Abstract
Objectives
The mainstay treatment for Degenerative Cervical Myelopathy (DCM) is surgical decompression. Not all cases, however, are suitable for surgery. Recent international guidelines advise surgery for moderate to severe disease as well as progressive mild disease. The goal of this study was to examine the factors in current practice that drive the decision to operate in DCM.
Study design
Retrospective cohort study.
Methods
1 year of cervical spine MRI scans (N = 1123) were reviewed to identify patients with DCM with sufficient clinical documentation (N = 39). Variables at surgical assessment were recorded: age, sex, clinical signs and symptoms of DCM, disease severity, and quantitative MRI measures of cord compression. Bivariate correlations were used to compare each variable with the decision to offer the patient an operation. Subsequent multivariable analysis incorporated all significant bivariate correlations.
Results
Of the 39 patients identified, 25 (64%) were offered an operation. The decision to operate was significantly associated with narrower non-pathological canal and cord diameters as well as cord compression ratio, explaining 50% of the variance. In a multivariable model, only cord compression ratio was significant (p = 0.017). Examination findings, symptoms, functional disability, disease severity, disease progression, and demographic factors were all non-significant.
Conclusions
Cord compression emerged as the main factor in surgical decision-making prior to the publication of recent guidelines. Newly identified predictors of post-operative outcome were not significantly associated with decision to operate.
Introduction
Degenerative Cervical Myelopathy (DCM) is a progressive condition characterized by degenerative changes in the cervical spine leading to chronic spinal cord compression. Pathological changes include osteophytosis, intervertebral disc bulging, and ligament ossification and hypertrophy leading to static and dynamic injury to the spinal cord [1]. DCM is the commonest cause of spinal cord dysfunction with an estimated minimum incidence and prevalence of 41 and 605 per 1,000,000 respectively in North America. The actual prevalence is likely to be much higher given under and misdiagnosis is common [2]. Estimates based on imaging series could put the prevalence as high as 5% in over 40 year olds [3,4]. In an aging population, DCM looks to become an increasing healthcare burden.
DCM is diagnosed through a combination of signs and symptoms consistent with a clinical diagnosis of myelopathy alongside an MRI scan showing the aforementioned pathological changes. Myelopathic features include: loss of manual dexterity, imbalance and falls, urinary and bowel dysfunction, hyper-reflexia, and limb weakness and spasticity [5]. DCM progresses in a step-wise or continuous manner and leads to significant reductions in a patient’s quality of life [6,7]. Given the non-specific and insidious presentation of DCM, assessment may prove challenging and significant delays have been noted with regards to diagnosis and treatment [8,9].
The mainstay of DCM management is surgery, but the optimal timing of surgery can be challenging, as the rate of disease progression is highly variable and cannot currently be predicted [2]. Current evidence does not demonstrate an ‘optimal’ surgical approach, and it should instead be tailored to the individual case depending on factors such as the location of the pathology, number of cervical levels affected, and the baseline cervical sagittal alignment [10].
In the largest prospective series of DCM patients undergoing surgery, Tetreault et al. found the following factors predict a better post-operative functional status: younger age, milder pre-operative myelopathy, non-smoker, fewer co-morbidities, non-impaired gait, shorter pre-operative symptom duration [11]. Other studies have highlighted the importance of short time duration between symptom onset and surgery in maximising patients’ postoperative function [12,13,14]. So far, pre-operative MRI factors have not shown to add further predictive power but do influence surgeons with regards to operative approach [15,16]. In their latest update on outcome prediction, Tetreault et al (2018) demonstrate that symptom duration and baseline disease severity remain the strongest and most consistent indicators [17].
In order to standardise management of DCM patients, an international group involving multiple stakeholders recently proposed the first set of guidelines [18]. These guidelines are based around an internationally accepted 18-point scale for the severity of DCM, the Modified Japanese Orthopaedic Association (mJOA) score. The guidelines recommend:
Surgery for cases of moderate (mJOA 12–14) or severe (mJOA <12) DCM.
Surgery or supervised non-operative treatment for mild (mJOA 15–17) DCM.
Surgery is strongly recommended in the case of progressive deterioration.
No surgery for asymptomatic cord compression.
Surgery or close follow-up for non-myelopathic patients with cord compression with radiculopathy symptoms.
