Abstract
Background: Coronary slow flow (CSF) is characterized by delayed opacification of coronary arteries in the absence of epicardial occlusive disease. In this study, we aimed to determine endothelin‐1 (ET‐1), nitric oxide (NOx) levels and time domain heart rate variability (HRV) parameters in patients with CSF and relationship among these parameters.
Methods: Thirty‐three patients with CSF detected in the coronary angiography (17 females; mean age 55 ± 7) and 19 patients with normal coronary flow (10 females; mean age 54 ± 11) as a control group were enrolled in the study. Patients were divided into two groups according to exercise testing as if positive (group A, n = 8) or negative (group B, n = 25).
Results: Plasma ET‐1 levels were higher in the group A patients (28.7 ± 17.4 pg/ml) than that of group B (15.9 ± 10.6 pg/ml) and control group (6.0 ± 5.7 pg/ml); and higher in group B patients than that of control group (P < 0.05). Although groups A and B did not differ according to plasma NOx levels (23.4 ± 13.5 μmol/L vs. 32.8 ± 22.7 μmol/L, P > 0.05), NOx levels in group A were lower than the control group (23.4 ± 13.5 μmol/L versus 42.5 ± 15.9 μmol/L, P < 0.05). Time domain HRV parameters were decreased in all patient groups. This was more prominent in group A. Additionally, HRV parameters were negatively correlated with ET‐1 and TIMI frame counts. TIMI frame count was also significantly correlated with ET‐1 and NOx levels (r = 0.61, P < 0.0001, r =−0.30, P < 0.05). Upon intravascular ultrasonography investigation, the common finding was longitudinally extended massive calcification throughout the epicardial arteries. Mean intimal thickness was 0.50 ± 0.13 mm (group A; 0.58 ± 0.11 mm, group B 0.47 ± 0.12 mm, P = 0.029).
Conclusions: The present study demonstrated that in patients with CSF, both increased plasma ET‐1, decreased plasma NOx and diffuse atherosclerosis may cause the decrease in HRV by effecting myocardial blood flow.
Keywords: coronary slow flow, endothelin‐1, nitric oxide, atherosclerosis, heart rate variability
Coronary slow flow (CSF) is a phenomenon characterized by delayed opacification of coronary arteries in the absence of epicardial occlusive disease. 1 Many etiological factors such as endothelial dysfunction, coronary vasospasm, and small vessel disease have been implicated in CSF. 1 , 2 , 3 , 4 , 5 It is well known that endothelin‐1 (ET‐1) and nitric oxide (NOx) are key molecules in normal autoregulatory mechanisms such as modulating coronary circulation. 6 , 7 The endothelial dysfunction in the small coronary arteries (increased ET‐1 levels) in patients with angina pectoris and normal coronary angiography may increase coronary resistance and may cause myocardial ischemia. 8 , 9
Time domain heart rate variability (HRV) is a reliable method, which can be used as an index of cardiac autonomic balance. 8 , 9 It is a noninvasive technique, based on electrocardiogram (ECG) sampling of RR interval variation, thus providing a dynamic assessment of sympathetic and parasympathetic components of the autonomic nervous system. 10 In clinical practice, indices of HRV have been proved to be good predictors of arrhythmic events, sudden death, acute ischemic syndromes, suggesting that low HRV parameters are associated with increased risk of cardiovascular morbidity and mortality. 11 Moreover, low HRV predicts progression of coronary artery disease, providing information beyond that obtained by traditional risk markers of atherosclerosis. 12 Rosano et al. 13 using the analysis of HRV, showed a sympathetic predominance in syndrome X. Autonomic imbalance may influence vasomotor control of the coronary circulation. In the same way, autonomic imbalance would contribute to the pathogenesis of the CSF. In the present study, we investigated relationship between ET‐1, NOx plasma levels and measures of cardiac autonomic function, obtained by time domain HRV analysis, an accepted clinical indicator of cardiac autonomic activity. 10 Additionally, we investigated diffuse atherosclerotic changes in these patients by using intravascular ultrasonography (IVUS).
