Abstract
The role that interferon-γ (IFNγ) plays during herpetic stromal keratitis (HSK) has not been definitively determined. In primary HSK most reports suggest that IFNγ may help control viral replication and contribute to corneal pathology. However, its role in recurrent HSK has not been directly addressed. The present study addresses its role in recurrent HSK by comparing HSK in latently infected normal and IFNγ gene knockout (GKO) on the C57BL/6 background. We initially evaluated HSK following primary infection and observed that GKO mice had higher tear film virus titers, but virtually identical ocular disease as normal mice. In contrast, following reactivation of latent virus, GKO mice had a greater incidence and severity of opacity, neovascularization, and blepharitis. Interestingly, the incidence of reactivation after UV-B exposure was equivalent in GKO and normal mice, but virus shedding was increased in the GKO groups. We also observed diminished delayed-type hypersensitivity responses in GKO mice, as expected. These data indicate that IFNγ is important for the control of virus replication in both primary and recurrent ocular HSV infection in C57BL/6 mice. The enhanced recurrent disease seen in GKO mice may be the result of increased viral titers and persistence in these mice which act to prolong the stimulation of an inflammatory response.
Keywords: Herpes simplex, Herpetic stromal keratitis, Cytokines, Interferon-γ, Inflammation, T cells, Viral reactivation
Introduction
Herpetic stromal keratitis (HSK) is a potentially blinding corneal inflammation that accompanies herpes simplex virus (HSV) infection of the eye. The disease course in HSK begins with a primary infection by HSV followed by a period during which the virus enters latency in sensory and autonomic ganglia. Many studies have shown that clinical manifestations of the disease are the result of a cocktail of inflammatory cells, consisting of PMN’s, macrophages and T cells (both CD4+ and CD8+) (Maertzdorf et al., 2003; Pepose et al., 1996; Thomas and Rouse, 1997; Youinou et al., 1985, 1986). These cells are recruited to the site of infection by the release of inflammatory factors, including cytokines and chemokines (Maertzdorf et al., 2002; Su et al., 1996; Thomas et al., 1998). Once there, these cells appear to be responsible for the majority of corneal damage (Streilein et al., 1997). Primary models of this disease have indicated that the process resulting in corneal damage is due to the presence of CD4+ Th1 whose cytokines dominate during the preclinical and clinical phases of acute HSK (Niemialtowski and Rouse 1992; Thomas et al., 1998). These cytokines include IL-2, IL-12, and IFN-γ (Babu et al., 1995; Thomas et al., 1998; He et al., 1999).
Studies addressing the role that these Th1 cytokines play following HSV-1 infection can be categorized in two ways: Survival of the animal, in which IFNγ plays a minor role that is subordinate to that played by type 1 interferons (Ghiasi et al., 2000; Vollstedt et al., 2004) Secondly, evaluation of corneal disease has indicated that IL-12 and IFNγ are not only found early in infection (Stumpf et al., 2002; Thomas et al., 1998), but that targeting them significantly altered disease (Bouley et al., 1995; Lee et al., 2002; Osorio et al., 2002). Thus, during acute ocular HSV infection, treatment of mice with neutralizing anti-IFNγ antibodies resulted in diminished HSK lesions (Hendricks et al., 1992; Smith et al., 1994). In contrast, a study of primary infection in IFNγ knock out (GKO) mice showed that HSK lesions may be generated in the absence of IFNγ, potentially due to the influence of other pro-inflammatory cytokines or the prolonged presence of the virus itself (Bouley et al., 1995).
However these reports have been restricted to primary models of disease. Interestingly, HSK in humans most often occurs long after primary infection abates, when reactivated virus returns to the eye from latency sites in neuronal tissues (Miller et al., 1996; Rinne et al., 1992). Renewed virus presence in the cornea initiates a recurrent HSK response, with the potential to cause impaired vision, including permanent corneal opacities and neovascularization. It has previously been reported that IFNγ and TNF-α are found in infected trigeminal ganglia in both humans (Theil et al., 2003) and mice (Liu et al., 1996). The role of these cytokines in the trigeminal ganglia is one of maintenance of latency (Liu et al., 2001; Khanna et al.,2003). However it has been reported that GKO mice establish latency as efficiently as do wild-type mice (Ellison et al., 2000). Studies investigating reactivation, both in humans and mice have shown that both the cells and the cytokines found in corneas display a mixed phenotype, namely that both Th1 and Th2 cytokines are present, though there might be slightly more Th1 cells than Th2 cells (He et al., 1999; Keadle et al., 2001; Stumpf et al., 2001). However, when it comes to evaluation of the role these cytokines play during recurrent disease, there is a paucity of information. Hence, our desire was to address the role that these cytokines play during recurrent HSK in mice. We previously reported that both TNF-α and IL-1 are required for recurrent HSK (Keadle et al., 2000). More recently we reported that IL-10 appears to be involved in ameliorating recurrent disease (Keadle and Stuart, 2005).
Due to the role that IFNγ seems to play in primary HSK as well as maintenance of latency, we wanted to determine what role it plays during recurrent HSK. IFNγ is a multifunctional cytokine with antiviral and pro-inflammatory properties that may contribute to the ocular pathology observed during primary HSK. Since recurrent HSK has the greatest potential to cause blindness in humans, this work examines the role of IFNγ in a mouse model of recurrent HSK utilizing GKO mice (Laycock et al., 1991). We present data demonstrating that in contrast to what has been reported during primary disease, GKO mice display increased severity of ocular disease following UV-B induced reactivation of latently infected mice. We also demonstrate that viral titers were increased for GKO mice and that virus persisted in these mice longer than in wild-type mice. These data suggest that recurrent HSK in GKO mice might be the result of viral persistence.
