Abstract
Background
Accurate markers of atrial fibrillation (AF) recurrences after electrical cardioversion (ECV) are lacking. This study was conducted to assess the value of P‐wave signal averaging (SAPW) for predicting AF recurrences in a nonselected patients population submitted to ECV.
Methods
A total of 133 patients (107 males, 26 females, mean age 66 ± 9 years) were included after successful ECV for persistent AF (mean duration of AF 3.6 ± 2.2 months). The mean ejection fraction (EF) was 60 ± 9%, and left atrial (LA) diameter was 44 ± 6 mm. SAPW ECG was obtained immediately after ECV and patients were prospectively followed.
Results
During a mean follow‐up of 8.9 ± 5.2 months, AF recurrences occurred in 40.6% (54/133). No SAPW parameters was statistically different between the group of patients with and the group without recurrences. Recurrences were less often observed in patients with a total P‐wave duration <150 ms (16/52 or 31% vs 38/81 or 47% in patients with total P‐wave duration ≥150 ms) but the difference was not statistically different (P = 0.07). P‐wave duration was correlated with age (r = 0.32; P < 0.001) and left atrial diameter (r = 0.19; P = 0.02). Age, sex, structural heart disease, amiodarone therapy, or hypertension were not associated with AF recurrences but patients without recurrences had a shorter AF duration (P = 0.001) and more often had a history of previous ablation (P = 0.027).
Conclusion
In this unselected “real‐life” group of patients submitted to ECV for persistent AF, none of the SAPW parameters, including total filtered P‐wave duration, was able to predict AF recurrences.
Keywords: atrial fibrillation, signal‐averaging ECG, recurrences
INTRODUCTION
Atrial fibrillation (AF) is the most frequently encountered sustained arrhythmia in clinical practice, occurring in 1–2% of the general population and this prevalence will increase in the next decades because of the ageing of the population. The natural course of AF is progression from a paroxysmal form to a persistent or permanent form, and at the beginning of the disease, attempts are usually made to keep the patient into sinus rhythm with antiarrhythmic drugs and electrical therapy. Electrical cardioversion (ECV) is the most effective method for converting AF to sinus rhythm. However, recurrences are frequently observed and until now only few markers of recurrences have been identified, such as AF duration, size of left atrium, age and left ventricular hypertrophy.1, 2, 3, 4 The signal‐averaged electrocardiogram (SAECG) is a simple noninvasive tool, which has been used for years, initially to evaluate the presence of ventricular late potentials, and the technology has been extended to analyse the P wave in order to provide a more accurate evaluation of atrial conduction. Several studies have used this method and the related P‐wave parameters to predict AF recurrences after electrical cardioversion but results have been controversial essentially because the design of the published trials and the SAECG systems used have been different.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 In this study, we hypothesized that the signal‐averaged P‐wave (SAPW) ECG could be a useful, inexpensive and simple predictive marker of AF recurrences in an unselected “real‐world” patients population submitted to direct current electrical cardioversion.
METHODS
Patients
After informed consent, 133 consecutive patients (107 male, 26 female, mean age 66 ± 9 years) with persistent AF and referred for direct current electrical cardioversion were prospectively enrolled. Indication for electrical cardioversion was persistent symptomatic AF despite rate control (class IIa, level B indication).19 Patients were treated with the anticoagulant acenocoumarol for at least 4 weeks before the procedure with a targeted INR of 2.0–3.0. On the day of the procedure, all patients had an INR control and a transoesophageal echocardiography was performed before ECV in case of inadequate anticoagulation. Patients with permanent pacemakers or defibrillators were excluded from the study because of the possibility of atrial pacing during the SAPW recording. Direct current electrical cardioversion was performed under general anaesthesia with 150–200 J (biphasic waveform), with conversion to sinus rhythm at the first (n = 106), second (n = 24) or third attempt (n = 3). The P‐wave signal‐averaged ECG was obtained in the first 2 hours following ECV. Patients were followed with continuous ECG monitoring in the ICU during at least 3 hours and all patients were discharged from the hospital on the day of the procedure. No complication occurred in relation with ECV. Patients were prospectively followed for 10 months or until recurrences occurred. Recurrences were defined as documented AF (duration > 1 min) occurring after the ECV. To detect AF recurrences, serial clinical evaluations and 12‐lead ECG recordings were obtained at 3 months interval as well as at least one 24‐hour Holter ECG at 1 month after the procedure or in case of symptoms. Antiarrhythmic drug treatment was led to the physician's discretion in charge of the patient and anticoagulation was maintained for at least 1 month in the absence of recurrence and longer in the presence of recurrences or in patients with a CHA2DS2VASc score >1.
