Abstract
Purpose: The identification of subjects with systemic sarcoidosis at higher risk for sudden death is an unresolved issue. An influence of the autonomic activity on the genesis of ventricular arrhythmias was postulated. Heart rate variability (HRV) analysis provides a useful method to measure autonomic activity, and is a predictor of increased risk of death in various conditions. Therefore, the aim of the study was to evaluate HRV in patients with systemic sarcoidosis.
Methods: The study included 35 patients with biopsy proven systemic sarcoidosis who were not taking antiarrhythmic medications. Thallium scintigraphy was performed to all patients with systemic sarcoidosis. The cardiac sarcoidosis was accepted in 16 patients as abnormal thallium scintigraphy and normal coronary arteriography. The time‐domain analysis of HRV was expressed as the standard deviation of all normal to normal NN intervals (SDNN) detected during 24‐hour Holter monitoring. Twenty‐four healthy subjects represented a control group for HRV analysis.
Results: There were no differences in age (44 ± 13 years for cardiac sarcoidosis, 42 ± 15 years for noncardiac sarcoidosis, and 40 ± 10 years for control group; P = NS), sex (the ratio of female; 63%, 68%, and 55%, respectively; P = NS), and echocardiographic ejection fraction (63 ± 10%, 67 ± 8%, and 69 ± 6%, respectively; P = NS) among study groups. The mean SDNN value of the group with cardiac sarcoidosis was significantly lower than both the group with noncardiac sarcoidosis and the control group (72 ± 32 ms vs 110 ± 46 ms and 152 ± 36 ms; P < 0.05, respectively).
Conclusion: HRV is decreased in patients with systemic sarcoidosis compared to the control group. This decreasing is more obvious in patients with cardiac sarcoidosis.
Keywords: sarcoidosis, HRV, cardiac involvement
Sarcoidosis is a multisystem granulomatous disorder. Clinically recognizable sarcoid involvement of the heart occurs in less than 10% of the patients, although cardiac granulomas are found in as many as 30% at autopsy. 1 Sarcoidosis, in general, has a mortality rate of only 0.2/10 2 , 5 but the prognosis when the heart involved is very much worse, being about 40% by 5 years. 3 In Japan, approximately 73% of the patients had died of myocardial sarcoidosis 4 and sudden death was accepted as the most common manifestation of clinically significant myocardial involvement. 5 , 6 , 7
Autonomic dysfunction is associated with an increased mortality risk in patients with diabetes mellitus, hypertension, and coronary artery disease that may be explained by the fact that autonomic imbalance predisposes individuals to cardiac arrhythmias. 8 , 9 , 10 Measurement of 24‐hour heart rate variability (HRV) is a practical noninvasive method for studying cardiac autonomic function. 11 , 12 , 13 Fluctuations in the interval between normal heart beats, because they are mediated by autonomic inputs to the sinus node, provide information about cardiac autonomic modulation. Thus, low HRV values could reflect a lack of central modulation of heart rate or a lack of response of the sinus node; the latter could even be caused by saturation from highly elevated levels of autonomic tone. 14
It is not dependent on patient's cooperation and has proven reproducible 15 , 16 and more sensitive 17 , 18 than the conventional reflex tests. Furthermore, decreased HRV has been shown to be associated with increased mortality in various populations, including patient with myocardial infarction 19 , 20 and the elderly. 21
The aim of this study was to evaluate HRV as a tool to assess cardiovascular autonomic nervous system (ANS) function in patients with systemic sarcoidosis compared to healthy subjects.
MATERIALS AND METHODS
Study Groups
Forty‐one consecutive outpatients with transbronchial biopsy specimen–proved pulmonary sarcoidosis were recruited from a specialized chest disease hospital clinic. Six patients were excluded from the study because of the presence of one or more of the following exclusion criteria: presence of lung disease other than sarcoidosis, the presence of known intrinsic heart disease, systemic hypertension, anemia, diabetes mellitus, pregnancy, alcoholism, or any routine medication including steroid use. Thus, patient group was composed of 35 patients, who had mean 21 ± 12 (range 1–43) month's disease duration. Patients with systemic sarcoidosis were further divided into two subgroups depending on the myocardial thallium involvement. The cardiac sarcoidosis was accepted in 16 patients as abnormal thallium scintigraphy and normal coronary arteriography. The control group consisted of 24 healthy volunteers with no previous history of cardiac disease or hypertension.
