Summary
New evidence has stirred up a long-standing but undeservedly forgotten interest in the role of erythrocytes, or red blood cells (RBCs), in blood clotting and its disorders. This review summarizes the most recent research that describes the involvement of RBCs in hemostasis and thrombosis. There are both quantitative and qualitative changes in RBCs that affect bleeding and thrombosis, as well as interactions of RBCs with cellular and molecular components of the hemostatic system. The changes in RBCs that affect hemostasis and thrombosis include RBC counts or hematocrit (modulating blood rheology through viscosity) and qualitative changes, such as deformability, aggregation, expression of adhesive proteins and phosphatidylserine, release of extracellular microvesicles, and hemolysis. The pathogenic mechanisms implicated in thrombotic and hemorrhagic risk include variable adherence of RBCs to the vessel wall that depends on the functional state of RBCs and/or endothelium, modulation of platelet reactivity and platelet margination, alterations of fibrin structure and reduced susceptibility to fibrinolysis, modulation of nitric oxide availability, and the levels of von Willebrand factor and factor VIII in blood related to the ABO blood group system. RBCs are involved in platelet-driven contraction of clots and thrombi that results in formation of a tightly packed array of polyhedral erythrocytes, or polyhedrocytes, which comprises a nearly impermeable barrier important for hemostasis and wound healing. The revisited notion on the importance of RBCs is largely based on clinical and experimental associations between RBCs and thrombosis or bleeding, implying that RBCs are a prospective therapeutic target in hemostatic and thrombotic disorders.
Keywords: red blood cells, erythrocytes, hemostasis, thrombosis, blood clotting
Introduction
Studies of erythrocytes, or red blood cells (RBCs), has been a major focus of hematology, as has been hemostasis and thrombosis, but until recently, there has been little overlap in these two areas, since most scientists and clinicians have assumed that RBCs play a largely passive and relatively unimportant role in thrombosis and hemostasis. However, now it has become apparent that RBCs have a variety of important functions and exert substantial influences on blood clotting, hemostasis and thrombosis that are clinically significant (Table 1). This notion is based on the major observations that include reduced bleeding at a high hematocrit irrespective of the platelet count and predisposition to thrombosis associated with an increase in the RBC count, congenital erythroid diseases, as well as various acquired pathological conditions that change the properties of RBCs (Table 2). A relatively high incidence of thrombotic complications after RBC transfusions provides another strong argument for the involvement of RBCs in blood clotting disorders, although the thrombosis risk could also be ascribed to the underlying disease, which may dampen the causality of blood transfusion for thrombosis. In addition to clinical observations and experimental studies, computational modeling of thrombosis with a focus on the effects of RBCs has provided quantitative and mechanistic insights [1]. This review will briefly summarize what is currently known about the involvement of RBCs in hemostasis and thrombosis and its underappreciated importance.
Table 1.
Effects of RBCs related to thrombosis and hemostasis and underlying mechanisms
| Effects | Mechanisms | Pro- or antithrombotic | References ## |
|---|---|---|---|
| Hemorheological effects | RBCs increase blood viscosity because of a rise in hematocrit, an increase in RBC aggregation, or a decrease in RBC deformability (increasing flow resistance) | Pro | 2, 3, 4, 5 |
| Conversely, anemia is associated with low blood viscosity and bleeding tendency due to reduced platelet margination toward endothelium and enhanced NO availability | Anti | 2, 3, 4, 5 | |
| RBCs undergo shear-dependent reversible aggregation mediated by plasma proteins (mainly fibrinogen, immunoglobulins) and/or local osmotic gradient | Pro | 14, 15, 16, 70, 71, 72, 73, 74 | |
| RBCs with increased rigidity occlude small vessels | Pro | 11, 12 | |
| Deformability of RBCs reduces frictional resistance to flow | Anti | 8, 11, 12, 13 | |
| RBC maintain biconcave shape and a high surface-to-volume ratio due to cytoskeleton and water/ions balance | Pro or Anti | 5 | |
| RBCs migrate to the center of blood flow and push platelets toward the endothelium (margination) in a hematocrit- and shear-dependent manner | Pro | 59, 60, 61 | |
| Effects on platelet reactivity | RBCs increase platelet adhesion and aggregation by release of ADP and thromboxane A2 | Pro | 66, 67 |
| RBC form aggregates with platelets via adhesive molecules (ICAM-4 and fibrinogen with αIIbβ3) | Pro | 62, 63, 64 | |
| Free hemoglobin released during hemolysis scavenges nitric oxide, a platelet inhibitor and vasodilator | Pro | 50, 51, 68, 69 | |
| Free hemoglobin suppresses platelet activation by release of S-nitrosothiols, functional equivalents of NO | Anti | 48, 53 | |
| Interactions with vessel wall | RBCs bind directly to endothelium via adhesive molecules (Lutheran blood group/basal cell adhesion molecule/band 3, integrin α4Bβ1, CD36, ICAM-4, phosphatidylserine, etc.) | Pro | 10, 54, 55 |
| In FeCl3-induced thrombosis RBCs bind to endothelium via unknown mechanisms | Pro | 57 | |
| RBCs modulate endotheliocyte activation through release of NO, NO equivalents, and ATP | Anti | 49, 52 | |
| Thrombin generation | Phosphatidylserine is exposed on RBCs by Ca2+-dependent scramblase in response to high-shear stress, complement attack, oxidative stress, apoptosis, etc. | Pro | 18, 19, 20, 21, 22, 23, 24, 25 |
| RBCs release membrane-derived procoagulant microvesicles bearing phosphatidylserine during in vivo aging and in vitro storage | Pro | 28, 29, 30, 31, 33, 34 | |
| Meizothrombin, a protein C activator with low fibrinogen-cleaving activity, is formed on RBCs and released into the blood | Anti | 20 | |
| Factor IX is activated directly by an elastase-like enzyme on the RBC membrane | Pro | ||
| Structure and properties of clot and thrombi | RBCs make the fibrin network more porous | Anti | 65, 79, 80 |
| Variable deformability of RBCs affect blood clot mechanics | Pro or Anti | ||
| Factor XIIIa-mediated RBC retention increases thrombus size | Pro | 81, 82, 83 | |
| Effects on fibrinolysis and thrombolysis | RBCs reduce clot permeability | Pro | 84, 86, 87 |
| RBCs suppress tPA-induced plasminogen activation | Pro | ||
| RBCs decrease fibrin fiber dimeter and change the network structure, thus reducing susceptibility to fibrinolysis | Pro | ||
| RBCs are potential transportation cargo for targeted delivery of thrombolytic drugs | Anti | 45 | |
| Effects on clot contraction | Compacted RBCs form impermeable seal | Pro or Anti | 88, 89 |
| RBCs undergo compressive deformation from biconcave to polyhedral and intermediate forms | Pro or Anti | 91, 92, 93 | |
| RBCs are redistributed in contracted clots toward the middle | Pro or Anti | 89 | |
| Hemostatic effects of RBC transfusions | RBC transfusion stops bleeding associated with anemia and thrombocytopenia | Pro | 39, 47 |
| RBC transfusion improves platelet responsiveness to stimulation | Pro | ||
| Complications of RBC transfusions | “Storage lesion” of RBCs includes: - oxidative stress and membrane damage - phosphatidylserine exposure - release of microvesicles - hemolysis - increased membrane rigidity - release of free hemoglobin - activation of complement - depletion of NO and its functional equivalents - apoptosis (eryptosis) |
Pro | 31, 32, 34, 36, 37, 40, 41, 42, 43, 44, 45, 47, 48, 49 |
Table 2.
