Abstract
Dofetilide, a new class III antiarrhythmic agent, has been approved as an antiarrhythmic agent for the treatment of atrial fibrillation and atrial flutter. Dofetilide selectively inhibits the rapid component of the delayed rectifier potassium current resulting in a prolongation of the effective refractory period. Like other drugs that affect potassium currents, the prolonged QT interval occurring in the patients treated with dofetilide can be complicated by torsades de pointes. We report four cases of dofetilide‐induced QT prolongation and torsades de pointes. We discuss the risk factors for the development of dofetilide‐induced long QT and torsades de pointes and review the current literature.
Keywords: dofetilide, Tikosyn, torsades de pointes, atrial fibrillation
Dofetilide is a novel class III antiarrhythmic drug that selectively inhibits the rapid component of the delayed rectifier potassium current and prolongs the refractory period without any significant effect on the atrioventricular nodal conduction system. 1 , 2 As a pure class III agent, it has no negative inotropic effect even in patients with markedly reduced left ventricular function. 3 In patients with atrial fibrillation (AF), dofetilide has been shown to frequently restore and maintain sinus rhythm. 4 , 5 In patients with left‐ventricular dysfunction with recent myocardial infarction and congestive heart failure with left ventricular dysfunction, treatment with dofetilide does not affect all cause mortality, cardiac mortality, or total arrhythmic deaths. 6 , 7 , 8 Dofetilide is effective in treating AF and atrial flutter (AFL) in this population. Like other drugs that affect potassium currents, dofetilide is known to prolong the QT interval and induce malignant ventricular tachyarrhythmias including torsade de pointes. 2 , 5 , 7 , 8 We present a series of patients, admitted to Strong Memorial Hospital, with dofetilide‐induced QT prolongation and torsades de pointes.
CASE PRESENTATION
Patients started on dofetilide at our institution are closely monitored by the Heart Station for QT prolongation and/or torsades de pointes. Since October 2001, 268 patients with AF or AFL have been treated with dofetilide. Four patients developed prolonged QT and torsades de pointes corresponding to an incidence of 1.5%.
The first patient is a 51‐year‐old man with nonischemic cardiomyopathy (NICM), a left ventricular ejection fraction (LVEF) of 20%, chronic left bundle branch block (LBBB), who developed symptomatic AF and was admitted to the hospital for dofetilide initiation. His baseline QTc was 0.46 seconds and he was started on dofetilide, 500 micrograms (mcg) twice daily. He converted to normal sinus rhythm (NSR) after two doses. After the fourth dose of dofetilide, he developed multiple runs of nonsustained ventricular tachycardia (VT) during which time his QTc was calculated to be 0.8 seconds (Fig. 1). His potassium level was 3.7 meq/L and his magnesium level was 1.8 meq/L, which were both corrected intravenously. Dofetilide was discontinued and his QTc normalized and the patient did not have any further episodes of VT.
Figure 1.
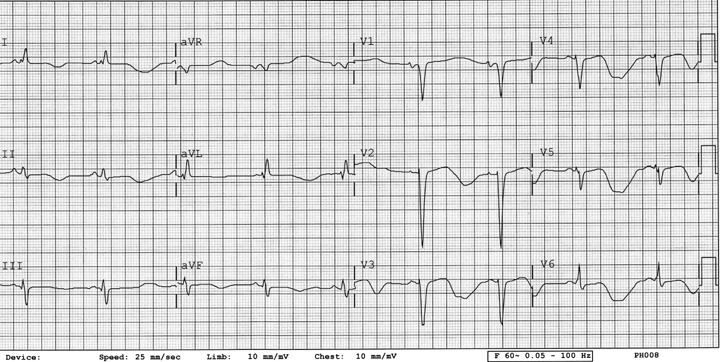
ECG 1. Patient 1. Twelve‐lead ECG showing sinus bradycardia, left atrial enlargement, left bundle branch block, and severe QT prolongation with a QTc of 0.8 seconds. Telemetry 1. Patient 1. Telemetry showing prolonged QTc and polymorphic ventricular tachycardia.