Study Protocol
Patients were informed about the study protocol and consent was obtained from each patient and local ethical committee approval was undertaken. All patients were performed echocardiographic examination and thallium scintigraphy, then 24‐hours Holter monitorization. Coronary angiography was performed to patients with abnormal thallium scintigraphy.
Transthoracic echocardiography was performed by one of the authors, who did not have any information of the patients' clinical data, using a system V (Vingmed, GE Healthcare) with a 2.5 MHz phased‐array transducer. Recordings were taken on patients positioned in the left lateral decubitus position. The left ventricular ejection fraction was measured using modified Simpson's rule. 22
All patients underwent 24‐hour ECG monitoring. The Holter tapes were analyzed by a SyneView 1.02, Ela Medical System, 1997–1999. As parameters of HRV, the mean of all normal R‐R intervals and the standard deviation of all normal to normal RR intervals (SDNN) during 24 hours were calculated as previously described. 23
Myocardial thallium‐201 scintigraphy was performed in all patients. After an overnight fast, 1.5 mCi of 201TI was administered intravenously at the peak exercise and scans were recorded with a high‐resolution scintillation camera and a high‐resolution, low‐energy, parallel‐hole collimator. Images were obtained in the anterior, 45‐degree left anterior oblique, and left lateral projections. Rest images were taken 2 hours later with the same protocol and the tomographic images of vertical long axis, horizontal long axis, and short axis were reconstructed. Scans were evaluated visually by two physicians. In the presence of normal coronary arteries, the perfusion defects, especially reverse redistribution on 201TI imaging in a patient with biopsy proven systemic sarcoidosis were accepted as a sign of cardiac involvement. 24
Statistical Analysis
Data are expressed as mean ± SD. All numeric variables showed normal distribution and the variance between the study groups was similar. Thus, comparison among the study groups for various numeric parameters was carried out by one‐way ANOVA and the post hoc Scheffé test for multiple comparisons. Comparisons of proportions were performed using a 3 × 2 cross‐table and chi‐square test. A P value < 0.05 was considered statistically significant. The SPSS 7.5 program for Windows was utilized for the entire statistical work‐up.
RESULTS
Demographics and Patient Characteristics
There were no differences in age (44 ± 13 years for cardiac sarcoidosis, 42 ± 15 years for noncardiac sarcoidosis, and 40 ± 10 years for control group), sex (the ratio of female; 63%, 68%, and 55%, respectively), and echocardiographic ejection fraction (63 ± 10%, 67 ± 8%, and 69 ± 6%, respectively) among study groups (Table 1). Radiological stages were not significantly different between patients with thallium involvement (46% stage 1, 48% stage 2, and 8% stage 3) and without thallium involvement (30% stage 1, 55% stage 2, and 15% stage 3). There was no difference in disease duration between groups cardiac and noncardiac sarcoidosis (21 ± 13 months vs 22 ± 12 months). Pulmonary function tests such as forced expiratory volume (FEV1), forced vital capacity (FVC), angiotensin converting enzyme (ACE) level, and Ca++ levels were not different between sarcoidosis patients with thallium involvement and without thallium (Table 2).
Table 1.
The Patients Characteristics and 24‐Hour Holter Monitoring Results
| Thallium (+) (n = 16) | Thallium (−) (n = 19) | Control (n = 24) | |
|---|---|---|---|
| Age (years) | 44 ± 13 | 42 ± 15 | 40 ± 10 |
| Female (n) | 10 | 13 | 13 |
| LV EF (%) | 63 ± 10 | 67 ± 10 | 69 ± 6 |
| HR (bpm) | 88 ± 9a | 83 ± 13 | 75 ± 10 |
| SDNN (ms) | 72 ± 32a,b | 110 ± 46a,b | 152 ± 36 |
LV EF = left ventricular ejection fraction; HR = heart rate; SDNN = standard deviation of all normal to normal NN intervals.
aComparison with control group (P < 0.017).
bComparison between two patients groups (P < 0.017).