Results
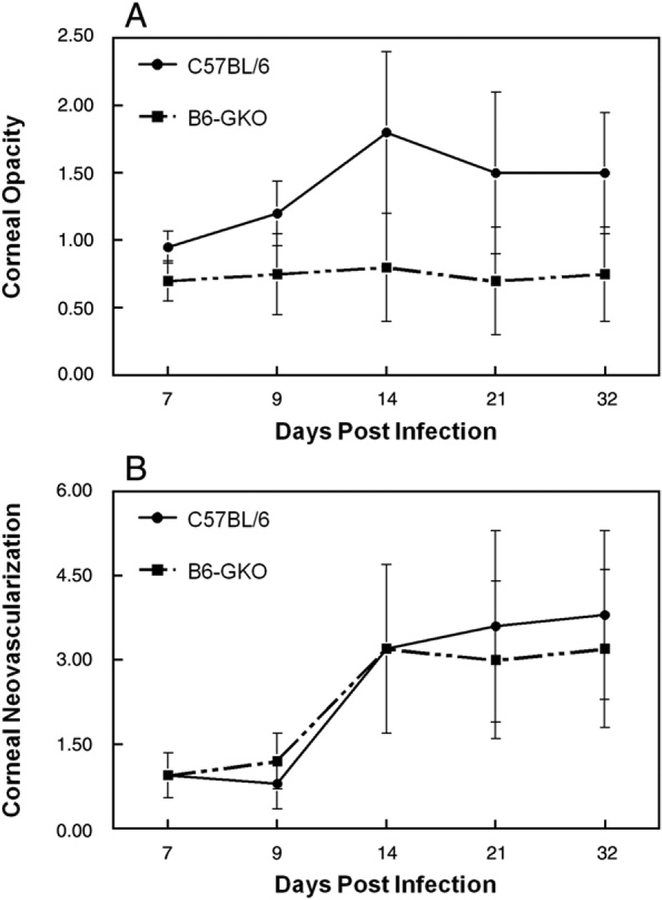
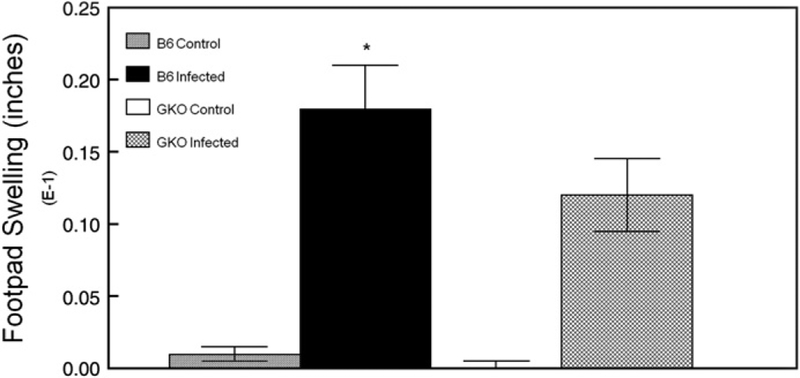
Previous studies investigating the role that IFNγ plays during primary HSK used C.129S7 (B6)-Ifngtm1Ts/J (Bouley et al., 1995), which is on the HSV-sensitive BALB/C background. And since Lekstrom-Himes’ study using the same strain of GKO mice that we did, did not examine clinical disease (Lekstrom-Himes et al., 2000), we decided to perform an examination of primary HSK in normal and GKO mice on the C57/BL6 background. Post infection corneal opacity tended to be greater in normal B6 mice than in GKO mice, but it was not statistically significant (Fig. 1A). Neovascularization was indistinguishable between normal and GKO mice (Fig. 1B). Blepharitis scores were similar to opacity scores in that normal B6 tended to be higher than GKO mice, but day 21 was significant (Fig. 1C). Virologic analysis indicated that GKO mice had elevated tear film titers at day 3 post infection (normal 3161±652, n=15; GKO 7450±2118, n=15; P=0.005). Viral titers for other time points, while elevated for GKO mice, were not statistically significant (data not shown). As anticipated, when delayed-type hypersensitivity (DTH) responses were measured two weeks post infection were significantly reduced in GKO mice as compared to normal B6 mice (Fig. 2). Thus the lack of IFNγ during acute HSV-1 infection in mice with the B6 background did not result in a significantly altered disease phenotype.
Fig. 1.
Corneal disease in B6 mouse strains lacking IFNγ following HSV-1 infection. Following infection, mice were monitored for corneal opacity (A) corneal neovascularization (B) and blepharitis for 32 days. The numbers of mice used for these studies were as follows: B6, n=20; GKO, n=20. Results indicate mean±SEM. *P<.05. ○, B6; ●, GKO.
Fig. 2.
Delayed-type hypersensitivity (DTH) responses 2 weeks following primary infection with HSV-1. Following HSV-1 infection, mice were tested for DTH responses. The numbers of mice used in this study were as follows: B6 infected, n=20; B6 naive, n=5; GKO infected, n=20; GKO, n=5. Results indicate mean±SEM. *P=0.004 infected vs. naive. **P=0.009 infected B6 vs. infected GKO. ***P<0.001 infected vs. naive.
