Abstract
Background: Premature ventricular contractions (PVC) at rest are frequently seen in heart failure (HF) patients but conflicting data exist regarding their importance for cardiovascular (CV) mortality. This study aims to evaluate the prognostic value of rest PVCs on an electrocardiogram (ECG) in patients with a history of clinical HF.
Methods and Results: We considered 352 patients (64 ± 11 years; 7 females) with a history of clinical HF undergoing treadmill testing for clinical reasons at the Veterans Affairs Palo Alto Health Care System (VAPAHCS) (1987–2007). Patients with rest PVCs were defined as having ≥1 PVC on the ECG prior to testing (n = 29; 8%). During a median follow‐up period of 6.2 years, there were 178 deaths of which 76 (42.6%) were due to CV causes. At baseline, compared to patients without rest PVCs, those with rest PVCs had a lower ejection fraction (EF) (30% vs 45%) and the prevalence of EF ≤ 35% was higher (75% vs 41%). They were more likely to have smoked (76% vs 55%).The all‐cause and CV mortality rates were significantly higher in the rest PVCs group (72% vs 49%, P = 0.01 and 45% vs 20%, P = 0.002; respectively). After adjusting for age, beta‐blocker use, rest ECG findings, resting heart rate (HR), EF, maximal systolic blood pressure, peak HR, and exercise capacity, rest PVC was associated with a 5.5‐fold increased risk of CV mortality (P = 0.004). Considering the presence of PVCs during exercise and/or recovery did not affect our results.
Conclusion: The presence of PVC on an ECG is a powerful predictor of CV mortality even after adjusting for confounding factors.
Ann Noninvasive Electrocardiol 2010;15(1):56–62
Keywords: rest premature ventricular contractions, heart failure, cardiovascular mortality
Our group has recently reported the prognostic value of premature ventricular contractions (PVC) noted on a routine electrocardiogram (ECG) in a general population of veterans 1 and then expanded these observations including a quantitative technique for assessing PVCs during exercise testing. 2 Patients with heart failure (HF) were excluded from this latter study and so attention is now directed to this important group using these technologies. The subset of those with HF and exercise testing enabled comparison of the rest PVC findings to quantified PVCs during exercise as well as to established risk predictors including exercise capacity and ejection fraction (EF). While the initial aim of this study was to study the prognostic impact of exercise‐induced PVCs, preliminary analyses directed our attention to the prognostic value of rest PVCs on a single resting 10‐second ECG in patients with clinical HF referred for exercise treadmill testing.
METHODS
Study Population
We studied 374 subjects referred to the Palo Alto Veterans Affairs Health Care System between 1997 and 2004 for clinical symptom‐limited treadmill testing who were tested on a device, QUEST (Burdick Corp, Deerfield, WI, USA) that enabled continuous digital ECG recording. We included all patients who were referred by their physician for exercise testing following a clinical presentation of heart failure. All patients needed to be in a stable and compensated cardiopulmonary condition prior to testing. We excluded patients with incomplete data (n = 22), leaving 352 subjects for analysis. Data on coronary risk factors, symptoms, medications, and prior cardiac events were gathered prior to exercise testing. The EF was assessed by echocardiography in standard fashions within 12 months of exercise testing. All subjects gave written informed consent and the study was approved by the Stanford University Institutional Review Board.
Exercise Testing
Subjects underwent symptom‐limited treadmill testing using an individualized ramp treadmill protocol and exercised to maximum exertion. 3 All tests began at a uniform speed of 2 miles/h and 0% grade. A pretest questionnaire was used to predict a target maximal MET level that would be reached within 10 minutes. 4 Heart rate targets were not used as an endpoint or to judge the adequacy of the test. Blood pressure (BP) was taken manually every 2 minutes and exercise capacity (in metabolic equivalents, METs) was calculated from treadmill speed and grade. 5 Subjects were encouraged to assume a supine position after exercise without a cool‐down walk. 6 No medications were changed or stopped prior to testing and the senior authors (VF, JM) overread all exercise studies.
