Abstract
Congenital disorders, such as dextrocardia and persistent left superior vena cava, are rare. However, their presence is often associated with other cardiac anomalies, and may lead to lethal ventricular tachyarrhythmias, which result in sudden cardiac death. Treating patients with these disorders can present a challenge to clinicians, as it may cause technical difficulties during interventional procedures, and more often, altered defibrillation techniques in a setting of prehospital sudden cardiac arrest. This report describes the first case of successful defibrillation therapy delivered by the wearable cardioverter defibrillator to a patient with dextrocardia and persistent left superior vena cava during a ventricular tachycardia arrest.
Keywords: electrophysiology ‐ cardiac arrest/sudden death, electrophysiology ‐ ventricular tachycardia, cardiac anatomy
CASE SUMMARY
A 44‐year‐old male presented to the hospital with a fever and was admitted with endocarditis involving an implantable cardioverter defibrillator (ICD). The ICD was implanted six years prior for sustained ventricular tachycardia (VT) in the setting of valvular cardiomyopathy. He had a history of dextrocardia with a persistent left superior vena cava (PLSVC), aortic valve disease, obesity and obstructive sleep apnea, and dilated cardiomyopathy. The patient underwent aortic valve replacement and repair of an ascending aortic aneurysm six years before admission. Several months before the admission his ICD was replaced after receiving inappropriate shocks due to a lead malfunction. One month before admission he was admitted due to a gastrointestinal disturbance and developed methicillin‐sensitive Staphylococcus aureus (MSSA) bacteremia. A tranesophageal echocardiogram during the current admission showed vegetation on the defibrillator lead and he underwent extraction of the entire ICD system.
At the time explantation, his transthoracic echocardiogram demonstrated a reduced left ventricular ejection fraction of ≤20%. His ECG showed atrial fibrillation with premature ventricular or aberrantly conducted complexes, and a prolonged QT interval. His chest x‐ray is shown in Figure 1. In light of these findings, he was prescribed a wearable cardioverter defibrillator (WCD) to protect him from sudden cardiac death while his infection cleared.
Figure 1.
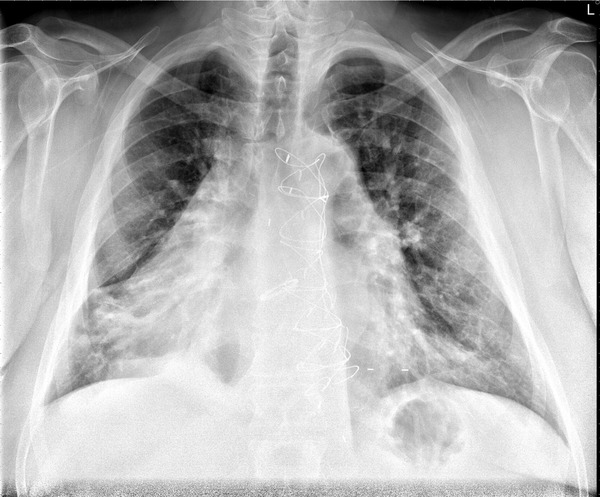
Chest x‐Ray showing a dextroversion of the heart (portable semi‐upright anterior‐posterior view of the chest).
Description of the Event
One month after discharge, the patient collapsed suddenly at home. He was in the bathroom when he experienced a sudden onset of lightheadedness followed by syncope. The electrocardiogram (ECG; Fig. 2) at the time of the event revealed atrial fibrillation with a rapid wide‐QRS ventricular response, at a rate of 115 beats per minute (BPM). Fifteen seconds later, the ECG revealed monomorphic VT at 179 BPM. The WCD detected the VT within 14 seconds of onset and defibrillated the patient 33 seconds later (no response button use during the alarm sequence) with a 150 joule shock.
Figure 2.
