Abstract
Objectives
To describe the frequency of sleep-disordered breathing (SDB) in pediatric cystic fibrosis (CF) and to study associations between polysomnographic respiratory parameters and available clinical information.
Methods
This was a retrospective, cross-sectional study. The sample data were obtained from information recorded on patient charts in 2015 and 2016. The study included all individuals with CF aged from 2 to 20 years for whom records were available for polysomnography performed within the previous two years.
Results
Sixteen individuals with CF (mean age 11 ± 5.6 years old) were included. Polysomnographic respiratory parameter abnormalities were defined as an apnea-hypopnea index (AHI) exceeding one event per hour of sleep or an oxyhemoglobin saturation (SpO2) nadir below 90%; observed in 10 subjects (62.5%). Forced expiratory volume in first second (FEV1) was correlated (r=0.602, p=0.023) with mean sleep SpO2. FEV1 was also negatively correlated with sleep peak end-tidal carbon dioxide (EtpCO2) (r=-0.645, p=0.024). Additionally, chronic airway colonization by Pseudomonas aeruginosa was associated with mean EtpCO2 in non-REM sleep (p=0.024).
Discussion
SDB was frequently observed in this sample of children with CF. There was an association between CF respiratory disease progression markers and sleep breathing parameters in children. Sleep studies appear to be an important tool for assessment of the respiratory status of these individuals with CF, although further studies are needed, especially with carbon dioxide sleep analysis.
Keywords: Cystic Fibrosis, Pediatrics, Polysomnograph, Sleep Apnea Syndromes
INTRODUCTION
Cystic fibrosis (CF) is a genetic disease that compromises the normal physiology of several different organs. The main cause of morbidity and mortality in these patients is respiratory system involvement1. As with other chronic respiratory disorders, ventilation impairment begins during sleep, especially during the rapid eye movement (REM) stage2,3. In children, the REM stage of sleep is of longer duration than in adults. Although overnight polysomnography is costly and access is limited, it is considered the gold standard test for evaluation of respiratory abnormalities during sleep4,5.
Cystic fibrosis patients exhibit decreases in sleep oxyhemoglobin saturation (SpO2) that are associated with reduced intercostal muscle activity, irregular breathing patterns, and hypoventilation caused by reduced tidal volume and minute ventilation2. Hypoxemia and hypercapnia during sleep are common findings in patients with advanced lung disease, but they are also described in some individuals with mild or moderate disease6-8. Even in the absence of frank hypoxemia, nocturnal SpO2 is lower in the CF pediatric population than in healthy controls5,7,9,10. For nocturnal carbon dioxide (CO2) estimation, a study comparing CF children with healthy controls found an association between peak CO2 and CF11. Polysomnography (PSG) findings are conflicting with regard to the greater frequency of obstructive sleep apnea in CF children compared to controls5,7,9,11,12.
There are also previous studies that have investigated associations between sleep-disordered breathing (SDB) and waking clinical and functional variables (such as spirometry data). These studies have reported different and even conflicting results7,13-15. Furthermore, there are studies that have described improvements in CF disease after treatment of obstructive sleep apnea-hypopnea syndrome (OSAHS)16-18.
Although relevant, studies of SDB in CF pediatric populations have reported discrepant findings and are subject to limitations19. Therefore, the objectives of this study were to describe SDB frequency and to study associations between changes in polysomnography respiratory indexes and available clinical data.
MATERIAL AND METHODS
This was a cross-sectional study performed by review of medical records for 2015 and 2016 from a CF center. The project was approved by the Institutional Ethics Committee (number 1.294.834). Participants were recruited from among patients in follow-up at the multidisciplinary CF outpatient clinic at a tertiary hospital in Porto Alegre (Southern Brazil). Subjects included were aged from 2 to 20 years and had undergone nocturnal PSG during the previous two years. Exclusion criteria were use of nocturnal ventilatory support, prior lung transplantation, unavailability of medical records and irregular follow-up.
