Abstract
Background: Although predictive value of heart rate recovery (HRR) has been tested in large populations, the reproducibility of HRR in treadmill exercise test has not been assessed prospectively. This prospective study examined whether HRR index has test–retest stability in the short term.
Methods: A total of 52 healthy volunteers without cardiovascular risk factors (mean age, 30 ± 10 years, 30 females) underwent standardized graded treadmill exercise test, and the test was repeated on the 7th and the 30th days. The subjects’ maximal heart rates and the decrease of heart rate from the peak exercise level to the level of 1, 2, 3, 4, and 5 minutes after the termination of the exercise were examined on each test, and heart rates for each minute from the first, second, and third tests were compared for each individual.
Results: The maximal heart rates on the 1st, 7th, and the 30th days were 179 ± 11, 177 ± 10, 178 ± 10 beats/min, respectively [P = 0.07, intraclass correlation coefficient (ICC) = 0.92], and the 1st minute HRR indices after peak exercise were 33 ± 10, 33 ± 10, 33 ± 11, respectively (P = 0.66, ICC = 0.88). There was no statistical difference in the 2nd, 3rd, 4th, and 5th minute heart rates of the recovery phase among the 1st, 7th, and 30th day treadmill exercise tests, either.
Conclusion: Maximal heart rates and the decline of heart rate to the 5th minute on recovery phase after treadmill exercise test have short‐term reproducibility.
Ann Noninvasive Electrocardiol 2011;16(4):365–372
Keywords: heart rate recovery, reproducibility, treadmill exercise test
Heart rate (HR) profiles during exercise testing are easy to perform and useful predictors of cardiovascular mortality. Heart rate recovery (HRR) index shows the rate of decline in the heart rate after the cessation of exercise test and is defined as heart rate difference between the maximal heart rate on exercise and the heart rate of the 1st or the 2nd minute on recovery phase. The rise in heart rate during exercise period is a consequence of an increase in sympathetic activity and a decrease in parasympathetic activity 1 and the decline in heart rate during recovery is principally due to a reactivation of parasympathetic nervous system, mostly in the early recovery period. 2 It is well known that the imbalance in autonomic nervous system is a cardiovascular risk factor and increased parasympathetic activity is associated with a reduction in the risk of sudden death in postinfarction patients. 3 , 4 HRR can be used as a measure of autonomic imbalance, particularly as a function of parasympathetic activity and HRR has been shown to be a useful criterion for predicting cardiac and all‐cause mortality even after adjustment for ischemia, the Duke treadmill score, and the angiographic severity of coronary artery disease. 5 In addition, our group has shown that HRR index might have a supportive role in the diagnosis of vasovagal syncope as it reflects autonomic imbalance. 6 Nonetheless, there is no agreement regarding a single numerical value that denotes abnormal HRR and the sensitivity and specificity of an abnormal HRR for an individual patient remains unknown. 7 , 8 , 9 , 10 , 11 Moreover, for any test to be a reliable predictor of mortality risk, its measurement should be stable and reproducible in the individual patient unless any intervention or cardiac events have occurred. If the HRR is not stable or reproducible at least in short term, its clinical utility and role as prognostic tool would be of very limited value or would be worthless. The aim of this study is to assess the reproducibility of HRR in short term on healthy subjects prospectively, thereby the reliability of the HRR as a prognostic tool.
METHODS
Fifty‐two voluntary healthy subjects (mean age, 30 ± 10 years, 30 females) without risk factors for coronary heart disease were enrolled in the study. None of the subjects had known heart disease, peripheral vascular disease, history of stroke or transient ischemic attack, symptoms suggestive of cardiac disease, any drug use, uninterpretable ST segments due to left bundle‐branch block, pre‐excitation syndrome, left ventricular hypertrophy, or more than 1 mm of resting ST‐segment depression. Furthermore, none of the subjects was a smoker or had a disease that could have an effect on heart rate or cause an autonomic dysfunction such as hypothyroidism, hyperthyroidism, or anemia. All subjects underwent echocardiographic evaluation to exclude any subclinical structural or congenital heart disease.