Electrocardiography
Twelve‐lead ECG data were recorded continuously during the exercise test. Resting PVCs were considered to be present if any PVC was detected in the 10‐second ECG prior to exercise. PVCs were defined as at least one QRS complex that was premature, ectopic‐shaped and had a QRS duration ≥120 ms. All ECGs classified as having PVCs were manually overread by two cardiologists. The Q‐wave score represents anatomically based regions of the ECG and is used to evaluate myocardial damage. It is obtained by adding the number of myocardial territory (anterior, lateral, inferior) with pathological Q waves and has been shown to have significant predictive value for CV mortality. 1 Visual ST‐segment depression was measured at the J junction and ST slope was measured over the following 60 ms. Heart rate (HR) was recorded from the ECG standing at rest, at each 60‐second interval during the test and at peak exercise. Resting HR was recorded in the standing position. Arrhythmias during exercise testing were classified as previously defined. 2
Follow‐Up
We only considered cardiovascular (CV) mortality outcomes. Mortality data were gathered from the Social Security Death index and California Death registry. Cause of death was determined from the registry classification and confirmed using the VA medical record. CV mortality was defined as death of subjects from a clearly identifiable CV cause or death of subjects with a history of CV disease and no identifiable non‐CV cause for death. Classification was made by consensus of two observers blinded to the exercise test results with conflicts resolved by the senior author (VFF). The median follow‐up time for the total study group was 6.2 years (range 5.5–6.7 years).
Statistical Analysis
Clinical and exercise variables according to the presence of PVCs during rest and according to outcomes were compared using chi‐square tests (categorical variables) and unpaired t‐tests (continuous variables). Nonnormally distributed variables were compared using the Mann‐Whitney U test.
Age‐adjusted Cox regression analysis was used to assess the independent prognostic value of clinical variables and exercise variables. Multivariate Cox proportional hazards analyses were calculated to evaluate the combined ability of various rest and exercise variables to predict CV mortality.
Kaplan‐Meier analysis was used to evaluate the relationship between rest PVCs and CV mortality.
Statistical analyses were performed using NCSS (NCSS Inc., Salt Lake City, UT, USA). A two‐sided P value less than 0.05 was considered statistically significant.
RESULTS
Baseline Characteristics
The study cohort consisted of 352 patients (mean age ± SD: 64 ± 11 years) of whom 2% were females. There were 178 (50.6%) deaths during follow‐up and 76 (42.7%) of these deaths were due to CV causes.
Baseline characteristics and test variables according to the presence of rest PVCs are presented in Table 1. Twenty‐nine subjects (8.2%) presented ≥1 PVC on the rest ECG prior to treadmill testing. At baseline, compared to patients without rest PVCs, those with rest PVCs had a lower EF [30 (25–50%) vs 45 (40–45%); P = 0.04] and the prevalence of EF ≤ 35% was higher (75.0% vs 41.1%; P = 0.02). They were more likely to have smoked at some point in their lives (75.9% vs 54.8%; P = 0.03) and to be receiving digoxin treatment (51.7% vs 31.0%; P = 0.02), but were less likely to be using beta‐blockers (10.3% vs 29.4%; P = 0.03). The all‐cause and CV mortality rates are significantly higher in the rest PVCs group than in those without rest PVCs (72.4% vs 48.6%, P = 0.01 and 44.8% vs 19.7%, P = 0.002; respectively).
Table 1.