(Pro)thrombotic pathologies with RBCs as a (major) pathogenic factor
| Pathologies | References |
|---|---|
| Erythroid diseases | |
| Polycythemia vera | J Intern Med 1998, 244: 49; Curr Opin Hematol 2014, 21: 186 |
| Hereditary elliptocytosis | Int J Lab Hematol 2017; 39 Suppl 1: 47 |
| Hereditary stomatocytosis | Br J Haematol 1996, 93: 303; Blood 1997, 89: 3451 |
| Hereditary spherocytosis | Blood 2009; 114: 2861; J Thromb Haemost 2008; 6: 1289; Curr Opin Hematol 2014, 21: 186 |
| Hereditary xerocytosis | Int J Lab Hematol 2017; 39 Suppl 1: 47; Rev Med Interne 2007; 28: 879 |
| (Beta)-thalassemia | Acta Hematol 1992, 87: 71; Stroke 1990, 21: 812; Am J Physiol 1996, 270: H1951 |
| Sickle cell disease | Thromb Haemost 1996, 76: 322; Curr Opin Hematol 1996, 3: 118; Microcirculation 2009, 16: 97 |
| Paroxysmal nocturnal haemoglobinuria | Blood Cells Mol Dis 2017, 65: 29; Br J Haematol 2011; 152: 631 |
| Glucose-6-phosphate dehydrogenase deficiency (favism) | Vox Sang 2013; 105: 271 |
| Secondary erythrocytosis | Sleep Breath 2010; 14: 193 |
| Non-erythroid diseases | |
| Immune hemolytic anemias | Br J Haematol 2016, 172: 144 |
| Atherosclerotic vascular disease | Coron Artery Dis 1998, 9: 113; Clin Hemorheol Microcirc 2004, 31: 185 |
| Cerebral infarction | Lancet 1981, 2: 114 |
| Coronary heart disease | Blood 1997, 89: 4236 |
| Myocardial infarction | Clin Hemorheol Microcirc 1999, 20: 111 |
| Complications of RBC transfusion | Thromb Res 2015, 136: 1204 |
| Retinal venous occlusions | Am J Ophthalmol 1983, 96: 399; Br J Haematol 1990, 75: 127; Curr Opin Hematol 2014, 21: 186 |
| Hypertension | J Hypertens 1992, 10: S69; Clin Hemorheol Microcirc 1997, 17: 193 |
| Diabetes mellitus | J Biomed Eng 1993, 15: 155; Rom J Intern Med 2004, 42: 407; Biorheology 2009, 46: 63 |
| Leg vein thrombosis | Br J Haematol 1994, 88: 174 |
| Stroke | Curr Opin Neurol Neurosurg 1992, 5: 44 |
| Malaria | Science 1994, 264: 1878; Cell Microbiol 2013, 15: 1976 |
| Acquired dysfibrinogenemia | J Vasc Surg 1997, 26: 1061 |
| Systemic inflammation (hypergammaglobulinemia) | BBA Clin 2016, 5, 186; Cell Death Dis 2012, 3: 410 |
| Bacterial sepsis | J Mol Med 2007, 85: 269; Am J Respir Crit Care Med 1998, 157: 421 |
| Gaucher disease | Br J Haematol 2006, 134: 432; Curr Opin Hematol 2014, 21: 186 |
Quantitative and qualitative changes in RBCs related to bleeding and thrombosis
Hematocrit and rheological effects
It has long been known that low hematocrits are associated with prolonged bleeding times, even if the platelet counts are normal [2]. Consequently, many bleeding disorders have been corrected by transfusion of RBCs, despite normal or even low platelet levels. Conversely, patients with an abnormally high hematocrit, such as those with polycythemia vera or taking erythropoietin, including doping by healthy athletes [3], are more susceptible to thrombotic disorders [4]. Thus, for some time there has been indirect but solid evidence that RBCs do play some role in hemostasis and thrombosis and can be procoagulant or prothrombotic.
RBCs contribute to blood viscosity, which increases non-linearly with hematocrit and comprises a pathogenic mechanism for thrombosis (Fig. 1). The increased viscosity slows down the flow and can be a strong prothrombotic factor as a component of Virchow’s triad, which accounts for the pathophysiological mechanisms of thrombosis as a combination of endothelial damage, hypercoagulability, and disturbance of blood flow. Such increases in blood viscosity may promote platelet margination and have physical effects on the interaction between platelets and the blood vessel walls, since platelet adhesion increases with hematocrit. Therefore, physical effects of RBCs on hemostasis and thrombosis depend on both the hematocrit and flow conditions [5].
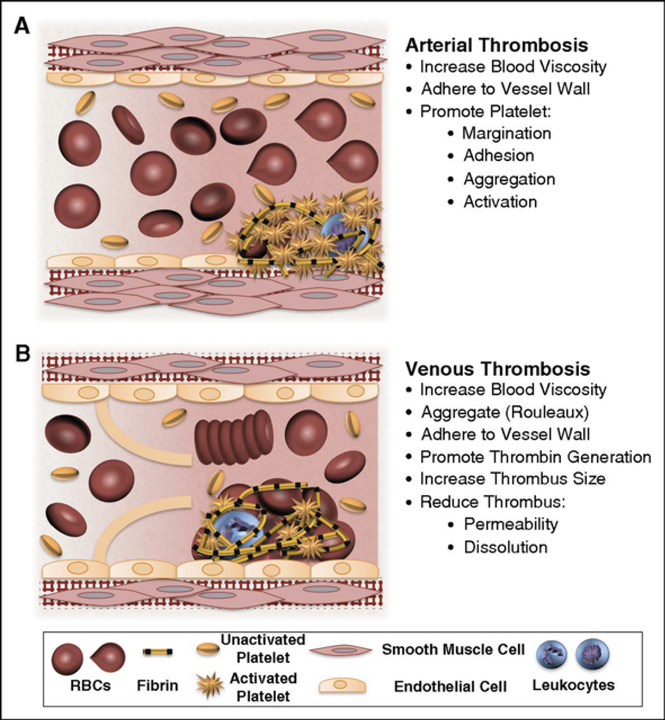
Figure 1.
Potential contributions of normal and abnormal RBCs to arterial and venous thrombosis/thromboembolism. (A) Arterial thrombi arise in vessels with high shear rates, which promotes the rapid formation of platelet-rich thrombi. During arterial thrombosis, RBCs promote platelet margination, increase platelet-thrombus interactions, and enhance platelet adhesion and activation. Although RBCs increase blood viscosity, this effect is lessened in arteries by high shear-induced shape change. (B) Venous thrombi form slowly in stasis or low flow (frequently in venous valve pockets) and are RBC and fibrin rich. In veins, RBC aggregation into stacked rouleaux structures increases blood viscosity. RBCs can also directly or indirectly adhere to the vessel wall and may contribute to thrombin generation within thrombi. Once incorporated into venous thrombi, RBCs increase thrombus size and reduce thrombus permeability and susceptibility to lysis. In disease states, abnormal RBCs and RBC-derived microvesicles may also adhere to the endothelium or extracellular matrix, activate platelets and other cells, and enhance local thrombin generation during thrombosis.
[With permission from: Byrnes JR, Wolberg AS. Red blood cells in thrombosis. Blood 2017, 130: 1795]
The commonly observed direct correlation between hematocrit and the prothrombotic phenotype has exceptions. Elevated hematocrit in animal models of polycythemia vera or erythropoietin-induced erythrocytosis did not correlate with thrombosis. Moreover, enhanced FeCl3-induced thrombosis in polycythemia vera mice was associated with an increased tail bleeding time, perhaps due to simultaneous deficiency of GPVI and impaired multimerization of von Willebrand factor [6]. The same bleeding tendency was revealed in mice with extremely high hematocrit (85%), while animals with a lower hematocrit were indistinguishable from controls in a thrombosis model, suggesting that the prothrombotic effects of RBCs may be compensated for with other mechanisms [7]. Therefore, the relationship of the RBC content to thrombosis may be not straightforward and so worth further investigation.
Deformability
Physiologically, RBCs that are 7–8 μm in size must change from their native biconcave shape to a bullet-like shape every time they squeeze through 1–3-μm blood vessels to maintain a high surface area necessary for efficient exchange of oxygen and carbon dioxide between blood and tissues. The efficacy of this diffusive exchange is determined by maximizing the active contact area between a RBC and the vessel wall, due to the deformation of RBCs and a high surface-to-volume ratio. The biconcave discoid shape compared to spherical provides approximately 40 μm2 (43%) of additional surface area. Deformability of RBCs depends mainly on cytoskeletal proteins and intracellular viscosity [8]. RBCs have a remarkably soft cytoskeleton under the plasma membrane that has a special dynamical molecular structure comprised of non-covalent association of proteins, namely spectrin, actin, ankyrin, Band 3, Band 4.1, and glycophorin C [9]. Structural alterations of transmembrane or cytoskeletal proteins or composition of membrane phospholipids result in rupture of the RBC membrane (hemolysis) or an increase in membrane stiffness. In addition to a decrease in membrane flexibility, increased RBC rigidity can be caused by changes in the viscosity of the cytoplasm due to an increase in hemoglobin concentration or a decrease in hemoglobin solubility [10]. The intracellular content of ATP used by the ion pumps to maintain the RBC volume through water-ion balance content, as well as increased Ca2+ concentration, also reduce RBC deformability. Irrespective of the underlying mechanisms, more rigid RBCs are associated with thrombogenic potential, since they can hardly squeeze through the microvasculature and they also enhance platelet margination (Fig. 2).