The second patient is a 46‐year‐old man with an NICM, LVEF of 8%, who was admitted with decompensated, New York Heart Association Class IV heart failure. He had a chronic LBBB, persistent AF and underwent a successful transesophageal echocardiography guided cardioversion. He eventually received a biventricular‐implantable cardioverter defibrillator (BiV‐ICD) for cardiac resynchronization therapy but continued to have intermittent AF. He was started on dofetilide 500 mcg twice daily with a baseline QTc of 0.46 seconds (Fig. 2). Following the third dose of dofetilide, his QTc increased to 0.56 seconds and he developed several episodes of torsades de pointes requiring multiple defibrillations (Fig. 3). His electrolytes were within the normal range. Dofetilide was discontinued and his BiV‐ICD was reprogrammed to pace at 100 beats/minute to decrease the risk of torsades de pointes. Two days later, his BiV‐ICD was reprogrammed to its nominal setting and the patient was discharged home. He has since had no further ventricular arrhythmias at over 18 months of follow‐up.
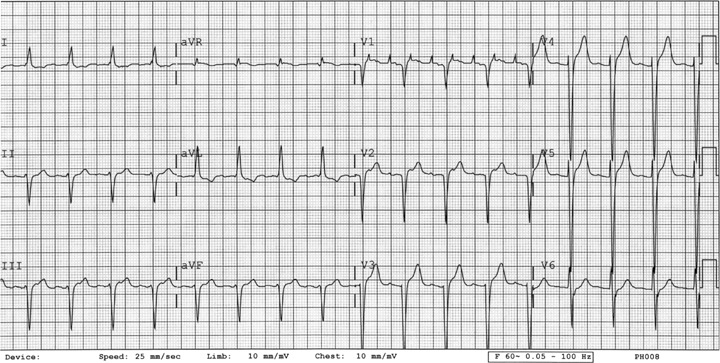
Figure 2.
ECG 2. Patient 2. Before initiation of dofetilide. Twelve‐lead ECG showing atrial fibrillation, left bundle branch block, and QTc of 0.46 seconds.
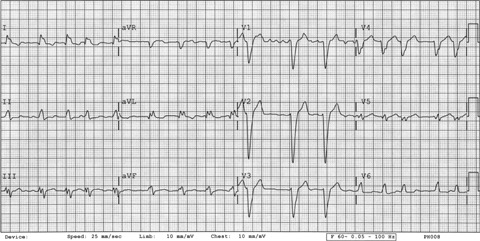
Figure 3.
ECG 3. Patient 2. After third dose of dofetilide. Twelve‐lead ECG showing NSR with ventricular capture spikes and a QTc of 0.57 seconds. Telemetry 2. Patient 2. Telemetry showing torsades de pointes.
The third patient is a 64‐year‐old woman with rheumatic valvular heart disease, with an LVEF of 10% who was admitted with decompensated heart failure. ECG on admission showed atrial tachycardia with 2:1 atrioventricular block, a QRS duration of 0.11 seconds and a QTc of 0.43 seconds (Fig. 4). She was started on dofetilide at 125 mcg twice daily due to renal insufficiency and she converted to NSR. On hospital day 7, while on dofetilide, she was started on milrinone due to worsening pulmonary edema. On hospital day 8 she developed torsades de pointes requiring multiple external defibrillations (Telemetry 3). She was successfully resuscitated and both dofetilide and milrinone were discontinued. The QTc measured immediately after the cardiac arrest was 0.67 seconds (Fig. 5). Laboratory evaluation revealed normal potassium and magnesium levels. An ICD was implanted for the primary prevention of sudden cardiac death and she was eventually discharged to home. She has not had any further ventricular arrhythmias during 10 months of follow‐up.
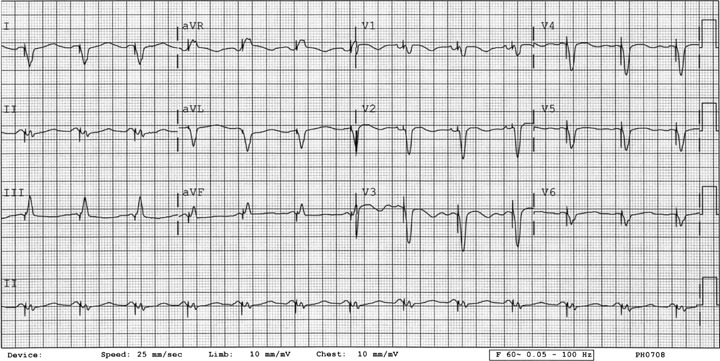
Figure 4.