MATERIAL AND METHODS
The study population consisted of 33 patients with slow coronary flow (17 females; mean age = 55 ± 7 years), who underwent coronary angiography to determine whether or not obstructive coronary artery disease existed because of typical and quasi‐typical symptoms of angina and ECG changes between January 2001 and June 2002 at Cardiology Clinic of Mersin University. The patients with CSF had angiographically normal coronary arteries without luminal irregularities. The patients who suffered from one of the following diseases or associated disorders were excluded from this study; myocardial and/or valvular heart disease, tortuous coronary vessels, myocardial bridge, coronary ectasia, a proximal lumen diameter less than 3 mm, and left ventricular hypertrophy. The intravascular ultrasonography was performed on all patients with coronary angiography, then, all medications were stopped and at the end of a week, 24‐hour ECG Holter monitorization was performed. Patients were divided into two groups according to exercise testing as if positive (group A, n = 8) or negative (group B, n = 25). Nineteen patients with normal coronary flow in the coronary angiography and normal exercise testing (10 females; mean age 54 ± 11) as a control group were enrolled in the study. The study was carried out according to the principles of the Declaration of Helsinki and approved by Mersin University, School of Medicine, investigational review board.
Coronary Angiography and TIMI Frame Count
Coronary angiography was applied by femoral approach using standard Judkins technique. Coronary arteries in left and right oblique planes, and cranial and caudal angles were demonstrated. Left ventricular and aortic pressures were obtained. During the coronary angiography, lopromide (Ultravist‐370, Schering AG) was used as contrast agent and was manually injected (6–8 ml contrast agent at each position). Proximal coronary lumen diameter was measured by quantitative computer‐assisted (QCA) facility and those with a caliber of 3 mm or more were enrolled for further CSF measurements. For the quantitative measurement of coronary blood flow, the time elapsed from the appearance till the contrast agent reached the distal end of coronary artery in terms of cineframe count was considered to be the TIMI frame count. 4 , 14 Thereafter, the final count was substracted from the initial and the exact TIMI frame was calculated for the given artery. However, it was divided by 1.7 when left anterior descending coronary artery was the case, for adjusted correction. TIMI frame counting was undertaken by two different cardiologists. In case of conflict, the frames were referred to a third one. The corrected cut‐off values due to the length, for normal visualization of coronary arteries were 36.2 ± 2.6 frames for left anterior descending coronary artery, 22.2 ± 4.1 frames for left circumflex coronary artery, and 20.4 ± 3 frames for right coronary artery. 14 Any values obtained above these thresholds were considered to be CSF. All TIMI frame counts were measured in matched projections with use of Medcon Telemedicine Technology (version 1.900, Israel).
Intravascular Ultrasound
All patients enrolled in the study underwent IVUS investigation; 7Fr guiding catheter was positioned in the given coronary artery. Two micrograms of nitroglycerine was administered via intracoronary injection. “Endosonics in Visions Imaging System” was utilized during IVUS. The imaging catheter (The Endosonic Visions Five‐64 F/X catheter) had a 30 frames/second maximum frame rate and 20‐MHz single‐piezoelectric crystal transducer mechanically rotating at 1,800 rpm within a 3.5‐F monorail catheter, was advanced over the 0.014‐inch guide wire into the coronary artery as distally as possible and was then carefully pulled back to continuously image the wall morphology. The size of Judkins catheter was used to calibrate the length of the coronary segment. Images were analyzed frame‐by‐frame having the external elastic lamina border manually traced, maximal and minimal intimal thicknesses were measured within the same segment. The following criteria 15 were chosen for lesion characteristics and severity; Atherosclerotic plaque; in any segment intimal thickness ≥5 mm, eccentric lesion; if maximal thickening exceeded two‐fold minimal thickening, calcified lesion; focal or diffuse calcification leading to acoustic shadowing. All images were recorded on recordable compact disk for subsequent data analysis. Each IVUS image was analyzed off‐line by two independent experienced IVUS analysts.
Exercise Testing
All patients performed the modified Bruce protocol. Electrocardiograms were recorded using an exercise system (T2ooo Treadmill, Marquette Electronics Systems Inc., Millwaukee, WI, USA). The treadmill exercise end point was defined as: (1) reaching the maximum heart rate (220‐age), (2) tipical angina pectoris, (3) ST segment depression in the sequential two derivation with or without angina pectoris (>1.0 mm after J point, 60 ms horizontal or downsloping ST segment depression), (4) severe dispnea or syncope, (5) repeated ventricular arythmia, (6) >10 mmHg fall in systolic blood pressure at any stage.