Figure 2.
Pro-thrombotic alteration of RBCs in various disease states and during storage. S/V = surface to volume ratio.
There are a number of acquired pathological conditions and inherited diseases with reduced RBC deformability, such as autoimmune hemolytic anemia, sickle cell disease, thalassemia, hereditary spherocytosis and xerocytosis. In sickle cell disease patients, the membrane of RBCs is much stiffer than in normal cells [11]. Besides the increased membrane rigidity, the overall stiffness of cells increases dramatically due to intracellular polymerization of mutated hemoglobin S, resulting in formation of sickled RBCs. Increased stiffness of RBC membranes combined with prothrombotic properties of RBCs have been reported also in β-thalassemia, immune hemolytic anemias, hereditary stomatocytosis, coronary heart disease, hypertension, diabetes, deep vein thrombosis (Table 2). RBC membrane viscosity and rigidity have been shown to correlate directly with RBC-derived reactive oxygen species lipid peroxidation [12]; however, the RBC deformability in conditions of oxidative stress is preserved by nitric oxide [13]. During storage, the rigidity of RBCs increase over time, this may be partly responsible for thrombotic complications of RBC transfusions along with other RBC alterations and with respect to thrombotic risk associated with the primary disease.
RBC aggregation
Another example of the significance of locally altered blood rheology is the formation of roleaux (linear arrays of stacked cells) or three-dimensional aggregates with stasis of blood or at low shear rates [14]. These aggregates increase the blood viscosity and hydrodynamic resistance in larger blood vessels with low shear, such as the veins in the lower limbs [15], again confirming Virchow’s triad, as these RBC aggregates promote venous thrombosis (Fig. 1). In very small vessels, aggregated RBCs concentrate along the flow axis and enhance platelet margination, and cause a decreased local viscosity and reduced flow resistance (Fahraeus effect) [16].
Two alternative mechanisms of RBC aggregation have been conceived, namely a bridging model and local osmotic gradient model [10]. The former model implies that the intercellular interactions are mediated by plasma proteins, mainly fibrinogen and immunoglobulins. The local osmotic gradient or depletion model attributes aggregation of RBCs to a lower protein concentration near the cell membrane compared with the ambient solution, resulting in an osmotic gradient or local depletion interaction [17]. There is increasing evidence supporting the osmotic gradient mechanism of RBC aggregation.
Phosphatidylserine exposure in RBC membrane
An essential component of blood clotting is a procoagulant cellular or cell-derived phospholipid membrane with exposed negatively charged phosphatidylserine that provides a matrix for assembly of coagulation complexes, namely the intrinsic tenase and prothrombinase (Fig. 1). In normal and quiescent cells, phosphatidylserine is located in the inner leaflet of the plasma membrane to separate this procoagulant phospholipid from plasma coagulation factors. Phosphatidylserine becomes exposed by the protein scramblase that abolishes natural membrane phospholipid asymmetry in response to Ca2+-induced inactivation of translocase and flippase that sustain this asymmetry [18]. The exposure of phosphatidylserine and its role in blood clotting has been mostly studied on platelets, but recent data provide evidence that RBCs can expose phosphatidylserine on their membrane and promote thrombin formation [19]. However, it has been proposed that RBCs may have dual pro- and anticoagulant activity, because they, unlike platelets, generate α-thrombin through an intermediate meizothrombin, a strong protein C activator with low fibrinogen- and PAR-cleaving activity, although the relative significance of the procoagulant effects is greater [19, 20].
RBCs lose membrane asymmetry and expose phosphatidylserine under conditions of cell damage induced by high shear rates, inflammation, or oxidative stress [21]. The exposure and shedding of phosphatidylserine mediated by the intracellular influx of Ca2+ is a part of RBC apoptosis and natural cell senescence [22]. The prothrombotic potential of phosphatidylserine exposure in RBCs is large due to the high RBCs count. While ~0.5–0.6% of the RBC population normally expresses phosphatidylserine in healthy subjects and induces some thrombin generation, the contribution of RBCs in pathological conditions may reach 40% of the thrombin-generating potential of whole blood [23].
A substantial amount of phosphatidylserine is exposed on RBC membranes in patients with sickle cell disease and thalassemia [24]. In sickle cell disease, this exposure results from the repeated cell deformation into sickled shapes and back to the biconcave shape due to reversible polymerization of sickle hemoglobin. Interestingly, in the blood of sickle cell disease patients, thrombin generation has been shown to correlate inversely to RBC phosphatidylserine exposure [25], implying that in sickle cell disease, additional procoagulant mechanisms exist unrelated to phosphatidylserine that are described elsewhere [26]. In β-thalassemia, increased phosphatidylserine exposure on the surface of RBCs is associated with the cell death pathway named eryptosis [27].
RBC-derived microvesicles
Many cells, including RBCs, generate microscopic extracellular membranous structures called microvesicles (MVs) or microparticles as a result of activation, apoptosis or aging. Membrane blebbing and formation of MVs is a consequence of the loss of membrane phospholipid asymmetry on RBCs, which is why MVs bare phosphatidylserine on their surface [28]. MV generation in RBCs results from a disturbance of the membrane-cytoskeleton interactions [29]. While MVs were once thought to be an undesirable byproduct of these processes, it is now known that they represent a means for intercellular communications in vivo, an important regulatory mechanism of physiologic reactions, and a pathogenic component in many thrombotic and hemostatic disorders (Fig. 2) [30]. Furthermore, MVs from RBCs accumulate during storage of whole blood [31] which might be partially responsible for an increased incidence of deep vein thrombosis or other thrombotic conditions after transfusion of RBCs stored for longer times [32]. MVs from RBCs increase in sickle cell disease and hemolytic anemia, and other prothrombotic states associated with RBCs [33]. Higher levels of MVs in the plasma are associated with a dose- and time-dependent increase in generation of thrombin and a reduction in clotting time, suggesting that they enhance hypercoagulability [34]. This enhanced thrombin generation has been associated with expression of phosphatidylserine [28]. Alternatively, RBC-derived MVs can initiate thrombin formation via a factor XII-dependent pathway without tissue factor activity [35]. The circulating MVs can also promote vaso-occlusion by internalizing free heme and transferring it to vascular endothelium, or activate the complement system [36]. Altogether, RBC-derived MVs, either formed in vivo or infused along with stored RBCs, have prothrombotic effects with multiple underlying mechanisms [37, 38]. With all of these procoagulant activities, RBC-derived MVs could be a target for treatment of thrombotic disorders [39].
RBC storage
During storage for transfusions, RBC preparations develop multiple and diverse changes in their structure and metabolism caused by the accumulation of their own waste products, by enzymatic and oxidative injury, and by programmed cell death [40]. These alterations are altogether designated as “storage lesion” and include a decrease in the content of 2,3-diphosphoglycerate and ATP concentrations, membrane loss, shape changes, formation of MVs, and release of toxic products, such as extracellular hemoglobin associated with hemolysis, lysophospholipids, and iron ions [41]. Storage of RBCs is accompanied by strong procoagulant changes, such as exposure of phosphatidylserine on cells as well as on the abundant RBC-derived MVs (Fig. 2) [40]. High concentrations of procoagulant phosphatidylserine-expressing MVs formed in preparation of stored RBCs exaggerate thrombotic complications after RBC transfusion [42]. In addition, free extracellular hemoglobin resulting from storage-related hemolysis binds and inactivates nitric oxide in blood, a potent vasodilator and inhibitor of platelet activation, which is another prothrombotic consequence of RBC infusions [43]. Therefore, infusions of RBCs, especially old ones, have adverse thrombotic effects, among which deep vein thrombosis is one of the most common [32]. In particular, perioperative RBC transfusions have been shown to be associated with a higher incidence of postoperative venous thromboembolism on top of the risk associated with surgery itself [44]. In addition to transfusions of normal stored RBCs, chemically modified and loaded RBCs have been studied intensively as cargo for targeted drug delivery [45].
