Abstract
Background: Prolonged ventricular repolarization duration confers increased risk for malignant ventricular arrhythmias. We sought to clarify the optimal method of QT/JT interval assessment in patients with complete bundle branch block (BBB).
Methods: Study patients (n = 71) were dual‐chamber device recipients with baseline left or right BBB who preserved intrinsic ventricular activation during incremental atrial pacing. Patients were classified according to the presence or not of structural heart disease. The former group received chronic amiodarone therapy. QT and JT intervals were recorded at baseline heart rate of 51 ± 4 beats/min and during atrial pacing at 60, 80, and 100 beats/min. We used linear mixed‐effects models to assess the effect of heart rate on the derived QTc and JTc values with the use of six different heart rate correction formulae.
Results: Heart rate had a significant effect on the QTc and the JTc intervals regardless of the correction formula used (P < 0.001 for all formulae). The formula of Hodges demonstrated the least variability in QTc and JTc measurements across the different heart rates in both patients groups without (F = 15.05 and F = 13.53, respectively) and with structural heart disease (F = 5.71 and F = 7.69, respectively), followed by the Nomogram and Framingham methods, whereas the uncorrected QT and JT intervals showed comparable heart rate–dependency. The application of Bazett's JTc and QTc led to the most pronounced interval variations in any case with BBB.
Conclusions: The Hodges, Nomogram and Framingham correction methods provide best assessment of QT/JT intervals in BBB, whereas Bazett's formula exaggerates heart rate–dependency of ventricular repolarization intervals.
Keywords: bundle branch block, QT/JT interval, heart rate correction
Assessment of ventricular repolarization in patients with bundle branch block (BBB) poses a significant challenge in electrocardiography (ECG). 1 Besides inherent and technical difficulties in defining the QT interval as a measure of ventricular repolarization, even in patients with narrow QRS complex, 2 , 3 there are additional unresolved problems in cases with BBB. 1 , 4 At present, correction methods for ventricular repolarization that account for heart rate and QRS duration in patients with BBB present key questions, which directly pertain to prognosis and therapy. 5 , 6 Recommendations to use the JT interval instead of the QT interval and linear regression heart rate correction methods are often not implemented in practice, 7 whereas Bazett's correction formula, despite constant criticism, continues to be used as a standard clinical tool. 8 Several explanations may be offered for the underutilization of alternative heart rate correction methods in practice, including complexity, limited data on heart rate‐dependence, insufficient clinical validation, and inadequate physician familiarity and experience. In all, optimal assessment of ventricular repolarization in patients with BBB remains uncertain.
In this prospective study we evaluated the reliability of uncorrected QT and JT intervals as well as of six different heart rate correction formulae in assessing ventricular repolarization in patients with BBB across various heart rates. For this purpose, we studied patients with and without structural heart disease who were implanted with dual‐chamber devices, and preserved intrinsic BBB ventricular activation throughout incremental atrial pacing.
METHODS
Patients
The study population consisted of 71 ambulatory patients implanted with a dual‐chamber pacemaker or an implantable cardioverter defibrillator (ICD) due to symptomatic sinus bradycardia or ventricular tachyarrhythmias, respectively. Eligible patients were clinically stable outpatients who were in sinus rhythm in the long term and had their medications unchanged during the last month before investigation. Only patients with preserved intrinsic ventricular activation and complete left or right BBB (LBBB or RBBB) with prolonged QRS duration ≥120 ms were enrolled. Patients were excluded if they showed ventricular pacing and had symptoms or signs of heart failure on the basis of physical examination and chest x‐ray. In all patients the ventricular leads were positioned near the right ventricular apex. The stability of sinus rhythm and underlying atrioventricular conduction was documented by previous 12‐lead ECGs, 24‐hour Holter recording, and retrieved data from device event counters and histograms, when possible, at planned follow‐up visits.
The patients were classified into two groups on the basis of the presence of underlying structural heart disease. Patients without structural heart disease were not on medications with a known potential for ventricular repolarization prolongation. Most patients with structural heart disease were receiving long‐term amiodarone due to ventricular tachyarrhythmias. The etiology of cardiomyopathy was classified into ischemic, dilated, or valvular, on the basis of medical history, echocardiography, and coronary angiography.
Complete BBB abnormalities were defined according to AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. 7 Two‐dimensional echocardiographic measurements were performed in standard fashion to obtain cardiac dimensions, left ventricular systolic function (LVSF), and wall contraction abnormalities.
Study Protocol
The patients were examined during the routine follow‐up service of our institution in supine position under standardized conditions and continuous ECG monitoring. Devices had been programmed at implantation in DDD mode with a fixed long atrioventricular delay >250 ms to allow as much as possible intrinsic ventricular activity. Baseline ECG measurements were taken after a minimal 5‐minute period at the slowest sinus rhythm available or at the fixed lower pacing rate. For atrial pacing, devices were programmed in AAI mode first at the lower rate of 40 beats/min, to allow the lowest nonpaced intrinsic heart rate, and then at progressively increasing pacing rates of 60 beats/min, 80 beats/min, and 100 beats/min to allow intrinsic ventricular conduction with no ventricular capture or fusion and stable 1:1 atrioventricular conduction. ECG measurements at each pacing stage were obtained after approximately 5 minutes of constant atrial pacing to obtain stable hemodynamics and steady state ECG intervals. Atrial pacing was suspended for approximately 5 minutes between each pacing rate stage. The study protocol was approved by the ethics committee of our hospital, and all patients gave informed consent.