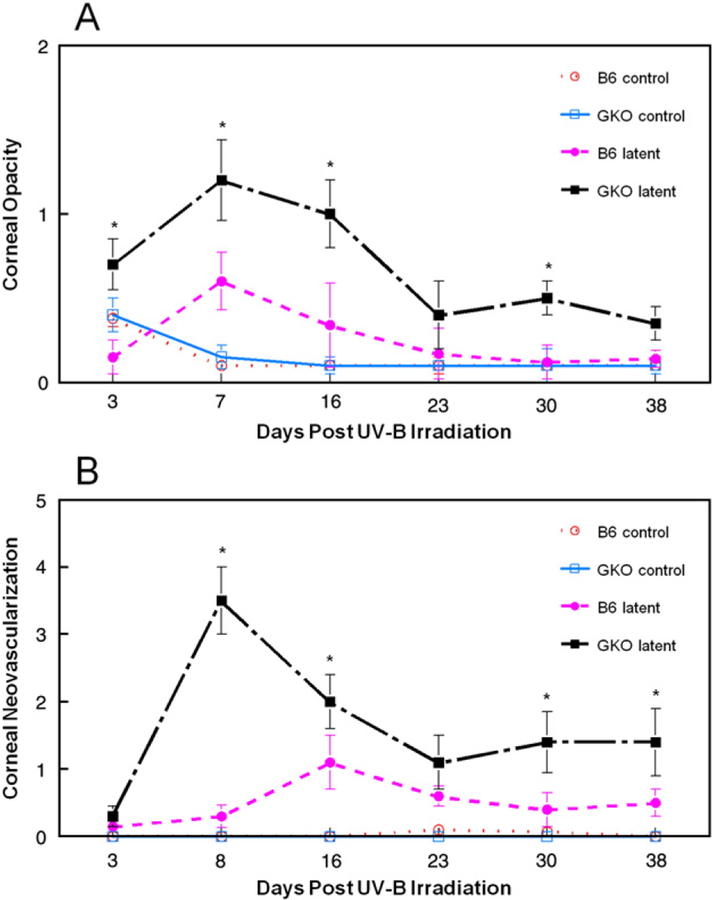
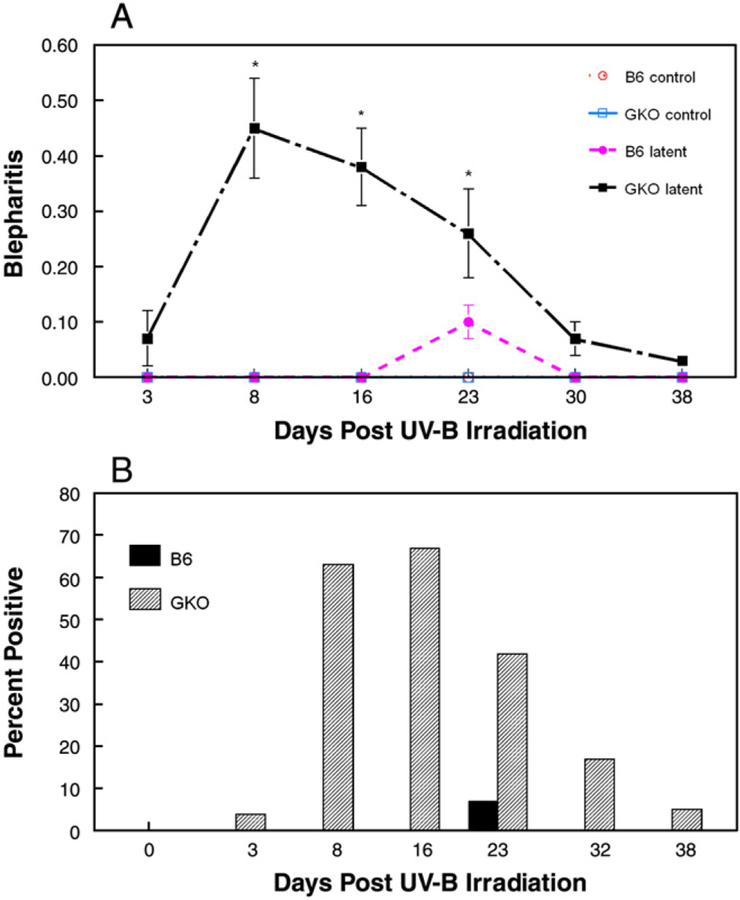
Next we examined the role of IFNγ in recurrent HSK. In this model, we ocularly infect both normal and IFN-γ knock out (GKO) C57BL/6 mice with HSV-1 McKrae strain with concurrent administration of protective anti-HSV antibodies. These antibodies have been shown to prevent mortality while preserving corneal clarity. Five weeks after infection, eyes of latently infected normal C57BL/6 and GKO mice were UV-B irradiated and monitored for signs of ocular disease. Examination of corneal opacity (Fig. 3A) and neovascularization (Fig. 3B) surprisingly revealed that GKO mice displayed increased or similar disease scores as compared to normal mice at all time points measured. Differences in disease were more pronounced when blepharitis was examined. GKO mice displayed increased severity (Fig. 4A) and incidence (Fig. 4B) compared to normal mice from days 8 to 23. Interestingly, when the corneas of representative mice from these groups were examined histologically by H & E staining, the inflammatory infiltrate was not significantly different between wild-type and GKO mice at Day 7 and 11 post UV-B irradiation (data not shown).
Fig. 3.
Corneal disease in B6 mouse strains lacking IFNγ following UV-B induced reactivation. Latently infected mice were induced to reactivate with UV-B irradiation and mice were monitored for corneal opacity (A) and corneal neovascularization (B) for 38 days. The numbers of mice used for these studies were as follows: B6, n=35; GKO, n=30. Results indicate mean±SEM. *There was significant virus-induced disease (compared to strain matched uninfected UV-B controls) in GKO eyes for days 3–16 in corneal opacity and days 8–38 for neovascularization (P =0.001 to 0.01). There was significant virus-induced disease in B6 mice only on days 3 and 8 for corneal opacity (P<0.02) and none for neovascularization. Disease scores for infected GKO mice exceeded those of infected B6 mice on days 3–16, 32 for corneal opacity (P<0.001 to 0.05) and days 8, 16, 32, 38 for neovascularization (P<0.001 to 0.05). Incidence of corneal opacity score >1 was: GKO-31%, normal=9% (P=0.02). Incidence for neovascularization scores >1 was: GKO=50%, normal=24% (P=0.02). ○, B6 control; ●, B6 latently infected; □, GKO control; ■, GKO latently infected.
Fig. 4.
Blepharitis in B6 mouse strains lacking IFNγ following UV-B induced reactivation. Latently infected mice were induced to reactivate with UV-B irradiation and scored for blepharitis (A) and percent mice showing positive blepharitis (B) for 32 days. The numbers of mice used for these studies were as follows: B6, n=35; GKO, n=30. Results indicate mean±SEM. GKO mice displayed significantly greater blepharitis scores and incidence than normal B6 mice, which did not display significant blepharitis above their UV-B controls, for days 8 to 23 (P<.001 to 0.03). ○, B6 control; ●, B6 latently infected; □, GKO control; ■, GKO latently infected.