Hemolysis
Hereditary and acquired hemolytic anemias, of which immune hemolysis is the most common, arise from hemolysis, with release of free, extracellular hemoglobin into the blood (Fig. 2). Massive hemolysis followed by thrombotic complications is a major pathogenic mechanism in paroxysmal nocturnal hemoglobinuria [46] and of adverse effects of RBC transfusions, provided they are not caused by the underlying disease [47, 48]. Hemolysis is accompanied by (pro)thrombotic conditions that can range from mild hypercoagulability detected by laboratory signs to life-threatening complications, such as disseminated intravascular coagulation and venous thromboembolism [49]. Hemolysis may result in such prothrombotic conditions via several pathogenic mechanisms. Hemolysis is commonly accompanied by a large release of RBC-derived MVs, with all of the effects described above [35]. Free hemoglobin and heme, which are toxic to many cells and tissues, are released [50]. Furthermore, extracellular hemoglobin sequesters NO and thus enhances adhesion/aggregation of platelets and activation of endothelial cells [51]. Free heme also generates reactive oxygen species, upregulates heme oxygenase activity, and directly activates macrophages and endothelial cells [52]. Finally, immune hemolysis is accompanied by activation of the complement cascade and production of TNF-α, which induces tissue factor expression in endothelial cells and decreases the endothelial expression of thrombomodulin, down-regulating the anti-coagulant pathway [49].
Although extracellular hemoglobin release during hemolysis has been considered as a transporter and scavenger of nitric oxide (NO), an inhibitor of endothelial cells and platelets, and a vasodilator, it has been shown recently that hemoglobin can preserve functional effects of NO by formation of S-nitrosothiols bound reversibly to the Cys-93 residue of the β-chain [53]. S-nitrosothiols have antiplatelet activity similar to NO and therefore lysed RBCs can either enhance platelet aggregation by scavenging NO or inhibiting platelets through release of functional equivalents of NO [48].
Interactions of RBCs with cellular and molecular components of the hemostatic system
Vessel wall
The interaction of RBCs with endothelium under physiological conditions is minimal, but they become adhesive when RBCs and/or endothelial cells undergo pathological perturbations, resulting in occlusion of the microvasculature, often associated with thrombotic conditions. Increased RBC adhesion to endothelium is mediated by a number of adhesive molecules, such VCAM-1, α4β1, Lu/BCAM, ICAM-4, etc. [10, 54]. In addition to the interaction of RBCs with activated endothelial cells, they can be exposed and bind to subendothelial matrix when the endothelium is damaged. The RBC-endothelium adhesive interaction causing occlusion of small vessels has been shown in a number of pathological conditions, such as retinal venous occlusion, hypertension, diabetic mellitus, and stroke (Table 2). RBC adhesion to endothelium in central retinal vein occlusion is mediated by the interaction between phosphatidylserine exposed on the surface of RBCs and endothelial phosphatidylserine receptor [55]. Prolonged storage followed by time-dependent alterations of RBCs enhance the ability of infused RBCs to bind to the endothelium and form micro-aggregates that impair blood flow in the microvasculature [56]. In one of the most commonly used vascular injury models in mice for thrombosis using FeCl3, RBCs were shown to be the first cells to adhere to the chemically injured endothelium, but this interaction is an artifact of the FeCl3 [57]. It has been shown recently that dysfunction or loss of CD59, a protective glycoprotein that prevents formation of the complement-dependent membrane attack complex, is a major arterial prothrombotic factor in paroxysmal nocturnal hemoglobinuria. To alleviate consequences of massive hemolysis under this pathological condition, CD59 reduces endothelial damage and platelet activation, as well as their aggregation with leukocytes that exaggerate vascular occlusion [58].
Platelets
A purely rheological effect of RBCs is that they preferentially move down the center of blood vessels, causing margination of platelets, so that they are adjacent to the vessel wall, where they can interact to form a temporary plug in case of injury [59]. This peripheral layer also contains plasma with clotting factors and neutrophils, important for hemostasis. As a result of the RBCs being in the center of the channel and plasma at the periphery, there is a decrease in viscosity at lower vessel diameters, except in capillaries that are smaller than RBCs, where the viscosity of the RBC-free layer increases because of the presence of platelets, which have a greater viscosity than RBCs [60]. One consequence of an elevated hematocrit is increased margination of platelets, enhancing their interactions with the endothelium, perhaps accounting for increased thrombotic complications. Another consequence of the reduced viscosity near the vessel wall and decreased wall shear stress is a reduction in NO release [61]. Since NO prevents activation of endothelial cells and platelets, this NO deficiency results in increased cellular activation.
RBCs can interact directly with platelets at venous shear rates [62], which may be important in prothrombotic pathological conditions, such as thalassemia [63] or sickle cell disease [64]. The ability of RBCs to directly bind activated platelets may play some role in the unexpected prevalence of RBCs in arterial thrombi that have been traditionally called “white” thrombi made mainly of activated platelets and fibrin [65].
RBCs can also modulate platelet reactivity directly through chemical signaling [66]. Under low oxygen pressure, low pH, and in response to mechanical deformation, RBCs release ATP and ADP, which activate platelets [67]. Release of extracellular hemoglobin from damage to RBCs enhances platelet activation by lowering NO bioavailability [68], since the hemoglobin is a strong NO scavenger, preventing the suppressive effect of nitric oxide on platelet activation [69]. An additional effect is the release from damaged RBCs of arginase, which cleaves L-arginine, a substrate for NO production [68].
Fibrinogen and fibrin
Hyperfibrinogenemia is associated with aggregation of RBCs in a form of roleaux, a morphological sign of (pro)thrombotic conditions and a pathogenic mechanism of microthrombosis [70]. The association between the fibrinogen plasma concentration and the erythrocyte tendency to aggregate was reported in metabolic, inflammatory and vascular diseases [71]. Formation of the aggregates of RBCs occurs through fibrinogen that bridges adjacent cells, similar to the role of fibrinogen in platelet aggregation. It has been shown that binding of fibrinogen to the RBC membrane may be mediated by an integrin-like receptor [72, 73] or an integrin-associated protein (CD47) [74]. The involvement of a β3 integrin of RBCs is supported by the observation that in a patient with mutated αIIbβ3 (Glanzmann’s thrombasthenia) interaction of RBCs with fibrinogen is impaired [73]. Remarkably, the interactions of fibrinogen with RBCs circulating in the blood for a longer time are gradually reduced, perhaps due to desialization of membrane proteins in older RBCs [75]. A recent paper showed that the minor heterozygous fibrinogen variant containing two splicing variants of the γ chain called γA and γ′ bound stronger to RBCs that the major homozygous fibrinogen fraction γAγA [76]. Because fibrinogen and fibrin share binding sites to the integrin αIIbβ3 [77], the specificity of fibrinogen binding to RBCs can be similar to RBC-fibrin interactions in blood clots and thrombi. In addition, retention of RBCs in venous thrombi at a low flow speed and stasis can be mediated by their interaction with von Willebrand factor located either on fibrin on another surface [78]. Understanding the molecular nature of the RBC binding to fibrinogen and fibrin is important because prevention and/or disruption of this interaction may be a novel antithrombotic therapeutic target similar to platelet-fibrin(ogen) interactions.
Clot structure and fibrinolysis
RBCs are incorporated into all types of clots and thrombi formed in vivo, especially in the venous system [79] but even in arterial thrombi [65]. Thus, the effects of RBCs on clot structure have been studied in vitro. Intermediate RBC concentrations cause considerable heterogeneity in the fiber network, with pockets of densely packed fibers alongside regions where fibers are sparse [80]. With higher levels of RBCs, fibers are more uniformly but loosely arranged around the cells, and fiber diameters are larger. RBCs embedded into a blood clot exclude fibrin and platelets and thereby enlarge the pores of the fibrin network making it more permeable if they are washed out, but at same time they can make the entire clot less permeable because those RBCs that remain (especially those compressed during contraction) make a physical barrier or a seal that hampers diffusion or perfusion. The modulation in clot structure and mechanical properties from RBCs affects clot stability, embolization, and the efficacy of anticoagulation and therapeutic thrombolysis [80]. In a venous model, RBC retention within clots determines thrombus size dependent on factor XIIIa activity [81, 82], via crosslinking of the fibrin α chains [83].