Baseline Characteristics According to the Presence of Rest PVCs
| Characteristics | Entire Population n = 352 | No Rest PVCs n = 323 (91.8) | Rest PVCs n = 29 (8.2) | p Value |
|---|---|---|---|---|
| Age (years) | 64 ± 10 | 64 ± 11 | 66 ± 8 | 0.18 |
| BMI (kg/m2) | 27.8 (26.6–28.3) | 28.0 (26.6–28.5) | 25.9 (24–29.7) | 0.18 |
| Females – no. (%) | 7 (2.0) | 7 (2.2) | 0 | 0.42 |
| Diabetes – no. (%) | 77 (21.9) | 68 (21.1) | 9 (31.0) | 0.21 |
| Claudication – no. (%) | 45 (12.8) | 42 (13.0) | 3 (10.3) | 0.68 |
| Hypertension – no. (%) | 240 (68.2) | 216 (66.9) | 24 (82.8) | 0.08 |
| CAD – no. (%) | 248 (70.5) | 224 (69.3) | 24 (82.8) | 0.13 |
| Stroke – no. (%) | 31 (8.8) | 29 (9.0) | 2 (6.9) | 0.7 |
| Smoking | ||||
| Ever – no. (%) | 199 (56.5) | 177 (54.8) | 22 (75.9) | 0.03 |
| Currently – no. (%) | 72 (20.5) | 68 (21.1) | 4 (13.8) | 0.35 |
| Angina Pectoris | ||||
| Typical – no. (%) | 79 (22.4) | 71 (22.0) | 8 (27.6) | 0.49 |
| Atypical – no. (%) | 70 (20.0) | 64 (19.8) | 6 (20.7) | 0.91 |
| COPD – no. (%) | 38 (10.8) | 33 (10.2) | 5 (17.2) | 0.24 |
| Beta‐blocker – no. (%) | 98 (27.8) | 95 (29.4) | 3 (10.3) | 0.03 |
| ACEI/ARB – no. (%) | 130 (36.9) | 119 (36.8) | 11 (37.9) | 0.91 |
| Diuretic – no. (%) | 43 (12.2) | 41 (12.7) | 2 (6.9) | 0.36 |
| Nitrate – no. (%) | 123 (34.9) | 109 (33.7) | 14 (48.3) | 0.12 |
| Digoxin – no. (%) | 115 (32.7) | 100 (31.0) | 15 (51.7) | 0.02 |
| Statin – no. (%) | 40 (11.4) | 38 (11.8) | 2 (6.9) | 0.43 |
| Anticoagulant – no. (%)* | 128 (36.4) | 122 (37.8) | 6 (20.7) | 0.07 |
| Calcium‐ Channel B – no. (%) | 101 (28.7) | 95 (29.4) | 6 (20.7) | 0.32 |
| LVEF (%) | 40 (35–45) | 45 (40–45) | 30 (25–50) | 0.04 |
| LV EF >50%– no. (%) | 69 (19.6) | 66 (20.4) | 3 (10.3) | 0.25 |
| LVEF <35%– no. (%) | 74 (20.7) | 65 (20.1) | 9 (31.0) | 0.02 |
| Q score† | 0.31 | |||
| 0 | 259 (73.6) | 241 (74.6) | 18 (62.1) | |
| 1 | 70 (20) | 61 (18.9) | 9 (31.0) | |
| 2 | 16 (4.6) | 14 (4.3) | 2 (6.9) | |
| 3 | 7 (2) | 7 (2.2) | 0 (0) | |
| Deaths | ||||
| – All‐cause – no. (%) | 178 (50.6) | 157 (48.6) | 21 (72.4) | 0.01 |
| – CV deaths – no. (%) | 76 (21.8) | 63 (19.7) | 13 (44.8) | 0.002 |
Results are presented as number (%), mean (±SD) or median (95%CI). t‐test or chi‐square tests between groups for continuous and categorical variables, respectively. Mann‐Whitney test used for nonnormally distributed variables. PVC = premature ventricular contraction; BMI = body mass index; CAD = coronary artery disease; COPD = chronic obstructive pulmonary disease; ACEI = angiotensin‐conversion ezyme inhibitor; ARB = angiotensin‐receptor blocker; LVEF = left ventricular ejection fraction; CV = cardiovascular. *included asa, coumadin, plavix. †number of myocardial territories with pathological Q waves on a 12‐lead rest ECG.
Exercise variables according to the presence of rest PVCs are presented in Table 2. Subjects with rest PVCs had higher standing rest HR (84 ± 15 vs 78 ± 17 beats/min; P = 0.04) and at peak exercise, they demonstrated a lower exercise capacity [5.0 (3.2–5.3) vs 5.1 (5.0–5.8) METs; P = 0.05].
Table 2.