Twelve‐lead electrocardiography was recorded at 25 mm/s paper speed, and transthoracic echocardiographic examination was performed on all subjects by using a SystemFive (GE Vingmed Ultrasound, Horten, Norway) cardiac ultrasound scanner with 2.5–3.5 MHz transducers. Subjects were asked to attend the study in as near to identical state as possible with exercise, diet, and sleep in the 24 hours before testing being similar for all tests. The subjects were individually tested at the same time of day (as their first test) to control for any within‐subject physiological variation due to circadian rhythms. For calculation of HRR indices all patients underwent an exercise stress test with an active cool‐down period (slow walk at 1.5 mph and 2.5% grade for 1 min) with Bruce protocol aiming to reach at least 85% of age predicted heart rates in the absence of symptoms. Blood pressures at baseline and on maximal exercise, arrhythmias, symptoms, exercise duration, and maximal workload as METs (metabolic equivalent) were recorded. ECG tracings were obtained at baseline on the beginning of every stage, on peak exercise, and on 1st, 2nd, 3rd, 4th, and 5th minutes on recovery period. Heart rates were taken from the computerized reports on which the heart rate was the average of the last five RR intervals to prevent any false result due to sinus arrhythmias. Afterward, HRR indices were calculated by subtracting 1st, 2nd, 3rd, 4th, and 5th minute heart rates on recovery period from the maximal heart rate obtained during stress testing and designated as HRR1, HRR2, HRR3, HRR4, and HRR5, respectively. The test was repeated on the 7th and the 30th days at the same time of day, and HRR1, HRR2, HRR3, HRR4, and HRR5 were recorded on each test for analyses.
The study was approved by the local ethics committee, and subjects gave informed written consent.
STATISTICAL ANALYSIS
Results were presented as mean ± standard deviation (SD). The degree of agreement among repeated measurements of HR for each minute and HRR on each day was presented in terms of a coefficient of variation and intraclass correlation coefficient (ICC; with 95% confidence interval). Bland‐Altman plots with 95% limits of agreement were also derived. 12 Two‐factor repeated measures ANOVA with minute and day as the two repeated factors was used.
The ICC value ranges from 0 to 1, with values closer to 1 representing stronger reproducibility. A high ICC with value greater than 0.75 was interpreted as excellent reproducibility. 13 , 14 P values < 0.05 were considered significant. Statistical analyses were performed using Statistical Package for Social Science (SPSS) for Windows version 15.0 (SPSS, Chicago, IL, USA) and STATISTICA for Windows version 11 (StatSoft Inc., Tulsa, OK, USA).
RESULTS
Baseline characteristics of the study subjects are shown in Table 1. All subjects had normal ejection fraction without any structural or congenital heart disease.
Table 1.
Baseline characteristics of study subjects (1st day)
| Variable | |
|---|---|
| Number of subjects | 52 |
| Sex (male/female) | 22/30 |
| Age (years)a | 30 ± 10 |
| Systolic blood pressure (mmHg)a | 117 ± 10 |
| Diastolic blood pressure (mmHg)a | 67 ± 7 |
| Heart rate (beats/min)a | 75 ± 11 |
| Body mass index (kg/m2)a | 23 ± 2 |
| Ejection fraction (%)a | 69 ± 5 |
aValues are mean ± SD.
All subjects had normal rest 12‐lead ECG and were in sinus rhythm. Ischemic changes were not observed during ECG stress test and all subjects were asymptomatic on exercise but there were rare/infrequent uniformic ventricular premature beats in two subjects and one subject had rate dependent left bundle branch block on maximal exercise returning to normal within 2 minutes on recovery phase only on the 1st day. All subjects completed the exercise stress test and reached at least 85% of age predicted heart rates without any complication on 1st, 7th, and 30th days. The duration of treadmill exercise test, baseline heart rate, maximal systolic and diastolic blood pressure, and maximal metabolic equivalents during the 1st, 7th, and 30th day exercise treadmill stress tests were similar and are shown in Table 2.
Table 2.
The Comparison of Baseline Heart Rate, Peak Exercise Systolic and Diastolic Blood Pressure, Exercise Duration, and Exercise Workload on 1st, 7th, and 30th Days
| Parameter | 1st Day | 7th Day | 30th Day | P Value |
|---|---|---|---|---|
| Heart rate, baseline (beats/min) | 75 ± 11 | 76 ± 10 | 75 ± 10 | 0.41 |
| Systolic blood pressure (mmHg)a | 168 ± 10 | 169 ± 10 | 169 ± 10 | 0.36 |
| Diastolic blood pressure (mmHg)a | 94 ± 5 | 94 ± 4 | 94 ± 5 | 0.72 |
| Maximal METsb | 11.1 ± 2.2 | 11.3 ± 2.0 | 11.3 ± 2.2 | 0.33 |
| Exercise duration (minute) | 11.9 ± 2.2 | 11.9 ± 2.3 | 12.0 ± 2.2 | 0.12 |
aPeak exercise; bMETs = metabolic equivalent.