Subjects had been referred to the outpatient clinic for CF investigation, either because of symptoms of CF (such as recurrent respiratory infections, low weight gain, and fatty stools)1 or because of an abnormal newborn CF screening test. Sweat testing and/or CFTR genotyping was used to confirm CF.
The following information was extracted from medical records and used for patients' clinical, functional and sleep evaluation:
1. Date of birth, sex, age of diagnosis;
2. Anthropometric data (weight and height) and indicators of nutritional status (weight for age, height for age and body mass index - BMI), which were classified by percentiles and Z scores, according to National Center for Health Statistics charts20;
3. Comorbidities, treatment, number of exacerbations per year, Shwachman score15,21 and bacterial airway colonization (chronic colonization by Pseudomonas aeruginosa [PA] was defined as PA present in at least 50% of cultures over one year)22,23;
4. Lung function data, such as forced expiratory volume in first second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio and forced expiratory flow from 25% to 75% of FVC (FEF25-75). The Global Lung Function Initiative international equation was used as the reference for percentiles and for Z scores for age, height, and sex24. Examinations were performed using Koko equipment (PDS Instrumentation, Inc., Louisville, CO, USA) and all procedures were conducted in accordance with American Thoracic Society criteria25;
5. Polysomnography data included: sleep efficiency, sleep latency for sleep and REM sleep onset, apnea-hypopnea index per hour of sleep (AHI), obstructive AHI index per hour of sleep (OAHI, where central apneas were discounted in the numerator calculation), baseline and minimal oxyhemoglobin saturation, desaturation index (which was defined as at least a 3% reduction from baseline SpO2), mean values of exhaled CO2 (EtpCO2) in REM and NREM sleep and when awake, total time and percentage of total sleep time (TST) with measured value of high carbon dioxide, and highest (peak) sleep EtpCO2 value. Examination data were recorded using Alice 5® (Philips Respironics) equipment, with the exception of one examination (which was performed with Bio-Logic®). In all subjects, the test was conducted at night with spontaneous breathing and the study was performed according to international American Academy of Sleep Medicine recommendations.
For statistical analysis, categorical variables were described as absolute and relative frequencies, and continuous variables as mean and standard deviation. Continuous variables outcomes were compared using the t-test. The fisher's exact test or chi-square test were used for associations between categorical variables. Spearman and Pearson tests were used for correlation analysis, as appropriate for distribution of variables. A 0.05 significance (p) cutoff was used.
RESULTS
Ninety-one subjects are followed at the PUCRS CF Center and 16 cases had undergone PSG. Among these individuals with PSG records, 75% were male and mean age was 11 ± 5.6 years. Thirteen subjects had identified genotypes (> 80% had at least one allele for F508del). All subjects had pancreatic insufficiency and were on enzyme replacement therapy.
The mean AHI for the sample was 1.4 ± 1.8 events per hour of sleep and after exclusion of central apneas (OAHI), the mean value was 1.2 ± 1.7 events per hour of sleep. Subjects' mean sleep SpO2 was 95.7 ± .3%, with a minimum of 89.5 ± 3.9%. Fifteen subjects (93%) had zero percent of their TST with SpO2 below 90%. Of fourteen individuals for whom information about EtpCO2 measurements was available, just one individual had a CO2 peak higher than 50mmHg and remained 0.1% of TTS (approximately 0.4 minutes) with this range. Mean EtpCO2 values during NREM and REM sleep were 34.3 ± 3.1 mmHg and 35.1 ± 2.6 mmHg respectively and the whole-sample peak value was 42.1 ± 3.5 mmHg. When the parameters for normality were defined as AHI less than or equal to one hourly sleep event and an SpO2 nadir greater than or equal to 90%, only six subjects (37.5% of the total, mean age of 11.7 years and BMI Z score of - 0.18) met these criteria for normality. Table 1 lists additional characterization data and results broken down by respiratory findings (defined as normal for patients with AHI less than or equal to one event per hour of sleep and an SpO2 nadir greater than or equal to 90%).