Prior to the publication of the guidelines, the decision to operate was largely left to the treating surgeon. The present study therefore sought to establish which clinical or radiological features were used to guide treatment decisions prior to the release of these guidelines, whether these included features that were shown to be predictive of post-operative outcomes, and to provide a reference against which adoption of guidelines could be assessed. This is the first study to examine this critical moment in DCM management and to compare past practice with international best practice guidelines.
Methods
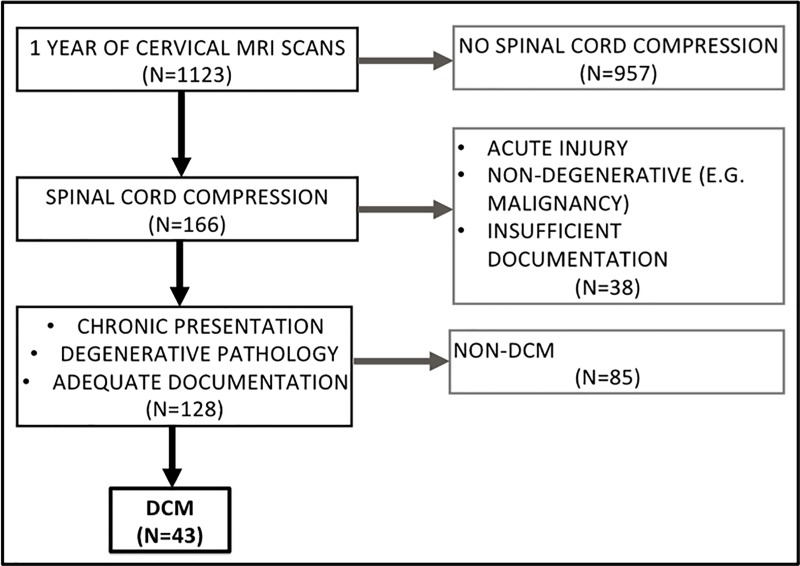
This was a retrospective, observational study based on a cohort of DCM patients (N = 39) identified from screening one year’s worth of cervical MRI scans (N = 1123) at a tertiary neurosciences centre. The case notes of patients with radiological cord compression were screened for a clinical diagnosis of DCM [8, 9] (Fig 1). All demographic and clinical data was collected from case notes as per the prior protocol. The inferred modified Japanese Orthopaedic Assessment (i-mJOA) was developed and used as a validated proxy for mJOA to assess disease severity as described [17]. The ‘decision to operate’ was defined by the clinician offering the patient an operation, regardless of intended surgical approach and if the patient decided to go ahead with surgery or not.
Fig 1. PRISMA flow diagram depicting cohort formation methodology.
Quantitative MRI measurements commonly used in DCM literature were collected as per previous methodology [19]. Measurements used in this study were: maximum canal compromise (MCC) [20], maximum spinal cord compression (MSCC) [19], spinal canal occupation ratio (SCOR)[21], compression ratio (CR) [22], normal canal diameter (NCaD), and normal cord diameter (NCoD). NCaD and NCoD were defined as the average anterior-posterior diameter of the spinal canal and cord respectively at the first non-degenerative spinal level above and below the diseased segment.
Statistical analysis was carried out using SPSS V22 (Chicago, IL, USA). The following statistical tests were applied depending on variable types involved in the analysis. Chi-squared test of homogeneity was used for and Fisher’s exact were applied for the relationship between clinical features (dichotomous, present or absent) and the decision to operate. Pearson’s correlations were used for i-mJOA (ordinal) relative to the decision to operate. Bivariate correlations followed by binomial logistic regression were applied for quantitative MRI measures (continuous) relative to the decision to operate.
All data was collected as part of a localised audit and quality improvement project. The work was formally registered and permission acquired in 2016 as a Service Evaluation project entitled “Degenerative cervical myelopathy: correlating clinical encounters with disease progression and radiological appearances using retrospective functional assessment”, Department of Clinical Neurosciences, Addenbrooke’s Hospital, University of Cambridge. All patient data was fully anonymised prior to collection and there was no active patient participation in this study.
Results
Cohort summary
43 cases of DCM were initially identified in this cohort. The average age at time of symptom onset was 61.4±13.9 years and the majority of patients (28, 65%) were male. Except for one patient, all cases were assessed by a spine surgeon. 48% (20/42) of these cases were new DCM while 52% (22/42) were recurrent DCM.