Results of maximal heart rate and HRR indices on 1st, 7th, and 30th days are shown in Table 3. All subjects had normal HRR1 (i.e., more than 12 beats 7 , 15 ) on 1st, 7th, and 30th days (33 ± 10, 33 ± 10, 33 ± 11, respectively) and HRR1s were similar of each subject on 1st, 7th, and 30th days. There were no significant differences for HRR1, HRR2, HRR3, HRR4, HRR5 among 1st, 7th, and 30th days (Table 3). Paired HRR values of each subject on 1st day versus 7th day; 1st day versus 30th day; and 7th day versus 30th day with different cutoff values are graphed for visual assessment (Fig. 1). We did not have any abnormal HRR1 result by using the criterion of abnormal HRR as a decrease of less than 12 beats/min. 9 By using the criterion of abnormal HRR as a decrease of less than 18 beats in 1 minute on recovery, reproducibility of abnormality was 100% between 1st and 7th day (i.e., both of the two patients who had abnormal HRR on 1st day had abnormal HRR on 7th day) (Fig. 1A). The reproducibility of abnormality was 50% between 1st and 30th day (i.e., only one of the two patients who had abnormal HRR on 1st day had abnormal HRR on 30th day) (Fig. 1B) and 50% between 7th and 30th day (Fig. 1C). By a criterion of abnormal HRR as a decrease of less than 42 beats between peak heart rate and heart rate of 2 minutes into recovery, there was no abnormal HRR on 1st day, two patients had abnormal HRR on 7th day, one patient had abnormal HRR on 30th day. Only one of the two patients who had abnormal HRR on 7th day had abnormal HRR on 30th day.
Table 3.
Heart Rate Recovery Indices of Subjects on 1st, 7th, and 30th Days
| Parameter | 1st Day | 7th Day | 30th Day | ICC/α (Lower‐Upper Bound)a | CV (Min‐Max) | P Valueb |
|---|---|---|---|---|---|---|
| HRR1c | 33.5 ± 10.4 | 33.8 ± 10.1 | 33.4 ± 11.0 | 0.88/0.95 | 0.9–23.2 | 0.000 |
| (30.6–36.3) | (30.9–36.6) | (30.3–36.4) | (0.81–0.92) | |||
| HRR2c | 59.3 ± 9.6 | 59.4 ± 10.4 | 59.4 ± 11.7 | 0.88/0.9 | 0–12.4 | 0.000 |
| (56.6–61.9) | (56.5–62.3) | (56.1–62.6) | (0.82–0.92) | |||
| HRR3c | 69.1 ± 10.3 | 70.2 ± 10.4 | 69.7 ± 10.6 | 0.85/0.94 | 0.6–10.9 | 0.000 |
| (66.2–71.9) | (67.3–73.1) | (66.8–72.7) | (0.78–0.91) | |||
| HRR4c | 72.7 ± 9.3 | 73.5 ± 8.4 | 73.7 ± 8.6 | 0.80/0.92 | 0.9–12.3 | 0.000 |
| (70.1–75.3) | (71.2–75.9) | (71.3–76.1) | (0.70–0.87) | |||
| HRR5c | 76.2 ± 9.4 | 76.7 ± 9.1 | 76.5 ± 8.2 | 0.82/0.93 | 0–10.1 | 0.000 |
| (73.6–78.8) | (74.1–79.2) | (74.2–78.8) | (0.73–0.88) |
aLower and upper bounds are for ICCs; bP values are for ICCs; cHRR = heart rate recovery; heart rate: beats/min, HRR values are mean ± SD (95% CI for mean).
Figure 1.