Table 1.
Clinical and functional characteristics of study participants.
| Information | All patients | Normal | Abnormal | p |
|---|---|---|---|---|
| Age; mean ± SD | 11.0 ± 5.6 | 11.7 ± 6.4 | 10.6 ± 5.4 | 0.738 |
| Boys; n (%) | 12/16 (75%) | 3/6 (50%) | 9/10 (90%) | 0.118 |
| BMI Z score; mean ± SD | 0.13 ± 0.61 | -0.18 ± 0.58 | 0.32 ± 0.57 | 0.125 |
| ≥ one DF508 allele; n (%) | 11/13 (84.6%) | 3/5 (60%) | 8/8 (100%) | 0.128 |
| Chronic PA airway colonization; n(%) | 7/16 (43.7%) | 4/6 (66.6%) | 3/10 (30%) | 0.302 |
| Respiratory exacerbations/year; mean ± SD | 3.1 ± 2.0 | 2.0 ± 1.3 | 4.0 ± 2.2 | 0.067 |
| FVC %predict; mean ± SD† | 92.22 ± 23.22 | 91.54 ± 27.74 | 92.60 ± 22.16 | 0.944 |
| FVC Z score; mean ± SD† | -0.67 ± 1.99 | -0.74 ± 2.39 | -0.64 ± 1.89 | 0.937 |
| FEV1 %predict; mean ± SD† | 87.95 ± 26.21 | 91.04 ± 27.03 | 86.23 ± 27.24 | 0.758 |
| FEV1 Z score; mean ± SD† | -0.99 ± 2.19 | -0.76 ± 2.34 | -1.12 ± 2.24 | 0.789 |
| FEV1/FVC Z score; mean ± SD† | -0.68 ± 1.30 | -0.04 ± 0.65 | -1.04 ± 1.47 | 0.106 |
| FEF25-75 Z score; mean ± SD† | -1.03 ± 1.92 | -0.30 ± 1.31 | -1.43 ± 2.15 | 0.247 |
| Snoring; n (%)‡ | 5/15 (33.3%) | 0/5 (0%) | 5/10 (50%) | 0.101 |
| AHI; mean ± SD | 1.4 ± 1.8 | 0.2 ± 0.3 | 2.1 ± 2.0 | 0.036 |
| OAHI; mean ± SD | 1.2 ± 1.7 | 0.2 ± 0.3 | 1.7 ± 1.9 | 0.078 |
| Mean SpO2; mean ± SD‡ | 95.7 ± 2.3 | 96.2 ± 0.4 | 95.5 ± 2.8 | 0.458 |
| SpO2 nadir; mean ± SD | 89.5 ± 3.9 | 92.7 ± 1.6 | 87.6 ± 3.7 | 0.002 |
| Mean NREM EtpCO2; mean ± SD§ | 34.3 ± 3.1 | 34.4 ± 3.4 | 34.3 ± 3.1 | 0.954 |
| Mean REM EtpCO2; mean ± SD§ | 35.1 ± 2.6 | 34.6 ± 3.0 | 35.4 ± 3.5 | 0.625 |
| Peak sleep EtpCO2; mean ± SD† | 42.1 ± 3.5 | 42.2 ± 2.9 | 42.1 ± 4.2 | 0.983 |
| Change CO2 REM-NREM; n (%)§ | 6/12 (50%) | 2/5 (40%) | 4/7 (57.1%) | 1.00 |
*n=13;
n=14;
n=15;
n=12.