ECG Measurements
Standard 12‐lead ECGs were recorded throughout the study using an MAC‐VU electrocardiograph (Marquette Medical Systems, Milwaukee, WI, USA) at a paper speed of 25 mm/s. Measurements of RR, QRS, JT, and QT intervals were performed manually by the same investigator (JC) blinded to clinical data using a electronic digitizer (Electronic Digital Caliber, Central Tools Inc., Cranston RI, USA). The width of QRS duration was measured in the precordial lead with the widest QRS interval as duration between the initial QRS depolarization and the J‐point. When the landmarks at the end of the QRS complex were distinct, the J‐point was determined as the beginning of the isoelectric ST segment. If the landmarks were not distinguishable, a tangent line was drawn on the descending part of the R wave or the ascending part of the S wave. The point where this tangent met the baseline was termed the J‐point. The QT interval was determined on the basis of the longest measured QT interval across all simultaneously recorded ECG leads from the earliest QRS deflection to the latest T wave end with sharp end point. When the end of the T wave was indistinct or if atrial pacing interfered with the T wave, a tangent line was drawn on the downward limb of the T wave to the point where the line joined the baseline. The maximum (QT) and the minimum QT (QTmin) value in any lead were used as the uncorrected intervals in each patient. The maximum JT intervals (JT) and the corrected JT intervals (JTc) were measured by subtracting the widest QRS complex from the QT and the corrected QT intervals, respectively. 4 , 5 , 7 Corrected QT (QTc) and JTc intervals were calculated using the following six correction formulae:
-
1
Bazett 8 : QTcB (JTcB) = QT (JT) /RR interval1/2.
-
2
Sagie‐Framingham 9 : QTcFa (JTcFa) = QT (JT) + 154 (1 − 60 /heart rate).
-
3
Fridericia 10 : QTcFi (JTcFi) = QT (JT) /RR interval1/3.
-
4
Hodges 11 : QTcH (JTcH) = QT (JT) + 1.75 (heart rate − 60).
-
5
Karjalainen‐Nomogram 12 : QTcN (JTcN) = QT (JT) + Nomogram correction factor.
-
6
Rautaharju QT 4 : QT − 155 x (60/HR − 1) − 0.93 x (QRS ‐139) + k (k =− 22 ms for men, and ‐34 ms for women).
Rautaharju JT : JT − 155 × (60/HR − 1) + k (k = 34 ms for men, and 22 ms for women).
Statistical Analysis
Descriptive statistics are presented as mean ± standard deviation for continuous variables and as number (percent) for categorical variables. Differences in baseline characteristics were assessed with the nonparametric rank‐sum test for continuous variables and Fisher's exact test for categorical variables. We used mixed‐effects linear models to evaluate the influence of the various correction formulae on QTc and JTc variation across heart rates. Specifically, for each formula, the pacing rate was treated as a fixed effect whereas the patient was treated as a random effect. 13 The corresponding F value for the effect of pacing rate (baseline, 60 beats/min, 80 beats/min, 100 beats/min) in the model represents the variability in QTc and JTc intervals explained by the pacing rate; ideally, this variability should be nonsignificant, i.e., an ideal QTc or JTc correction formula should have no residual dependency on the heart rate. A large F value for the effect of pacing rate indicates a statistically significant variation of QTc or JTc across the various pacing rates. We also obtained the t statistic for linear trend across pacing rates for each formula; a positive t value signifies an increasing trend across pacing rates while a negative t value signifies a decreasing trend. For post‐hoc comparisons of the QTc and the JTc intervals, we used the Sidak correction method. The effect of type of block (LBBB vs RBBB) and QRS duration on the QTc and the JTc was examined by adding the corresponding terms as fixed effects in mixed‐effects linear models with pacing rate and patient entered as described above. A P value <0.05 was considered to represent statistical significance. All analyses were performed with Stata 11 (StataCorp, College Station, TX, USA).