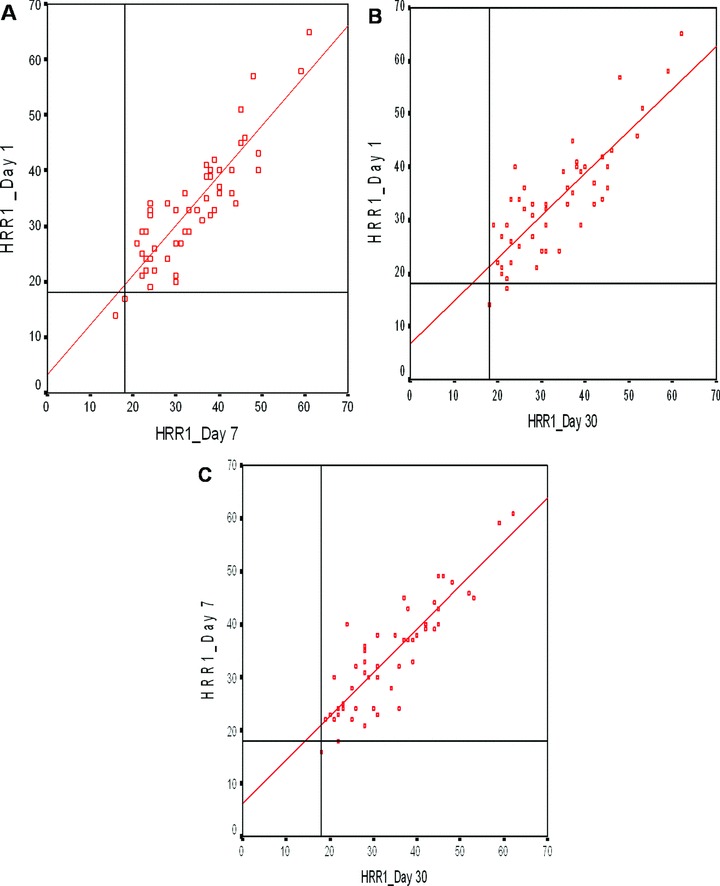
Linear regression between 1st and 7th day (A), 1st and 30th day (B), and 7th and 30th day (C) exercise tests for one‐minute heart rate recovery (HRR1) with cutoff value of 18. Only two patients had abnormal test on 1st and 7th days (Fig. 2A), two patients on 1st day and one patient on 30th day (Fig. 2B), and two patients on 7th day and one patient on 30th day (Fig. 2C).
Since we have very few abnormal HRR results, statistical analysis for reproducibility of HRR in terms of abnormality or normality was not done. Therefore, we assessed the reproducibility of HRR values, maximal heart rates, and heart rates on each minute of recovery. ICC values for all HRRs are shown in Table 3 and for maximal heart rates and heart rates on recovery are shown in Table 4. ICC values for HRR1, HRR2, HRR3, HRR4, and HRR5 were equal or greater than 0.80 (95% confidence interval) suggesting all HRR indices had excellent reproducibility (P < 0.01). Bland‐Altman plots of difference between 1st and 7th day, 1st and 30th day, and 7th and 30th day exercise test for HRR1 are shown in Fig. 2. Bland‐Altman plots of difference between 1st and 7th day, 1st and 30th day, and 7th and 30th day for all HRR indices revealed all indices are reproducible (data are not shown for HRR2, HRR3, HRR4, and HRR5). The measurement errors according to Bland and Altman in the intrasubject studies ranged from 9.7 to 12.1.
Table 4.
The Maximal Heart Rates and 1st, 2nd, 3rd, 4th, and 5th Minute Heart Rates on 1st, 7th, and 30th Days
| Parameter | 1st Day | 7th Day | 30th Day | ICC/α (Lower‐Upper Bound)a | CV (Min‐Max) | P Valueb |
|---|---|---|---|---|---|---|
| Maximal HRc | 179 ± 11 | 178 ± 10 | 179 ± 10 | 0.92/0.97 | 0–4.4 | 0.000 |
| (147–199) | (150–195) | (154–199) | (0.88–0.95) | |||
| −1 minute HRc | 145 ± 13 | 144 ± 14 | 145 ± 15 | 0.89/0.96 | 0–5.7 | 0.000 |
| (111–170) | (110–170) | (111–173) | (0.84–0.93) | |||
| −2 minute HRc | 119 ± 13 | 118 ± 14 | 119 ± 14 | 0.88/0.95 | 0–11.1 | 0.000 |
| (78–142) | (79–142) | (78–147) | (0.83–0.93) | |||
| −3 minute HRc | 110 ± 11 | 107 ± 12 | 108 ± 12 | 0.83/0.93 | 0.4–10.4 | 0.000 |
| (78–130) | (76–130) | (78–130) | (0.75–0.89) | |||
| −4 minute HRc | 106 ± 10 | 104 ± 11 | 104 ± 10 | 0.80/0.92 | 0–10.7 | 0.000 |
| (79–125) | (76–125) | (76–125) | (0.70–0.87) | |||
| −5 minute HRc | 102 ± 10 | 101 ± 11 | 101 ± 12 | 0.80/0.92 | 0.5–10.2 | 0.000 |
| (77–123) | (76–124) | (56–120) | (0.71–0.87) |
aLower and upper bounds are for ICCs; bP values are for ICCs; cHeart rate: beat/min; values are mean ± SD (Min HR‐Max HR).