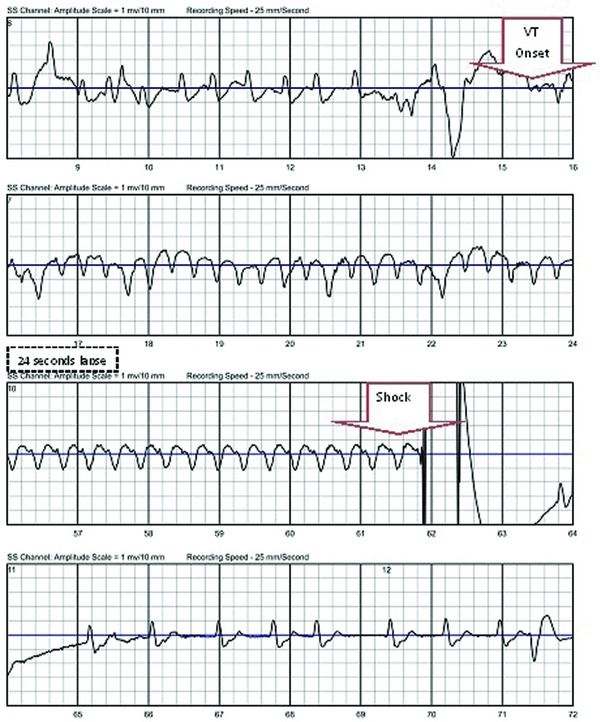
Treatment electrocardiogram recorded by wearable cardioverter defibrillator.
After a brief period of electrical stunning, the patient's rhythm converted into atrial fibrillation with a wide‐QRS, having a ventricular rate of 61 BPM. The patient regained consciousness and pressed the WCD's response buttons briefly six minutes after the defibrillation. He was found by his father; then sent to the emergency room and admitted on the same day.
DISCUSSION
This has been the first case of dextrocardia patient who has been treated by a wearable defibrillator.
Dextrocardia is a rare congenital structural abnormality defined as having the apex of the heart directed towards the right. It has a reported incidence of approximately 1–2 per 10,000 live births.1 Based on the position of atria and visceral organs, dextrocardia has three variations: situs solitus, situs inversus, and situs ambiguous. In situs solitus, also called dextroversion, atriums, visceral organs are in their normal positions but the cardiac apex is pivoting to the right side of the chest.1 In our case report, the patient's clinical presentation is consistent with dextroversion and situs solitus. The heart chambers were in normal relationship with the apex pointing to the right.
PLSVC is a congenital thoracic venous anomaly with a prevalence of 0.3–0.5% in the general population.2 It is characterized by a left‐side superior vena cava draining into the right atrium through a dilated coronary sinus (mostly seen), or draining directly into the left atrium producing a right‐to‐left shunt, or directly into the right atrium.3, 4 Our case falls into the first subtype. All the other great vessels were normally connected.
Both dextrocardia and PLSVC are frequently associated with congenital heart diseases. Grayburn et al.5 reported that 90% of patients with dextrocardia with situs solitus are also diagnosed with other cardiac anomalies. Hence, patients are often predisposed to lethal ventricular arrhythmias.
Even though a patient warrants an ICD, the potential vascular anomalies can make interventional procedures challenging. However, they are still at high risk of sudden cardiac death; and their unique congenital anatomic abnormalities may affect the outcome of a conventional external defibrillation because of an altered electrical axis and twisted critical mass of the heart muscle. Currently there have been no standard defibrillation/cardioversion techniques for dextrocardia patients, especially in a setting of prehospital cardiac arrest. There have been four equally effective pad positions recommended for the general population: anterolateral, anteroposterior, anterior‐left infrascapular, and anterior‐right infrascapular; with the anterolateral position considered as the default electrode placement.6 Customized pad positions may be considered based on individual patient characteristics. Cattermole et al.7 used conventional right sternal and left apical positions in a case of successful defibrillation involving a dextrocardiac ventricular fibrillation arrest. In another case, Gorenek et al.8 reported a successful emergency cardioversion of a dextrocardia patient in atrial fibrillation, utilizing a mirror‐like fashion to the conventional placement of paddles in the normal heart arrangement. In our case, the WCD utilized an apex (left chest wall) to posterior placement and achieved successful conversion by a single 150‐joule shock. Figure 3 demonstrates the WCD electrode positions and electric current vectors for both normal and dextrocardia patient. The WCD has nonadhesive, self‐gelling defibrillation therapy pads incorporated into a chest strap assembly (Fig. 4). It provides autonomous monitoring and defibrillation for ventricular tachyarrhythmias. The patient's state of consciousness is identified through response button use during a series of alarms. If the response buttons are not used, the patient is assumed to be unconscious and a shock is delivered without the need for a bystander to be present. Although this single case is not sufficient to claim a standard solution to sudden death for dextrocardia patients, it does demonstrate that WCD can be successfully used in dextrocardia patients without customizing the electrode belt and paddle positions.
Figure 3.