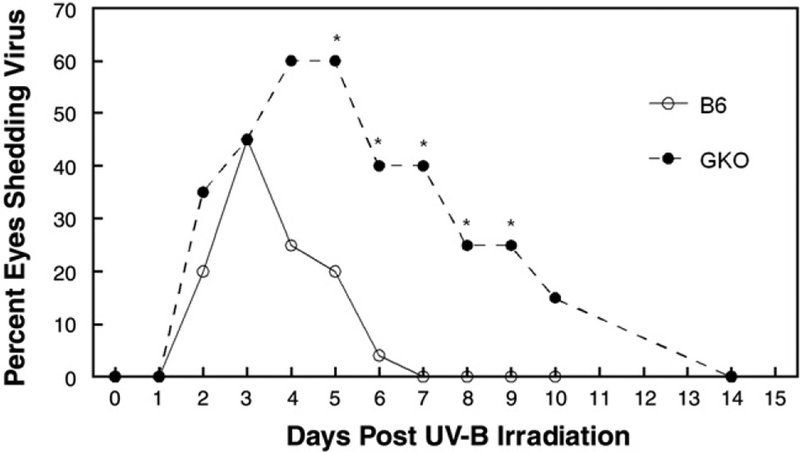
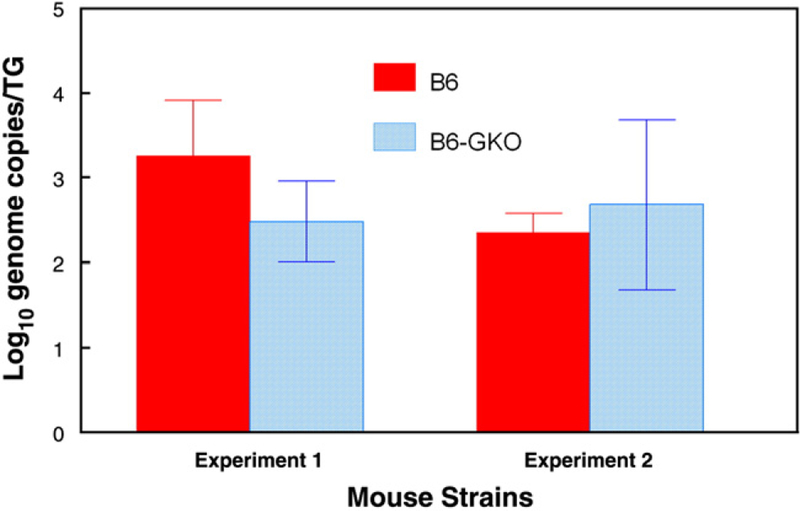
Post UV-B virus shedding in tears was assessed along with ocular disease. The reactivation rate was greater for GKO mice (80%) than normal mice (60%), but this difference did not prove to be statistically significant (P>.05). However, virus was shed significantly longer in GKO eyes than in normal eyes with detectable virus found in GKO mice up to Day 10 while virus could not be found in normal eyes beyond Day 5 post UV-B (Fig. 5). In addition, peak virus shedding occurred later in GKO mice (normal 3.09±0.211 versus GKO 4.79±0.38 days; Table 1) and virus titers were greater in GKO than in normal mice (normal 1716±781, GKO 44,643±15,593 Table 1). Because differences in viral titer could have been the direct result of increased viral load in the trigeminal ganglia of GKO vs. normal mice we decided to perform real-time PCR analysis of trigeminal ganglia to determine relative genome copies in these mouse strains following re-establishment of latency after reactivation. Results of such analyses did not reveal significant differences in genome loads between latently infected normal and GKO mice (Fig. 6). These data argue that differences in viral shedding following UV-B-induced reactivation are likely not due to increased viral load in trigeminal ganglia of GKO mice.
Fig. 5.
Incidence of mice shedding virus following UV-B reactivation. The numbers of mice used in this study were as follows: B6, n=35; GKO, n=30. Results indicate percent mice shedding virus at that time point. Percent GKO mice shedding virus exceeded B6 on days 5 to 9 (P=0.003 to 0.045). Mean number of days shedding virus was GKO=4.25, B6=2.0, (P=0.002). ○, B6 latently infected; ●, GKO latently infected.
Table 1.
HSV-1 shedding following UV-B reactivation
| Normal C57BL/6 | γKO C57BL/6 | |
|---|---|---|
| EYESa | ||
| 3 Days Plb (n) | 1367+567 (21) | 695+158 (27) |
| Post react peakc (n) | 1716+781 (16) | 44,643+15,593 (24)d |
| Days sheddinge | 2.0+0.3 | 3.88+0.4f |
| % Reactivation rate | 60% (21+/35 total) | 80% (24+/30 total) |
| Trigeminal gangliag | ||
| Days to positive (n)h | 4.5+0.3 (4) | 4+0 (3) |
| PFU/TG at positive (n)i | 45+17 (4) | 21+9 (3) |
| Titer at positive× 106 (n)j | 34+12 (4) | 7+5 (3) |
All values are mean±SEM unless noted. 2 experiments for eyes except primary model.
Eye swab virus titers taken 3 days after primary infection of reactivation model mice (anti-HSV antibodies administered on day of primary infection).
Peak tear film virus titer for all mice shedding virus after UV-B irradiation.
P=0.005.
Average number of days shedding for positive mice.
P<0.001.
Trigeminal ganglia of latently infected (non-irradiated mice) were cultured on Vero cells until viral plaques appeared; 1 experiment for TGs.
Number of days in culture before appearance of plaques.
Number of viral plaques observed on Vero monolayer on day of first appearance of plaques.
Titer of HSV obtained from TG of positive wells plated on Vero cells.
Fig. 6.
Analysis of genome copies in latently infected trigeminal ganglia. Real-time PCR for the HSV-1 tk gene was performed on individual trigeminal ganglia from latently infected mice. All samples were normalized to the single-copy mouse adipsin gene. The data are reported as the logarithmic mean of the number of genome copies per trigeminal ganglion. Trigeminal ganglia from latently infected mice (n=8 for all groups) were dissociated from a single-cell suspension and then plated in twofold dilutions.