Tables 2 and 3 show associations between clinical variables (lung function and chronic PA airway colonization) and PSG findings. FEV1 was associated with mean nocturnal SpO2 (96.87 ± 1.12% when FEV1≥90% and 94.16 ± 2.78% when FEV1 < 90%; p=0.027). Chronic PA colonization was associated with mean EtpCO2 value during NREM sleep (33 ± 2.77 mmHg in those free from PA and 37 ± 1.41 mmHg in individuals with chronic PA; p=0.024). Except for significant associations between Z scores for BMI and Z scores for lung function variables (FEV1 and FEF25-75) and PA colonization, other clinical data (Shwachman score, number of exacerbations) were not significantly associated with FEV1 or PA.
Table 2.
Comparison between patients with FEV1 ≥ 90% and < 90% of predicted.
| Information | FEV1≥90% (n=8) | FEV1<90% (n=6) | p |
|---|---|---|---|
| mean±SD | mean±SD | ||
| Age | 11.75±4.74 | 11.83±5.6 | 0.976 |
| BMI Z score | 0.16±0.57 | 0.11±0.59 | 0.897 |
| Shwachman score† | 93.71±5.02 | 85.5±12.12 | 0.273 |
| AHI | 1±1.85 | 1.5±1.87 | 0.628 |
| OAHI | 0.75±1.48 | 1.16±1.94 | 0.656 |
| Mean SpO2 | 96.87±1.12 | 94.16±2.78 | 0.027* |
| SpO2 nadir | 89±3.77 | 89±4.42 | 1.00 |
| Mean NREM EtpCO2† | 33.86±3.48 | 35.75±2.21 | 0.358 |
| Mean REM EtpCO2† | 34.85±3.02 | 36.25±1.25 | 0.410 |
| Peak sleep EtpCO2‡ | 41.14±2.41 | 43.4±5.31 | 0.340 |
Significance cutoff (p)<0.05;
n=7 for FEV1≥90% and n=4 for FEV1 <90% of predicted;
n=7 for FEV1≥90% and n=5 for FEV1<90% of predicted.
FEV1=forced expiratory volume in one second SD=standard deviation; BMI=body mass index; AHI=apnea-hypopnea index; OIAH=obstructive AHI index - without central apneas; SpO2=oxyhemoglobin saturation; EtpCO2=end-tidal carbon dioxide; NREM=non rapid eye movement (REM) stage.
Table 3.
Comparison between patients with and without chronic Pseudomonas aeruginosa (PA) airway colonization.
| Information | With PA (n=9) | Without PA (n=7) | p |
|---|---|---|---|
| mean ± SD | mean ± SD | ||
| Age | 9.44 ± 5.05 | 12.14 ± 6.22 | 0.354 |
| BMI Z score | 0.40 ± 0.55 | -0.22 ± 0.49 | 0.034* |
| Shwachman score† | 95.71 ± 3.54 | 87 ± 10.17 | 0.093 |
| FEV1 Z score‡ | 0.21 ± 1.38 | -2.59 ± 2.09 | 0.011* |
| FEV1/FVC Z score‡ | -0.18 ± 1.1 | -1.34 ± 1.34 | 0.102 |
| FEF25-75 Z score‡ | 0 ± 1.34 | -2.4 ± 1.74 | 0.013* |
| AHI | 1.33 ± 1.73 | 1 ± 1.82 | 0.715 |
| OAHI | 0.77 ± 1.39 | 1 ± 1.82 | 0.786 |
| Mean SpO2§ | 96.77 ± 1.09 | 94.16 ± 2.78 | 0.071 |
| SpO2 nadir | 88.88 ± 3.33 | 90.28 ± 4.78 | 0.502 |
| Mean NREM EtpCO2|| | 33 ± 2.77 | 37 ± 1.41 | 0.024* |
| Mean REM EtpCO2|| | 34.12 ± 2.64 | 37 ± 1.15 | 0.068 |
| Peak sleep EtpCO2‡ | 41 ± 2.44 | 43.66 ± 4.41 | 0.173 |
Significance cutoff (p)<0.05;
n=7 without and 6 with chronic airway colonization by Pseudomonas aeruginosa (PA);
n=8 without and 6 with PA;
n=6 with PA;
n=8 without and 4 with PA.