The overall times between symptom onset and surgical assessment for new and recurrent cases of DCM were 17.7±16.0 and 9.5±9.0 months respectively. Of the new cases of DCM, 45% (9/20) received an operation, 35% (7/20) received a follow-up appointment, and 20% (4/20) were discharged. Of the recurrent cases of DCM, 50% (11/22) received an operation, 23% (5/22) received a follow-up appointment, and 27% (6/22) were discharged. The details of 3 surgical assessments were not included beyond this descriptive cohort analysis. The reasons for this were: one case also suffered from severe Parkinson’s disease thus blurring symptomatic origin, one case did not attend their appointment, and one case’s assessment notes were inaccessible. 77% (30/39) of cases had sufficient documentation available to track disease progression prior to arrival at surgical assessment.
Correction between MRI measurements and clinical features of DCM
In order to investigate how objective measures of spinal cord compression impact on functional deficits, every clinical feature recorded at surgical assessment was examined relative to every MRI measurement (Table 1). A narrower NCaD was associated with corticospinal motor deficits (p = 0.04), clonus (p = 0.001), upper limb paraesthesia (p = 0.027), and lower limb paraesthesia (p = 0.014). A narrower NCoD and SCOR each showed one significant association with clonus (p = 0.02) and limb pain (p = 0.017) respectively. MCC, MSCC, and CR were not significantly associated with the presence of any clinical feature of DCM.
Table 1. The relationships between MRI measurements and clinical features at surgical assessment.
| Clinical feature | MCC | MSCC | NCaD | NCoD | SCOR | CR |
|---|---|---|---|---|---|---|
| Paraesthesia (upper limb) | 0.97 | 0.66 | 0.27 | 0.23 | 0.55 | |
| Weakness (upper limb) | 0.43 | 0.88 | 0.84 | 0.40 | 0.17 | 0.32 |
| Paraesthesia (lower limb) | 0.94 | 0.29 | 0.20 | 0.11 | 0.60 | |
| Weakness (lower limb) | 0.88 | 0.47 | 0.44 | 0.87 | 0.30 | 0.92 |
| Limb pain | 0.21 | 0.46 | 0.36 | 0.41 | 0.74 | |
| Neck pain/stiffness | 0.29 | 0.53 | 0.46 | 0.78 | 0.40 | 0.94 |
| Sphincter dysfunction | 0.35 | 0.84 | 0.62 | 0.38 | 0.49 | 0.34 |
| Instability | 0.24 | 0.09 | 0.84 | 0.66 | 0.61 | 0.61 |
| Falls | 0.96 | 0.45 | 0.65 | 0.91 | 0.52 | 0.61 |
| Corticospinal motor deficits | 0.41 | 0.77 | 0.44 | 0.08 | 0.77 | |
| Hyper-reflexia | 0.99 | 0.92 | 0.22 | 0.18 | 0.93 | 0.66 |
| Positive hoffmann | 0.99 | 0.27 | 0.89 | 0.48 | 0.32 | 0.68 |
| Upgoing plantars | 0.31 | 0.51 | 0.07 | 0.11 | 0.95 | 0.35 |
| Clonus | 0.61 | 0.26 | 0.10 | 0.46 | ||
| Unstable gait | 0.56 | 0.91 | 0.55 | 0.51 | 0.93 | 0.63 |
| Upper limb motor i-mJOA | 0.19 | 0.13 | 0.89 | 0.94 | 0.79 | 0.19 |
| Lower limb motor i-mJOA | 0.37 | 0.59 | 0.52 | 0.37 | 0.66 | 0.60 |
| Sensory i-mJOA | 0.60 | 0.95 | 0.99 | 0.44 | 0.25 | 0.78 |
| Sphincter i-mJOA | 0.59 | 0.52 | 0.91 | 0.59 | 0.52 | 0.52 |
| Total i-mJOA | 0.94 | 0.91 | 0.64 | 0.67 | 0.73 | 0.34 |
* = significant at 95%.
The relationship between MRI features and an imputed version of the main clinical tool for assessing dysfunction in DCM (i-mJOA) was examined based on total scores and broken down by subcategory (Table 1). Neither subcategories of i-mJOA nor total i-mJOA score were significantly related to any of the MRI measures. Taken together, morphological features of spinal cord compression did not correlate well with clinical findings.