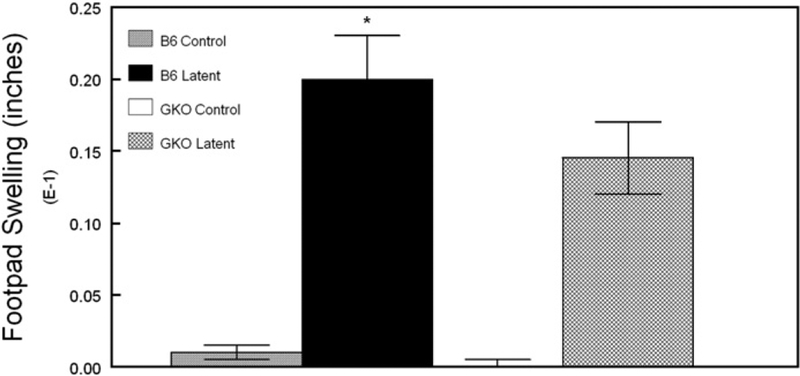
To functionally evaluate the lack of IFNγ in GKO mice, we again performed DTH testing on mice two weeks after the reactivation stimulus. As shown in Fig. 7, DTH responses in latently infected GKO mice were significantly less than that of normal latently infected mice but were significantly greater than that of naive control mice.
Fig. 7.
Delayed-type hypersensitivity (DTH) responses 2 weeks following UV-B induced reactivation. Following UV-B reactivation, mice were tested for DTH responses. The numbers of mice used in this study were as follows: B6 infected, n=35; B6 naive, n=5; GKO infected, n=30; GKO, n=5. Results indicate mean±SEM. *P=0.001 infected vs. naive. **P=0.022 infected B6 vs. infected GKO.
Discussion
This work examines for the first time the role of IFNγ in recurrent HSK using KO mice of the C57BL/6 background. Since mouse strains are known to vary in susceptibility to HSV infection (Streilein et al., 1997), we also included a study of primary HSK in C57BL/6 mice as the most relevant comparison. Our findings highlight the importance of IFNγ in controlling viral replication, while demonstrating the redundancy of IFNγ in generating immunopathologic lesions in GKO mice.
IFNγ control of viral replication in primary HSK was first demonstrated for GKO mice on the BALB/c background (Bouley et al., 1995). These findings were extended to include the ability of virus to establish latency in trigeminal ganglia, in which the viral load was similar for normal and GKO mice as well as their ability to reactivate (Lekstrom-Himes et al., 2000). In contrast to the Lekstrom-Himes report for primary infection (Lekstrom-Himes et al., 2000), we observed higher post infection tear film virus titers in GKO eyes compared to normal C57BL/6 eyes (Table 1).
In the ocular reactivation model, viral growth is a more complex issue, because these mice have already been exposed to virus during primary infection. Thus, factors to consider include: 1) Local immune responses in the trigeminal ganglia that will restrict both viral reactivation and the ability of virus to traffic from the trigeminal ganglia to the periphery (Liu et al., 2001; Khanna et al., 2003, Mikloska and Cunningham 2001). 2) A cornea that possesses T cells that are likely able to respond to HSV-1 (Stumpf et al., 2001). 3) Circulating HSV-1 specific cells and antibodies that will migrate to the cornea upon re-emergence of virus (Miller et al., 1996; Stumpf et al., 2001). Consequently, the first hurdle that the virus must overcome is the presence of CD8+ T cells and their production of IFNγ in latently infected trigeminal ganglia (Liu et al., 1996; Liu et al., 2001; Khanna et al., 2003). Therefore, in order to compare shedding of virus at the cornea, one must presuppose relative equivalent latency in trigeminal ganglia and the absence of significant ganglion-level IFNγ effects on reactivation from latency. Our data suggests that is the case. First, day 3 postinfection tear film titers are the same for normal and GKO mice later used in reactivation studies (see Table 1). Second, in vitro ganglionic reactivation rates and titers were not different between groups (see Table 1). Third, for normal and GKO groups, an equal number of days was required for virus to appear in in vitro ganglia explant cultures, as well as, post UV-B irradiation tear films (Days to Positive Eye Swab, Table 1). Finally, the number of latent genomes in the trigeminal ganglia of normal and GKO mice was relatively equivalent for wild-type and GKO mice that had re-established latency following reactivation (see Fig. 6). These findings are similar to that of Lekstrom-Himes et al. who reported equivalent latent HSV genome copies in the trigeminal ganglia of C57BL/6 normal and GKO mice after primary infection (Lekstrom-Himes et al., 2000). On this basis, we conclude that IFNγ control of viral replication during reactivation is exerted primarily at the level of the eye and skin. Thus, while both normal and GKO eyes took about 2.5 days to begin shedding virus, eyes lacking IFNγ (GKO) shed longer (days shedding, Table 1) with higher virus titers (Table 1). These findings are in accord with a proposed role of IFNγ in limiting replication of newly reactivated virus rather than limiting reactivation itself (Cantin et al., 1999a,b; Presti et al., 1998; He et al., 1999). It should also be pointed out that while we generally never detect spontaneous corneal shedding of virus from B6 mice we did detect spontaneous corneal shedding in 2 of the 70 total B6-GKO mice that were latently infected.
IFN-γ appears to be superfluous for the generation of corneal lesions in GKO mice during acute infection (see Fig. 1). This finding is consistent with previous reports of primary infection of both BALB/c GKO mice (Bouley et al., 1995) and C57BL/6 GKO mice (Ghiasi et al., 2000). These findings were later confirmed when CD154 KO mice were infected and in spite of significant reductions in IFNγ following HSV-1 infection, their disease was equivalent to that seen in normal BALB/c mice (Xu et al., 2004). Those authors suggested that while primary HSK does require the presence of Th1 cells that other Th1 cytokines could compensate for the lack of IFNγ (Bouley et al., 1995). Support for this hypothesis has recently been put forward by studies comparing Stat4 KO mice to normal mice, in which Stat4 KO mice displayed significantly reduced disease (Banerjee et al., 2007). Stat 4 is intracellular protein whose activation polarizes T cells to a Th1 phenotype and so disruption of this pathway significantly reduces the ability of these cells to take on a Th1 phenotype and the accompanying production of all Th1-associated cytokines (Kaplan, 2005).
Here we have examined HSK in both a primary infection model as well as a recurrent model of HSK in C57BL/6 mice utilizing equal virus infection loads. We found that GKO mice had no difficulty manifesting corneal opacity and neovascularization equal to, or even greater than controls (Figs. 1, 3), after primary and recurrent ocular infection. Interestingly, we saw an even more pronounced increase in blepharitis following UV-B irradiation (see Fig. 4). This could be due to either more efficient spread of virus to perioccular tissues in the absence of IFNγ or that once again mice lacking IFNγ have impaired ability to clear virus in the periphery leading to increased inflammation due to prolonged and increased levels of virus. We are currently performing studies to address these alternatives.