RESULTS
Patient Characteristics
The baseline characteristics of the study patients are presented in Table 1. Patients without structural heart disease (n = 28) had normal left ventricular ejection fraction, did not take any ventricular repolarization‐prolonging drugs, and most of them had implanted pacemakers. Patients with structural heart disease (n = 43) had decreased left ventricular ejection fraction, had been on long‐term oral amiodarone at maintenance dose 200 mg/day daily for at least one month, and most of them were implanted with an ICD. In the group of patients without structural heart disease, patients with LBBB [10 patients (36%)] and RBBB [18 patients (64%)] had similar clinical and echocardiographic characteristics except for the LVSF, which was lower in patients with LBBB (55 ± 3% vs 60 ± 4%, P = 0.001). In the group of patients with structural heart disease, patients with LBBB [23 patients (53.5%)] and RBBB [20 patients (46.5%)] had similar clinical and echocardiographic characteristics except for the LVSF, which was lower in patients with LBBB (31 ± 10% vs 39 ± 11%, P = 0.026). Both patients groups without and with structural heart disease had similar heart rates at baseline (50 ± 4 beats/min vs 51±3 beats/min, P = 0.38), but different QRS durations at 60 beats/min (147 ± 11 vs 159 ± 28, P = 0.01) and QT intervals (436 ± 30 ms vs 485 ± 41 ms, P < 0.001) (Tables 2 and 3). At each heart rate stage, the patients without structural heart disease patients had significantly shorter QT and JT durations compared with the patients with structural heart disease (P < 0.001).
Table 1.
Patient Baseline Characteristics
| Patients without Structural Heart Disease | Patients with Structural Heart Disease | |||||
|---|---|---|---|---|---|---|
| Total (n = 28) | LBBB (n = 10) | RBBB (n = 18) | Total (n = 43) | LBBB (n = 23) | RBBB (n = 20) | |
| Age (years) | 71 ± 10 | 70 ± 10 | 71 ± 11 | 70 ± 9 | 69 ± 9 | 70 ± 10 |
| Men, n (%) | 19 (68) | 6 (−) | 13 (−) | 36 (84) | 19 (−) | 17 (−) |
| BMI (kg/m2) | 27 ± 3 | 28 ± 3 | 27 ± 3 | 28 ± 3 | 28 ± 3 | 27 ± 3 |
| Heart disease, n (%) | (0) | (100) | (53) | (47) | ||
| CAD | 25 | 11 | 14 | |||
| DCM | 17 | 12 | 5 | |||
| VHD | 1 | 0 | 1 | |||
| Echocardiography | ||||||
| LVEDD (mm) | 51 ± 3 | 52 ± 3 | 50 ± 3 | 62 ± 7 | 62 ± 7 | 61 ± 8 |
| LA (mm) | 40 ± 4 | 41 ± 3 | 40 ± 5 | 44 ± 4 | 44 ± 5 | 45 ± 4 |
| LVEF (%) | 58 ± 4 | 55 ± 2 | 60 ± 4 | 35 ± 11 | 31 ± 10 | 39 ± 11 |
| Devices, n (%) | ||||||
| Pacemaker | 26 (93) | 10 | 16 | 8 (19) | 3 | 5 |
| ICD | 2 (7) | 0 | 2 | 35 (81) | 20 | 15 |
| Medications, n (%) | ||||||
| Amiodarone | 0 | 0 | 0 | 34 (79) | 18 | 16 |
| Beta‐blockers | 7 (25) | 2 | 5 | 37 (86) | 20 | 17 |
CAD = coronary artery disease; DCM = dilated cardiomyopathy; ICD = implantable cardioverter defibrillator; LA = left atrium; LVEDD = left ventricular end‐diastolic diameter; LVEF = left ventricular ejection fraction; VHD = valvular heart disease.
Table 2.
Patients without Structural Heart Disease: ECG Intervals at Different Heart Rates
| BL | 60 beats/min | 80 beats/min | 100 beats/min | F | P Value | t | P Value | |
|---|---|---|---|---|---|---|---|---|
| Group A (Total) | ||||||||
| RR (ms) | 1205 ± 96 | 998 ± 3 | 749 ± 3 | 598 ± 3 | – | <0.0001 | – | – |
| PR (ms) | 182 ± 31 | 192 ± 35 | 216 ± 39 | 244 ± 47 | – | <0.0001 | – | – |
| QRS (ms) | 147 ± 11 | 147 ± 11 | 147 ± 13 | 147 ± 14 | – | 0.99 | – | – |
| QT (ms) | 437 ± 30∥ | 436 ± 30 | 417 ± 25 #** | 395 ± 18* | 15.06 | <0.0001 | −6.34 | <0.0001 |
| QTmin (ms) | 417 ± 27∥ | 410 ± 36 | 398 ± 21** | 377 ± 18* | 11.83 | <0.0001 | −5.77 | <0.0001 |
| JT (ms) | 290 ± 31∥ | 289 ± 31 | 271 ± 26 | 249 ± 21* | 12.95 | <0.0001 | −5.86 | <0.0001 |
| QTcB (ms) | 399 ± 31*§§# | 436 ± 31 | 482 ± 29*†† | 511 ± 23* | 78.02 | <0.0001 | 15.25 | <0.0001 |