Relationship between clinical features and decision to operate
Current guidelines base the decision to operate mainly on clinical presentation of DCM. We therefore investigated the individual relationships between clinical features and the decision to operate prior to publication of the guidelines (Table 2). No clinical feature reached statistical significance relative to the decision to operate. Age (p = 0.19) and sex (p = 0.45) were also both found to have no significant influence on clinical management.
Table 2. The relationships between clinical features and the decision to operate ordered by ascending p-value.
Number of DCM cases the feature was recorded as present in as a percentage of total cases.
| Clinical Feature | Cases present (%) | p-value |
|---|---|---|
| Paraesthesia (lower limb) | 38 | 0.12 |
| Clonus | 16 | 0.12 |
| Limb pain | 53 | 0.22 |
| Lower limb spasticity | 3 | 0.25 |
| Subjective weakness (lower limb) | 33 | 0.30 |
| Hyper-reflexia | 80 | 0.34 |
| Paraesthesia (upper limb) | 65 | 0.48 |
| Unstable gait | 28 | 0.63 |
| Subjective weakness (upper limb) | 38 | 0.68 |
| Falls | 10 | 0.75 |
| Sphincter dysfunction | 5 | 0.83 |
| Subjective imbalance | 35 | 0.85 |
| Positive Hoffmann reflex | 47 | 0.89 |
| Objective corticospinal motor deficits | 40 | 0.90 |
| Neck pain/stiffness | 35 | 0.97 |
| Upgoing plantars | 47 | 0.98 |
The relationship between the decision to operate and the i-mJOA score, total and broken down by subcategory was investigated (Table 3). No subcategory of i-mJOA or total i-mJOA score was significantly related to the decision to operate.
Table 3. The relationships between i-mJOA scores, change in i-mJOA scores, and the decision to operate.
| i-mJOA category | Average +/- SD | p-value |
|---|---|---|
| Upper limb motor | 4.1±0.9 | 0.52 |
| Lower limb motor | 5.7±1.5 | 0.16 |
| Sensory | 2.1±0.7 | 0.91 |
| Sphincter | 2.9±0.3 | 0.79 |
| Total | 14.8±2.5 | 0.24 |
| Upper limb motor change | -0.2±0.7 | 0.61 |
| Lower limb motor change | -0.3±0.8 | 0.07 |
| Sensory change | -0.2±0.8 | 0.88 |
| Sphincter change | 0±0.3 | 0.61 |
| Total change | -0.8±2.0 | 0.20 |
| Total deterioration* | n/a | 0.28 |
*The relationship between if the patient had deteriorated at all, regardless of degree, with the decision to operate.
Progression of DCM reflected in deterioration of mJOA scores are now thought to provide a strong indication for surgery. Change in i-mJOA between the first available assessment (primary or secondary) and pre-operative assessment by a surgeon were therefore calculated and examined relative to the decision to operate (Table 3). Deterioration in i-mJOA, subcategory or total, was not significantly associated with an increased likelihood of being offered an operation.
Relationship between MRI measurements and the decision to operate
To investigate whether imaging parameters influence treatment, the relationships between individual MRI measurements and the decision to operate were studied (Table 4). Bivariate correlations showed significant relationship between decision to operate and NCaD (p = 0.024), NCoD (p = 0.019), and CR (p<0.001). Nagelkerke R2 = 0.495. Binomial logistic regression using these three significant variables showed only compression ratio as a significant predictor of decision to operate (p = 0.017).
Table 4. The relationships between MRI measurements and the decision to operate.
| MRI measurements | p-value |
|---|---|
| MCC | 0.50 |
| MSCC | 0.53 |
| NCaD | |
| NCoD | |
| SCOR | 0.97 |
| CR |
* = significant at 95%
** = significant at 99%.
Relationship between decision to operate and international guidelines
Finally, we examined the decision to operate in this cohort relative to the new international guidance outlines above [5]. 29/39 cases (74%) were managed in line with these guidelines. Of the remaining 10 cases: 4 had severe myelopathy (i-mJOA <12) and were not offered surgery, 4 had moderate myelopathy (i-mJOA 12–14) and were not offered surgery, and 2 had asymptomatic cord compression (i-mJOA 18) and were offered surgery.