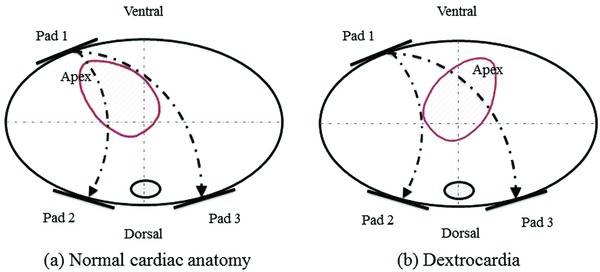
Electrode positions on the thorax (pad 1–3) and electric current vectors.
Figure 4.
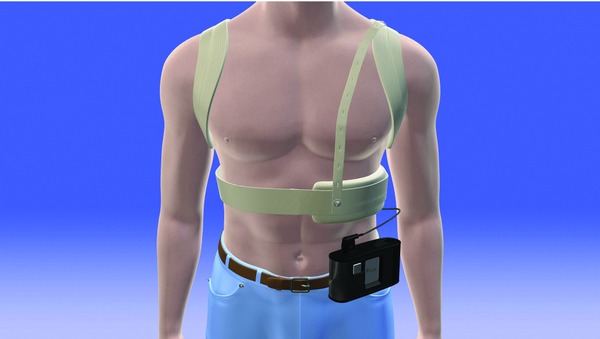
Wearable cardioverter defibrillator.
Transthoracic impedance (TTI) is another important determinant of shock outcome. When TTI is too high, a low‐energy shock will not generate sufficient current to depolarize a critical mass of myocardium. The measured TTI for WCD patients at EP lab was reported as 64–71 Ω.9 It is affected by energy level, electrode size, paddle‐skin coupling material, number of and time interval between previous shocks, distance between electrodes, and paddle electrode pressure.10 If abnormal anatomies result in higher impedances at conventional paddles placement then it is possible that an impedance‐compensating waveform would fully or partially compensate for the reduction in current. The WCD does in fact use a biphasic truncated exponential impulse that adapts to the patient's impedance. In this instance, however, the patient's TTI measured within the normal range (72 Ω)
CONCLUSION
Patients with congenital disorders, such as dextrocardia and PLSVC, can be at risk of sudden cardiac arrest. Our current case demonstrates that the WCD may be an option for this patient group without modifying the default defibrillation pad positioning.
REFERENCES
- 1. Freed MD PW. The pathology, pathophysiology, recognition and treatment of congenital heart disease In Alexander RW, Fuster SR, Hurst V. (eds.): The Heart. 9th edition New York: The McGraw‐Hill Company, 1998, pp. 1925–1993. [Google Scholar]
- 2. Goyal SK, Punnam SR, Verma G, et al. Persistent left superior vena cava: A case report and review of literature. Cardiovasc Ultrasound 2008;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gonzalez‐Juanatey C, Testa A, Vidan J, et al. Persistent left superior vena cava draining into the coronary sinus: Report of 10 cases and literature review. Clin Cardiol 2004;27(9):515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Konstantino Y, Kusniec J, Shohat‐Zabarski R, et al. Cardiac defibrillator implantation via persistent left superior vena cava facilitated by a coronary sinus delivery system. Europace 2009;11(1):119–120. [DOI] [PubMed] [Google Scholar]
- 5. Grayburn R, Singh D, Paydak H, et al. Urgent biventricular implantable cardioverter defibrillator implantation in a patient with situs inversus totalis and dextrocardia. Congest Heart Fail 2009;15(6):293–294. [DOI] [PubMed] [Google Scholar]
- 6. Link MS, Atkins DL, Passman RS, et al. Part 6: Electrical therapies: Automated external defibrillators, defibrillation, cardioversion, and pacing: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122(18 Suppl 3):S706–S719. [DOI] [PubMed] [Google Scholar]
- 7. Cattermole G, McKay N. A case of dextrocardiac ventricular fibrillation arrest. Emerg Med J 2006;23(2):147–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gorenek B, Kuskus S, Kudaiberdieva G, et al. Electrical cardioversion of atrial fibrillation in a case of dextrocardia. Can J Cardiol 2004;20(8):819–821. [PubMed] [Google Scholar]
- 9. Reek S, Geller JC, Meltendorf U, et al. Clinical efficacy of a wearable defibrillator in acutely terminating episodes of ventricular fibrillation using biphasic shocks. Pacing Clin Electrophysiol 2003;26(10):2016–2022. [DOI] [PubMed] [Google Scholar]
- 10. Walsh SJ, Bedi A. Successful defibrillation in the prone position. Br J Anaesth 2002;89(5):799; author reply 799–800. [DOI] [PubMed] [Google Scholar]