Holter ECG Monitoring
Patients and controls were monitored for 24 hours using version 1.04 Flash Card digital Holter recorder. Recordings were evaluated on Holter for Windows version 3.6F computer (North East Monitoring Inc., USA) and time domain analyses of HRV are derived from statistical calculations performed on the set of normal‐to‐normal (NN) interbeat intervals: the average heart rate in beats per minute, standard deviation of all NN intervals (SDNN, ms), standard deviations of all NN intervals for all 5‐minute segments of the entire recording (SDNN index, ms), percentage of the differences of adjacent NN intervals greater 50 ms normalized to all differences within the interval (pNN50,%), the root mean square of adjacent differences of NN intervals (RMSSD, ms), total number of NN intervals divided by the height of the histogram of all NN intervals measured on discrete scale with bins of 7.8125 ms (1/128 s) (triangular index) and standard deviation of averages of NN intervals in all 5‐minute segments of the entire recording (SDANN, ms) were calculated. RMSSD and pNN50 primarily reflect parasympathetically mediated changes in heart rate. 16 The other time domain variables reflect a mixture of parasympathetic, sympathetic, and other physiologic influences. 16 Subjects with less than 22‐hour recordings or normal sinus beats less than 80% of total beats were excluded.
Analysis of Endothelin‐1
Plasma samples were drawn into chilled EDTA tubes (1 mg/ml blood) containing aprotinin (500 KIU/ml of blood). The whole blood samples were centrifuged at 1600 xg for 15 minutes at 0°C. The plasma fractions were transferred to a plastic tube and stored at −70°C for long term storage. After a short incubation period, the excess sample was washed out and a polyclonal antibody to ET‐1 was labeled and the enzyme horseradish peroxidase was added. This labeled antibody was bound to the ET‐1 and was captured on the plate. After a short incubation period, the excess labeled antibody was washed out and substrate was added. The substrate, which reacted with the labeled antibody was bound to the ET‐1 and was captured on the plate. The color generated with the substrate was read at 450 nm, and was directly proportional to the concentration of ET‐1 in the sample (Human Endothelin‐1, catalog no EIA‐3111, DRG International Inc., USA).
Analysis of Serum Nitrite and Nitrate
The levels of nitrite and nitrate were determined using a procedure based on the Griess reaction. Blood samples were centrifuged at 4000 rpm for 10 minutes. Serum samples were then separated and stored at −70°C until used for assay. Equal volumes of serum and potassium phosphate buffer were placed in an ultrafilter and centrifuged at 4000 rpm for 45 minutes. The ultrafiltrate was collected and used in the test. Nitrates were quantitatively converted to nitrites for analysis. Enzymatic reduction of nitrate to nitrite was carried out using coenzymes (NADPH, FAD) in the presence of nitrate reductase in step of incubation assay. N‐1‐(naphthyl) ethylenediamine dihydrochloride, sulfanilamide and incubation solutions were mixed at a ratio of 1:1:2 (v/v). These mixtures were incubated for 5 minutes at room temperature and measured at 540 nm. Sodium nitrite of 1.00 mM was used as standard for determination of nitrite and potassium nitrate of 80 mM was used as standard for determination.
Statistical Analysis
Statistical analysis was performed using SPSS software package (version 10.0, SPSS Inc., Chicago, Illinois, USA). Categoric variables were expressed as counts and percentages. Comparison of categoric variables was made by Pearson Chi‐square test. Continuous variables were defined as means ±SD. The time domain HRV parameters and other continuous variables of the patient groups and control group were compared with one‐way ANOVA test. The posthoc analysis was done by Bonferroni's test. Correlations were examined by Pearson's correlation. All hypothesis testing was 2‐tailed. The P values <0.05 were considered to be significant.
RESULTS
The clinical characteristics of study groups were given in Table 1. Groups were similar according to age, gender, systolic and diastolic blood pressures, total cholesterol level, current smoking, and family history of coronary heart disease. Two patients in group A had left bundle branch block. During contrast agent injection in the coronary angiography, ECG of seven patients (six patients in group A, one patient in group B) have showed 2–3 mm ST segment depression and five of them (all in group A) have developed angina pectoris. The exercise testings were stopped in eight patients (involving all patients in group A) because of ST segment depression over 2 mm and angina pectoris. In the control subjects, angina pectoris and ST segment depression were not observed during the coronary angiography and exercise testing.
Table 1.