Discussion
Overall, this study found that the only factor significantly associated with offering a patient an operation was the compression ratio of the cervical spinal cord. Features such as disease severity (assessed through i-mJOA functional scoring) and disease progression were not significantly associated with offering a patient an operation. This finding suggests that surgeons are basing their interventions on images and not on the patients in front of them. Although compression ratio is not routinely undertaken in a clinical setting, it can feasibly act as a proxy for how compressed the cord appears upon examining sagittal MRI slices. Indeed, compression ratio alone appeared to capture 50% of the variability in the decision to offer a patient an operation. It has been previously observed through a survey of 689 international spinal professionals that a strong belief exists that MRI is a valuable prognostic tool in DCM. Our study affirms this observation in routine clinical practice.
It is known that degenerative changes in the spine are increasingly common with age [19]. Whilst the natural history of asymptomatic cervical spinal cord compressions secondary to degenerative changes is not fully understood, the risk of developing myelopathy in the short-term appears low [23]. In one of the few studies to prospectively observe asymptomatic cord compression, Bednarik et al identified that abnormal electrophysiology, hyperintense lesions on T2-weighted MRI, and radiculopathic symptoms [24] predicted progression to myelopathy. The amount of pre-operative spinal cord compression has not been shown to be good indicator of post-operative outcomes. As has been previously demonstrated, the degree of spinal cord compression on MRI correlates poorly with clinical signs of myelopathy [25–27]. Our study further supports this point. The degree of spinal cord compression on MRI also correlates poorly with symptomatology and patients’ functional disability [21,28]. Patients with minimal cord compression may experience debilitating symptomatology and those with substantial cord compression may experience little to no symptoms at all. Therefore, amount of cord compression on MRI is not a suitable factor to hold such influence over the decision to operate in DCM.
Imaging techniques to predict response to surgery remain a significant research focus, with an emphasis on new techniques and technology. A potential issue blurring the correlation between MRI appearance and symptomatology is that the vast majority of cervical MRI scans are static. They are taken with the patient supine in a cervical position that may not correspond to their normal erect cervical alignment. A study found that the cervical canal narrowed at C5/6 and C6/7 when a force was applied cranially that simulated body weight whilst in the supine position [29]. Dynamic MRI, taken in flexed and extended neck positions, may yield a more accurate representation of cord compression occurring in a patient’s day-to-day life. Dynamic MRI has shown improved visualisation of significant canal stenosis and T2 hyperintense lesions on the spinal cord [30–32]. Furthermore, dynamic injury though lesions underestimated on static MRI may cause repetitive neurovascular trauma to the spinal cord and lead to significant symptomatology and disability for patients. Unfortunately, this type of imaging is not widely available for general use. Other forms of advanced imaging such as DTI and PET also show promise but are again currently a long way from routine clinical use [33–35].
A systematic literature review carried out this year found that the two factors most predictive of post-operative outcome in DCM are duration of symptoms prior to surgery and disease severity [36]. Current international guidance proposes that surgery be offered to all patients with moderate DCM or progressive disease. No mention of imaging characteristics is mentioned in these guidelines for offering an operation. We observed 74% concordance with these guidelines. However, it appears that degree of spinal cord compression is driving this decision-making, rather than patients’ functional disability or disease progression. This approach is problematic as may offer operations for patients who are asymptomatic and put them under undue risk for prophylactic treatment of disease that may never progress. Furthermore, static MRI imaging may underestimate the degree of cord and nerve impingement present in an erect position and may thus mislead surgeons as to the most problematic cervical levels to target surgically.
With regards to the discrepancy between best practice guidelines and observed practice, it is important to note that the guidelines were published after the cohort used for this study. However, the difficulties in changing established surgical practice have been well described [37]. Two particular issues that Meshikhes proposes seem especially pertinent here: individual clinical experiences and publication bias. Firstly, if cord compression has been driving DCM operative decisions for years, surgeons will inevitably see patients with significant cord compression who make good improvement post-operatively. Such cases were observed in our cohort. Current knowledge suggests that this is likely coincidence rather than a link between MRI findings and post-operative outcomes. However, such clinical observations may distort surgeons’ perceptions of appropriate indications for surgery. Furthermore, these observations may be passed down to trainee surgeons before they have even established their own clinical observations. And secondly, given the heavy focus on surgery as the central research interest in DCM [38], there may well exist a reluctance to publish data showing poor improvement post-operatively or stable patients not requiring surgery. Such issues must be confronted in order to align current practice with evidence-based best practice guidelines.