The presence of increased corneal lesions in the absence of IFNγ during recurrent HSK may reflect the influence of other compensatory pro-inflammatory cytokines (e.g. IL-2, Hendricks et al., 1992), and redundant cytokine pathways in GKO mice, autoimmune processes (Bouley et al., 1995), and/or alternative IFNγ receptor ligands (Cantin et al., 1999a,b). Indeed, reduced but significant DTH responses in GKO mice (Figs. 2 and 6) suggest that other HSV-directed pro-inflammatory immune responses are at work in these mice. Absent immunologic causes, the most likely explanation for HSK lesions in GKO mice is the virus itself. Thus, increased ocular disease could result from higher levels of virus (Table 1) and prolonged viral replication (Fig. 5, Table 1) that exacerbates corneal damage. Likewise, increased blepharitis is probably the result of uncontrolled virus replication in the skin as previously reported (Bouley et al., 1995; He et al., 1999; Smith et al., 1994).
In summary, we have affirmed the importance of IFNγ in controlling viral replication in the mouse model of recurrent ocular HSV. Importantly, viral and immunologic factors other than IFNγ may contribute to the genesis of HSK in both primary and recurrent ocular HSV infection.
Methods
Virus and tissue culture
The virus used in these studies was the human isolate HSV-1 McKrae strain. A plaque-purified stock was grown and assayed on VERO cells in minimum essential medium with Earle’s balanced salts (MEM-EBS) containing 5% fetal bovine serum, 100 U/ml Penicillin and 100 μg/ml Streptomycin. Cells were cultured at 37 °C in a humidified incubator containing 5% CO2. To detect recurrent ocular virus shedding at the cornea, eye swabs were performed by the same person, who swipes each eye 10 times in order to maintain consistency of results. The material from eye swabs was plated onto VERO cells, which were then monitored for cytopathic effects (CPE) 48 and 96 h later. Those swabs demonstrating CPE were titered in using serial dilution and standard plaque assays (Keadle et al., 1997). For in vitro reactivation studies, trigeminal ganglia were removed from latently infected (nonirradiated) mice, physically disrupted and placed into 2 wells of a 6-well plate containing a VERO cell monolayer. At the first appearance of viral plaques, a visual count of plaques was performed (both wells) and the remaining ganglia tissue, VERO cells and media were removed from the 2 wells for each trigeminal ganglia and combined. A virus-containing supernatant was obtained after 3 freeze/thaw cycles, sonication, and centrifugation of the well contents. Virus titers in the supernatant were then obtained by serial dilution and plaque assays.
Infection of mice
Investigations with mice conformed to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. In the reactivation model, 6–12 week old C57BL/6 (B6), or C57BL/6 background IFN γ (IFN γ −/−) gene knock out (GKO) mice (Jackson Laboratories; Strain B6129S7-Ifngtm1Ts/J) were infected on the scarified cornea with 106 PFU HSV-1 McKrae strain as previously described (Keadle et al., 1997). Each mouse received an intra-peritoneal (IP) injection of 0.5 ml pooled human serum (Sigma Chemicals, St. Louis MO; ED50 for virus neutralization=1:1600) concurrent with infection. Administration of anti-HSV antibodies at the time of ocular infection has been shown to protect mice from death and corneal disease during primary infection, while allowing for the establishment of latency and subsequent reactivation of virus after corneal UV-B exposure. These antibodies are undetectable at the time of UV-B irradiation 5 weeks after primary infection. HSV positive eye swabs obtained three days after treatment confirm primary infection.
In primary infection studies, B6 and GKO mice were infected with 5×105 PFU of the McKrae strain of HSV-1 as described above without administration of human serum.
UV-B irradiation and virus reactivation
The eyes of all latently infected mice were examined for corneal opacity before irradiation, and only animals with clear corneas were used. At least 5 weeks after primary infection, the eyes of latently infected and control mock-infected mice (B6 and GKO) were exposed to 250 mJ/cm2 of UV-B light using a TM20 Chromato-Vu transilluminator (UVP, Inc., San Gabriel, CA), which emits UV-B at a peak wavelength of 302 nm. Before (day 0), and on days 1 to 10+day 14 post-UV-B irradiation, the eyes of mice were swabbed with surgical spears (Weckcel, Xomed-Treace, Jacksonville, FL) saturated with tissue culture medium. The swab material was cultured on VERO cells, as described above, in order to detect recurrent virus shedding from the cornea. Reactivation was defined as the finding of any HSV positive eye swab on days 1 to 14 post-UV-B exposure, with day 0 swabs serving as a control. Normal C57BL/6 mice experience low and variable virus shedding (5–40%) with accompanying low levels of corneal pathology (Foster et al., 1986; Keadle et al., 2002).
Clinical evaluation
On the designated days after primary infection UV-B irradiation of latently infected mice, a masked observer examined mouse eyes through a binocular-dissecting microscope in order to score clinical disease. Stromal opacification was rated on a scale of 0 to 4, where 0 indicates clear stroma, 1 indicates mild stromal opacification, 2 indicates moderate opacity with discernible iris features, 3 indicates dense opacity with loss of defined iris detail except pupil margins, and 4 indicates total opacity with no posterior view. Neovascularization was rated on a scale from 0 to 2 for 4 corneal quadrants, with a maximum score of 8. A score of 0 indicates no new blood vessel incursion into the cornea, a score of 1 indicates blood vessel incursion up to 50% of the distance from the corneal limbus to the center of the pupil, and a score of 2 indicates blood vessel growth traversing more than 50% of the distance between limbus and pupil. Blepharitis was scored from 0 to 2 where 0 is normal eye lid morphology and 2 is necrotizing dermatitis.