Exercise Test Variables According to the Presence of Rest and Exercise PVCs
| Variables | Entire Population n = 352 | No Rest PVCs n = 323 (91.8) | Rest PVCs n = 29 (8.2) | p Value | No Exercise PVCs n = 318 (90.3) | Exercise PVCs N = 34 (9.7) | p Value |
|---|---|---|---|---|---|---|---|
| Standing resting HR (beats/min) | 78 ± 16 | 78 ± 17 | 84 ± 15 | 0.04 | 78± 17 | 79 ± 15 | 0.75 |
| Peak HR (beats/min) | 128 ± 26 | 128 ± 26 | 128 ± 28 | 0.91 | 127 ± 26 | 129 ± 26 | 0.68 |
| Heart rate reserve (beats/min) | 49 ± 22 | 49 ± 22 | 44 ± 21 | 0.18 | 49 ±22 | 50 ± 23 | 0.78 |
| Resting SBP (mm Hg) | 130 ± 21 | 130 ± 21 | 131 ± 22 | 0.73 | 129 ± 21 | 135 ± 18 | 0.13 |
| Peak SBP (mm Hg) | 162 ± 33 | 162 ± 33 | 162 ± 31 | 0.96 | 162 ± 33 | 163 ± 28 | 0.81 |
| Angina | |||||||
| Stopped the test – no. (%) | 40 (11.4) | 39 (12.1) | 1 (3.4) | 0.16 | 36 (11.3) | 4 (11.8) | 0.94 |
| ST depression | |||||||
| Stopped the test – no. (%) | 19 (5.4) | 19 (5.9) | 0 | 0.18 | 16 (5.0) | 3 (8.8) | 0.35 |
| Borg's Exertion Score | 17 (17–18) | 17 (!7–18) | 17 (15–19) | 0.68 | 17 (17–18) | 17 (15–18) | 0.23 |
| Exercise capacity (METs) | 5.0 (5.0–5.4) | 5.1 (5.0–5.8) | 5.0 (3.2–5.3) | 0.05 | 5 (5–5.7) | 4.8 (3.6–5.4) | 0.12 |
Results are presented as number (%), mean (±SD) or median (95%CI). t ‐test or chi‐square tests used between groups for continuous and categorical variables, respectively. Mann‐Whitney test used for nonnormally distributed variables. PVC = premature ventricular contraction; HR = heart rate; SBP = systolic blood pressure.
Outcomes According to the Occurrence of Rest and Exercise PVCs
Results of Kaplan‐Meier survival analysis for CV mortality outcomes according to the presence of rest PVCs are presented in Figure 1. Results of multivariate Cox regression analysis for CV mortality outcomes are presented in Table 3. Rest EF, the Q‐Wave Score, the presence of rest PVCs, and the absence of rest and exercise PVCs were all significant age‐adjusted predictors of CV mortality (not shown in tables). After adjusting for age, beta‐blocker use, rest ECG findings, resting and peak HR, rest EF, maximal systolic BP, and exercise capacity, the presence of rest PVC was associated with a 5.48‐fold increased risk of CV mortality (Table 3; P = 0.004). Considering the presence of PVCs during exercise testing in the model significantly increased the risk of CV mortality associated with rest PVCs (Table 4). After adjusting for age, beta‐blocker use, rest ECG findings, resting and peak HR, rest EF ≤ 35%, maximal systolic BP, and exercise capacity, only a rest EF ≤ 35% (HzR = 4.63; P = 0.0001) and the presence of rest PVCs (HzR = 2.09; P = 0.03) were predictive of CV mortality (not shown in table).
Figure 1.
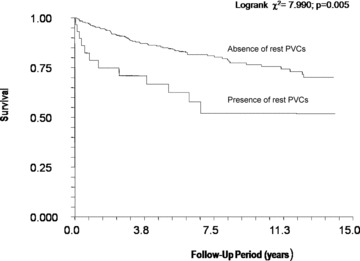
Kaplan‐Meier analysis of the associations between rest premature ventricular contractions and cardiovascular mortality.
Table 3.