Table 2.
Pulmonary Function Tests, ACE and Urinary Ca++ Levels in Patients with Systemic Sarcoidosis
| Thallium (+), n = 16 | Thallium (−), n = 19 | P value | |
|---|---|---|---|
| FEV1 (mL) | 2626 ± 767 | 2685 ± 750 | NS |
| FVC (mL) | 3001 ± 1116 | 3124 ± 788 | NS |
| ACE (U/L) | 83 ± 33 | 75 ± 37 | NS |
| Ca++ (mg/dL) | 10 ± 0.8 | 10 ± 0.6 | NS |
FEV1= forced expiratory volume; FVC = forced vital capacity; ACE = angiotensin converting enzyme; Ca++= Calcium; NS = not significant.
HRV Analysis
The mean heart rate during 24‐hour Holter monitorization in control group was lower than patient group with cardiac sarcoidosis (75 ± 10 bpm vs 88 ± 9 bpm, P < 0.017) but it did not differ between controls and patients with noncardiac sarcoidosis (75 ± 10 bpm vs 83 ± 13 bpm), (Table 1). The mean SDNN value of the group with cardiac sarcoidosis was significantly lower than both the group with noncardiac sarcoidosis and the control group (72 ± 32 ms vs 110 ± 46 ms and 152 ± 36 ms; P < 0.05, respectively) (Figure 1). After adjusted for heart rate, the mean SDNN value of the group with cardiac sarcoidosis was significantly lower than both the group with noncardiac sarcoidosis and the control group (85 ± 11 ms vs 112 ± 9 ms and 146 ± 8 ms; P < 0.001, respectively).
Figure 1.
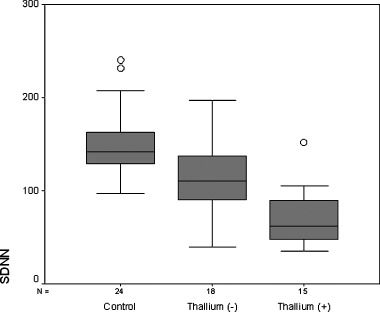
Heart rate variability represented as standard deviation of all normal to normal NN intervals (SDNN (ms)) in controls and patients groups.
DISCUSSION
To our knowledge, the present study is the first to use HRV for evaluation of ANS function in patients with sarcoidosis. This study shows that HRV is affected in patients with sarcoidosis
ANS dysfunction has been described in autoimmune diseases, such as diabetes 14 , 19 , 20 and rheumatic conditions, including rheumatoid arthritis, 25 systemic lupus erythematosus, 26 and scleroderma. 27 Sarcoidosis can involve all components of nervous system and neurologic findings including peripheral neuropathy are observed in 5% of patients. But the prevalence of autonomic dysfunction in systemic sarcoidosis is unknown. HRV measurements from electrocardiographic recordings are used as markers of autonomic modulation of heart. 13 Spectral analysis of HRV allows assessment of frequency specific fluctuations in heart rate behavior. Modern computerized techniques have now permitted the analysis of beat‐to‐beat variability in heart rate, and have enabled the HRV to be used as a noninvasive tool to study the function of ANS and to test its sympathetic and parasympathetic integrity. 23
Twenty‐four‐hour Holter monitorization has been used to screen arrhythmias and conduction defects in suspected sarcoidosis 28 but the usefulness as a noninvasive indicator of sudden death in sarcoidosis has not been established or investigated. Two‐thirds of patients with cardiac sarcoidosis die suddenly. Ventricular tachycardia is one of the most frequently reported cardiac arrhythmias and, together with complete heart block, is presumed to be the cause of sudden death in most patients with myocardial sarcoidosis. 6 , 29 They accepted that granulomas and development of ventricular aneurysms provide a substrate for ventricular tachycardia and ventricular fibrillation. In cardiac sarcoidosis, with alteration of the normal kinetic of involved area of the heart, could induce an abnormal mechanical stimulation of the receptors in the myocardial wall with a reflex increase of the sympathetic activity. A reduction of SDNN was shown to be associated with a high mortality after acute myocardial infarction 19 and elderly. 21 In our study, a reduction of SDNN in patients with sarcoidosis was found in comparison with a control group of healthy subjects, reflecting a reduction of vagal activity with a concominant sympathic dominance. Decreased HRV might be a risk factor for sudden death in patients with sarcoidosis and HRV analysis might be used as a part of risk stratification for these patients. Prospective studies will be done to examine this phenomenon.