| QTcFi (ms) | 412 ± 30†§∥ | 436 ± 31 | 459 ± 28 | 469 ± 21∥ | 22.74 | <0.0001 | 8.11 | <0.0001 |
| QTcFa (ms) | 406 ± 32‡§∥ | 436 ± 31 | 456 ± 25† | 457 ± 18† | 20.51 | <0.0001 | 7.11 | <0.0001 |
| QTcH (ms) | 420 ± 30§∥ | 436 ± 31 | 453 ± 25 | 466 ± 18* | 15.05 | <0.0001 | 6.77 | <0.0001 |
| QTcRa (ms) | 372 ± 34‡§∥ | 403 ± 31 | 423 ± 25 | 425 ± 19† | 20.03 | <0.0001 | 7.04 | <0.0001 |
| QTcN (ms) | 413 ± 31†§∥ | 436 ± 30 | 457 ± 25† | 461 ± 17† | 18.10 | <0.0001 | 6.98 | <0.0001 |
| JTcB (ms) | 252 ± 34†§∥ | 289 ± 32 | 336 ± 30*,†† | 364 ± 26∥ | 69.87 | <0.0001 | 14.44 | <0.0001 |
| JTcFi (ms) | 264 ± 32†§∥ | 289 ± 32 | 313 ± 29† | 322 ± 24∥ | 20.61 | <0.0001 | 7.72 | <0.0001 |
| JTcFa (ms) | 258 ± 35‡§∥ | 289 ± 32 | 309 ± 26 | 310 ± 21 | 18.34 | <0.0001 | 6.74 | <0.0001 |
| JTcH (ms) | 272 ± 32§∥ | 289 ± 32 | 306 ± 26 | 319 ± 22* | 13.53 | <0.0001 | 6.42 | <0.0001 |
| JTcRa (ms) | 288 ± 34‡§∥ | 320 ± 31 | 340 ± 26 | 341 ± 203† | 19.77 | <0.0001 | 6.98 | <0.0001 |
| JTcN (ms) | 265 ± 34†§∥ | 289 ± 31 | 311 ± 26 | 314 ± 21† | 16.21 | <0.0001 | 6.62 | <0.0001 |
Values are mean ± SD. BL = baseline. QTc formulae: QTcB = Bazett, QTcFi = Fridericia, QTcFa = Framingham, QTcH = Hodges, QTcN = Nomogram, QTcRa = Rautaharju. JTc formulas abbreviations analogous to the QTc formulas. The F statistic refers to the overall difference of values between pacing rates, whereas the t statistic refers to linear trend across pacing rates; a positive t value signifies an increasing trend across pacing rates while a negative t value signifies a decreasing trend.
*P < 0.001, †P < 0.05, †P < 0.01 versus 60 beats/min; §P < 0.001,P < 0.05, #P < 0.01 versus 80 beats/min; P < 0.001, **P < 0.05, ††P < 0.01 versus 100 beats/min.
Table 3.
Patients with Structural Heart Disease: ECG Intervals in at Different Heart Rates
| BL | 60 beats/min | 80 beats/min | 100 beats/min | F | P Value | t | P Value | |
|---|---|---|---|---|---|---|---|---|
| Group B (Total) | ||||||||
| RR (ms) | 1182 ± 75 | 998 ± 5 | 751 ± 2 | 600 ± 2 | – | <0.0001 | – | – |
| PR (ms) | 204 ± 27 | 206 ± 27 | 239 ± 38 | 281 ± 34 | – | <0.0001 | – | – |
| QRS (ms) | 159 ± 28 | 159 ± 28 | 157 ± 29 | 160 ± 31 | – | 0.98 | – | – |
| QT (ms) | 489 ± 40§** | 485 ± 41 | 462 ± 38‡†† | 437 ± 36† | 16.04 | <0.0001 | −6.64 | <0.0001 |
| QTmin (ms) | 465 ± 37§** | 461 ± 37 | 437 ± 31*# | 414 ± 31† | 19.97 | <0.0001 | −7.43 | <0.0001 |
| JT (ms) | l330 ± 37§** | 327 ± 37 | 304 ± 31∥ | 276 ± 28† | 22.17 | <0.0001 | −7.68 | <0.0001 |
| QTcB (ms) | 450 ± 40*** | 486 ± 41 | 533 ± 44∥ | 564 ± 47** | 56.08 | <0.0001 | 12.97 | <0.0001 |
| QTcFi (ms) | 463 ± 40 ** | 486 ± 41 | 508 ± 41 | 518 ± 43* | 14.59 | <0.0001 | 6.54 | <0.0001 |
| QTcFa (ms) | 461 ± 42‡** | 486 ± 41 | 500 ± 38 | 498 ± 36 | 8.72 | <0.0001 | 4.60 | <0.0001 |
| QTcH (ms) | 473 ± 41#** | 485 ± 41 | 497 ± 38 | 507 ± 36 | 5.71 | <0.0001 | 4.16 | <0.0001 |
| QTcRa (ms) | 418 ± 38*** | 444 ± 37 | 459 ± 30 | 455 ± 28 | 12.56 | <0.0001 | 5.32 | <0.0001 |
| QTcN (ms) | 467 ± 42,** | 485 ± 41 | 502 ± 38 | 502 ± 36 | 7.31 | <0.0001 | 4.40 | <0.0001 |
| JTcB (ms) | 291 ± 36†** | 327 ± 38 | 375 ± 35†∥ | 404 ± 36† | 79.80 | <0.0001 | 15.39 | <0.0001 |
| JTcFi (ms) | 303 ± 35†** | 327 ± 37 | 351 ± 33† | 358 ± 33* | 20.62 | <0.0001 | 7.66 | <0.0001 |
| JTcFa (ms) | 302 ± 37*** | 327 ± 37 | 343 ± 31 | 338 ± 28 | 12.37 | <0.0001 | 5.25 | <0.0001 |
| JTcH (ms) | 314 ± 37§** | 327 ± 37 | 339 ± 31 | 347 ± 29† | 7.69 | <0.0001 | 4.80 | <0.0001 |
| JTcRa (ms) | 334 ± 38*** | 358 ± 39 | 375 ± 31 | 371 ± 29 | 12.04 | <0.0001 | 5.23 | <0.0001 |
| JTcN (ms) | 310 ± 39 ** | 327 ± 37 | 346 ± 32 | 342 ± 28 | 9.28 | <0.0001 | 4.69 | <0.0001 |
*P < 0.01, †P < 0.001, and †P < 0.05 versus 60 beats/min; §P < 0.01,P < 0.001, #P < 0.05 versus 80 beats/min; ∥P < 0.01, **P < 0.001, ††P < 0.05 versus 100 beats/min.