P‐wave Signal Averaging
A P‐wave signal averaged recording was obtained in the first 2 hours after ECV in all patients using the Phi‐Res analysis module from Marquette Medical system (GE Healthcare, Waukesha, WI, USA). The software uses a P‐wave trigger and provides filtering and automatic delineation of the averaged P wave. Automatic measurements include total filtered and unfiltered P‐wave duration (FPD), P‐wave integral, and root mean squared (RMS) voltages of the terminal 20, 30, and 40 ms. Electrocardiographic data are obtained in the X, Y, Z planes and the three leads are combined into a vector magnitude VM (VM = √ (X2 + Y2 + Z2). Qualified P waves are correlated with a P‐wave template and only P waves with a correlation coefficient >0.95 are taken into account for the averaging process for filtering (bidirectional Butterworth high‐pass filter of 40 Hz) and for analysis. The endpoint for the averaging process is predetermined (250 beats) and only recordings with a residual noise level <0.6 microvolt are used. Two independent observers verified all tracings and automatic measurements were corrected according to visual delineation of the beginning and of the end of the P wave (Fig. 1). Normal values for the P‐wave signal‐averaged ECG were determined in 34 normal subjects (22 males; 12 females; mean age 44 ± 17 years) without any history of AF, structural heart disease or hypertension. In this group, the mean FPD was 122 ± 28 ms, the mean RMS‐20 3.8 ± 1.9 μV, the mean RMS‐30 4.5 ± 2.0 μV, the mean RMS‐40 4.9 ± 2.1 μV, and the mean P‐wave integral 507 ± 158 μVs, with a residual noise of 0.4 ± 0.2 μV. Investigators and patients as well as attending physicians were blinded as to the results of SAPW ECG.
Figure 1.
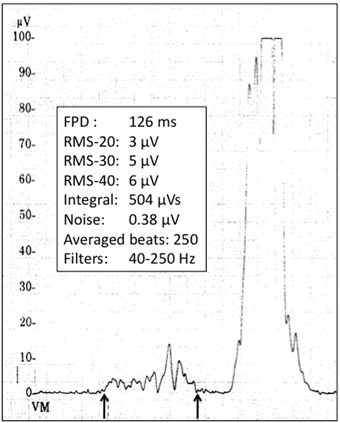
Example of P‐wave signal averaged recording with measurement of total filtered P‐wave duration and other SAECG parameters.
Statistical Analysis
Data are expressed as mean ± standard deviation. Differences in continuous variables between patients with recurrences and patients without recurrences were performed using Student's unpaired t‐test. Differences in categorical data were performed using chi‐square analysis or Fisher's exact test. A P value of less than 0.05 was considered significant. The correlation between P‐wave duration and other variables was assessed using Pearson's correlation coefficient.
RESULTS
Among the 133 patients with successful ECV, 107 were males and 26 females, with a mean age of 66 ± 9 years. Structural heart disease was identified in 52/133 (39%; coronary artery disease in 20; valvulopathy in 16; dilated cardiomyopathy in 5; others—atrial septal defect, hypertrophic cardiomyopathy, hypertensive heart disease—in 11) and hypertension was present in 62/133 (47%). Mean duration of the current AF episode was 3.6 ± 2.2 months. Beta‐blockers were used at the time of cardioversion in 65/133 patients (49%), alone in 32/65 and in combination with antiarrhythmics in 33/65. Antiarrhythmic drug therapy was used at the time of ECV in 79/133 patients (59%) (amiodarone in 61; flecainide in 18) (see Table 1).