Figure 2.
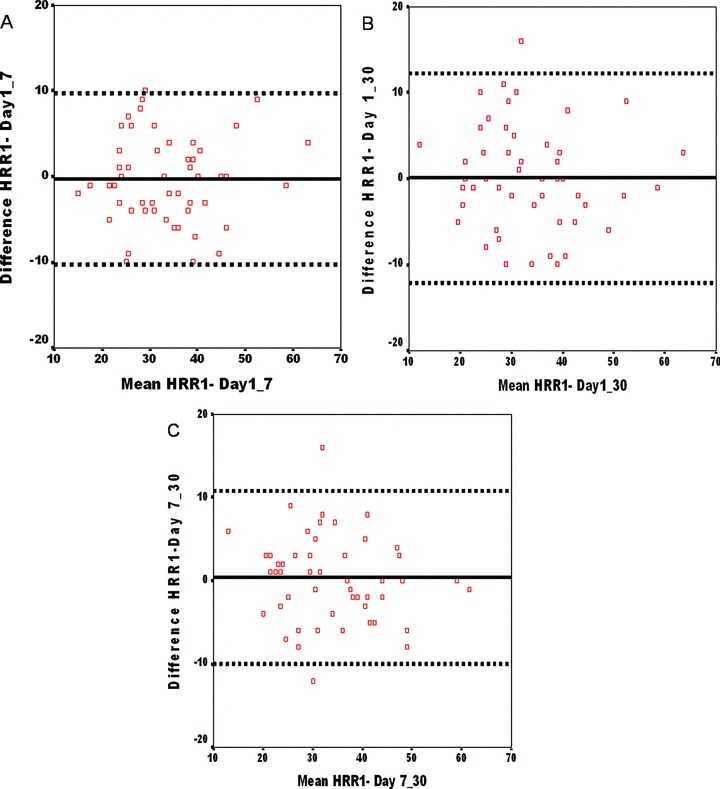
Bland‐Altman plot between 1st and 7th day (A), 1st and 30th day (B), and 7th and 30th day (C) exercise tests for HRR1. The biases (mean difference between the two paired means) (——) were 0.2 (A), 0.1 (B), 0.4 (C) and the limits of agreement (▪▪▪▪▪▪) were −9.8 and 10.2 (A), −12.1 and 12.3 (B), −10.0 and 10.8 (C). The standard deviation of the difference in HRR for the two tests [5.0 for (A), 6.1 for (B), and 5.4 for (C)] is not large compared with the cutoff of 12 beats/min used to define an abnormal 1‐minute HRR.
There was no statistical difference between maximal heart rates on exercise, 1st to 5th minute recovery heart rates and HRR1 to HRR5 among 1st, 7th, and 30th days suggesting that not only the HRR1 but also the profile of heart rate decline from maximal heart rate on recovery period after treadmill exercise test is reproducible in short term.
DISCUSSION
We demonstrated that HRR in 1st, 2nd, and 3rd minutes and the profile of heart rate decline in recovery period after exercise stress test was reproducible in short and mid terms in this sample of healthy young subjects. There was no significant difference in the HRRs reached at three time points separated by 7th and 30th days. The intraclass correlation coefficient was excellent for the maximal heart rates and for HRRs. HRR measurements appear to be characterized by good intrasubject reproducibility and excellent reliability.