SD=standard deviation; BMI=body mass index; FVC=forced vital capacity; FEV1=forced expiratory volume in one second; FEF25-75=forced expiratory flow from 25% to 75% of FVC; AHI=apnea-hypopnea index; OIAH=obstructive AHI index - without central apneas; SpO2=oxyhemoglobin saturation; EtpCO2=end-tidal carbon dioxide; NREM=non rapid eye movement (REM) stage.
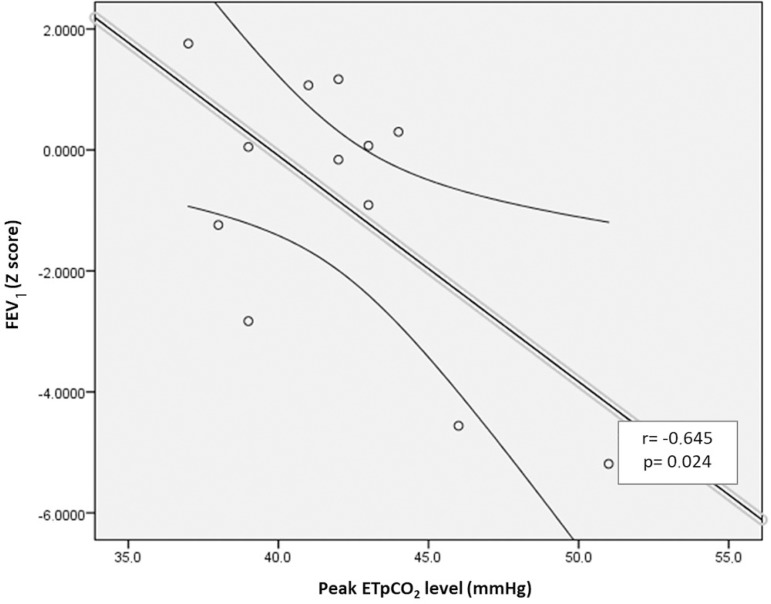
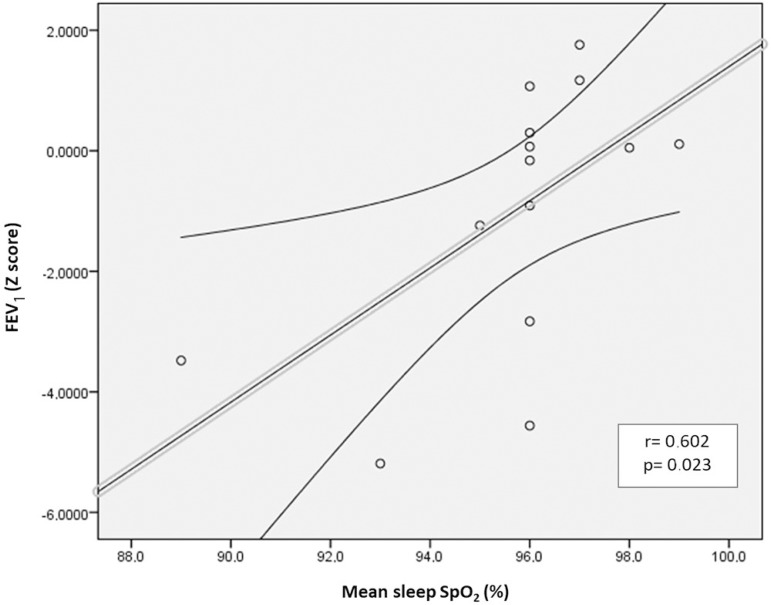
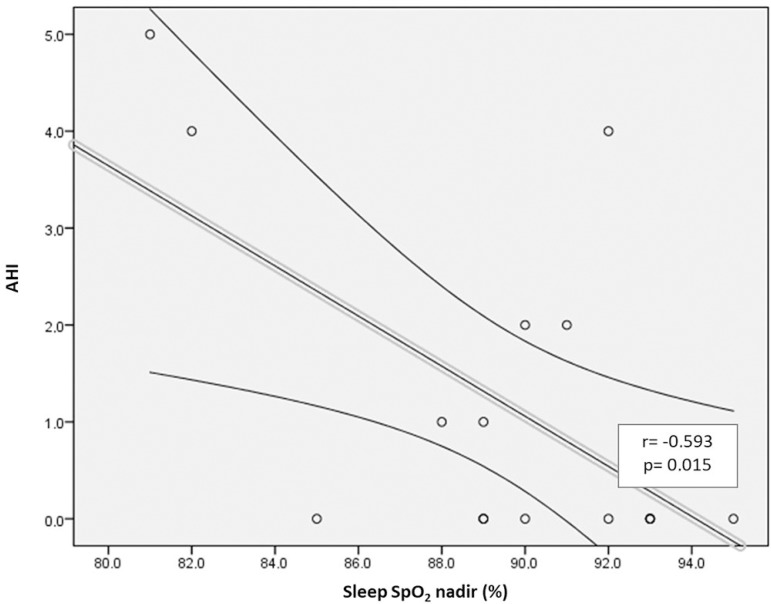
Correlations were observed between variables associated with CF lung diseases and PSG respiratory variables. There was a correlation between FEV1 Z score and peak CO2 level (Figure 1A). Moreover, FEV1 Z score was correlated with mean sleep SpO2 (Figure 1B). Additionally, a correlation was observed between AHI and SpO2 nadir (Figure 1C). None of the other correlations were statistically significant.
Figure 1A.
Correlation between FEV1 (Z score) and the EtpCO2 peak.
Figure 1B.
Correlation between FEV1 (Z score) and mean sleep SpO2.
Figure 1C.
Correlation between AHI and sleep SpO2 nadir.
DISCUSSION
We observed that FEV1 was correlated (r=0.602, p = 0.023) with mean sleep SpO2. Furthermore, FEV1 was negatively correlated with peak EtpCO2 at night in our pediatric CF population sample. Interestingly, we found that the presence of airway PA colonization exhibited an association with mean EtpCO2 during NREM sleep (33 ± 2.77 mmHg vs. 37 ± 1.41 mmHg, p=0.024). We also found that chronic PA airway colonization exhibited associations with BMI and with lung function parameters, as previously described26. Finally, AHI was negatively correlated with SpO2 nadir.