Table 1.
Patient Characteristics
| No Recurrence | Recurrence | P Value | |
|---|---|---|---|
| (n = 79) | (n = 54) | ||
| Age, years | 65.4 ± 9.3 | 66.7 ± 8.3 | 0.39 |
| Male sex | 64/79 (81%) | 43/54 (80%) | 0.84 |
| Structural heart disease | 27/79 (34%) | 25/54 (41%) | 0.16 |
| Drug therapy | |||
| Beta‐blockers alone | 19/79 (24%) | 14/54 (26%) | 0.83 |
| Beta‐blockers + amiodarone | 16/79 (20%) | 7/54 (13%) | 0.27 |
| Amiodarone alone | 22/79 (28%) | 16/54 (30%) | 0.82 |
| Flecainide + beta‐blockers | 6/79 (8%) | 3/54 (6%) | 0.73 |
| Flecainide alone | 6/79 (8%) | 3/54 (6%) | 0.73 |
| Hypertension | 24/79 (51%) | 22/54 (41%) | 0.26 |
| Atrial fibrillation duration (months) | 2.6 ± 2.7 | 6.9 ± 11.1 | 0.001* |
| Left atrial diameter (mm) | 43.8 ± 5.5 | 45.0 ± 7.2 | 0.27 |
| Left ventricular ejection fraction (%) | 60.6 ± 10.0 | 61.4 ± 8.7 | 0.61 |
| Previous atrial fibrillation ablation‐pulmonary vein isolation (%) | 32/79 (41%) | 12/54 (22%) | 0.027* |
Compared to controls, patients with persistent AF had a prolonged FPD after electrical cardioversion (161 ± 25 ms vs 122 ± 28 ms, P < 0.0001) but all other SA‐ECG parameters were not statistically different from controls. FPD was not statistically different in patients with compared to patients without antiarrhythmics at the time of cardioversion (167 ± 29 ms vs 158 ± 21 ms, P = 0.13).
During a mean follow‐up of 8.9 ± 5.2 months, recurrences occurred in 40.6% of the patients (54/133). Clinical characteristics of patients with and without AF recurrences during follow‐up are summarized in Table 1 and the various P‐wave signal‐averaged ECG parameters in patients with or without AF recurrences are shown in Table 2. Although a trend toward a longer P‐wave duration was observed in patients with AF recurrences, none of the SAPW parameters was statistically different between patients with and patients without recurrences, including total FPD (164.8 ± 24.9 ms vs 157.9 ± 24.1 ms, P = 0.11) and RMS‐20 (4.5 ± 8.0 μV vs 4.0 ± 2.7 μV, P = 0.65), two parameters found to be powerful predictors of AF recurrences in some other studies. In a univariate analysis, only AF duration (P = 0.001) and a history of previous radiofrequency catheter ablation (P = 0.27) were found to be significantly different between patients with and patients without AF recurrences. None of the clinical variables such as age, sex, presence of structural heart disease, amiodarone therapy, hypertension or left atrial diameter was significantly associated with AF recurrences (Table 1). Patients with a FPD of <150 ms were found to have less recurrences (16/52 or 31%) than patients with a PFD ≥150 ms (38/81 or 47%) but the difference was not statistically significant (P = 0.07) and sensitivity, specificity, and predictive values of this parameter were low (sensitivity 47%, specificity 69%, positive predictive value 70%, negative predictive value 46%). A weak but significant correlation was observed between FPD and age (r = 0.32; P < 0.0001) (Fig. 2A) and between FPD and left atrial diameter (r = 0.19, P = 0.02) (Fig. 2B), but no significant correlation was observed between age and left atrial diameter (r = 0.15; P = 0.08).
Table 2.