LIMITATION OF STUDY
We used thallium‐201 scintigraphy for the detection of cardiac involvement. Myocardial thallium involvement with normal coronary arteries was accepted as cardiac sarcoidosis. Unfortunately, a normal finding on the 201TI scan does not exclude the presence of cardiac involvement. 30 And Kinney and Coldwell reported that the 201TI scan was too sensitive and very nonspecific test to diagnose cardiac involvement in sarcoid patients without cardiac symptoms. 31 This criterion may be criticized, because the endomyocardial biopsy or autopsy was suggested for definitive diagnosis of cardiac sarcoidosis. 32 However, we studied live patients and endomyocardial biopsy has a low yield of diagnostic accuracy. 32 Moreover, we did not aim primarily to detect cardiac involvement rather we targeted to evaluate autonomic function in patients with sarcoidosis. Another limitation of this study is that we determined HRV over 24 hours without standardization of patient activities.
CONCLUSION
HRV is decreased in patients with systemic sarcoidosis compared to the control group. This decreasing is more obvious in patients with cardiac sarcoidosis.
REFERENCES
- 1. Haywood LJ, Sharma OP, Siegel ME, et al Detection of myocardial sarcoidosis by thallium 201 imaging. J Natl Med Assoc 1982;74: 959–964. [PMC free article] [PubMed] [Google Scholar]
- 2. National Center for Health Statistics , 1984, Unpublished data.
- 3. Flaming HA, Bailey SM. The prognosis of sarcoid heart disease in the United Kingdom. Ann N Y Acad Sci 1986;465: 543–550. [DOI] [PubMed] [Google Scholar]
- 4. Tachibana T, Ohmori F, Ueda E. Clinical study of cardiac sarcoidosis. Ann N Y Acad Sci 1986;465: 530–542. [DOI] [PubMed] [Google Scholar]
- 5. Matsui Y, Iwai K, Tachibana T, et al Clinicopathologic study on fatal myocardial sarcoidosis. Ann N Y Acad Sci 1976;278: 455–469 [DOI] [PubMed] [Google Scholar]
- 6. Robert WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of heart. Am J Med 1977;63: 86–108. [DOI] [PubMed] [Google Scholar]
- 7. Fleming HA, Bailey SM. Sarcoid heart disease. J R Coll Physcians Lond 1981;15(4):245–253. [PMC free article] [PubMed] [Google Scholar]
- 8. Gerritsen J, Dekker JM, TenVoorde BJ, et al Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension or a history of cardiacvascular disease. Diabetes Care 2001;24: 1973–1978 [DOI] [PubMed] [Google Scholar]
- 9. Dekker JM, Schouten EG, Klootwijk P, et al Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle aged and elderly man: The Zurphen study. Am J Epidemiol 1997;145: 889–908. [DOI] [PubMed] [Google Scholar]
- 10. Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med 1980;49: 95–108. [PubMed] [Google Scholar]
- 11. Akselrod S, Gordon D, Ubel FA, et al Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat‐to‐beat cardiovascular control. Science 1981;213: 220–222. [DOI] [PubMed] [Google Scholar]
- 12. Stein PK, Bosner MS, Kleiger RE, et al Heart rate variability: A measure of cardiac autonomic tone. Am Heart J 1994;127: 1376–1381. [DOI] [PubMed] [Google Scholar]
- 13. Huikiri HV, Mäkikallio T, Airaksinen KEJ, et al Measurement of heart arte variability: A clinical tool or a research toy? J Am Coll Cardiol 1999;34: 1878–1883. [DOI] [PubMed] [Google Scholar]
- 14. Malik M, Camm AJ. Components of heart rate variability: What they really mean and what we really measure. Am J Cardiol 1993;72: 821–822. [DOI] [PubMed] [Google Scholar]