Although recommended in pediatric PSG27, capnography is not always performed for patients with CF. Also, results can be described in different forms: peak and average in sleep (REM and NREM)10,11; during wakefulness5; percent of sleep time with CO2 above a cutoff19; and, more recently, by comparing CO2 increase in REM sleep in relation to the NREM value (change CO2)15. Unlike Waters et al.15, in our study the change CO2 ranged from -1 to +3 (CO2 in REM sleep increased in relation to NREM in half of the 12 subjects for whom these data were available and remained the same or reduced in the other six subjects) and was not associated with any clinical or functional variables. In our study, FEV1 Z score correlated with peak EtpCO2 and chronic PA infection was associated with mean CO2 in NREM sleep; findings that have not been described previously. When using exhaled CO2 measurements, values may be underestimated (compared with transcutaneous CO2) in patients with respiratory disease5,11,16. Therefore, we can speculate that if we had used transcutaneous measurement and a larger sample size, we could have found even more consistent findings.
We also observed a correlation between AHI and sleep SpO2 nadir, which is expected when evaluating associated respiratory disorders (CF and OSAHS). Most studies seem to agree with our finding6,9, even those employing different definitions for OSAHS. One example is a study Ramos et al.28 conducted with 67 CF patients aged from 2 to 14 years, in which they observed a relationship between an apnea index (number of obstructive and mixed apneas by TST) and lower SpO2 values.