Baseline clinical and laboratory characteristics of patients with coronary slow flow and controls
| Group A (n = 8) | Group B (n = 25) | Control group (n = 19) | |
|---|---|---|---|
| Age (year) | 50 ± 6 | 56 ± 9 | 54 ± 11 |
| Gender (female) n(%) | 3(37.5) | 14(56.0) | 10(52.6) |
| Total cholesterol (mg/dl) | 214.4 ± 39.0 | 228.4 ± 37.8 | 212.7 ± 32.1 |
| Diabetes mellitus n (%) | 1(12.5) | 2(8) | – |
| Smoking n (%) | 5(62.5) | 8(32) | 7(36.8) |
| Family history n (%) | 2(25) | 4(16) | 9(47.4) |
| Sistolic blood pressure (mmHg) | 140 ± 16 | 142 ± 14 | 132 ± 13 |
| Diastolic blood pressure (mmHg) | 81 ± 16 | 77 ± 20 | 75 ± 18 |
| End‐diastolic pressure (mmHg) | 11 ± 3 | 10 ± 5 | 10 ± 4 |
| TIMI frame counta (frame) | 55.3 ± 10.9 | 52.6 ± 11.8 | 26.2 ± 4.6 |
| Left ventricule ejection fraction (%) | 63.6 ± 6.5 | 66.8 ± 4.3 | 65.8 ± 6.4 |
| Endothelin 1 (pg/ml)b,c,d | 28.7 ± 17.4 | 15.9 ± 10.6 | 6.0 ± 5.7 |
| Nitric oxide (μmol/L)e | 23.4 ± 13.5 | 32.8 ± 22.7 | 42.5 ± 15.9 |
aP < 0.0001 (group A vs. control and group B vs. control); bP < 0.05 (group A vs. group B), cP < 0.0001 (group A vs. control), dP < 0.05 (group B and control), eP < 0.05 (group A vs. control).
Plasma ET‐1 levels were higher in the group A patients than that of group B and control group and higher in group B patients than that of control group. Although NOx levels in group A were lower than control group, the difference of NOx levels in the other groups was statistically insignificant (Table 1).
Time domain HRV parameters were decreased in all patient groups. This was more prominent in group A (Table 1). All time domain HRV parameters of patient groups and control group and their analysis with Bonferroni's test are shown in the Table 2. Additionally, HRV parameters were negatively correlated with ET‐1 levels and TIMI frame counts (Figs. 1 and 2, respectively). However, any correlation between HRV parameters and NOx levels was not detected. While, a positive correlation was observed between TIMI frame count and plasma ET‐1 levels, correlation between TIMI frame count and NOx levels was negative (r = 0.61, P = 0.0001, r =−0.31, P = 0.028, respectively).
Table 2.
Time domain heart rate variability (HRV) parameters of patients with coronary slow flow and controls
| HRV parameters | Group A (n = 8) | Group B (n = 25) | Control (n = 19) |
|---|---|---|---|
| Heart rate (beat/min) | 72 ± 13 | 71 ± 12 | 74 ± 15 |
| SDNN (ms)a,b | 103.0 ± 19.5 | 136.3 ± 44.7 | 170.4 ± 38.9 |
| SDANN (ms)c,d,e | 92.4 ± 12.8 | 121.3 ± 29.6 | 143.7 ± 24.4 |
| RMSSD (ms)f | 21.9 ± 9.5 | 28.3 ± 8.6 | 34.8 ± 12.1 |
| SDNN index (ms)c,d,e | 40.8 ± 13.8 | 70.1 ± 29.2 | 90.3 ± 18.0 |
| PNN 50 (%)d,g | 13.3 ± 5.7 | 18.4 ± 6.9 | 27.1 ± 8.9 |
| Triangular indexa,b | 18.5 ± 9.6 | 24.8 ± 8.3 | 33.7 ± 9.3 |
aP < 0.001 (group A vs. control); bP < 0.05 (group B vs. control); cP < 0.05 (group A vs. group B); dP < 0.0001 (group A vs. control), eP < 0.05 (group B vs. control), fP < 0.05 (group A vs. control), gP < 0.001 (group B vs. control).
Figure 1.
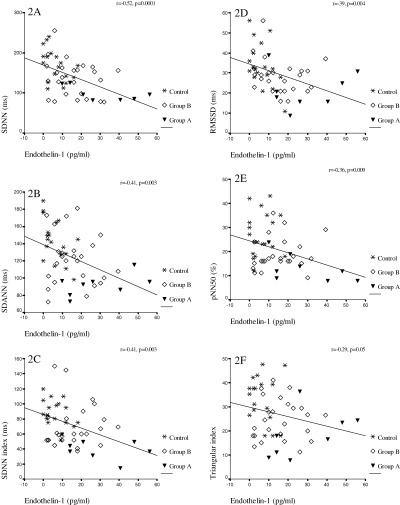
Correlation among HRV paremeters and endothelin‐1 (ET‐1) in patients' coronary slow flow. (A) between SDNN and ET‐1, (B) between SDANN and ET‐1, (C) between SDNN index and ET‐1 (D) between RMSSD and ET‐1, (E) between pNN50 and ET‐1, (F) between triangular index and ET‐1.
Figure 2.