Ultimately, surgery is the current mainstay treatment for DCM, by anterior or posterior approach, and should be based on clinical assessment, not imaging findings. Current surgical management can therefore be easily improved by more evidence-based stratification of operative candidates: moderate and severe myelopathy, any progressive disease, and consideration in radiculopathic patients with cord compression.
Limitations
This study was conducted in a single tertiary neurosciences centre. Thus, it is possible that decision-making may be more homogeneous than would be observed in an inter-centre study. However, as the decision to operate was not MDT-guided, there is a strong case to believe that surgeons were freely expressing their individual clinical judgments when deciding whether to operate or not. Furthermore, although smaller than recent international studies, the sample size used in this study should adequately display correlations in decision-making tendencies. Future multi-centre studies of greater sample size would provide further insight into factors currently influencing the decision to operate in DCM.
Conclusion
This work demonstrates that the decision to operate is multi-faceted. However, despite its poor representation of disease severity and response to treatment, cord compression is a major factor in surgical decision-making. Whilst imaging features are required to make a diagnosis, they are not recommended for use in selecting surgical candidates. Current international best practice guidelines recommend surgery for: moderate and severe myelopathy, any progressive disease, and consideration in radiculopathic patients with cord compression. Further work is needed to evaluate the integration of these guidelines into wider clinical practice.
Supporting information
Excel file containing raw data collected in this research and used for analysis.
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Research in the senior author’s laboratory is supported by a core support grant from the Wellcome Trust and MRC to the Wellcome Trust-Medical Research Council Cambridge Stem Cell Institute to MK. Mark R. N. Kotter is supported by a NIHR Clinician Scientist Award. Disclaimer: This report is independent research arising from a Clinician Scientist Award, CS-2015-15-023, supported by the National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine (Phila Pa 1976). 2015. June 15;40(12):E675–93 [DOI] [PubMed] [Google Scholar]
- 2.Davies BM, Mowforth OD, Smith EK, Kotter MR. Degenerative cervical myelopathy. BMJ. 2018. February 22;360:k186 10.1136/bmj.k186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovalova I, Kerkovsky M, Kadanka Z, et al. Prevalence and Imaging Characteristics of Nonmyelopathic and Myelopathic Spondylotic Cervical Cord Compression. Spine (Phila Pa 1976). 2016. December 15;41(24):1908–1916. [DOI] [PubMed] [Google Scholar]
- 4.Kadanka Z Jr, Adamova B, Kerkovsky M, et al. Predictors of symptomatic myelopathy in degenerative cervical spinal cord compression. Brain Behav. 2017. August 11;7(9):e00797 10.1002/brb3.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Oliveira Vilaça C, Orsini M, Leite MA, de Freitas MR, Davidovich E, Fiorelli R, Fiorelli S, Fiorelli C, Oliveira AB, Pessoa BL. Cervical Spondylotic Myelopathy: What the Neurologist Should Know. Neurol Int. 2016. November 23;8(4):6330 10.4081/ni.2016.6330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matz PG, Anderson PA, Holly LT, Groff MW, Heary RF, Kaiser MG, et al. : The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 2009. August;11(2):104–11. 10.3171/2009.1.SPINE08716 [DOI] [PubMed] [Google Scholar]
- 7.King JTJ, McGinnis KA, Roberts MS. Quality of Life Assessment with the Medical Outcomes Study Short Form-36 among Patients with Cervical Spondylotic Myelopathy. Neurosurgery. 2003. January 1;52(1):113 10.1097/00006123-200301000-00014 [DOI] [PubMed] [Google Scholar]