It is known that pulmonary function assessment is an essential tool in CF follow-up29, and it has been evaluated in other studies of sleep in CF subjects. Comparing our results to Uyan et al.14, we observed different findings. They did not find a correlation between mean SpO2 and FEV1 in 24 children with CF (mean age 9.5 years and FEV1> 40%), but did describe an association between SpO2 nadir and FEV1. In a more recent publication, Spicuzza et al.9 also found no correlation between mean SpO2 and FEV1 (r2 =0.03, p = 0.14). On the other hand, de Castro-Silva et al.7 observed a similar result to ours (correlation between FEV1 and mean SpO2; p < 0.001). Moreover, Waters et al.15 detected a correlation with baseline SpO2, but not with its nadir. They did not report the sleep mean SpO2 in their sample of 42 CF patients aged 8 to 12 years15. The reasons for this lack of uniformity may be due to different methodologies used by the studies: 1) regarding the SpO2 parameter (mean, nadir, baseline, time and percentage of TST with SpO2 below a certain value)30, as in the case of Waters et al.15; 2) regarding the pulmonary function predictive equation used, such as Knudson et al.31 in Uyan and Polgar32 in Waters et al.15 and Spicuzza et al.9; and 3) differences between subjects' severity profiles in the studies.
One potential implication of our study is the possible inclusion of PSG as part of a routine annual check-up at CF centers. Although there is no consensus on performing PSG in individuals with mild and/or stable disease, there are recent studies suggesting early SDB investigation and PSG may be useful in CF patients5,6,9. In our sample of 16 subjects, only six subjects (37.5%) were classified as normal considering AHI less than or equal to one event per hour of sleep and SpO2 nadir greater than or equal to 90% as criteria for normality. Thus, in addition to other results, our impression is that SBD evaluation by PSG has a potential positive impact in CF patients and this benefit may be even greater when considering some subgroups, such as those colonized by PA and those with persistent reductions in pulmonary function parameters, especially FEV1.
For instance, it is difficult to describe the immediate clinical relevance of differences observed in our study. Both values were within the normal range, even in subjects colonized by PA. However, we believe these findings may serve as markers for CF respiratory disease progression, rather than revealing a respiratory disturbance.
Small sample size was the most relevant limitation of our study. However, it is known that PSG is not readily available, especially for the pediatric population33,34. Even in countries with greater PSG availability, CF pediatric population studies rarely include more than 40 subjects. Interestingly, Brazilian studies by Ramos et al. have the largest number of individuals (more than 60)6,28,35, but they did not conduct sleep CO2 assessment. Nevertheless, we believe our sample size may have been restricted by both the study methodology adopted (a retrospective study) and the PSG access difficulties faced by patients. Ultimately, there are few sleep centers that conduct PSG on children available. Although our study is restricted in quantity, our subjects' ages and clinical manifestations are comprehensive. This raises two observations: 1) our sample encompasses subjects with different severities; 2) whether it is possible to predict disease progression using PSG.
Finally, we agree with a previous study5 that suggested sleep analysis could serve as an early indicator for CF pulmonary disease progression, although further multicenter studies are necessary to assess PSG findings including nocturnal capnography. The potential positive impact of PSG for pediatric CF patients may be even greater within some subgroups, such as those colonized by PA and those with persistent reductions in pulmonary function parameters, especially FEV1.