All of these effects of RBCs on the physical and chemical properties of clots have striking effects on clot dissolution via fibrinolysis. Overall, incorporation of RBCs increases the resistance to lysis and decreases the permeability of clots in a dose-dependent manner [84]. As expected from in vitro studies demonstrating an increase in mechanical stability and retardation of fibrinolysis, similar effects were shown for thrombi in experimental cerebral ischemia [85]. Alternatively, RBC-derived MVs have a prominent fibrinolytic activity in vitro due to the presence of plasminogen on their surface [86]. Moreover, a higher fraction of RBCs in cerebral thrombi has been shown to correlate with better responsiveness to intravenous thrombolysis [87]. Collectively, the effects of RBCs on fibrinolysis are controversial and need further attention.
Clot contraction
Clot contraction, also known as retraction, is the volume shrinkage of the blood clot that occurs when activated platelets pull on the fibrin network. Non-muscle myosin IIa inside the platelet interacts with actin filaments attached to the membrane-associated adhesive integrin αIIbβ3 via talin and kindlin. Fibrin or fibrinogen bind to αIIbβ3 outside the platelet to link other platelets into a platelet-fibrin meshwork that undergoes mechanical compaction, causing compression of RBCs embedded into the clot [88]. Clot contraction has a number of potential pathophysiological implications. First, it is biologically important that compaction of RBCs reduces the space between cells, due to more efficient packing, which helps to create an impermeable seal at the site of vessel injury to prevent bleeding [89]. Second, contraction pulls clots or thrombi closer to the vessel wall so that they are less obstructive. Third, contraction can modulate effects of the fibrinolytic enzymes by changing clot permeability and spatial proximity of the fibrin fibers. Fourth, clot contraction pulls together wound edges making this process important at the early stages of wound healing.
The kinetics and the extent of clot contraction depend on variations in the molecular and cellular blood composition, including fibrinogen concentration, RBC and platelet count [90]. Unlike thrombin activity, factor XIIIa-catalyzed crosslinking of fibrin, and platelet count, which all enhance the rate and efficacy of clot contraction, RBCs delay and reduce the process of clot compaction, while increasing the mechanical force generated by the activated platelets due to viscoelastic properties and mechanical resilience of RBCs [82, 83, 90]. The examination of how the presence of RBCs affects the compaction of a thrombus, and consequently the blood flow past the thrombus, has the potential to guide future therapeutic applications and has clinical implications in patients with pathological conditions associated with increased (polycythemia) or reduced (anemia, hemodilution) RBC count in the blood.
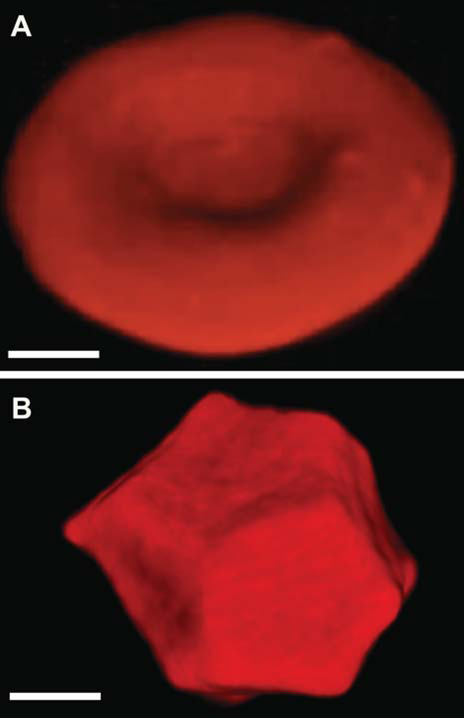
Platelet contraction propagated via the fibrin network results in the compaction of erythrocytes to the core of the clots and redistribution of platelets and fibrin toward the outside of the clot [89]. The erythrocytes amassed in the core of the contracting clot undergo a shape transformation from their native biconcave shape to that of polyhedral (Fig. 3), hence named polyhedrocytes [89]. This remarkable polyhedral shape of erythrocytes is a natural morphological form of erythrocytes in addition to echinocytes, acanthocytes, spheroechinocytes, ovalocytes, elliptocytes, stomatocytes, and more. Polyhedrocytes have been observed in ex vivo clots and thrombi obtained from human and murine samples and can be used a morphological sign of intravital contraction of clots, thrombi, and thrombotic emboli. In particular, polyhedrocytes have been observed in human arterial and especially venous thrombi, and pulmonary emboli, taken from patients [89, 91–93]. Unexpectedly, the rate and extent of clot contraction has been found to be impaired in a number of (pro)thrombotic conditions, such as ischemic stroke [94], deep vein thrombosis [95], and systemic lupus erythematosus [96], and may be considered an underappreciated factor that increases the risk and/or exaggerates the course and outcome of thrombosis. Altogether these data suggest that the extent of clot compaction and the formation of compressed RBCs (polyhedrocytes) comprise an important pathogenic mechanism of thrombosis and could have a diagnostic and/or predictive value in (pro)thrombotic states.
Figure 3.
Three-dimensional confocal microscopy images of a native biconcave RBC (A) and a compressed multi-faceted polyhedral RBC, or polyhedrocyte (B), formed as a result of blood clot contraction. Magnification bars = 1 μm.
Factor VIII and von Willebrand factor
An understudied and underappreciated mechanism of the effects of RBCs on bleeding and thrombosis is modulation of the levels of von Willebrand factor and factor VIII in blood, which is related to the ABO blood group system [97]. In subjects that have A and B antigens, the concentrations of factor VIII and, especially, von Willebrand factor are substantially higher than in the O group individuals. This difference has been attributed to the posttranslational glycosylation of the proteins stimulated by the A and B antigens via an unknown mechanism [98]. Presumably, the extent of glycosylation could determine the clearance rate of factor VIII and von Willebrand factor, which results in faster elimination in O blood type subjects than in non-O individuals [99]. The higher levels of von Willebrand factor (and to a lesser extent of factor VIII) might underlie the association between the ABO blood group and the risk of thrombosis or bleeding [100].
Conclusions
There are both quantitative and qualitative changes in RBCs that affect bleeding and thrombosis, as well as interactions of RBCs with cellular and molecular components of the hemostatic system. Low hematocrits are associated with bleeding while high hematocrits are associated with thrombosis, as is the formation of RBC aggregates. Both the stiffness of RBCs and the exposure of phosphatidylserine to form a procoagulant surface that enhances thrombin generation can contribute to thrombosis. MVs from stored RBCs or from some pathological conditions have strong procoagulant effects, as do extracellular hemoglobin and heme as a result of hemolysis. Rheological effects of RBCs include their being concentrated in the center of blood vessels with consequent platelet margination, including effects on viscosity. RBCs interact with endothelial cells and platelets, both of which may be significant for thrombosis. RBCs also interact with fibrin(ogen) and affect the structure, mechanical properties, and hence the lytic resistance of clots and thrombi. Clot contraction may be important for hemostasis and wound healing because contracted clots form an impermeable barrier made of tightly packed polyhedrocytes. Furthermore, clot contraction restores blood flow past otherwise obstructive thrombi, though in some prothrombotic conditions platelet activation and exhaustion leads to a lower extent of clot contraction. In summary, the ability of RBCs to affect hemostasis and thrombosis is multifactorial and has multiple underlying mechanisms: modulating blood viscosity via hematocrit, deformability, and aggregation; variable adherence to the vessel wall that depends on the functional state of RBCs and/or endothelium; modulation of platelet reactivity; platelet margination; release of MVs; membrane composition (expression of adhesive proteins and phosphatidylserine); modulation of nitric oxide availability; expression of blood group antigens implicated in thrombotic and hemorrhagic risk. Most of these effects (summarized in Figs. 1 and 2) are prothrombotic and result in promotion of arterial and venous thrombosis. However, the RBC-related influences are much more complex and may be either pro- or antithrombotic (Table 1), making analysis of the biological role of RBCs not straightforward and emphasizing the need for further investigation.