Real-time PCR analysis for herpes genome
DNA was isolated using a DNeasy tissue preparation kit (Qiagen, Valencia, CA). The number of latent genomes per trigeminal ganglia was determined by real-time PCR essentially as described (Strand and Leib 2004). Briefly, a 70 bp fragment of the thymidine kinase (tk) gene was amplified from trigeminal ganglia DNA and 10-fold dilutions of HSV-1 bacterial artificial chromosome DNA, 17–49 BAC (Gierasch et al., 2006). BAC DNA was used to generate a standard curve to determine the number of genome copies per trigeminal ganglion since BAC DNA models the episomal, latent genome. To control for total DNA content of each sample, the single-copy mouse adipsin gene was amplified in each sample along with dilutions of mouse genomic DNA to generate a standard curve. The values for tk copy number were normalized to the lowest value of mouse adipsin copy number to yield the normalized genome copy per ganglion, which were expressed on a log scale.
Delayed type hypersensitivity (DTH) responses
DTH responses were measured in uninfected (naive) and infected mice, 2 weeks after primary infection or UV-B irradiation of latently infected animals. As previously described, 5×106 PFU (before inactivation) of UV-inactivated HSV-1 McKrae in 30 μl of medium was injected into the right rear foot pad of each mouse (Keadle et al., 1997). The left rear foot pad was injected with the same amount of virus-free tissue culture medium. Foot pad swelling was measured with a micrometer (Mitutoyo Manufacturing, Tokyo, Japan) immediately prior to and 24 h after injection. HSV-specific footpad swelling was determined by the formula: [right foot pad swelling at 24 h–right footpad swelling before injection]–[left footpad swelling at 24 h after injection–left footpad swelling before injection].
Statistical analysis
All statistical analyses were performed with the aid of Sigma Stat for Windows version 2.0 (Jandel, Corte Madera, CA). The Student’s T test and the Mann–Whitney rank sum test were used as appropriate to analyze opacity, DTH responses, and virus titer data Fisher’s exact or Chi square tests were used to compare reactivation rates.
Acknowledgments
This work was supported by NIH grants to Patrick M. Stuart (RO1 EY11850) and David A. Leib (RO1 EY09083), and the Department of Ophthalmology and Visual Sciences (P30 EY02687), and a National Institutes of Health, Institutional National Research Service Award (5-T32-EY13360-06) from the National Eye Institute and Research to Prevent Blindness. Support from Research to Prevent Blindness to the department and a Lew Wasserman Scholarship to David A. Leib is gratefully acknowledged. We also wish to acknowledge the technical assistance of Dr. Xiao-Teng Yin and Stephanie Brosius.
References
- Babu JS, Kanangat S, Rouse BT, 1995. T cell cytokine mRNA expression during the course of the immunopathologic ocular disease herpetic stromal keratitis. J. Immunol 154, 4822–4829. [PubMed] [Google Scholar]
- Banerjee K, Biswas PS, Rouse BT, 2007. Role of Stat4-mediated signal transduction events in the generation of aggressor CD4+ T cells in herpetic stromal keratitis pathogenesis. J. Interfer. Cytokin. Res 27, 65–75. [DOI] [PubMed] [Google Scholar]
- Bouley DM, Kanangat S, Wire W, Rouse BT, 1995. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFNg Knockout mice. J. Immunol 155, 3964–3971. [PubMed] [Google Scholar]
- Cantin E, Tanamachi B, Openshaw H, 1999a. Role for gamma interferon in control of herpes simplex virus type 1 reactivation. J. Virol 73, 3418–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin E, Tanamachi B, Openshaw H, Mann J, Clarke K, 1999b. Gamma interferon receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFNg ligand null mutant mice. J. Virol 73, 5196–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison AR, Yang L, Voytek C, Margolis TP, 2000. Establishment of latent herpes simplex virus type 1 infection in resistant, sensitive, and immunodeficient mouse strains. Virology 268, 17–28. [DOI] [PubMed] [Google Scholar]
- Foster CS, Tsai Y, Monroe JG, Campbell R, Cestari M, Wetzig R, Knipe D, Greene MI, 1986. Genetic studies on murine susceptibility to herpes simplex keratitis. Clin. Immunol. Immunopathol 40, 313–325. [DOI] [PubMed] [Google Scholar]
- Ghiasi H, Cai S, Perng GC, Nesburn AB, Wechsler SL, 2000. The role of natural killer cells in protection of mice against death and corneal scarring following ocular HSV-1 infection. Antiviral Res. 45, 33–45. [DOI] [PubMed] [Google Scholar]
- Gierasch WW, Zimmerman DL, Ward SL, Vanheyningen TK, Romine JD, Leib DA, 2006. Construction and characterization of bacterial artificial chromosomes containing HSV-1 strains 17 and KOS. J. Virol. Methods 135, 197–206. [DOI] [PubMed] [Google Scholar]
- He J, Ichimura H, Iida T, Minami M, Kobalyashi K, Kita M, Sotozono C, Tagawa YI, Iwakura Y, Imanishi J, 1999. Kinetics of cytokine production in the cornea and trigeminal ganglion of C57BL/6 mice after corneal HSV-1 infection. J. Interfer. Cyto. Res 19, 609–615. [DOI] [PubMed] [Google Scholar]
- Hendricks RL, Tumpey TM, Finnegan A, 1992. IFN-g and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J. Immunol 149, 3023–3028. [PubMed] [Google Scholar]
- Kaplan MH, 2005. Stat4: a critical regulator of inflammation in vivo. Immunol. Res 31, 231–242. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Stuart PM, 2005. IL-10 ameliorates corneal disease in a mouse model of recurrent herpetic keratitis. Microbial Path. 38, 13–21. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Laycock KA, Miller JK, Hook KK, Fenoglio ED, Francotte M, Slaoui M, Stuart PM, Pepose JS, 1997. Efficacy of a recombinant glycoprotein D subunit vaccine on the development of primary and recurrent ocular infection with herpes simplex virus type 1 in mice. J.Inf. Dis 176, 331–338. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Laycock KA, Pepose JS, Stuart PM, 2000. Proinflammatory cytokines IL-1 and TNF-a are required for recurrent herpetic keratitis in NIH mice. Invest. Ophth. Vis. Sci 41, 96–102. [PubMed] [Google Scholar]