SAPW Parameters in Patients with and without Recurrences
| No Recurrence | Recurrence | P Value | |
|---|---|---|---|
| (n = 79) | (n = 54) | ||
| FPD (ms) | 157.9 ± 24.1 | 164.8 ± 24.9 | 0.11 |
| RMS20 (μV) | 4.5 ± 8.1 | 4.0 ± 2.7 | 0.65 |
| RMS30 (μV) | 4.9 ± 7.5 | 4.3 ± 2.5 | 0.60 |
| RMS40 (μV) | 5.4 ± 6.5 | 4.4 ± 2.4 | 0.31 |
| P‐wave integral (mV) | 746 ± 361 | 691 ± 254 | 0.39 |
FPD = total filtered P‐wave duration; RMS = root mean square voltage.
Figure 2.
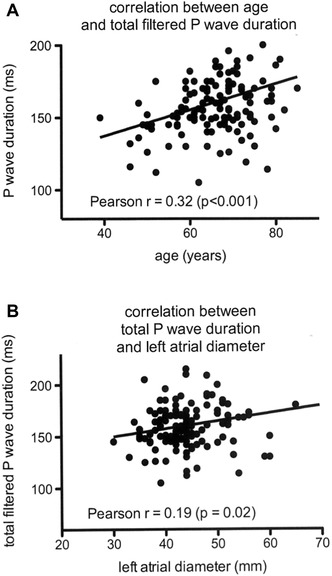
(A) Correlation between total filtered P‐wave duration during signal averaging and age. (B) Correlation between total filtered P‐wave duration during signal‐averaging and left atrial diameter measured during transthoracic echocardiography.
DISCUSSION
Several independent predictors of AF recurrences after ECV have been identified, such as age, AF duration, number of episodes, hypertension, LA size, coronary heart disease, mitral valve disease, and C‐reactive protein levels1, 2, 3, 4 but quantitative noninvasive markers are still lacking. In this study, we evaluated the value of SAPW using a well‐established and commercially available SAECG methodology but we failed to demonstrate that the SAPW parameters could be useful predictors of AF recurrences after ECV in an unselected group of patients.
In the literature, several studies have been published on that subject5, 6, 7, 8, 9, 10, 11, 14, 15, 16, 17, 18. Results have been conflicting because these studies had different designs, different methodologies, different patient populations, and different endpoints. Some studies have concluded that FPD was able to identify patients prone to AF recurrences after electrical cardioversion7, 8, 11, 12, 14, 15, 16, 17 whereas others showed that FPD was not useful to assess the risk of AF recurrence.5, 9, 10 Chalfoun et al.5 studied the reverse electrical remodeling after ECV using the SAPW. In agreement with our data, they found that the baseline FPD was not different between patients with or without recurrences but they observed that the FPD in patients remaining in sinus rhythm decreased significantly over time compared to patients with recurrences. This observation was confirmed by Elesber et al.6 in the first hours after successful ECV, but these authors did not study the relationship to AF recurrences. Guo et al.7 followed a group of 60 patients for 6 months after successful ECV for AF: they observed a high rate of AF recurrences (67%) and found that, at the beginning of the follow‐up, all the SAPW parameters were statistically different in patients with compared to patients without AF recurrences. However, at 1 week, only RMS and integral of the entire P wave were statistically different between the two groups of patients suggesting early electrical remodeling. A prolonged FPD (>160 ms) was found by Dixen et al.18 to be a risk factor for recurrences with an OR of 2.22 (95% CI 1.07–4.60) but the statistical significance was borderline (P = 0.03), sensitivity (48%) and specificity (71%) scores were low (comparable to what was observed in the present study), and the follow‐up was very short (1 month). Interestingly, the study of Raitt et al.17 showed that FPD was useful only in patients not taking antiarrhythmic medication whereas FPD had a poor predictive value in patients taking antiarrhythmic drugs. It should be remembered, however, that most patients submitted to electrical cardioversion in daily practice are treated with antiarrhythmics in order to enhance maintenance of sinus rhythm and therefore, such information is of limited clinical value. In the present report, FPD was not statistically different in patients with or without antiarrhythmic drug therapy.