Acknowledgments
Disclosure of Conflict of Interest
J.W. Weisel’s work was supported by NIH grants UO1HL116330, HL135254, NSF grant DMR1505662, and a grant from Boston Scientific. R.I. Litvinov has received support from the Program for Competitive Growth at Kazan Federal University.
References
- 1.Wang W, Diacovo TG, Chen J, Freund JB, King MR. Simulation of platelet, thrombus and erythrocyte hydrodynamic interactions in a 3D arteriole with in vivo comparison. PLoS One 2013; 8: e76949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duke WW. The relation of blood platelets to hemorrhagic disease. JAMA 1910; 60: 1185–92. [PubMed] [Google Scholar]
- 3.Tokish JM, Kocher MS, Hawkins RJ. Ergogenic aids: a review of basic science, performance, side effects, and status in sports. Am J Sports Med 2004; 32: 1543–53. [DOI] [PubMed] [Google Scholar]
- 4.Kroll MH, Michaelis LC, Verstovsek S. Mechanisms of thrombogenesis in polycythemia vera. Blood Rev 2015; 29: 215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barshtein G, Ben-Ami R, Yedgar S. Role of red blood cell flow behavior in hemodynamics and hemostasis. Expert Rev Cardiovasc Ther 2007; 5: 743–52. [DOI] [PubMed] [Google Scholar]
- 6.Lamrani L, Lacout C, Ollivier V, Denis CV, Gardiner E, Ho Tin Noe B, Vainchenker W, Villeval JL, Jandrot-Perrus M. Hemostatic disorders in a JAK2V617F-driven mouse model of myeloproliferative neoplasm. Blood 2014; 124: 1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata J, Hasegawa J, Siemens HJ, Wolber E, Dibbelt L, Li D, Katschinski DM, Fandrey J, Jelkmann W, Gassmann M, Wenger RH, Wagner KF. Hemostasis and coagulation at a hematocrit level of 0.85: functional consequences of erythrocytosis. Blood 2003; 101: 4416–22. [DOI] [PubMed] [Google Scholar]
- 8.Huisjes R, Bogdanova A, van Solinge WW, Schiffelers RM, Kaestner L, van Wijk R. Squeezing for life - properties of red blood cell deformability. Front Physiol 2018; 9: 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki T A comprehensive review of our current understanding of red blood cell (RBC) glycoproteins. Membranes (Basel) 2017; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du VX, Huskens D, Maas C, Al Dieri R, de Groot PG, de Laat B. New insights into the role of erythrocytes in thrombus formation. Semin Thromb Hemost 2014; 40: 72–80. [DOI] [PubMed] [Google Scholar]
- 11.Barabino GA, Platt MO, Kaul DK. Sickle cell biomechanics. Annu Rev Biomed Eng 2010; 12: 345–67. [DOI] [PubMed] [Google Scholar]
- 12.Becatti M, Marcucci R, Gori AM, Mannini L, Grifoni E, Alessandrello Liotta A, Sodi A, Tartaro R, Taddei N, Rizzo S, Prisco D, Abbate R, Fiorillo C. Erythrocyte oxidative stress is associated with cell deformability in patients with retinal vein occlusion. J Thromb Haemost 2016; 14: 2287–97. [DOI] [PubMed] [Google Scholar]
- 13.Diederich L, Suvorava T, Sansone R, Keller TCSt, Barbarino F, Sutton TR, Kramer CM, Luckstadt W, Isakson BE, Gohlke H, Feelisch M, Kelm M, Cortese-Krott MM On the effects of reactive oxygen species and nitric oxide on red blood cell deformability. Front Physiol 2018; 9: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumler H, Neu B, Donath E, Kiesewetter H. Basic phenomena of red blood cell rouleaux formation. Biorheology 1999; 36: 439–42. [PubMed] [Google Scholar]
- 15.Yu FT, Armstrong JK, Tripette J, Meiselman HJ, Cloutier G. A local increase in red blood cell aggregation can trigger deep vein thrombosis: evidence based on quantitative cellular ultrasound imaging. J Thromb Haemost 2011; 9: 481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahraeus R The influence of the rouleau formation of the erythrocytes on the rheology of the blood. Acta Med Scand 1958; 161: 151–65. [PubMed] [Google Scholar]
- 17.Baskurt OK, Meiselman HJ. Erythrocyte aggregation: basic aspects and clinical importance. Clin Hemorheol Microcirc 2013; 53: 23–37. [DOI] [PubMed] [Google Scholar]
- 18.Kay JG, Grinstein S. Phosphatidylserine-mediated cellular signaling. Adv Exp Med Biol 2013; 991: 177–93. [DOI] [PubMed] [Google Scholar]
- 19.Whelihan MF, Mann KG. The role of the red cell membrane in thrombin generation. Thromb Res 2013; 131: 377–82. [DOI] [PubMed] [Google Scholar]
- 20.Whelihan MF, Mooberry MJ, Zachary V, Bradford RL, Ataga KI, Mann KG, Key NS. The contribution of red blood cells to thrombin generation in sickle cell disease: meizothrombin generation on sickled red blood cells. J Thromb Haemost 2013; 11: 2187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi J, Shi Y, Waehrens LN, Rasmussen JT, Heegaard CW, Gilbert GE. Lactadherin detects early phosphatidylserine exposure on immortalized leukemia cells undergoing programmed cell death. Cytometry A 2006; 69: 1193–201. [DOI] [PubMed] [Google Scholar]
- 22.Freikman I, Fibach E. Distribution and shedding of the membrane phosphatidylserine during maturation and aging of erythroid cells. Biochim Biophys Acta 2011; 1808: 2773–80. [DOI] [PubMed] [Google Scholar]
- 23.Whelihan MF, Zachary V, Orfeo T, Mann KG. Prothrombin activation in blood coagulation: the erythrocyte contribution to thrombin generation. Blood 2012; 120: 3837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noubouossie D, Key NS, Ataga KI. Coagulation abnormalities of sickle cell disease: Relationship with clinical outcomes and the effect of disease modifying therapies. Blood Rev 2016; 30: 245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whelihan MF, Lim MY, Mooberry MJ, Piegore MG, Ilich A, Wogu A, Cai J, Monroe DM, Ataga KI, Mann KG, Key NS. Thrombin generation and cell-dependent hypercoagulability in sickle cell disease. J Thromb Haemost 2016; 14: 1941–52. [DOI] [PubMed] [Google Scholar]
- 26.De Franceschi L, Cappellini MD, Olivieri O. Thrombosis and sickle cell disease. Semin Thromb Hemost 2011; 37: 226–36. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim HA, Fouda MI, Yahya RS, Abousamra NK, Abd Elazim RA. Erythrocyte phosphatidylserine exposure in beta-thalassemia. Lab Hematol 2014; 20: 9–14. [DOI] [PubMed] [Google Scholar]
- 28.Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol 2011; 31: 15–26. [DOI] [PubMed] [Google Scholar]
- 29.Leal JKF, Adjobo-Hermans MJW, Bosman G. Red Blood Cell Homeostasis: Mechanisms and effects of microvesicle generation in health and disease. Front Physiol 2018; 9: 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morel O, Toti F, Hugel B, Bakouboula B, Camoin-Jau L, Dignat-George F, Freyssinet JM. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol 2006; 26: 2594–604. [DOI] [PubMed] [Google Scholar]
- 31.Rubin O, Crettaz D, Canellini G, Tissot JD, Lion N. Microparticles in stored red blood cells: an approach using flow cytometry and proteomic tools. Vox Sang 2008; 95: 288–97. [DOI] [PubMed] [Google Scholar]
- 32.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008; 358: 1229–39. [DOI] [PubMed] [Google Scholar]
- 33.van Beers EJ, Schaap MC, Berckmans RJ, Nieuwland R, Sturk A, van Doormaal FF, Meijers JC, Biemond BJ. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica 2009; 94: 1513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, Xia BT, Jung AD, Chang AL, Abplanalp WA, Caldwell CC, Goodman MD, Pritts TA. Microparticles from stored red blood cells promote a hypercoagulable state in a murine model of transfusion. Surgery 2018; 163: 423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Der Meijden PE, Van Schilfgaarde M, Van Oerle R, Renne T, ten Cate H, Spronk HM. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost 2012; 10: 1355–62. [DOI] [PubMed] [Google Scholar]