Multivariate COX Regression Analysis for CV Mortality*
| Test Variables | Hazard Ratio | p Value |
|---|---|---|
| Rest EF | 0.94 | 0.006 |
| Rest PVCs | 5.48 | 0.004 |
| Age | 1.01 | 0.73 |
| Standing resting HR (beats/min) | 1 | 0.99 |
| Peak HR (beats/min) | 1 | 0.98 |
| Peak SBP (mm Hg) | 0.99 | 0.08 |
| Exercise capacity (METs) | 0.15 | 0.72 |
| Q score | 0.9 | 0.8 |
| Beta‐blocker Use | 0.58 | 0.37 |
*From Cox regression analysis. EF = ejection fraction; HR = heart rate; SBP = systolic blood pressure; PVC = premature ventricular contraction.
Table 4.
Multivariate COX Regression Analysis for CV Mortality*
| Test Variables | Hazard Ratio | p Value |
|---|---|---|
| Rest EF | 0.94 | 0.004 |
| Rest PVCs | 8.98 | 0.003 |
| Presence of exercise/recovery PVCs | 0.78 | 0.3 |
| Age | 1.01 | 0.71 |
| Standing resting HR (beats/min) | 1 | 0.89 |
| Peak HR (beats/min) | 1 | 0.86 |
| Peak SBP (mm Hg) | 0.99 | 0.16 |
| Exercise capacity (METs) | 0.15 | 0.71 |
| Q score | 0.86 | 0.7 |
| Beta‐blocker Use | 0.55 | 0.34 |
*From Cox regression analysis. EF = ejection fraction; HR = heart rate; SBP = systolic blood pressure; PVC = premature ventricular contraction.
In the group without rest PVCs, the presence of exercise and/or recovery PVCs did not increase the CV mortality risk. The absence of exercise and/or recovery PVCs in this group was not associated with a significantly decrease risk of CV mortality in multivariate analyses (not shown in table, HzR = 0.57; P = 0.24).
DISCUSSION
PVCs at rest are common findings in patients with and without structural heart diseases. Since sudden cardiac death in patients with myocardial infarct (MI) was thought to be due to ventricular fibrillation, it has been hypothesized that the detection of PVCs and its subsequent drug suppression can prevent these fatal events. 7 However, due to disappointing CAST studies results in the late 1980s, 8 and the lack of evidence supporting the benefit of their suppression with antiarrhythmic medications, 9 PVCs are now generally ignored on the routine ECG.
Still, conflicting data exist regarding their prognostic importance for the prediction of CV mortality in various cardiac populations. 9 , 10 , 11 , 12 , 13 The most studied group was patients with a recent MI. The presence of PVC in this group has been related to ischemia, 14 degree of LV involvement, 15 , 16 and degree of coronary artery stenosis. 17 Marked heterogeneity of myocardial electrophysiological properties due to stretching and scarring, increased activity of the sympathetic system, and electrolyte abnormalities are among the numerous mechanisms implicated in the genesis of ventricular arrhythmia. 18 , 19 Since these abnormalities are particularly common in HF, it could be hypothesized that PVCs could help identify patients at higher CV mortality risk.
Rest PVCs were observed in 8% of our HF clinical cohort, which is comparable with the rates of 2–7% in previous studies. 9 , 20 , 21 , 22 In an unpublished study conducted at our institution on routine preparticipation ECG study and college athletes, 4% had rest PVCs on 12‐lead ECG. Compared to the general population, the prevalence of rest PVCs is therefore higher in HF patients.