- 15. Huikuri HV, Kessler KM, Terracall E, et al Reproducibility and circadian rhythm of heart rate variability in healthy subjects. Am J Cardiol 1990;65: 391–393. [DOI] [PubMed] [Google Scholar]
- 16. Kleiger RE, Bigger JT, Bosner MS, et al Stability over time of variables measuring heart rate variability in normal subjects. Am J Cardiol 1991;68: 626–630. [DOI] [PubMed] [Google Scholar]
- 17. Ewing DJ, Neilson JMM, Travis P. New method for assessing cardiac parasympathetic activity using 24 hour electrocardiograms. Br Heart J 1984;52: 396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weston PJ, James MA, Panerai RB, et al Evidence of defective cardiovascular regulation in insulin dependent diabetic patients without clinical autonomic dysfunction. Diabetes Res Clin Pract 1998;42: 141–148. [DOI] [PubMed] [Google Scholar]
- 19. Kleiger RE, Miller JP, Bigger JT, et al, the multicenter postinfarct research group . Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987;59: 256–262. [DOI] [PubMed] [Google Scholar]
- 20. Bigger JT Jr, Fleiss JL, Steinman RC, et al Frequency domain measures of heart rate variability and mortality after myocardial infarction. Circulation 1992;85: 164–171. [DOI] [PubMed] [Google Scholar]
- 21. Huikuri HV, Mäkikallio TH, Airaksinen KEJ, et al Power‐law relationship of heart rate variability as a predictor of mortality in the elderly. Circulation 1998;97: 2031–2036. [DOI] [PubMed] [Google Scholar]
- 22. Otterstad JE. Measuring left ventricular volume and ejection fraction with the biplane Simpson's method. Heart 2002;88: 559–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996;93: 1043–1065. [PubMed] [Google Scholar]
- 24. Fields G, Sharma O, Siegel M. Detection of myocardial sarcoidosis by thallium‐201 imaging. J Natl Med Assoc 1983;63: 478–482. [PMC free article] [PubMed] [Google Scholar]
- 25. Maule S, Quadri R, Mirante D, et al Autonomic nervous dysfunction in systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA): Possible pathogenic role of autoantibodies to autonomic nervous structures. Clin Exp Immunol 1997;110: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gamez‐Nava JI, Gonzalez‐Lopez L, Ramos‐Remus C, et al Autonomic dysfunction in patients with systemic lupus erythematosus. J Rheumatol 1998;25: 1092–1096. [PubMed] [Google Scholar]
- 27. Ferri C, Emdin M, Giuggioli D, et al Autonomic dysfunction in systemic sclerosis: Time and frequency domain 24 hour heart rate variability analysis. Br J Rheumatol 1997;36: 669–676. [DOI] [PubMed] [Google Scholar]
- 28. Jain A, Starek PJ, Delany DL. Ventricular tachycardia and ventricular aneurysm due to unrecognized sarcoidosis. Clin Cardiol 1990;13: 738–740. [DOI] [PubMed] [Google Scholar]
- 29. Fleming HA. Sarcoid heart disease: A review and an appeal. Thorax 1980;35: 641–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mana J. Nuclear imaging: 67 Gallium, 201 thallium, 18 F labeled fluoro‐2 deoxy‐D glucose position emission tomography. Clin Chest Med 1997;18: 799–811. [DOI] [PubMed] [Google Scholar]
- 31. Kinney EL, Caldwell JW. Do thallium myocardial perfusion scan abnormalities predict survival in sarcoid patients without cardiac symptoms? Angiology 1990;July 41: 573–576. [DOI] [PubMed] [Google Scholar]
- 32. Uemura A, Morimoto S, Hiramitsu S, et al Histologic diagnostic rate of cardiac sarcoidosis: Evaluation of endomyocardial biopsies. Am Heart J 1999;138: 299–302. [DOI] [PubMed] [Google Scholar]