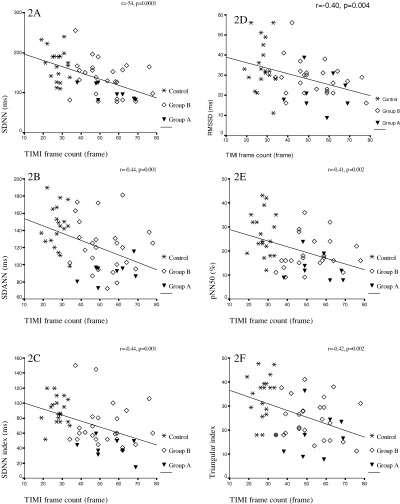
Correlation among HRV paremeters and TIMI frame count in patients with coronary slow flow. (A) between SDNN and TIMI frame count, (B) between SDANN and TIMI frame count, (C) between SDNN index and TIMI frame count (D) between RMSSD and TIMI frame count, (E) between pNN50 and TIMI frame count, (F) between triangular index and TIMI frame count.
Upon IVUS investigation, the common finding was longitudinally extended massive calcification throughout the epicardial arteries in 15 patients and regional calcification in eight patients. Mean intimal thickness was 0.50 ± 0.13 mm (group A; 0.58 ± 0.11 mm, group B 0.47 ± 0.12 mm P = 0.029) and in 14 patients eccentric lesions were observed (Fig. 3). All patients in group A had eccentric lesions and diffuse calcification.
Figure 3.
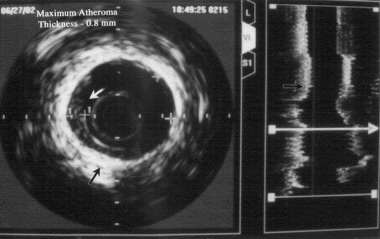
Intravascular ultrasound image in a patient with coronary slow flow showing longitudinally extended diffuse calcification throughout the epicardial artery and increased intimal thickness.
DISCUSSION
In many studies, 1 , 2 , 3 , 4 pathophysiology of CSF phenomenon is especially focused on microvascular disease. Furthermore, it is discusses as if it is a subgroup of syndrome X. However, in the present study, we found diffuse calcification and intimal thickening in all segments of the vessels by using IVUS despite the absence of focal stenosis or plaques in coronary angiography of CSF patients. According to these findings, we speculate that CSF may be a form or preliminary phase of diffuse atherosclerotic process that involve both small vessels and epicardial coronary arteries. Accordingly, some postmortem studies 17 , 18 revealed a coincidence of epicardial and small vessels disease, which supports our opinion. According to preliminary data, in the early phase of atherosclerosis or with intensive coronary artery disease risk factors, vasodilation capacity of coronary resistive arteriolles by pharmacologic and physical stress was disturbed before development of angiographic atherosclerotic disease. 19 , 20 Besides, IVUS imaging can detect intimal thickening and is suitable for detection of early atherosclerosis, which cannot be detected by conventional angiography. 15 , 21 , 22
Experimental studies on monkeys that fed with an atherogenic diet have demonstrated a relationship between resting heart rate and progression of coronary atherosclerosis, 23 , 24 and there was also a strong relationship between heart rate and arterial stiffness. 25 Reduced HRV and elevated heart rate result from altered cardiac autonomic regulation with sympathetic predominance and/or reduced vagal tone. Increased sympathetic tone with elevated catecholamine levels may have direct effects on vascular smooth muscle cells, 26 or it may affect other factors promoting the progression of atherosclerosis. 27
Endothelin‐1 and NOx play an important role in cardiovascular regulation and pathophysiology. 6 , 7 Endothelin‐1 has been shown to aggravate ischemia‐induced ventricular arrhythmias. Proposed mechanisms for the arrhythmogenic effects of ET‐1 are prolongation or increased dispersion of monophasic action potential duration, QT prolongation, development of early afterdepolarizations, acidosis, and augmentation of cellular injury. 28 Endothelin contributes to the progression of atherosclerosis and increased ET‐1 may substantially contribute to cell growth and the regulation of vascular tone in the very early stages of plaque evolution, when a plaque is clinically still imperceptible. 29 All these data support the hypothesis that elevated ET levels may have pathophysiologic significance in angiographically normal coronary arteries. Considering the autonomic regulation of coronary flow, a report using positron emission tomography demonstrated a correlation between time domain HRV and myocardial perfusion. 30 These data suggest that a low HRV, indicative of deficient autonomic function, may be related to coronary endothelial dysfunction and/or vasomotor disorders. Thus, impaired HRV levels could be the result of a deficient coronary perfusion. The results of this study show that baseline ET‐1 plasma levels were higher in patients with CSF than in control subjects. Additionally, we found that the time domain HRV parameters reduction is correlated to the increase in plasma ET‐1 levels. Thus, the autonomic impairment was significantly correlated to the degree of endothelial dysfunction and diffuse atherosclerosis in our study.