It is fundamental to remind that SAPW methodology can greatly influence the results, and could explained in part the discrepancies observed between prior studies20: Holmqvist and al.21 showed that some parameters of the methodology, such as the noise level, could deeply influence the duration of the P wave and falsify the interpretation of the results. In practice, the most widely available systems are the Predictor system (Corazonix Corporation Oklahoma City, OK, USA) and the Phi‐Res analysis Marquette system that have been validated in several trials.22, 23 In the largest one, Dhala et al.24 established standard SAPW parameters in healthy subjects according to age and sex using the Phi‐Res Marquette system and found that FPD was sex‐dependent and longer in men, but not related to age. In this study, as in others,11 a weak but significant correlation with age was observed. In Dhala's study,24 FPD was shorter in healthy patients compared to patients with a history of paroxysmal AF and these observations have been confirmed by others.11, 25, 26
The changes of PFD observed after ECV or recurrences are not completely understood: P‐wave duration reflects inter‐ and intraatrial conduction time but is often difficult to diagnose accurately on a standard electrocardiogram.27 AF first induces this electrical abnormality, which is followed by structural alterations such as fibrosis and left atrial enlargement.28 This electroanatomic remodeling is involved in the initiation and maintenance of AF, resulting in electrical dissociation and local conduction heterogeneities.29, 30 After cardioversion, a reverse process may occur with recovery of the rate‐adaptation of atrial refractoriness30, 31, 32 and a reduction of FPD.12, 13 In fact, persistent AF may induce prolonged and perhaps irreversible electroanatomic changes that cannot be completely restored to normality and this phenomenon may be reflected by a longer FPD which could represent an “electrico‐anatomical” marker of recurrences.
CLINICAL IMPLICATIONS
Our findings indicate that SAECG parameters are not useful in the real world to predict AF recurrences after ECV in an unselected group of patients with persistent AF. More studies are needed to define subgroups of patients who may benefit the most from such a technology,33 to establish a correlation between electrical markers and echocardiographic parameters and to assess potential reversibility of electroanatomical modifications during the weeks after ECV.
STUDY LIMITATIONS
Preprocedure antiarrhythmic drug therapy was not discontinued before ECV. Drugs like amiodarone and flecainide may have influenced the total P‐wave duration. However, in this study, FPD was not statistically different in patients with or without antiarrhythmic drug therapy, and there were no significant differences in the use of antiarrhythmic agents between patients with and patients without recurrent AF. Moreover, antiarrhythmic drugs are frequently used in the real world to enhance the success rate of electrical cardioversion (class IIa, level B)19 and most previous studies have also included patients with antiarrhythmic drug therapy.15, 17, 18 The present results may not be applicable to other methods of P‐wave signal averaging. The SAPW ECG recording can only be performed during sinus rhythm: therefore, patients with early AF recurrences after ECV are not suitable for SAPW ECG recording. Asymptomatic recurrences of nonpersistent AF may have occurred during the follow‐up period and the rate of recurrence may therefore be underestimated. However, all patients had persistent symptomatic AF before ECV and according to the follow‐up protocol recurrences of persistent AF would have been detected. The sample size is relatively small. In this study, no attempt was made to obtain serial SAPW ECGs and serial echocardiographic examinations at 1, 3, and 6 months. Therefore, reverse electroanatomical remodeling cannot be assessed from the present data. Left atrial size was evaluated only by left atrial diameter at echocardiography and a more precise evaluation of left atrial dimension or function could be helpful.
CONCLUSION
In unselected “real world” patients submitted to ECV for persistent AF, SAECG parameters including FPD and RMS‐20 are not predictive of AF recurrences. Further research is required to find accurate risk factors for AF recurrences in patients with persistent AF in order to improve treatment strategy after ECV.
ACKNOWLEDGMENT
Warmest thanks to the nurses of the intensive care unit of the Hôpital de La Tour who performed the P‐wave signal averaged recordings.
Funding: Dr. Blanche was supported by a grant from the “Fondation de La Tour pour la Recherche Cardiovasculaire,” Meyrin, Geneva, Switzerland.
Disclosure: none.