- 8.Hilton B, Tempest-Mitchell J, Davies B, Kotter M. Route to diagnosis of degenerative cervical myelopathy in a UK healthcare system: a retrospective cohort study. BMJ Open. 2019. May 5;9(5):e027000 10.1136/bmjopen-2018-027000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilton B, Tempest-Mitchell J, Davies B, Kotter M. Assessment of degenerative cervical myelopathy differs between specialists and may influence time to diagnosis and clinical outcomes. PLoS One. 2018. December 17;13(12):e0207709 10.1371/journal.pone.0207709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakhsheshian J, Mehta VA, Liu JC. Current Diagnosis and Management of Cervical Spondylotic Myelopathy. Global Spine J. 2017. September;7(6):572–586. 10.1177/2192568217699208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tetreault L, Kopjar B, Côté P, Arnold P, Fehlings MG. A Clinical Prediction Rule for Functional Outcomes in Patients Undergoing Surgery for Degenerative Cervical Myelopathy: Analysis of an International Prospective Multicenter Data Set of 757 Subjects. J Bone Joint Surg Am. 2015. December 16;97(24):2038–46 10.2106/JBJS.O.00189 [DOI] [PubMed] [Google Scholar]
- 12.Matz PG, Anderson PA, Groff MW, et al. Cervical laminoplasty for the treatment of cervical degenerative myelopathy. J Neurosurg Spine 11:157–169, 2009. 10.3171/2009.1.SPINE08726 [DOI] [PubMed] [Google Scholar]
- 13.Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine 11:112–118, 2009. 10.3171/2009.1.SPINE08718 [DOI] [PubMed] [Google Scholar]
- 14.Zhang JT, Wang LF, Wang S, Li J, Shen Y. Risk factors for poor outcome of surgery for cervical spondylotic myelopathy. Spinal Cord. 2016. December;54(12):1127–1131 10.1038/sc.2016.64 [DOI] [PubMed] [Google Scholar]
- 15.Nouri A, Tetreault L, Côté P, Zamorano JJ, Dalzell K, Fehlings MG. Does Magnetic Resonance Imaging Improve the Predictive Performance of a Validated Clinical Prediction Rule Developed to Evaluate Surgical Outcome in Patients With Degenerative Cervical Myelopathy? Spine (Phila Pa 1976). 2015. July 15;40(14):1092–100. [DOI] [PubMed] [Google Scholar]
- 16.Nouri A, Martin AR, Nater A, et al. Influence of Magnetic Resonance Imaging Features on Surgical Decision-Making in Degenerative Cervical Myelopathy: Results from a Global Survey of AOSpine International Members. World Neurosurg. 2017. September;105:864–874. 10.1016/j.wneu.2017.06.025 [DOI] [PubMed] [Google Scholar]
- 17.Tetreault L, Palubiski LM, Kryshtalskyj M, et al. Significant Predictors of Outcome Following Surgery for the Treatment of Degenerative Cervical Myelopathy: A Systematic Review of the Literature. Neurosurg Clin N Am. 2018. January;29(1):115–127 10.1016/j.nec.2017.09.020 [DOI] [PubMed] [Google Scholar]
- 18.Fehlings MG, Tetreault L, Riew K, et al. A Clinical Practice Guideline for the Management of Patients With Degenerative Cervical Myelopathy: Recommendations for Patients With Mild, Moderate, and Severe Disease and Nonmyelopathic Patients With Evidence of Cord Compression. Global Spine Journal. 2017. September 7(3_suppl), 70S–83S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tempest-Mitchell J, Hilton B, Davies BM, Nouri A, Hutchinson PJ, Scoffings DJ,Mannion RJ, Trivedi R, Timofeev I, Crawford JR, Hay D, Laing RJ, Kotter MRN. A comparison of radiological descriptions of spinal cord compression with quantitative measures, and their role in non-specialist clinical management. PLoS One. 2019. July 22;14(7):e0219380 10.1371/journal.pone.0219380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fehlings M. G. et al. (1999) ‘The optimal radiologic method for assessing spinal canal compromise and cord compression in patients with cervical spinal cord injury. Part II: Results of a multicenter study.’, Spine, 24(6), pp. 605–13. 10.1097/00007632-199903150-00023 [DOI] [PubMed] [Google Scholar]
- 21.Nouri A, Martin AR, Mikulis D, Fehlings MG. Magnetic resonance imaging assessment of degenerative cervical myelopathy: a review of structural changes and measurement techniques. Neurosurg Focus. 2016;40:E5. [DOI] [PubMed] [Google Scholar]
- 22.Shin J. J. et al. (2010) ‘Intramedullary high signal intensity and neurological status as prognostic factors in cervical spondylotic myelopathy’, Acta Neurochirurgica, 152(10), pp. 1687–1694. 10.1007/s00701-010-0692-8 [DOI] [PubMed] [Google Scholar]