Angiographic evaluation of CSF was made visually since first defined by Temb et al. 1 Gibson et al. 14 found TIMI frame count system and especially used it to evaluate coronary artery patency and flow velocity after thrombolytic treatment in patients with acute myocardial infarction. Later, this system was used to evaluate flow velocity quantitatively in patients with CSF. 4 , 31 TIMI frame count system is an important method to detect a numeric value of anterograde blood flow objectively in patients with SCF.
In this study, HRV parameters were negatively correlated with ET‐1 and TIMI frame counts. While a positive correlation was observed between TIMI frame count and plasma ET‐1 levels, correlation between TIMI frame count and NOx was negative. This finding could indicate that TIMI frame count system is a valuable parameter showing correlation between increase in diffuse coronary arterial resistance and anterograde blood flow.
There is a dynamic interaction between sympathetic and parasympathetic effects on heart rate regulation. 32 However, time domain HRV parameters RMSSD and pNN50 primarily reflect vagal modulation. 16 In our study, RMSSD levels were lower in group A compared to control group. Also pNN50 levels were lower in both group A and group B compared to the control group. These findings may reflect that autonomic control of the cardiovascular system in patients with CSF is shifted toward a sympathetic predominance. Several previous studies reported indirect evidence of an increased adrenergic activity in patients with syndrome X,13,30,33,34 abnormalities in HRV and baroreflex sensitivity 13 , 30 , 34 , and prolonged QT and QTc interval. 33 , 35
The effects of adrenergic stimulation on coronary microvascular tone complex are controversial. Sympathetic activation increases coronary flow indirectly through metabolic vasodilation secondary to an increase in heart rate and myocardial contractility 36 and, in part, to endothelium‐mediated vasodilation. 37 On the other hand, it may have both direct vasodilator and vasoconstrictor effects through alfa‐receptor and beta‐receptor stimulation, respectively, 36 the net effect likely depending on the pathophysiologic state of small coronary arteries. Indeed, an increased sympathetic stimulation may cause of abnormal microvascular constriction in endothelial dysfunction. 38 Additionally, ET‐1 can modulate central and peripheral sympathetic outflow. 39 Aronson et al. have observed ET‐1 may play an important role in the autonomic dysfunction characteristic of congestive heart failure. 39 In the present study, we found the correlation between ET‐1 levels and time domain HRV parameters. These HRV parameters may show markers of neurohormonal activation in CSF. Autonomic imbalance may influence vasomotor control of the coronary circulation. This, in turn, may cause transient reductions in regional myocardial perfusion responsible for ischemia at rest but also may reduce coronary flow reserve and cause angina during effort40. Gould et al. 41 have observed a graded, longitudinal, base‐to‐apex myocardial perfusion gradient, indicating diffuse coronary arterial narrowing by noninvasive positron emission tomography in patients with diffuse atherosclerosis without dipyridamole‐induced segmental myocardial perfusion defects caused by flow‐limiting stenoses.
In conclusion, the present study demonstrated that increased plasma ET‐1 levels, decreased plasma NOx levels and diffuse atherosclerosis may decrease HRV parameters by decreasing myocardial blood flow in patients with CSF.