- Keadle TL, Usui N, Laycock KA, Kumano Y, Pepose JS, Stuart PM, 2001. Corneal cytokine expression in a murine model recurrent herpetic stromal keratitis. Ocular Immunol. Inflam 9, 193–205. [DOI] [PubMed] [Google Scholar]
- Keadle TL, Morris JL, Pepose JS, Stuart PM, 2002. CD4+ and CD8+ cells are key participants in the development of recurrent herpetic stromal keratitis in mice. Microbial Path. 32, 255–262. [DOI] [PubMed] [Google Scholar]
- Khanna K, Bonneau R, Kinchington P, Hendricks RL, 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18, 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laycock KA, Lee SF, Brady RH, Pepose JS, 1991. Characterization of a murine model of recurrent herpes simplex viral keratitis induced by ultraviolet B irradiation. Invest. Ophthalmol. Vis. Sci 32, 2741–2746. [PubMed] [Google Scholar]
- Lekstrom-Himes JA, LeBlanc RA, Pesnicak L, Godleski M, Straus SE, 2000. Gamma interferon impedes the establishment of herpes simplex virus type 1 latent infection but has no impact on its maintenance or reactivation in mice. J Virol. 74, 6680–6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Zheng M, Deshpande S, Eo SK, Hamilton TA, Rouse BT, 2002. IL-12 suppresses the expression of ocular immunoinflammatory lesions by effects on angiogenesis. J. Leukoc. Biol 71, 469–476. [PubMed] [Google Scholar]
- Liu T, Tang Q, Hendricks RL, 1996. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol 70, 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Khanna KM, Carriere BN, Hendricks RL, 2001. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol 75, 11178–11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertzdorf J, Osterhaus AD, Verjans GM, 2002. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J. Immunol 169, 5897–5903. [DOI] [PubMed] [Google Scholar]
- Maertzdorf J, Verjans GM, Remeijer L, van der Kooi A, Osterhaus AD, 2003. Restricted T cell receptor beta-chain variable region protein use by cornea-derived CD4+ and CD8+ herpes simplex virus-specific T cells in patients with herpetic stromal keratitis. J. Infect. Dis 187, 550–558. [DOI] [PubMed] [Google Scholar]
- Mikloska Z, Cunningham AL, 2001. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J. Virol 75, 11821–11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JK, Laycock KA, Umphress JA, Hook KK, Stuart PM, Pepose JS, 1996. A comparison of recurrent versus primary HSK in inbred mice. Cornea 15, 497–504. [PubMed] [Google Scholar]
- Niemialtowski MG, Rouse BT, 1992. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J. Immunol 149, 3035–3039. [PubMed] [Google Scholar]
- Osorio Y, Weschsler SL, Nesburn AB, Ghiasi H, 2002. Reduced severity of HSV-1-induced corneal scarring in IL-12-deficient mice. Virus Res. 90, 317–326. [DOI] [PubMed] [Google Scholar]
- Pepose JS, Leib DA, Stuart PM, Easty DL, 1996. Herpes simplex virus diseases: anterior segment of the eye In: Peposes JS, Holland GAN, Wilhelmus KR (Eds.), Ocular infection and immunity. Mosby, St. Louis, Mo, pp. 905–932. [Google Scholar]
- Presti RM, Pollock JL, Dal Canto AJ, O’Guin AK, Virgin HW, 1998. Interferon gamma regulates acute and latent murine cytomegalovirus infection and chronic disease of the great vessels. J. Exp. Med 188, 577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JR, Abghari SZ, Stulting RD, 1992. The severity of herpes simplex viral keratitis in mice does not reflect the severity of disease in humans. Invest. Ophthal. Vis. Sci 33, 268–272. [PubMed] [Google Scholar]
- Smith PM, Wolcott RM, Chervenak R, Jennings SR, 1994. Control of acute cutaneous herpes simplex virus infection: T cell mediated viral clearance is dependent upon interferon-gamma. Virology 202, 76–88. [DOI] [PubMed] [Google Scholar]
- Strand SS, Leib DA, 2004. Role of the VP16-binding domain of vhs in viral growth, host shutoff activity, and pathogenesis. J. Virol 78, 13562–13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streilein JW, Dana MR, Ksander BR, 1997. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol. Today 18, 443–449. [DOI] [PubMed] [Google Scholar]
- Stumpf TH, Shimeld C, Easty DL, Hill TJ, 2001. Cytokine production in a murine model of recurrent herpetic stromal keratitis. Invest. Ophthalmol. Vis. Sci 42, 372–378. [PubMed] [Google Scholar]
- Stumpf TH, Case R, Shimeld C, Easty DL, Hill TJ, 2002. Primary herpes simplex virus type 1 infection of the eye triggers similar immune responses in the cornea and the skin of the eyelids. J. Gen. Virol 83, 1579–1590. [DOI] [PubMed] [Google Scholar]
- Su YH, Yan XT, Oakes JE, Lausch RN, 1996. Protective antibody therapy is associated with reduced chemokine transcripts in herpes simplex virus type 1 corneal infection. J. Virol 70, 1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T, 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol 163, 2179–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Rouse BT, 1997. Immunopathogenesis of herpetic ocular disease. Immunol. Res 16, 375–386. [DOI] [PubMed] [Google Scholar]
- Thomas J, Kanangat S, Rouse BT, 1998. Herpes simplex virus replication-induced expression of chemokines and proinflammatory cytokines in the eye: implications in herpetic stromal keratitis. J. Interfer. Cyto. Res 18, 681–690. [DOI] [PubMed] [Google Scholar]
- Vollstedt S, Arnold S, Schwerdel C, Franchini M, Alber G, Di Santo JP, Ackermann M, Suter M, 2004. Interplay between alpha/beta and gamma interferons with B, T, and natural killer cells in the defense against herpes simplex virus type 1. J. Virol 78, 3846–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Lepisto AJ, Hendricks RL, 2004. CD154 signaling regulates the Th1 response to herpes simplex virus-1 and inflammation in infected corneas. J. Immunol 173, 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youinou P, Colin J, Mottier D, 1985. Immunological analysis of the cornea in herpetic stromal keratitis. J. Clin. Lab. Immunol 17, 105–106. [PubMed] [Google Scholar]
- Youinou P, Colin J, Ferec C, 1986. Monoclonal antibody analysis of blood and cornea T lymphocyte subpopulations in herpes simplex keratitis. Graefes Arch. Clin. Exp. Ophthalmol 224, 131–133. [DOI] [PubMed] [Google Scholar]