Abbreviations as in Table 2.
QT and JT Intervals at Different Heart Rates
The ECG intervals with the corresponding QTc and JTc responses to the various heart rates, and the effects of heart rate on the QT/JT and QTc/JTc interval variations for each correction formula, as expressed by the F and t statistic, are summarized in Tables 2 and 3. While the uncorrected QT/JT intervals progressively shortened as the heart rate increased, all correction methods yielded higher QTc/JTc values with increasing heart rates (i.e., there was an apparent overcorrection with increasing heart rate). In both patient groups without and with structural heart disease, following heart rate increase beyond 60 beats/min, there was a progressive significant shortening of the QT/QTmin and JT intervals (P < 0.001) and increase in PR interval (P < 0.001), whereas the QRS duration did not change significantly (P = 0.99 and P = 0.98, respectively). In contrast, in both patient groups, heart rate increase had a significant prolonging effect on the QTc/JTc values regardless of the correction formula used (P < 0.001).
Within each patient group with or without structural heart disease, both LBBB and RBBB patient subgroups showed similar trends for QT/QTc and JT/JTc (P < 0.05) data series, QRS duration (P = NS), and PR interval (P < 0.005). There was no significant interaction between the type of block (LBBB vs RBBB) and heart rate on the QTc or JTc changes, i.e., QTc and JTc demonstrated similar responses across the various heart rate stages on patients with LBBB and RBBB (F > 0.05; P > 0.05). This observation was consistent regardless of the correction formula used. Within the patient groups without and with structural heart disease the variation of QRS duration across the various heart rate stages was nonsignificant both for the entire groups (P = 0.99 and P = 0.98, respectively) as well as for the subgroups with LBBB (P = 0.94 and P = 0.98, respectively) and RBBB (P = 0.87 and P = 0.84, respectively).
We also tested for interaction of beta‐blockers and amiodarone with the effect of pacing rate on corrected QT and JT values. There was no interaction with beta‐blockers in the patient group without structural heart disease (all interaction terms P > 0.9), in the patient group with structural heart disease (all interaction terms P > 0.7), and in the entire cohort (all interaction terms P > 0.9). No patient in the patient group without structural heart disease was receiving amiodarone. In the patient group with structural heart disease, there was no interaction with amiodarone (all interaction terms P > 0.6). There was also no interaction with amiodarone when the entire cohort was considered (all interaction terms P > 0.3).
Overall, all correction methods resulted in significant heart rate–dependence (P ≤ 0.001) whether or not patients had structural heart disease taking amiodarone. However, in patients without structural heart disease, the QTc formulae were found to be more heart rate–dependent than the corresponding JTc formulae (Table 2). The JTc intervals for the different heart rates are demonstrated graphically in Figure 1. The least heart rate–dependent correction formula (smaller F statistic) for both QT and JT intervals was Hodges formula followed by the Nomogram and Framingham methods; however, no correction method was superior to uncorrected QT and JT intervals in terms of heart rate‐dependence (i.e., even the least rate‐dependent formulas yielded F values similar to uncorrected QT and JT interval). In contrast, the highest heart rate‐dependent variations were observed with Bazett's formula followed by the Fridericia formula. In patients with structural heart disease and prolonged repolarization, in contrast to what was observed in nonstructural heart disease patients, the JTc formulae showed overall higher heart rate–dependent variation (higher F values) relative to the corresponding QTc formulas (Table 3). This QTc interval behavior in response to the different heart rates is demonstrated graphically in Figure 2. The least heart rate–dependent correction formula (smaller F statistic) for both QT and JT intervals was the formula of Hodges followed by the Nomogram and Framingham methods. In contrast to patients without structural heart disease, the use of a correction formula reduced rate‐dependency of QT and JT interval (i.e., the QTc and the JTc values were more consistent across the various heart rates compared with the uncorrected QT and JT intervals), with the exception of Bazett's formula, which exaggerated heart rate–dependency.