In this study, the identification of ≥1 PVC on a routine 10‐second ECG was independently associated with a 5.48‐fold increased risk of CV mortality. Maggioni et al., 23 in the GISSI‐2 study, had reported that a PVC frequency using 24‐hour recordings of ≥10/hour independently predicted total and all‐cause mortality in patients after a MI in the fibrinolytic era and in patients with HF. It is plausible that the presence of PVCs on a single routine rest ECG, a less time‐consuming test than a 24‐hour Holter, likely reflects the high density of PVCs in those patients. The surprising finding in our HF patients was that resting PVCs on the pretest ECG were associated with a stronger prognostic value than any exercise‐associated PVC pattern contrary to our results in non‐HF patients. 2 In this study, when rest PVCs were present, neither induced ventricular ectopy during exercise nor in the recovery period was significantly associated with an adverse prognosis. This lack of association with outcomes demonstrated that exercise‐induced PVCs were of limited prognostic significance in our HF clinical population and the strong prognostic value of rest PVCs. O’Neill et al., 24 in a study of 2123 consecutive patients with LVEF ≤ 35%, demonstrated severe ventricular ectopy during recovery after exercise to be the only variable predictive of increased all‐cause mortality in patients with severe HF. In the study, the presence of severe ventricular ectopy at rest was correlated with severe ventricular ectopy during recovery. Our data did not allow us to analyze further outcomes according to the frequency and/or occurrence of PVCs during recovery period. It is possible that categorizing further the occurrence of exercise‐related PVCs could have affected our results.
The group with rest PVCs had a lower EF. Previous studies have evaluated the relation between LV function and ventricular dysrhythmia using 24‐hour Holter monitoring tests 15 , 25 , 26 PVCs have been shown to correlate with extent of wall motion abnormality, ejection fraction, category of disease and age. 26 The results obtained in this study are therefore concordant with previous findings.
Increased activity of sympathetic system, 27 , 28 transient electrolyte abnormalities, and marked heterogeneity of electrophysiological properties due to segmental myocardial wall abnormalities, have been implicated in the genesis of PVCs. 18 , 19 , 29 In our study, the group with rest PVCs had higher rest HR. Increased sympathetic activity has been implicated in the development of ventricular ectopy at rest and during exercise. 27 , 28 Moreover, ventricular arrhythmia can be due to reentry, triggered activity, and enhanced automaticity, all of which are markedly potentiated by the action of catecholamines. 30 Recently, work from our group has demonstrated the independent association of HR with rest PVCs. 22 Also, the combination of PVCs on a resting ECG and increased HR was shown to be associated with a dramatic increase of all‐cause and CV mortality, suggesting a hyperadrenergic influence in patients with rest PVCs. Thus, in this study with the HF population, the higher rest HR observed in the group with rest PVCs may also result from the increased sympathetic activity, which can contribute to the adverse prognosis of these patients.
Our study has several limitations. The first is that the data set was collected using specialized equipment that made it possible to quantify PVCs using Holter‐like software. This makes our study sample a convenience sample rather than a consecutive series of patients. Our study population comprised almost entirely older males, and therefore may not be generalizable to other age groups or to women. The small sample size must also be considered a limitation. It remains possible that this study was not adequately powered to detect associations of lesser magnitude in our clinical population. Also, the predictive value of rest PVCs in patients with EF ≥35% could not be assessed in this study because it was underpowered for this analysis.
Finally, the ECGs were obtained over the span of more than two decades during which time there were significant changes in practice patterns and drugs usage. Only 28% of the subjects were prescribed a beta‐blocking agent on the test date, reflecting the era of the data. However, rest PVCs remained a powerful predictor of CV mortality after adjusting for drugs use.
Despite the advances in therapy, HF carries a high annual mortality and management of HF patients continues to present a challenge. Therefore, identifying patients at high risk of CV death is crucial to appropriately select patients for advanced therapies. Various clinical predictors such as gender, NYHA functional class, EF, and resting laboratory measures, such as natremia, blood urea nitrogen, and BNP have been integrated into HF scores to help clinicians identify patients at higher mortality risk. 31 This study demonstrates that the presence of any PVC on a routine 10‐second ECG, a less time‐consuming test than a 24‐hour Holter, in patients with moderate HF is a powerful predictor of CV mortality even after adjusting for confounding factors such as age, exercise capacity, and EF. This finding should be considered as hypothesis generating and be validated in prospective studies before it is applied clinically.
Acknowledgments
Acknowledgment: The authors would like to thank their colleagues, patients, and staff in the Palo Alto Veterans Affairs Health Care System for their assistance in conducting this study.
Author Contributions: Vy‐Van Le and Teferi Mitiku both contributed equally to the manuscript. They had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Myers, Froelicher.