ECG 4. Patient 3. Baseline before dofetilide. Twelve‐lead ECG showing atrial tachycardia with 2:1 atrioventricular block and a QTc of 0.43 seconds.
Figure 5.
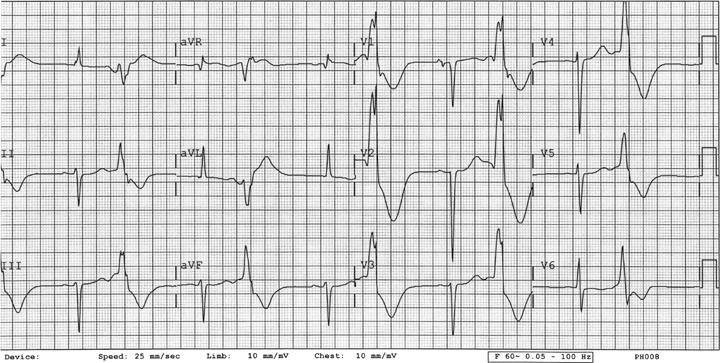
ECG 5. Patient 3. While on dofetilide and milrinone. Twelve‐lead ECG showing NSR with frequent ventricular premature contractions and a QTc of 0.67 seconds. Telemetry 3. Patient 3, Telemetry showing torsades de pointes.
The fourth patient, an 80‐year‐old woman with coronary artery disease and valvular heart disease but with preserved left ventricular systolic function was admitted with AF and a rapid ventricular response. She was started on dofetilide at which time her QTc was calculated as 0.45 seconds. Three hours after the first dose of dofetilide she had QT prolongation with a QTc calculated as 0.68 seconds and she subsequently developed recurrent but self‐terminating torsades de pointes (Fig. 6). Dofetilide was discontinued however she continued to have recurrent torsades de pointes. A temporary transvenous pacemaker was placed and she was paced at 85 beats/minute to decrease the risk of torsades de pointes. Eventually the pacer was removed and she had no further recurrence of torsades de pointes.
Figure 6.
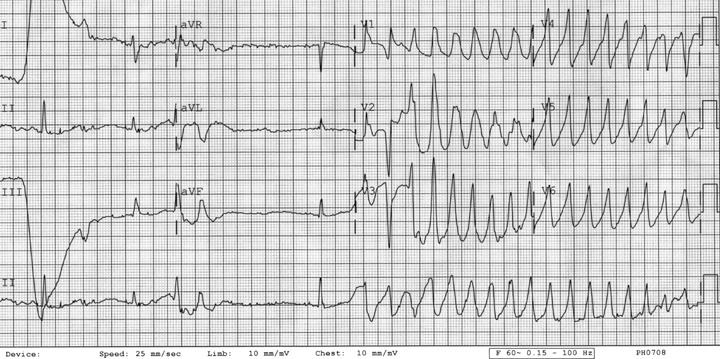
ECG 6. Patient 4. Twelve‐lead ECG showing NSR followed by torsades de pointes. Telemetry 4. Patient 4. Telemetry showing torsades de pointes.
DISCUSSION
Dofetilide has been used for the termination of supraventricular arrhythmias, 9 treatment of AF and AFL, 4 , 7 , 8 and the treatment of life‐threatening ventricular arrhythmias. 10 Although it is fairly effective in treating atrial arrhythmias, the major concern with dofetilide is its tendency to prolong the QT interval and its proarrhythmic effect. Dofetilide's effect on the potassium channel may result in an increase in the action potential duration, prolonging the QT, which can lead to torsades de pointes. 2 QT interval prolongation is directly related to the dose and plasma concentration of the drug. 1 Oral bioavailability of the drug is greater than 90% and peak concentrations are reached in 2–3 hours in an empty stomach and 3–4 hours when taken with food. 2 About 70–80% of the drug is excreted through the kidneys and the remainder is metabolized by the liver. 1
Several clinical trials were directed to the study of the efficacy and the safety of dofetilide. Dofetilide was associated with torsades de pointes in 1.6% of patients with a recent myocardial infarction and left ventricular dysfunction 7 and in 3.3% of patients with heart failure. 8 In the DIAMOND MI trial the frequency of torsades de pointes dropped from 3% to 0.6% after the protocol was changed to adjust the dose of dofetilide based on creatinine clearance. 6 A linear logistic regression analysis of the DIAMOND CHF study showed that only female sex (odds ratio, 3.2) and a NYHA class of III or IV (odds ratio, 3.9) were significantly associated with the occurrence of torsades de pointes. 8
Dofetilide did not increase mortality in multiple randomized clinical trials. 6 , 7 , 8 However, there was a difference in mortality when analyzed by baseline QTc interval. In a substudy of the DIAMOND‐CHF trial there was a significant mortality benefit when dofetilide was used in patients with baseline QTc intervals less than 429 ms (risk ratio 0.3), while an increase in mortality was seen with dofetilide therapy in patients with baseline QTc intervals greater than 429 ms (risk ratio 1.3). 11 All four of the patients presented in this series had baseline QTc intervals that were greater than 429 ms. Moreover, all four patients had a wide QRS duration at baseline; two patients had LBBB and the other two had intraventricular conduction delays. To our knowledge there are no studies showing an increased tendency to QT prolongation and/or torsades de pointes in patients with a wider baseline QRS complex. Our observation suggests that dofetilide induced torsades de pointes is more common after conversion to NSR, and therefore, patients should be observed for at least 24 hours after conversion to sinus rhythm. One reason for this observation could be that the relatively slower heart rates seen in NSR compared to AF may result in bradycardia‐related QT prolongation and torsades de pointes.