Figure 1.
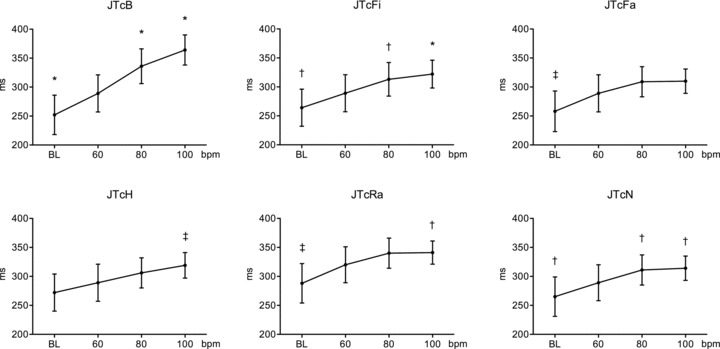
JTc intervals for the different heart rates obtained with the various correction formulas in patients without structural heart disease. *P < 0.001 versus 60 beats/min; †P < 0.05 versus 60 beats/min; †P < 0.01 versus 60 beats/min. BL = baseline heart rate at 50 ± 4 beats/min. JTc formulas: JTcB = Bazett; JTcFi = Fridericia; JTcFa = Framingham; JTcH = Hodges; JTcN = Nomogram; JTcRa = Rautaharju.
Figure 2.
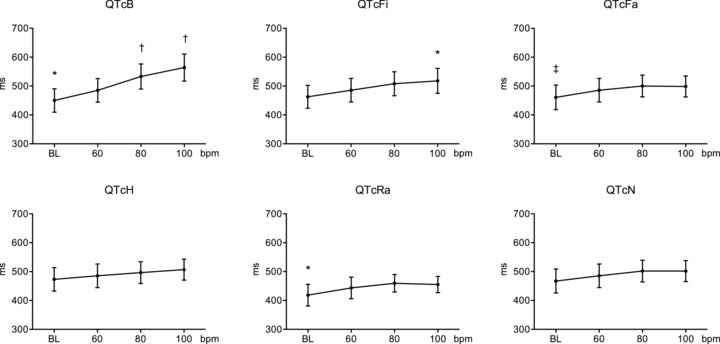
QTc intervals for the different heart rates obtained with the various correction formulas in patients with structural heart disease receiving amiodarone. *P < 0.01 versus 60 beats/min, †P < 0.001 beats/min 60 beats/min, and †P < 0.05 versus 60 beats/min. BL = baseline heart rate at 51 ± 3 beats/min. QTc formulas abbreviations analogous to the JTc formulas as in Figure 1.
DISCUSSION
This study focused on the determination of ventricular repolarization and its proper correction for a wide range of heart rates in patients with BBB. The major findings are as follows: (1) the Hodges formula, followed by the Nomogram and the Framingham correction methods produced the least heart rate–dependent QTc/JTc interval estimates in patients with or without structural heart disease; (2) Bazett's formula demonstrated the highest heart rate–dependency across the different heart rates; and (3) particularly in patients without structural heart disease, the raw JT and QT values provided satisfactory heart rate–independent interval estimates.
Abnormal ventricular repolarization duration confers increased risk for life‐threatening ventricular arrhythmias and sudden cardiac death. 14 , 15 Similarly, prolonged ventricular repolarization predicts mortality in patients with conduction disturbances after myocardial infarction or dilated cardiomyopathy. 5 , 6 Nevertheless, precise estimation of the ventricular repolarization in patients with BBB has been an arduous task. Since the QT interval represents both depolarization and repolarization, it is suggested to use the JT rather than the QT interval as a more accurate index of repolarization in the presence of wide QRS complex. 1 , 7 However, there are still concerns regarding the dependence of the corrected JT intervals on heart rate and QRS duration. Furthermore, although the proposed correction formulae aim to attenuate the inverse relationship between duration of ventricular repolarization and heart rate, all formulae more or less diverge from the theoretical “horizontal line,” i.e., lack of correlation between QTc/JTc interval and heart rate. A promising alternative would be to create a formula individually optimized for each subject based on individual relationships between QT and heart rate; however, such an approach would be too complex and impractical for routine ECG recording. 16 Although there is clearly no perfect solution, physicians, researchers, pharmaceutical agencies, and regulatory authorities are still in search of a correction method that would show minimal or no heart rate–dependency, thus better estimating the risk of life‐threatening ventricular arrhythmias.
This study is the first to examine the heart rate‐dependence of the QT and JT intervals as well as of different heart rate correction formulae in patients with BBB, in an intrapatient comparison, by exploiting the ability of the implanted devices to increase the patient's intrinsic heart rate stepwise with pacing while avoiding extraneous confounding influences. An advantage of our study design is the consideration of the ventricular repolarization hysteresis, 17 which refers to the gradual adaptation of ventricular repolarization to heart rate changes over a period of several minutes. The influence of hysteresis may not be appreciated properly in situations with dynamic variation, for example, during sinus arrhythmia or physical activity.