- 36.Zecher D, Cumpelik A, Schifferli JA. Erythrocyte-derived microvesicles amplify systemic inflammation by thrombin-dependent activation of complement. Arterioscler Thromb Vasc Biol 2014; 34: 313–20. [DOI] [PubMed] [Google Scholar]
- 37.Fischer D, Bussow J, Meybohm P, Weber CF, Zacharowski K, Urbschat A, Muller MM, Jennewein C. Microparticles from stored red blood cells enhance procoagulant and proinflammatory activity. Transfusion 2017; 57: 2701–11. [DOI] [PubMed] [Google Scholar]
- 38.Said AS, Rogers SC, Doctor A. Physiologic impact of circulating RBC microparticles upon blood-vascular interactions. Front Physiol 2017; 8: 1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jy W, Johansen ME, Bidot C Jr., Horstman LL, Ahn YS. Red cell-derived microparticles (RMP) as haemostatic agent. Thromb Haemost 2013; 110: 751–60. [DOI] [PubMed] [Google Scholar]
- 40.Hess JR. Measures of stored red blood cell quality. Vox Sang 2014; 107: 1–9. [DOI] [PubMed] [Google Scholar]
- 41.Grimshaw K, Sahler J, Spinelli SL, Phipps RP, Blumberg N. New frontiers in transfusion biology: identification and significance of mediators of morbidity and mortality in stored red blood cells. Transfusion 2011; 51: 874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Y, Lv L, Liu S, Ma G, Su Y. Elevated levels of thrombin-generating microparticles in stored red blood cells. Vox Sang 2013; 105: 11–7. [DOI] [PubMed] [Google Scholar]
- 43.Liu C, Liu X, Janes J, Stapley R, Patel RP, Gladwin MT, Kim-Shapiro DB. Mechanism of faster NO scavenging by older stored red blood cells. Redox Biol 2014; 2: 211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goel R, Patel EU, Cushing MM, Frank SM, Ness PM, Takemoto CM, Vasovic LV, Sheth S, Nellis ME, Shaz B, Tobian AAR. Association of perioperative red blood cell transfusions with venous thromboembolism in a North American registry. JAMA Surg 2018; 153: 826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villa CH, Pan DC, Johnston IH, Greineder CF, Walsh LR, Hood ED, Cines DB, Poncz M, Siegel DL, Muzykantov VR. Biocompatible coupling of therapeutic fusion proteins to human erythrocytes. Blood Adv 2018; 2: 165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peacock-Young B, Macrae FL, Newton DJ, Hill A, Ariens RAS. The prothrombotic state in paroxysmal nocturnal hemoglobinuria: a multifaceted source. Haematologica 2018; 103: 9–17. [DOI] [PubMed] [Google Scholar]
- 47.Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med 2017; 377: 1261–72. [DOI] [PubMed] [Google Scholar]
- 48.Dubovoy T, Engoren M. Thrombotic risks in red blood cell transfusions. Semin Thromb Hemost 2016; 42: 102–11. [DOI] [PubMed] [Google Scholar]
- 49.Davenport RD. Pathophysiology of hemolytic transfusion reactions. Semin Hematol 2005; 42: 165–8. [DOI] [PubMed] [Google Scholar]
- 50.Cubedo J, Suades R, Padro T, Martin-Yuste V, Sabate-Tenas M, Cinca J, Sans-Rosello J, Sionis A, Badimon L. Erythrocyte-heme proteins and STEMI: implications in prognosis. Thromb Haemost 2017; 117: 1970–80. [DOI] [PubMed] [Google Scholar]
- 51.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 2005; 293: 1653–62. [DOI] [PubMed] [Google Scholar]
- 52.Camus SM, De Moraes JA, Bonnin P, Abbyad P, Le Jeune S, Lionnet F, Loufrani L, Grimaud L, Lambry JC, Charue D, Kiger L, Renard JM, Larroque C, Le Clesiau H, Tedgui A, Bruneval P, Barja-Fidalgo C, Alexandrou A, Tharaux PL, Boulanger CM, Blanc-Brude OP. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood 2015; 125: 3805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gladwin MT, Lancaster JR Jr., Freeman BA, Schechter AN. Nitric oxide’s reactions with hemoglobin: a view through the SNO-storm. Nat Med 2003; 9: 496–500. [DOI] [PubMed] [Google Scholar]
- 54.Wautier JL, Wautier MP. Molecular basis of erythrocyte adhesion to endothelial cells in diseases. Clin Hemorheol Microcirc 2013; 53: 11–21. [DOI] [PubMed] [Google Scholar]
- 55.Wautier MP, Heron E, Picot J, Colin Y, Hermine O, Wautier JL. Red blood cell phosphatidylserine exposure is responsible for increased erythrocyte adhesion to endothelium in central retinal vein occlusion. J Thromb Haemost 2011; 9: 1049–55. [DOI] [PubMed] [Google Scholar]
- 56.Sparrow RL. Red blood cell storage duration and trauma. Transfus Med Rev 2015; 29: 120–6. [DOI] [PubMed] [Google Scholar]
- 57.Barr JD, Chauhan AK, Schaeffer GV, Hansen JK, Motto DG. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood 2013; 121: 3733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tabib A, Hindi I, Karbian N, Zelig O, Falach B, Mevorach D. Prothrombotic mechanisms in patients with congenital p.Cys89Tyr mutation in CD59. Thromb Res 2018; 168: 67–77. [DOI] [PubMed] [Google Scholar]
- 59.Flamm MH, Diamond SL. Multiscale systems biology and physics of thrombosis under flow. Ann Biomed Eng 2012; 40: 2355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dintenfass L Inversion of the Fahraeus-Lindqvist phenomenon in blood flow through capillaries of diminishing radius. Nature 1967; 215: 1099–100. [DOI] [PubMed] [Google Scholar]
- 61.Baskurt OK, Yalcin O, Ozdem S, Armstrong JK, Meiselman HJ. Modulation of endothelial nitric oxide synthase expression by red blood cell aggregation. Am J Physiol Heart Circ Physiol 2004; 286: H222–9. [DOI] [PubMed] [Google Scholar]
- 62.Goel MS, Diamond SL. Adhesion of normal erythrocytes at depressed venous shear rates to activated neutrophils, activated platelets, and fibrin polymerized from plasma. Blood 2002; 100: 3797–803. [DOI] [PubMed] [Google Scholar]
- 63.Thalassemia Sirachainan N. and the hypercoagulable state. Thromb Res 2013; 132: 637–41. [DOI] [PubMed] [Google Scholar]
- 64.White J, Lancelot M, Sarnaik S, Hines P. Increased erythrocyte adhesion to VCAM-1 during pulsatile flow: Application of a microfluidic flow adhesion bioassay. Clin Hemorheol Microcirc 2015; 60: 201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silvain J, Collet JP, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O, Montalescot G, Weisel JW. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol 2011; 57: 1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klatt C, Kruger I, Zey S, Krott KJ, Spelleken M, Gowert NS, Oberhuber A, Pfaff L, Luckstadt W, Jurk K, Schaller M, Al-Hasani H, Schrader J, Massberg S, Stark K, Schelzig H, Kelm M, Elvers M. Platelet-RBC interaction mediated by FasL-FasR induces procoagulant activity important for thrombosis. J Clin Invest 2018: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reimers RC, Sutera SP, Joist JH. Potentiation by red blood cells of shear-induced platelet aggregation: relative importance of chemical and physical mechanisms. Blood 1984; 64: 1200–6. [PubMed] [Google Scholar]
- 68.Helms CC, Marvel M, Zhao W, Stahle M, Vest R, Kato GJ, Lee JS, Christ G, Gladwin MT, Hantgan RR, Kim-Shapiro DB. Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost 2013; 11: 2148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tran PL, Pietropaolo MG, Valerio L, Brengle W, Wong RK, Kazui T, Khalpey ZI, Redaelli A, Sheriff J, Bluestein D, Slepian MJ. Hemolysate-mediated platelet aggregation: an additional risk mechanism contributing to thrombosis of continuous flow ventricular assist devices. Perfusion 2016; 31: 401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spengler MI, Svetaz MJ, Leroux MB, Bertoluzzo SM, Carrara P, Van Isseldyk F, Petrelli D, Parente FM, Bosch P. Erythrocyte aggregation in patients with systemic lupus erythematosus. Clin Hemorheol Microcirc 2011; 47: 279–85. [DOI] [PubMed] [Google Scholar]