Acquisition of data: Hadley, Myers, Froelicher.
Analysis and interpretation of data: Hadley, Froelicher.
Critical revision of the manuscript for important intellectual content: Myers, Froelicher.
Statistical analysis: Le, Mitiku, Myers, Froelicher.
Obtained funding: N/A.
Administrative, technical, or material support: Hadley, Myers, Froelicher.
Study supervision: Myers, Froelicher.
Financial Disclosures: Dr. David Hadley is a stock holder in Cardiac Science.
Funding/Support: None.
REFERENCES
- 1. Engel G, Beckerman JG, Froelicher VF, et al Electrocardiographic arrhythmia risk testing. Curr Probl Cardiol 2004;29:365–432. [DOI] [PubMed] [Google Scholar]
- 2. Dewey FE, Kapoor JR, Williams RS, et al Ventricular arrhythmias during clinical treadmill testing and prognosis. Arch Intern Med 2008;168:225–234. [DOI] [PubMed] [Google Scholar]
- 3. Myers J, Buchanan N, Walsh D, et al Comparison of the ramp versus standard exercise protocols. J Am Coll Cardiol 1991;17:1334–1342. [DOI] [PubMed] [Google Scholar]
- 4. Myers J, Do D, Herbert W, et al A nomogram to predict exercise capacity from a specific activity questionnaire and clinical data. Am J Cardiol 1994;73:591–596. [DOI] [PubMed] [Google Scholar]
- 5. American College of Sports Medicine . Guidelines for Exercise Testing and Prescription. Philadelphia , PA , Lippincott, Williams, and Wilkins, 2000. [Google Scholar]
- 6. Lachterman B, Lehmann KG, Abrahamson D, et al “Recovery only” ST‐segment depression and the predictive accuracy of the exercise test. Ann Intern Med 1990;112:11–16. [DOI] [PubMed] [Google Scholar]
- 7. Lown B, Wolf M. Approaches to sudden death from coronary heart disease. Circulation 1971;44:130–142. [DOI] [PubMed] [Google Scholar]
- 8. The Cardiac Arrhythmia Suppression Trial II Investigators. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction . N Engl J Med 1992;327:227–233. [DOI] [PubMed] [Google Scholar]
- 9. Singh SN, Fisher SG, Carson PE, et al Prevalence and significance of nonsustained ventricular tachycardia in patients with premature ventricular contractions and heart failure treated with vasodilator therapy. Department of Veterans Affairs CHF STAT Investigators. J Am Coll Cardiol 1998;32:942–947. [DOI] [PubMed] [Google Scholar]
- 10. Connolly SJ, Cairns JA. Comparison of one‐, six‐ and 24‐hour ambulatory electrocardiographic monitoring for ventricular arrhythmia as a predictor of mortality in survivors of acute myocardial infarction. CAMIAT Pilot Study Group. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial. Am J Cardiol 1992;69:308–313. [DOI] [PubMed] [Google Scholar]
- 11. Doval HC, Nul DR, Grancelli HO, et al Nonsustained ventricular tachycardia in severe heart failure. Independent marker of increased mortality due to sudden death. GESICA‐GEMA Investigators. Circulation 1996;94:3198–3203. [DOI] [PubMed] [Google Scholar]
- 12. Szabo BM, Van Veldhuisen DJ, Crijns HJ, et al Value of ambulatory electrocardiographic monitoring to identify increased risk of sudden death in patients with left ventricular dysfunction and heart failure. Eur Heart J 1994;15:928–933. [DOI] [PubMed] [Google Scholar]
- 13. Teerlink JR, Jalaluddin M, Anderson S, et al Ambulatory ventricular arrhythmias in patients with heart failure do not specifically predict an increased risk of sudden death. PROMISE (Prospective Randomized Milrinone Survival Evaluation) Investigators. Circulation 2000;101:40–46. [DOI] [PubMed] [Google Scholar]
- 14. Mukharji J, Rude RE, Poole WK, et al Risk factors for sudden death after acute myocardial infarction: Two‐year follow‐up. Am J Cardiol 1984;54:31–36. [DOI] [PubMed] [Google Scholar]
