report of drug‐induced cardiac arrhythmia. Br J Pharmacol 2002;137: 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pietilä EH, Kortelainen ML, Fodstad H, et al HERG K897T polymorphism and sudden arrhythmic death due to acute coronary event. Circulation 2001;104(17):II–463(Abstract). [Google Scholar]
- 21. Bedi M, Aleong R, Kinyanjui M, et al The HERG K897T polymorphism affects survival and sudden death in ischemic cardiomyopathy patients. Circulation 2003;108(17):IV–473 (Abstract). [Google Scholar]