The use of HRR as a prognostic marker of increased mortality has been confirmed across different populations. 5 , 9 , 10 , 16 Different cutoff values were derived and assessed for the prognostic value of HRR in these studies, such as, ≤12 beats after the 1st minute of recovery, 5 , 9 ≤18 beats/min for patients undergoing stress echocardiography, 5 <25 beats after the 1st minute of recovery, 16 and less than 42 beats decline from peak heart rate in 2 minutes of recovery. 10 In these studies, it was found that statistical evaluation of the data could produce a best measure of abnormal HRR which was associated with increased mortality for the study population. However, different numerical cutoff values of abnormal HRR, and also different time points for the measurement of HRR in these studies were causing some doubt about the validity of HRR. Yawn et al. 17 commented that these marked variations in the published definitions suggest a lack of reproducibility of a specific HRR measure. Nonetheless, different cutoff values used in these studies for the definition of abnormal HRR may not necessarily imply that HRR is not reproducible. The variation in defining the abnormal HRR might reflect the variation in the severity of autonomic dysfunction that led to increased mortality in the selected study population (i.e., high risk study population might have an increased mortality with a less severe autonomic dysfunction, and so, higher HRR cutoff value). In addition, this finding may indicate the need to use specific cutoff values of abnormal HRR for the selected study population but not the inefficacy of HRR index.
For any test, it is essential to demonstrate the methodology to obtain similar results and the reproducibility to validate as a prognostic or a diagnostic tool in specific populations. The test–retest stability of some cardiovascular responses (heart rate, blood pressure, cardiac output, total peripheral resistance) has been shown in several studies to physical or mental tasks within certain time periods. 18 , 19 , 20 , 21 Although HRR has been evaluated and found as a predictor of all‐cause mortality in several studies, only Yawn et al. 17 studied reproducibility and compared the medical records of 90 patients undergoing two exercise tests within 18 weeks or less, retrospectively. Investigators reported that there was a marked variation from first to second stress test in terms of abnormal HRR, and none of abnormal HRR definitions used more than 55% provided concordance between tests. This finding was interpreted as HRR which appears to have limited short‐term reproducibility, therefore it might not be a reliable addition to results of stress test and is not ready for routine use. Nevertheless, the study was limited by very few abnormal HRR results, retrospective nature of experiment, different study protocols used, and sample selection bias.
In our study, as we studied HRR on healthy subjects, none of them has an abnormal HRR defined as <12 beats/min decline in 1st minute on any test day, and very few had abnormal HRR when abnormality defined as <18 beats/min or <42 beats/2 min. 7 , 15 Since we had very few abnormal results, HRR was not assessed as reproducibility of normality or abnormality. We tried to find out whether HRR was reproducible as numerals (without cutoff values) with ICCs and Bland‐Altman 95% limits of agreement technique. ICC was found to be reliable for verifying reproducibility as it can measure the association strength among repetitions. 22 , 23 The reproducibility was considered as “good” if ICC ranged between 0.61 and 0.81, and “almost perfect” if it exceeded 0.81. In our study, we found that all HRR indices were well reproduced (ICCs > 0.80). Interpreting Bland‐Altman level of agreement in reproducibility is a challenge as the question, “what is an acceptable level of study‐to‐study variability?” needs to be addressed. The SD of the difference in HRR for the two tests (Fig. 2, SDs are 5.0 for A, 6.1 for B, and 5.4 for C) is not large compared with the cutoff of 12 beats/min used to define an abnormal 1‐minute HRR. This finding indicates that HRR indices, heart rate as a profile on recovery period, were reproducible in short term. However, if the SD of the difference was interpreted as ‘large’ compared to the specific numerical cutoff value of HRR, the reproducibility of abnormality/normality for that cutoff point (12 beats/min) would have a very limited value, although HRR values were reproducible as numerals. Future studies will need specific populations (diabetes mellitus, coronary artery disease, etc.) to specify and verify if there is a cutoff point that has reproducibility not only as numerals but also as normality/abnormality.
Limitations
We used Bruce protocol with an active cool down period and studied healthy subjects in short term. Since we had very few abnormal results as tests were performed on healthy young subjects, we could not assess the reproducibility of normality or abnormality. It is also possible that the results might be different with any other test protocol and with any other study population such as coronary artery disease, diabetes mellitus, and so on, that could affect autonomic nervous system. We also do not know whether the results would be affected if any intervention was performed between the tests or if the tests were repeated after a long time period.
CONCLUSION
In our study, we have demonstrated that HRR is reproducible when assessed as reproducibility of numerical HRR values (ICCs > 0.80). Moreover, we have demonstrated that baseline heart rates, maximal heart rates, and heart rate recoveries to 5th minute are reproducible, showing the heart rate as a profile when the treadmill exercise test is reproducible, and HRR has a test–retest stability and thereby reliability in a cohort of healthy adults within short term of period.
Disclosures: None.