- 15. Bigger JT, Jr ., Fleiss JL, Kleiger R, et al The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation 1984;69:250–258. [DOI] [PubMed] [Google Scholar]
- 16. Tracy CM, Winkler J, Brittain E, et al Determinants of ventricular arrhythmias in mildly symptomatic patients with coronary artery disease and influence of inducible left ventricular dysfunction on arrhythmia frequency. J Am Coll Cardiol 1987;9:483–488. [DOI] [PubMed] [Google Scholar]
- 17. Minisi AJ, Mukharji J, Rehr RB, et al Association between extent of coronary artery disease and ventricular premature beat frequency after myocardial infarction. Am Heart J 1988;115:1198–1201. [DOI] [PubMed] [Google Scholar]
- 18. Chakko CS, Gheorghiade M. Ventricular arrhythmias in severe heart failure: Incidence, significance, and effectiveness of antiarrhythmic therapy. Am Heart J 1985;109:497–504. [DOI] [PubMed] [Google Scholar]
- 19. Larsen L, Markham J, Haffajee CI. Sudden death in idiopathic dilated cardiomyopathy: Role of ventricular arrhythmias. Pacing Clin Electrophysiol 1993;16:1051–1059. [DOI] [PubMed] [Google Scholar]
- 20. Kostis JB, Byington R, Friedman LM, et al Prognostic significance of ventricular ectopic activity in survivors of acute myocardial infarction. J Am Coll Cardiol 1987;10:231–242. [DOI] [PubMed] [Google Scholar]
- 21. Simpson RJ, Jr ., Cascio WE, Schreiner PJ, et al Prevalence of premature ventricular contractions in a population of African American and white men and women: The Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002;143:535–540. [DOI] [PubMed] [Google Scholar]
- 22. Engel G, Cho S, Ghayoumi A, et al Prognostic significance of PVCs and resting heart rate. Ann Noninvasive Electrocardiol 2007;12:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maggioni AP, Zuanetti G, Franzosi MG, et al Prevalence and prognostic significance of ventricular arrhythmias after acute myocardial infarction in the fibrinolytic era. GISSI‐2 results. Circulation 1993;87:312–322. [DOI] [PubMed] [Google Scholar]
- 24. O’Neill JO, Young JB, Pothier CE, et al Severe frequent ventricular ectopy after exercise as a predictor of death in patients with heart failure. J Am Coll Cardiol 2004;44:820–826. [DOI] [PubMed] [Google Scholar]
- 25. Califf RM, Burks JM, Behar VS, et al Relationships among ventricular arrhythmias, coronary artery disease, and angiographic and electrocardiographic indicators of myocardial fibrosis. Circulation 1978;57:725–732. [DOI] [PubMed] [Google Scholar]
- 26. Uretz EF, Denes P, Ruggie N, et al Relation of ventricular premature beats to underlying heart disease. Am J Cardiol 1984;53:774–780. [DOI] [PubMed] [Google Scholar]
- 27. Lown BCA, Armington R, Ryan M. Monitoring for serious arrhythmias and high risk of sudden death. Circulation 1975;52:III189–III198. [PubMed] [Google Scholar]
- 28. Lown BVR. Neural activity and ventricular fibrillation. N Engl J Med 1976;294:1165–1170. [DOI] [PubMed] [Google Scholar]
- 29. Podrid PJ, Fogel RI, Fuchs TT. Ventricular arrhythmia in congestive heart failure. Am J Cardiol 1992;69:82G–95G; discussion 95G–96G. [DOI] [PubMed] [Google Scholar]
- 30. Dries DL, Verdino RJ, Kowal RC. Postexercise severe ventricular ectopy in heart failure patients: New marker for aggregate risk. J Am Coll Cardiol 2004;44:827–828. [DOI] [PubMed] [Google Scholar]
- 31. Myers JAR, Dewey F, Bensimhon D, et al A cardiopulmonary exercise testing score for predicting outcomes in patients with heart failure. Am Heart J 2008;156:1177–1183. [DOI] [PubMed] [Google Scholar]