There were several important observations in our study, irrespective of the underlying structural heart disease status, the pattern of BBB, or whether the patients were on amiodarone. First, we documented in patients with BBB the inverse relationship between the uncorrected QT and JT intervals and heart rate increase, similar to what is already known for patients with normal QRS duration. 17 , 18 Second, we confirmed that the correction formulae converged, as expected, at heart rates near 60 beats/min, but showed remarkable variability in QTc and JTc measurements at lower or higher heart rates, with substantial overcorrection at higher heart rates. 3 , 11 , 18 The observed major differences among the correction formulae underscore the difficulty in evaluating ventricular repolarization in patients with BBB, beginning with the uncertainty that even the recommended normal limits are based on scarce published data. Under these circumstances, the assessment of the drug‐induced ventricular repolarization prolongation in patients with BBB seems even more problematic, taking into account that prolongation of only 10 ms is considered alarming and that the level of proarrhythmia is defined as prolongation more than 20 ms. 7 , 19 Considering the large variations in measured JTc and QTc intervals with the different correction methods across the various heart rates, one realizes that drug safety evaluation may be altogether invalid when inappropriate approaches to ventricular repolarization estimation are used. Third, we verified that each JTc formula shared a similar heart rate–dependent behavior with its corresponding QTc formula. This was attributed to the overall negligible effect of heart rate on the QRS duration, which agrees with previous work showing lack of significant change of QRS duration with changes in heart rate, 1 and relative independence of JTc intervals as a measure of repolarization in patients with wide QRS complex. 20 Fourth, the Hodges formula presented the least heart rate–dependency, which worsened progressively when the Nomogram, Framingham, Rautaharju, Fridericia, and Bazett's methods were used for both JT and QT variants. Last, not surprisingly, Bazett's QT and JT formulae showed the highest heart rate–dependency, leading to the most pronounced interval variations as the heart rate diverged from 60 beats/min, with overt overcorrection at higher rates.
The present findings provide evidence that in patients without structural heart disease, the JTc formulae are less rate‐dependent compared to their corresponding QTc formulae. However, no JTc formula was better than the uncorrected QT and JT values, with the exception of Hodges QTc and JTc, which had comparable variation. In any case, Bazett's formula should be avoided since the Bazett‐corrected QTc and JTc intervals proved to be the most heart rate–dependent. Thus, in these patients, ventricular repolarization assessment could be significantly simplified by using uncorrected intervals at any heart rate, or if desired, to use Hodges JTc formula. The latter suggestion is in accord with the current recommendations to use the JT rather than the QT interval as a more appropriate measure of ventricular repolarization in patients with BBB. Our results show that this general suggestion might be particularly true for patients without evidence of structural heart disease, in whom the JT interval represents the specific repolarization time of the basal portions of the ventricles due to the concordance of the repolarization and depolarization process. However, physicians should keep in mind that uncorrected values tend to underestimate the ventricular repolarization interval with increasing heart rates, whereas corrected values tend to overestimate it.
This study indicates that in patients with structural heart disease taking amiodarone, the QTc formulas are less heart rate–dependent than the uncorrected JT and QT intervals. Surprisingly, the QTc formulas yielded more consistent intervals across the various heart rates relative to their corresponding JTc formulae, albeit the differences were small. The former result implies that the ventricular repolarization intervals should be adjusted as the heart rate deviates from 60 beats/min. The latter observation disagrees with current thinking that the JTc rather than the QTc interval might be preferred in patients with BBB. One likely explanation may involve the reverse or mixed patterns of repolarization sequences with respect to the excitation sequences in patients with structural heart disease, in whom the JT interval reflecting action potential duration at the endocardial regions of the ventricles may not be well distinguished from the slow phase of the repolarization process. In these patients, the repolarization process is more nonuniform, prolonged and complicated, because of the mixed activation and repolarization vectors and of the effect of amiodarone. As a consequence, it may be better represented by the whole QT interval. On this basis, the ventricular repolarization assessment should be based on the evaluation of the QTc intervals with preference to the Hodges formula, followed by the Normogram and Framingham methods. In any case, once again, the uncorrected QT and JT intervals should be preferred over Bazett's formula.
Study Limitations
This study evaluated ventricular repolarization with the use of QT/JT measurements, and lacked of other dynamic measures of repolarization behavior apart from influences of heart rate on the QT/JT intervals. We also acknowledge the high proportion of amiodarone use in the subgroup of patients with BBB and prolonged repolarization.
Clinical Implications
In light of our results, ventricular repolarization in patients with BBB without or with structural heart disease may be preferably assessed by the Hodges JTc or QTc intervals, or alternatively with the Normogram or Framingham corrections methods. Similar least heart rate–dependent results across different heart rates yields the evaluation of the uncorrected ventricular repolarization intervals. Our data suggest that the use of Bazett's formula should not be continued because of its significant heart rate–dependent effects on ventricular repolarization interval variability.