REFERENCES
- 1. Van Gelder IC, Crijns HJ, Tieleman RG, et al. Chronic atrial fibrillation. Success of serial cardioversion therapy and safety of oral anticoagulation. Arch Intern Med 1996;156:2585–2592. [DOI] [PubMed] [Google Scholar]
- 2. Kerr CR, Humphries KH, Talajic M, et al. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: Results from the Canadian Registry of Atrial Fibrillation. Am Heart J 2005;149(3):489–496. [DOI] [PubMed] [Google Scholar]
- 3. Suttorp MJ, Kingma JH, Koomen EM, et al. Recurrence of paroxysmal atrial fibrillation or flutter after successful cardioversion in patients with normal left ventricular function. Am J Cardiol 1993;71:710–713. [DOI] [PubMed] [Google Scholar]
- 4. Liu T, Li L, Korantzopoulos P, et al. Meta‐analysis of association between C‐reactive protein and immediate success of electrical cardioversion in persistent atrial fibrillation. Am J Cardiol 2008;101:1749–1752. [DOI] [PubMed] [Google Scholar]
- 5. Chalfoun N, Harnick D, Pe E, et al. Reverse electrical remodeling of the atria post cardioversion in patients who remain in sinus rhythm assessed by signal averaging of the P‐wave. PACE 2007;30:502–509. [DOI] [PubMed] [Google Scholar]
- 6. Elesber AA, Rosales AG, Shen WK, et al. Noninvasive assessment of acute changes in atrial electrophysiology after cardioversion by signal‐averaged P‐wave electrocardiography. PACE 2005;28:135–1359. [DOI] [PubMed] [Google Scholar]
- 7. Guo XH, Gahhagher MM, Poloniecki J, et al. prognostic significance of serial P wave signal‐averaged electrocardiograms following external electrical cardioversion for persistent atrial fibrillation: A prospective study. PACE 2003;26(Pt. IIJ:299–304. [DOI] [PubMed] [Google Scholar]
- 8. Budeus M, Hennersdorf M, Perings C, et al. Prediction of the recurrences of atrial fibrillation after successful cardioversion with P‐wave signal‐averaged ECG. A.N.E 2005;10(4):414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turitto G, Bandarizadeh B, Salciccioli L, et al. Risk stratification for recurrent tachyarrhythmias in patients with paroxysmal atrial fibrillation and flutter: Role of signal averaged electrocardiogram and echocardiography. PACE 1998;21:197–201. [DOI] [PubMed] [Google Scholar]
- 10. Stafford PJ, Kamalvand K, Tan K, et al. Prediction of maintenance of sinus rhythm after cardioversion of atrial fibrillation by analysis of serial signal‐averaged P waves. PACE 1998;21:1387–1395. [DOI] [PubMed] [Google Scholar]
- 11. Militaru C, Donoiu I, Ionescu DD. P wave signal‐averaged ECG in normal population and in patients with converted atrial fibrillation. Ann Noninvasive Electrocardiol 2011;16:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hegbom F, Tveit A, Grundvold I, et al. Effects of angiotensin receptor blockade on serial P‐wave signal‐averaged electrocardiograms after electrical cardioversion of persistent atrial fibrillation. Europace 2009;11:1301–1307. [DOI] [PubMed] [Google Scholar]
- 13. Correa Barbosa E, Benchimol‐Barbosa PR, de Souza Bonfim A, et al. Reversal atrial remodeling following cardioversion of long‐standing lone atrial fibrillation. Arq Bras Cardiol 2009;93:199–205. [DOI] [PubMed] [Google Scholar]
- 14. Aytemir K, Aksoyek S, Yildirir A, et al. Prediction of atrial fibrillation recurrence after cardioversion by P‐wave signal‐averaged electrocardiography. Int J Cardiol 1999;70:15–21. [DOI] [PubMed] [Google Scholar]
- 15. Opolski G, Scislo P, Stanislawska J, Gorecki A, et al. Detection of patients art risk for recurrence of atrial fibrillation after successful electrical cardioversion by signal‐averaged P‐wave ECG. Int J Cardiol 1997;60:181–185. [DOI] [PubMed] [Google Scholar]