REFERENCES
- 1. Tambe AA, Demany MA, Zimmerman HA, et al Angina pectoris and slow flow velocity of dye in coronary arteries: A new angiographic finding. Am Heart J 1972;84: 66–71. [DOI] [PubMed] [Google Scholar]
- 2. Mosseri M, Yarom R, Gotsman MS, et al Histologic evidence for small vessel coronary artery disease in patients with angina pectoris and patent large coronary arteries. Circulation 1986;74: 964–972. [DOI] [PubMed] [Google Scholar]
- 3. Mangieri E, Macchiarelli G, Ciavolella M, et al Slow coronary flow: Clinical and histopathological features in patients with otherwise normal epicardial coronary arteries. Cathet Cardiovasc Diag 1996;37: 375–381. [DOI] [PubMed] [Google Scholar]
- 4. Kurtoglu N, Akcay A, Dindar I. Usefulness of oral dipyridamole therapy for angiographic slow coronary artery flow. Am J Cardiol 2001;87: 777–779. [DOI] [PubMed] [Google Scholar]
- 5. Sezgin AT, Sigirci A, Barutcu I. Vascular endothelial function in patients with slow coronary flow. Coron Artery Dis 2003;14: 155–161. [DOI] [PubMed] [Google Scholar]
- 6. Highsmith RF, Blackburn K, Schmidt DJ. Endothelin and calcium dynamics in vascular smooth muscle. Annu Rev Physiol 1992;54: 257–277. [DOI] [PubMed] [Google Scholar]
- 7. Egashira K, Katsuda Y, Mohri M, et al Role of endothelium‐derived nitric oxide in coronary vasodilatation induced by pacing tachycardia in humans. Circulation Research 1996;79: 331–335. [DOI] [PubMed] [Google Scholar]
- 8. Buus NH, Bottcher M, Bottker HE, et al Reduced vasodilator capacity in syndrome X related to structure and function of resistance arteries. Am J Cardiol 1999;83: 149–154. [DOI] [PubMed] [Google Scholar]
- 9. Kaski JC, Cox ID, Crook JR, et al, for the Coronary Artery Disease Research Group. Differential plasma endothelin levels in subgroups of patients with angina and angiographically normal coronary arteries.. Am Heart J 1998;136: 412–417. [DOI] [PubMed] [Google Scholar]
- 10. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability : Standards of measurement, physiological interpretation, and clinical use. Circulation 1996;93: 1043–1065. [PubMed] [Google Scholar]
- 11. Quintana M, Storck N, Lindblad LE, et al Heart rate variability as a means of assessing prognosis after acute myocardial infarction. Eur Heart J 1997;18: 789–797. [DOI] [PubMed] [Google Scholar]
- 12. Huikuri HV, Jokinen V, Syvanne M, et al Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol 1999;19: 1979–1985. [DOI] [PubMed] [Google Scholar]
- 13. Rosano GMC, Ponikowski P, Adamopoulos S, et al Abnormal autonomic control of the cardiovascular system in syndrome X. Am J Cardiol 1994;73: 1174–1179. [DOI] [PubMed] [Google Scholar]
- 14. Gibson CM, Cannon CP, Daley WL, et al TIMI frame count: A quantitative method of assessing coronary artery flow. Circulation 1996;93: 879–888. [DOI] [PubMed] [Google Scholar]
- 15. Tuzcu EM, Kapadia SR, Tutar E, et al High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults evidence from intravascular ultrasound. Circulation 2001;103: 2705–2710. [DOI] [PubMed] [Google Scholar]
- 16. Kleiger RE, Stein PK, Bosner MS, et al Time domain measurements of heart rate variability. Cardiol Clin 1992;10: 487–498. [PubMed] [Google Scholar]
- 17. James TN. Small arteries of the heart. Circulation 1977;56: 2–14. [DOI] [PubMed] [Google Scholar]
- 18. Ratcliffe HL, Redfield E. Atherosclerotic stenosis of the extramural and intramural coronary arteries of man. Related lesions. Virchows Arch A Pathol Pathol Anat 1972;357: 1–10. [DOI] [PubMed] [Google Scholar]
- 19. Chilian WM, Dellsperger KC, Layne SM, et al Effects of atherosclerosis on the coronary microcirculation. Am J Physiol 1990;258: H529–H539. [DOI] [PubMed] [Google Scholar]
- 20. Zeiher AM, Schachinger V, Hohnloser SH, et al Coronary atherosclerotic wall thickening and vascular reactivity in humans: Elevated high‐density lipoprotein levels ameliorate abnormal vasoconstriction in early atherosclerosis. Circulation 1994;89: 2525–2532. [DOI] [PubMed] [Google Scholar]
- 21. Nakatani S, Yamagishi M, Tamai J, et al Assessment of coronary artery distensibility by intravascular ultrasound application of simultaneous measurements of luminal area and pressure. Circulation 1995;91: 2904–2910. [DOI] [PubMed] [Google Scholar]