REFERENCES
- 1. Arai Y, Saul JP, Albrecht P, et al Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol 1989;256:H132–H141. [DOI] [PubMed] [Google Scholar]
- 2. Imai K, Sato H, Hori M, et al Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 1994;24:1529–1535. [DOI] [PubMed] [Google Scholar]
- 3. Curtis BM, O’Keefe JH, Jr . Autonomic tone as a cardiovascular risk factor: The dangers of chronic fight or flight. Mayo Clin Proc 2002;77:45–54. [DOI] [PubMed] [Google Scholar]
- 4. Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post‐myocardial infarction risk stratification. Circulation 1992;85:I77–191. [PubMed] [Google Scholar]
- 5. Vivekananthan DP, Blackstone EH, Pothier CE, et al Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J Am Coll Cardiol 2003;42:831–838. [DOI] [PubMed] [Google Scholar]
- 6. Kocabas U, Kaya EB, Aytemir K, et al A novel method for the diagnosis of neurocardiogenic syncope: Heart rate recovery index. Cardiology 2009;114:50–55. [DOI] [PubMed] [Google Scholar]
- 7. Shetler K, Marcus R, Froelicher VF, et al Heart rate recovery: Validation and methodologic issues. J Am Coll Cardiol 2001;38:1980–1987. [DOI] [PubMed] [Google Scholar]
- 8. Desai MY, De la Pena‐Almaguer E, Mannting F. Abnormal heart rate recovery after exercise as a reflection of an abnormal chronotropic response. Am J Cardiol 2001;87:1164–1169. [DOI] [PubMed] [Google Scholar]
- 9. Cole CR, Blackstone EH, Pashkow FJ, et al Heart‐rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999;341:1351–1357. [DOI] [PubMed] [Google Scholar]
- 10. Cole CR, Foody JM, Blackstone EH, et al Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000;132:552–555. [DOI] [PubMed] [Google Scholar]
- 11. Nishime EO, Cole CR, Blackstone EH, et al Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA 2000;284:1392–1398. [DOI] [PubMed] [Google Scholar]
- 12. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 13. O’Keeffe ST, Lye M, Donnellan C, et al Reproducibility and responsiveness of quality of life assessment and six minute walk test in elderly heart failure patients. Heart 1998;80:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beaton DE, Hogg‐Johnson S, Bombardier C. Evaluating changes in health status: Reliability and responsiveness of five generic health status measures in workers with musculoskeletal disorders. J Clin Epidemiol 1997;50:79–93. [DOI] [PubMed] [Google Scholar]
- 15. Gibbons RJ. Abnormal heart‐rate recovery after exercise. Lancet 2002;359:1536–1537. [DOI] [PubMed] [Google Scholar]
- 16. Jouven X, Empana JP, Schwartz PJ, et al Heart‐rate profile during exercise as a predictor of sudden death. N Engl J Med 2005;352:1951–1958. [DOI] [PubMed] [Google Scholar]
- 17. Yawn BP, Ammar KA, Thomas R, et al Test‐retest reproducibility of heart rate recovery after treadmill exercise. Ann Fam Med 2003;1:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jern S, Wall U, Bergbrant A. Long‐term stability of blood pressure and pressor reactivity to mental stress in borderline hypertension. Am J Hypertens 1995;8:20–28. [DOI] [PubMed] [Google Scholar]
- 19. Barnes VA, Johnson MH, Treiber FA. Temporal stability of twenty‐four‐hour ambulatory hemodynamic bioimpedance measures in African American adolescents. Blood Press Monit 2004;9:173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu X, Iwanaga K, Shimomura Y, et al The reproducibility of cardiovascular response to a mental task. J Physiol Anthropol 2010;29;35–41. [DOI] [PubMed] [Google Scholar]
- 21. Hol AT, Eng JJ, Miller WC, et al Reliability and validity of the six‐minute arm test for the evaluation of cardiovascular fitness in people with spinal cord injury. Arch Phys Med Rehabil 2007;88:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carrasco S, Gonzalez R, Gaitan MJ, et al Reproducibility of heart rate variability from short‐term recordings during five manoeuvres in normal subjects. J Med Eng Technol 2003;27:241–248. [DOI] [PubMed] [Google Scholar]
- 23. Reland S, Ville NS, Wong S, et al Reliability of heart rate variability in healthy older women at rest and during orthostatic testing. Aging Clin Exp Res 2005;17:316–321. [DOI] [PubMed] [Google Scholar]