- 23.Witiw C, Mathieu F, Nouri A, Fehlings MG. Clinico-Radiographic Discordance: An Evidence-Based Commentary on the Management of Degenerative Cervical Spinal Cord Compression in the Absence of Symptoms or With Only Mild Symptoms of Myelopathy. Global Spine J. 1–8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bednarik J, Kadanka Z, Dusek L, et al. Presymptomatic spondyloticcervical myelopathy: an updated predictive model. Eur Spine J. 2008;17:421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagata K, Yoshimura N, Muraki S, et al. Prevalence of cervical cord compression and its association with physical performance in a population-based cohort in Japan: the Wakayama Spine Study. Spine (Phila Pa 1976). 2012. October 15;37(22):1892–8. [DOI] [PubMed] [Google Scholar]
- 26.Nemani VM, Kim HJ, Piyaskulkaew C, Nguyen JT, Riew KD. Correlation of cord signal change with physical examination findings in patients with cervical myelopathy. Spine (Phila Pa 1976). 2015. January 1;40(1):6–10 [DOI] [PubMed] [Google Scholar]
- 27.Rhee JM, Heflin JA, Hamasaki T, et al. Prevalence of physical signs in cervical myelopathy: a prospective, controlled study. Spine (Phila Pa 1976) 2009;34:890–5. [DOI] [PubMed] [Google Scholar]
- 28.Kovalova I, Kerkovsky M, Kadanka Z, et al. Prevalence and Imaging Characteristics of Nonmyelopathic and Myelopathic Spondylotic Cervical Cord Compression. Spine (Phila Pa 1976). 2016. December 15;41(24):1908–1916. [DOI] [PubMed] [Google Scholar]
- 29.Kimura S, Hesselink JR, Garfin SR, Kawaji Y, Hasegawa K, Hargens AR. Axial load-dependent cervical spinal alterations during simulated upright posture: a comparison of healthy controls and patients with cervical degenerative disease. J Neurosurg Spine. 2005. February;2(2):137–44. 10.3171/spi.2005.2.2.0137 [DOI] [PubMed] [Google Scholar]
- 30.Zeitoun D, El Hajj F, Sariali E, Catonné Y, Pascal-Moussellard H. Evaluation of spinal cord compression and hyperintense intramedullary lesions on T2-weighted sequences in patients with cervical spondylotic myelopathy using flexion-extension MRI protocol. Spine J. 2015. April 1;15(4):668–74. 10.1016/j.spinee.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 31.Bartlett RJ, Rigby AS, Joseph J, Raman A, Kunnacherry A, Hill CA. Extension MRI is clinically useful in cervical myelopathy. Neuroradiology. 2013. September;55(9):1081–8. 10.1007/s00234-013-1208-z [DOI] [PubMed] [Google Scholar]
- 32.Kim CH, Chung CK, Kim KJ, et al. Cervical extension magnetic resonance imaging in evaluating cervical spondylotic myelopathy. Acta Neurochir (Wien). 2014. February;156(2):259–66. 10.1007/s00701-013-1951-2 [DOI] [PubMed] [Google Scholar]
- 33.Uchida K, Nakajima H, Okazawa H, et al. Clinical significance of MRI/(18)F-FDG PET fusion imaging of the spinal cord in patients with cervical compressive myelopathy. Eur J Nucl Med Mol Imaging. 2012. October;39(10):1528–37. 10.1007/s00259-012-2192-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Floeth FW1, Stoffels G, Herdmann J, et al. Prognostic value of 18F-FDG PET in monosegmental stenosis and myelopathy of the cervical spinal cord. J Nucl Med. 2011. September;52(9):1385–91 10.2967/jnumed.111.091801 [DOI] [PubMed] [Google Scholar]
- 35.Ellingson BM, Salamon N, Holly LT. Advances in MR imaging for cervical spondylotic myelopathy. Eur Spine J. 2015. April;24 Suppl 2:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tetreault L, Palubiski LM, Kryshtalskyj M, et al. Significant Predictors of Outcome Following Surgery for the Treatment of Degenerative Cervical Myelopathy: A Systematic Review of the Literature. Neurosurg Clin N Am. 2018. January;29(1):115–127 10.1016/j.nec.2017.09.020 [DOI] [PubMed] [Google Scholar]
- 37.Meshikhes AW. Evidence-based surgery: The obstacles and solutions. Int J Surg. 2015. June;18:159–62 10.1016/j.ijsu.2015.04.071 [DOI] [PubMed] [Google Scholar]
- 38.Mowforth O, Davies BM, Goh S, O’Neill CP, Kotter MRN. Research Inefficiency in Degenerative Cervical Myelopathy: Findings of a Systematic Review on Research Activity Over the Past 20 Years. Global Spine Journal. June 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Excel file containing raw data collected in this research and used for analysis.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.