REFERENCES
- 1. Das G. QT interval and repolarization time in patients with intraventricular conduction delay. J Electrocardiol 1990;23:49–52. [DOI] [PubMed] [Google Scholar]
- 2. Spodick DH. Reduction of QT‐interval imprecision and variance by measuring the JT interval. Am J Cardiol 1992;70:628–629. [DOI] [PubMed] [Google Scholar]
- 3. Chiladakis J, Kalogeropoulos A, Arvanitis P, et al Heart rate‐dependence of QTc Intervals assessed by different correction methods in patients with normal or prolonged repolarization. Pacing Clin Electrophysiol 2010;33:553–560. [DOI] [PubMed] [Google Scholar]
- 4. Rautaharju PM, Zhang ZM, Prineas R, et al Assessment of prolonged QT and JT intervals in ventricular conduction defects. Am J Cardiol 2004;93:1017–1021. [DOI] [PubMed] [Google Scholar]
- 5. Crow RS, Hannan PJ, Folsom AR. Prognostic significance of corrected QT and corrected JT interval for incident coronary heart disease in a general population sample stratified by presence or absence of wide QRS complex: The ARIC Study with 13 years of follow‐up. Circulation 2003;108:1985–1989. [DOI] [PubMed] [Google Scholar]
- 6. Pelliccia F, Critelli G, Cianfrocca C, et al Electrocardiographic correlates with left ventricular morphology in idiopathic dilated cardiomyopathy. Am J Cardiol 1991;68:642–647. [DOI] [PubMed] [Google Scholar]
- 7. Surawicz B, Childers, R , Deal BJ, et al AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part III: Intraventricular Conduction Disturbances. J Am Coll Cardiol 2009;53:976–981. [DOI] [PubMed] [Google Scholar]
- 8. Bazett JC. An analysis of time relations of electrocardiograms. Heart 1920;7:353–367. [Google Scholar]
- 9. Sagie A, Larson MG, Goldberg RJ, et al An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol 1992;15:797–801. [DOI] [PubMed] [Google Scholar]
- 10. Fridericia LS. Die Systolendauer im Elektrokardiogramm bei normalem Menschen und bei Herzkranken. Acta Med Scand 1920;53:469–486. [Google Scholar]
- 11. Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: The effect of heart rate on the QTc of normal ECGs. J Electrocardiol 2004;37:81–90. [DOI] [PubMed] [Google Scholar]
- 12. Karjalainen J, Viitasalo M, Manttari M, et al Relation between QT intervals and heart rates from 40 to 120 beats per minute in rest electrocardiograms of men and a simple method to adjust QT interval values. J Am Coll Cardiol 1994;23:1547–1553. [DOI] [PubMed] [Google Scholar]
- 13. Fitzmaurice GM, Ravichandran C. A primer in longitudinal data analysis. Circulation 2008;118:2005–2010. [DOI] [PubMed] [Google Scholar]
- 14. Peters RW, Byington RP, Barker A, et al Prognostic value of prolonged ventricular repolarization following myocardial infarction: The BHAT experience. The BHAT Study Group. J Clin Epidemiol 1990;43:167–172. [DOI] [PubMed] [Google Scholar]
- 15. Perkiömäki J, Koistinen M, Huikuri H. Standard 12‐Lead and 24‐Hour Ambulatory Electrocardiographic Abnormalities in Survivors of Tachyarrhythmic Cardiac Arrest. Ann Noninvasive Electrocardiol 1999;4:158–166. [Google Scholar]
- 16. Malik M, Färbom P, Batchvarov V, et al Relation between QT and RR intervals is highly individual among healthy subjects: Implications for heart rate correction of the QT interval. Heart 2002;87:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lau CP, Freedman AR, Fleming S, et al Hysteresis of the ventricular paced QT interval in response to abrupt changes in pacing rate. Cardiovasc Res 1988;22:67–72. [DOI] [PubMed] [Google Scholar]
- 18. Aytemir K, Maarouf N, Gallagher MM, et al Comparison of formulae for heart rate correction of QT interval in exercise electrocardiograms. Pacing Clin Electrophysiol 1999;22:1397–1401. [DOI] [PubMed] [Google Scholar]
- 19. Haverkamp W, Breithardt G, Camm AJ, et al The potential for QT prolongation and pro‐arrhythmia by non‐anti‐arrhythmic drugs: Clinical and regulatory implications. Report on a Policy Conference of the European Society of Cardiology. Cardiovasc Res 2000;47:219–233. [DOI] [PubMed] [Google Scholar]
- 20. Salim MA, Case CL, Gillette PC. The JT interval as a depolarization independent measurement of repolarization: Lessons from catheter ablation of the Wolff‐Parkinson‐White syndrome. Pacing Clin Electrophysiol 1995;18:2158–2162. [DOI] [PubMed] [Google Scholar]