- 16. Censi F, Cacagnini G, Triventi M, et al. P‐wave characteristics after electrical external cardioversion: Predictive indexes of relapse. Conf Proc IEEE Eng Med Biol Soc 2010:3442–3445. [DOI] [PubMed] [Google Scholar]
- 17. Raitt MH, Ingram K, Thurman SM. Signal‐averaged P wave duration predicts early recurrences of atrial fibrillation after cardioversion. PACE 2000;23:259–265. [DOI] [PubMed] [Google Scholar]
- 18. Dixen U, Joens C, Parner J, et al. Prolonged signal‐averaged P wave duration after elective cardioversion increases the risk of recurrent atrial fibrillation. Scand Cardiovasc J 2004;38:147–151. [DOI] [PubMed] [Google Scholar]
- 19. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360–1420. [DOI] [PubMed] [Google Scholar]
- 20. Darbar D, Jahangir A, Hamill SC, et al. P wave signal‐averaged electrocardiography to identify risk for atrial fibrillation. PACE 2002;25:1447–1453. [DOI] [PubMed] [Google Scholar]
- 21. Holmqvist F, Platonov P, Havmöller R, et al. Signal‐averaged P wave analysis for delineation of interatrial conduction‐further validation method. BMC Cardiovascular Disorders 2007;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hofmann M, Goedel‐Meinen L, Beckhoff A, et al. Analysis of the P wave in the signal‐averaged electrocardiogram: Normal values and reproducibility. PACE 1996;19:1928–1932. [DOI] [PubMed] [Google Scholar]
- 23. Ehlert FA, Zaman N, Steinberg JS. Immediate and short‐term reproducibility of the P wave signal‐averaged electrocardiogram. PACE 1997;20:1636–1644. [DOI] [PubMed] [Google Scholar]
- 24. Dhala A, Underwood D, Leman R, et al. Signal‐averaged P wave analysis of normal controls and patients with paroxysmal atrial fibrillation: A study in gender differences, age, dependence, and reproducibility. Clin Cardiol 2002;25:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fukunami M, Yamada T, Ohmori M, et al. Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave‐triggered signal averaged electrocardiogram. Circulation 1991:83:162–169. [DOI] [PubMed] [Google Scholar]
- 26. Stafford PJ, Turner I, Vincent R. Quantitative analysis of signal‐averaged P waves in idiopathic paroxysmal atrial fibrillation. Am J Cardiol 1991;68:751–755. [DOI] [PubMed] [Google Scholar]
- 27. Spodick DH, Ariyarajah V. Interatrial block: The pandemic remains poorly perceived. PACE 2009;32:667–672. [DOI] [PubMed] [Google Scholar]
- 28. Ariyarajah V, Mercado K, Apiyasawat S, et al. Correlation of left atrial size with p‐wave duration in interatrial block. Chest 2005;128:2615–2618. [DOI] [PubMed] [Google Scholar]
- 29. Daoud EG, Bogun F, Goyal R, et al. Effect of atrial fibrillation on atrial refractoriness in humans. Circulation 1996;94:1600–1606. [DOI] [PubMed] [Google Scholar]
- 30. Kumagai K, Akimitsu S, Kawahira K, et al. Electrophysiological properties in chronic lone atrial fibrillation. Circulation 1991;84:1662–1668. [DOI] [PubMed] [Google Scholar]
- 31. Pandozi C, Bianconi L, Villani M, et al. Electrophysiological characteristics of the human atria after cardioversion of persistent atrial fibrillation. Circulation 1998;98(25):2860–2865. [DOI] [PubMed] [Google Scholar]
- 32. Raitt MH, Kusumoto W, Giraud G, et al. Reversal of electrical remodeling after cardioversion of persistent atrial fibrillation. J Cardiovasc Electrophysiol 2004;15(5):507–512. [DOI] [PubMed] [Google Scholar]
- 33. Blanche C, Tran N, Rigamonti, et al. Value of P‐wave signal averaging to predict atrial fibrillation recurrences after pulmonary vein isolation. Europace 2013;15(2):198–204. [DOI] [PubMed] [Google Scholar]