- 22. Mintz GS, Painter JA, Pichard AD, et al Atherosclerosis in angiographically “normal” coronary artery reference segments: An intravascular ultrasound study with clinical correlations. J Am Coll Cardiol 1995;25: 1479–1485. [DOI] [PubMed] [Google Scholar]
- 23. Kaplan JR, Manuck SB, Adams MR, et al Inhibition of coronary atherosclerosis by propranolol on behaviorally predisposed monkeys fed an atherogenic diet. Circulation 1987;86: 1364–1372. [DOI] [PubMed] [Google Scholar]
- 24. Kaplan JR, Manuck SB, Clarkson TB. The influence of heart rate on coronary atherosclerosis. J Cardiovasc Pharm 1987;10 (Suppl. 2): S100–S102. [PubMed] [Google Scholar]
- 25. Sa Cunha R, Pannier B, Benetos A, et al Association between high heart rate and high arterial rigidity in normotensive and hypertensive subjects. J Hypertension 1997;15: 1423–1430. [DOI] [PubMed] [Google Scholar]
- 26. Yu SM, Tsai SY, Guh JH, et al Mechanisms of catecholamine‐induced proliferation of vascular smooth muscle cells. Circulation 1996;94: 547–4. [DOI] [PubMed] [Google Scholar]
- 27. Dzau VJ, Sarbs FM. Regulation of lipoprotein metabolism by adrenergic mechanisms. J Cardiovasc Pharmacol 1987;10(Suppl. 9):S2–S6. [PubMed] [Google Scholar]
- 28. Duru F, Barton M, Luscher TF, et al Endothelin and cardiac arrhythmias: Do endothelin antagonists have a therapeutic potential as antiarrhythmic drugs Cardiovasc Res 2001;49: 272–280. [DOI] [PubMed] [Google Scholar]
- 29. Ihling C, Szombathy T, Bohrmann B, et al Coexpression of endothelin‐converting enzyme‐1 and endothelin‐1 in different stages of human atherosclerosis. Circulation 2001;104: 864–869. [DOI] [PubMed] [Google Scholar]
- 30. Meeder JG, Blanksma PK, Crijns HGJM, et al Mechanisms of angina pectoris in syndrome X assessed by myocardial perfusion dynamics and heart rate variability. Eur Heart J 1995;16: 1571–1577. [DOI] [PubMed] [Google Scholar]
- 31. Papadakis MC, Manginas A, Cotileas P, et al Documentation of slow coronary flow by the TIMI frame count in patients with coronary ectasia. Am J Cardiol 2001;88: 1030–1032. [DOI] [PubMed] [Google Scholar]
- 32. Kawada T, Sugimachi M, Shishido T, et al Simultaneous identification of static and dynamic vagosympathetic interactions in regulating heart rate. Am J Physiol 1999;276: R782–R789. [DOI] [PubMed] [Google Scholar]
- 33. Rosen SD, Dritsas A, Bourdillon PJ, et al Analysis of the electrocardiographic QT interval in patients with syndrome X. Am J Cardiol 1994;73: 971–972. [DOI] [PubMed] [Google Scholar]
- 34. Adamopoulos S, Rosano GMC, Ponikowski P, et al Impaired baroreflex sensitivity and sympathovagal balance in syndrome X. Am J Cardiol 1998;82: 862–868. [DOI] [PubMed] [Google Scholar]
- 35. Leonardo F, Fragasso G, Rosano GMC, et al Effect of atenolol on QT interval and dispersion in patients with syndrome X. Am J Cardiol 1994;73: 1174–1179. [DOI] [PubMed] [Google Scholar]
- 36. Feigl EO. Adrenergic control of transmural coronary blood flow. Basic Res Cardiol 1990;85(Suppl. I):167–176. [DOI] [PubMed] [Google Scholar]
- 37. Zehier AM, Drexler H, Wollschlaeger H, et al Coronary vasomotion in response to sympathetic stimulation in humans: Importance of the functional integrity of the endothelium. J Am Coll Cardiol 1989;14: 1181–1190. [DOI] [PubMed] [Google Scholar]
- 38. Cordero DL, Cagin NA, Natelson BH. Neurocardiology update: Role of the nervous system in coronary vasomotion. Cardiovasc Res 1995;29: 319–328. [PubMed] [Google Scholar]
- 39. Aronson D, Mittleman MA, Burger AJ. Role of endothelin in modulation of heart rate variability in patients with decompensated heart failure. Pacing Clin Electrophysiol 2001;24: 1607–1615. [DOI] [PubMed] [Google Scholar]
- 40. Ponikowski P, Rosano GM, Amadi AA, et al Transient autonomic dysfunction precedes ST‐segment depression in patients with syndrome X. Am J Cardiol 1996;77: 942–947. [DOI] [PubMed] [Google Scholar]
- 41. Gould KL, Nakagawa Y, Nakagawa K, et al Frequency and clinical implications of fluid dynamically significant diffuse coronary artery disease manifest as graded, longitudinal, base‐to‐apex myocardial perfusion abnormalities by noninvasive positron emission tomography. Circulation 2000;101: 1931–1939. [DOI] [PubMed] [Google Scholar]