- 71.Assayag EB, Bornstein N, Shapira I, Mardi T, Goldin Y, Tolshinski T, Vered Y, Zakuth V, Burke M, Berliner S, Bonet DS. Inflammation-sensitive proteins and erythrocyte aggregation in atherothrombosis. Int J Cardiol 2005; 98: 271–6. [DOI] [PubMed] [Google Scholar]
- 72.Lominadze D, Dean WL. Involvement of fibrinogen specific binding in erythrocyte aggregation. FEBS Lett 2002; 517: 41–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carvalho FA, Connell S, Miltenberger-Miltenyi G, Pereira SV, Tavares A, Ariens RA, Santos NC. Atomic force microscopy-based molecular recognition of a fibrinogen receptor on human erythrocytes. ACS Nano 2010; 4: 4609–20. [DOI] [PubMed] [Google Scholar]
- 74.De Oliveira S, Vitorino de Almeida V, Calado A, Rosario HS, Saldanha C. Integrin-associated protein (CD47) is a putative mediator for soluble fibrinogen interaction with human red blood cells membrane. Biochim Biophys Acta 2012; 1818: 481–90. [DOI] [PubMed] [Google Scholar]
- 75.Carvalho FA, de Oliveira S, Freitas T, Goncalves S, Santos NC. Variations on fibrinogen-erythrocyte interactions during cell aging. PLoS One 2011; 6: e18167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guedes AF, Carvalho FA, Domingues MM, Macrae FL, McPherson HR, Santos NC, Arismall io RRAS. Sensing adhesion forces between erythrocytes and gamma’ fibrinogen, modulating fibrin clot architecture and function. Nanomedicine 2018; 14: 909–18. [DOI] [PubMed] [Google Scholar]
- 77.Litvinov RI, Farrell DH, Weisel JW, Bennett JS. The platelet integrin alphaIIbbeta3 differentially interacts with fibrin versus fibrinogen. J Biol Chem 2016; 291: 7858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smeets MWJ, Mourik MJ, Niessen HWM, Hordijk PL. Stasis promotes erythrocyte adhesion to von Willebrand factor. Arterioscler Thromb Vasc Biol 2017; 37: 1618–27. [DOI] [PubMed] [Google Scholar]
- 79.Tratar G, Blinc A, Podbregar M, Kralj E, Balazic J, Sabovic M, Sersa I. Characterization of pulmonary emboli ex vivo by magnetic resonance imaging and ultrasound. Thromb Res 2007; 120: 763–71. [DOI] [PubMed] [Google Scholar]
- 80.Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost 2009; 102: 1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kattula S, Byrnes JR, Martin SM, Holle LA, Cooley BC, Flick MJ, Wolberg AS. Factor XIII in plasma, but not in platelets, mediates red blood cell retention in clots and venous thrombus size in mice. Blood Adv 2018; 2: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aleman MM, Byrnes JR, Wang JG, Tran R, Lam WA, Di Paola J, Mackman N, Degen JL, Flick MJ, Wolberg AS. Factor XIII activity mediates red blood cell retention in venous thrombi. J Clin Invest 2014; 124: 3590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Byrnes JR, Duval C, Wang Y, Hansen CE, Ahn B, Mooberry MJ, Clark MA, Johnsen JM, Lord ST, Lam WA, Meijers JC, Ni H, Ariens RA, Wolberg AS. Factor XIIIa-dependent retention of red blood cells in clots is mediated by fibrin alpha-chain crosslinking. Blood 2015; 126: 1940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wohner N, Sotonyi P, Machovich R, Szabo L, Tenekedjiev K, Silva MM, Longstaff C, Kolev K. Lytic resistance of fibrin containing red blood cells. Arterioscler Thromb Vasc Biol 2011; 31: 2306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van der Spuy WJ, Pretorius E. Interaction of red blood cells adjacent to and within a thrombus in experimental cerebral ischaemia. Thromb Res 2013; 132: 718–23. [DOI] [PubMed] [Google Scholar]
- 86.Levin G, Sukhareva E, Lavrentieva A. Impact of microparticles derived from erythrocytes on fibrinolysis. J Thromb Thrombolysis 2016; 41: 452–8. [DOI] [PubMed] [Google Scholar]
- 87.Choi MH, Park GH, Lee JS, Lee SE, Lee SJ, Kim JH, Hong JM. Erythrocyte fraction within retrieved thrombi contributes to thrombolytic response in acute ischemic stroke. Stroke 2018; 49: 652–9. [DOI] [PubMed] [Google Scholar]
- 88.Litvinov RI, Weisel JW. What is the biological and clinical relevance of fibrin? Semin Thromb Hemost 2016; 42: 333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cines DB, Lebedeva T, Nagaswami C, Hayes V, Massefski W, Litvinov RI, Rauova L, Lowery TJ, Weisel JW. Clot contraction: compression of erythrocytes into tightly packed polyhedra and redistribution of platelets and fibrin. Blood 2014; 123: 1596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tutwiler V, Litvinov RI, Lozhkin AP, Peshkova AD, Lebedeva T, Ataullakhanov FI, Spiller KL, Cines DB, Weisel JW. Kinetics and mechanics of clot contraction are governed by the molecular and cellular composition of the blood. Blood 2016; 127: 149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zabczyk M, Sadowski M, Zalewski J, Undas A. Polyhedrocytes in intracoronary thrombi from patients with ST-elevation myocardial infarction. Int J Cardiol 2015; 179: 186–7. [DOI] [PubMed] [Google Scholar]
- 92.Litvinov RI, Khismatullin RR, Shakirova AZ, Litvinov TR, Nagaswami C, Peshkova AD, Weisel JW. Morphological signs of intravital contraction (retraction) of pulmonary thrombotic emboli. BioNanoScience 2018; 8: 428–33. [Google Scholar]
- 93.Leong L, Chernysh IN, Xu Y, Sim D, Nagaswami C, de Lange Z, Kosolapova S, Cuker A, Kauser K, Weisel JW. Clot stability as a determinant of effective factor VIII replacement in hemophilia A. Res Pract Thromb Haemost 2017; 1: 231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tutwiler V, Peshkova AD, Andrianova IA, Khasanova DR, Weisel JW, Litvinov RI. Contraction of blood clots is impaired in acute ischemic stroke. Arterioscler Thromb Vasc Biol 2017; 37: 271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peshkova AD, Malyasyov DV, Bredikhin RA, Le Minh G, Andrianova IA, Tutwiler V, Nagaswami C, Weisel JW, Litvinov RI. Reduced contraction of blood clots in patients with venous thromboembolism is a possible thrombogenic and embologenic mechanism. TH Open 2018; 2: e104–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Le Minh G, Peshkova AD, Andrianova IA, Sibgatullin TB, Maksudova AN, Weisel JW, Litvinov RI. Impaired contraction of blood clots as a novel prothrombotic mechanism in systemic lupus erythematosus. Clin Sci (Lond) 2018; 132: 243–54. [DOI] [PubMed] [Google Scholar]
- 97.Jenkins PV, O’Donnell JS. ABO blood group determines plasma von Willebrand factor levels: a biologic function after all? Transfusion 2006; 46: 1836–44. [DOI] [PubMed] [Google Scholar]
- 98.Franchini M, Mannucci PM. ABO blood group and thrombotic vascular disease. Thromb Haemost 2014; 112: 1103–9. [DOI] [PubMed] [Google Scholar]
- 99.Casari C, Lenting PJ, Wohner N, Christophe OD, Denis CV. Clearance of von Willebrand factor. J Thromb Haemost 2013; 11 Suppl 1: 202–11. [DOI] [PubMed] [Google Scholar]
- 100.Franchini M, Lippi G. Relative risks of thrombosis and bleeding in different ABO blood groups. Semin Thromb Hemost 2016; 42: 112–7. [DOI] [PubMed] [Google Scholar]