Although dofetilide has been specifically studied and approved for the pharmacologic treatment of AF and AFL, it is associated with a real risk for QT prolongation and torsades de pointes. Appropriate patient selection and close monitoring of the QT interval, electrolytes, and creatinine clearance should be undertaken when administering dofetilide.
REFERENCES
- 1. Mounsey JP, DiMarco JP. Cardiovascular drugs. Dofetilide. Circulation 2000;102:2665–2670. [DOI] [PubMed] [Google Scholar]
- 2. Falk RH, Decara JM. Dofetilide: A new pure class III antiarrhythmic agent. Am Heart J 2000;140:697–706. [DOI] [PubMed] [Google Scholar]
- 3. Rousseau MF, Massart PE, Van Eyll C, et al Cardiac and hemodynamic effects of intravenous dofetilide in patients with heart failure. Am J Cardiol 2001;87:1250–1254. [DOI] [PubMed] [Google Scholar]
- 4. Singh S, Zoble RG, Yellen L, et al Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: The symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE‐D) study. Circulation 2000;102:2385–2390. [DOI] [PubMed] [Google Scholar]
- 5. Guanzon AV, Crouch MA. Phase IV trial evaluating the effectiveness and safety of dofetilide. Ann Pharmacother 2004;38(7–8):1142–1147. [DOI] [PubMed] [Google Scholar]
- 6. Kober L, Bloch Thomsen PE, Moller M, et al Effect of dofetilide in patients with recent myocardial infarction and left‐ventricular dysfunction: A randomised trial. Lancet. 2000;356:2052–2058. [DOI] [PubMed] [Google Scholar]
- 7. Pedersen OD, Bagger H, Keller N, Marchant B, et al Efficacy of dofetilide in the treatment of atrial fibrillation‐flutter in patients with reduced left ventricular function: A Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation 2001;104:292–296. [DOI] [PubMed] [Google Scholar]
- 8. Torp‐Pedersen C, Moller M, Bloch‐Thomsen PE, et al Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish investigations of arrhythmia and mortality on Dofetilide Study Group. N Engl J Med. 1999;341:857–865. [DOI] [PubMed] [Google Scholar]
- 9. Tendera M, Wnuk‐Wojnar AM, Kulakowski P, et al Efficacy and safety of dofetilide in the prevention of symptomatic episodes of paroxysmal supraventricular tachycardia: A 6‐month double‐blind comparison with propafenone and placebo. Am Heart J 2001;142:93–98. [DOI] [PubMed] [Google Scholar]
- 10. Boriani G, Biffi M, Bacchi L, et al A randomised cross‐over study on the haemodynamic effects of oral dofetilide compared with oral sotalol in patients with ischaemic heart disease and sustained ventricular tachycardia. Eur J Clin Pharmacol 2002;58:165–169. [DOI] [PubMed] [Google Scholar]
- 11. Brendorp B, Elming H, Jun L, et al QTc interval as a guide to select those patients with congestive heart failure and reduced left ventricular systolic function who will benefit from antiarrhythmic treatment with dofetilide. Circulation. 2001;103:1422–1427. [DOI] [PubMed] [Google Scholar]