REFERENCES
- 1.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Cystic Fibrosis Foundation Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153(2):S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballard RD, Sutarik JM, Clover CW, Suh BY. Effects of non-REM sleep on ventilation and respiratory mechanics in adults with cystic fibrosis. Am J Respir Crit Care Med. 1996;153(1):266–271. doi: 10.1164/ajrccm.153.1.8542127. [DOI] [PubMed] [Google Scholar]
- 3.Milross MA, Piper AJ, Dobbin CJ, Bye PT, Grunstein RR. Sleep disordered breathing in cystic fibrosis. Sleep Med Rev. 2004;8(4):295–308. doi: 10.1016/j.smrv.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Wise MS, Nichols CD, Grigg-Damberger MM, Marcus CL, Witmans MB, Kirk VG, et al. Executive summary of respiratory indications for polysomnography in children: an evidence-based review. Sleep. 2011;34(3):389–98AW. doi: 10.1093/sleep/34.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paranjape SM, McGinley BM, Braun AT, Schneider H. Polysomnographic Markers in Children With Cystic Fibrosis Lung Disease. Pediatrics. 2015;136(5):920–926. doi: 10.1542/peds.2015-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos RT, Santana MA, Almeida PC, Machado AS, Jr, Araújo-Filho JB, Salles C. Nocturnal hypoxemia in children and adolescents with cystic fibrosis. J Bras Pneumol. 2013;39(6):667–674. doi: 10.1590/S1806-37132013000600005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Castro-Silva C, de Bruin VM, Cavalcante AG, Bittencourt LR, de Bruin PF. Nocturnal hypoxia and sleep disturbances in cystic fibrosis. Pediatr Pulmonol. 2009;44(11):1143–1150. doi: 10.1002/ppul.21122. [DOI] [PubMed] [Google Scholar]
- 8.Bradley S, Solin P, Wilson J, Johns D, Walters EH, Naughton MT. Hypoxemia and hypercapnia during exercise and sleep in patients with cystic fibrosis. Chest. 1999;116(3):647–654. doi: 10.1378/chest.116.3.647. [DOI] [PubMed] [Google Scholar]
- 9.Spicuzza L, Sciuto C, Leonardi S, La Rosa M. Early occurrence of obstructive sleep apnea in infants and children with cystic fibrosis. Arch Pediatr Adolesc Med. 2012;166(12):1165–1169. doi: 10.1001/archpediatrics.2012.1177. [DOI] [PubMed] [Google Scholar]
- 10.Suratwala D, Chan JS, Kelly A, Meltzer LJ, Gallagher PR, Traylor J, et al. Nocturnal saturation and glucose tolerance in children with cystic fibrosis. Thorax. 2011;66(7):574–578. doi: 10.1136/thx.2010.142141. [DOI] [PubMed] [Google Scholar]
- 11.Naqvi SK, Sotelo C, Murry L, Simakajornboon N. Sleep architecture in children and adolescents with cystic fibrosis and the association with severity of lung disease. Sleep Breath. 2008;12(1):77–83. doi: 10.1007/s11325-007-0123-0. [DOI] [PubMed] [Google Scholar]
- 12.Villa MP, Pagani J, Lucidi V, Palamides S, Ronchetti R. Nocturnal oximetry in infants with cystic fibrosis. Arch Dis Child. 2001;84(1):50–54. doi: 10.1136/adc.84.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Versteegh FG, Bogaard JM, Raatgever JW, Stam H, Neijens HJ, Kerrebijn KF. Relationship between airway obstruction, desaturation during exercise and nocturnal hypoxaemia in cystic fibrosis patients. Eur Respir J. 1990;3(1):68–73. [PubMed] [Google Scholar]
- 14.Uyan ZS, Ozdemir N, Ersu R, Akpinar I, Keskin S, Cakir E, et al. Factors that correlate with sleep oxygenation in children with cystic fibrosis. Pediatr Pulmonol. 2007;42(8):716–722. doi: 10.1002/ppul.20643. [DOI] [PubMed] [Google Scholar]
- 15.Waters KA, Lowe A, Cooper P, Vella S, Selvadurai H. A cross-sectional analysis of daytime versus nocturnal polysomnographic respiratory parameters in cystic fibrosis during early adolescence. J Cyst Fibros. 2017;16(2):250–257. doi: 10.1016/j.jcf.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Katz ES. Cystic fibrosis and sleep. Clin Chest Med. 2014;35(3):495–504. doi: 10.1016/j.ccm.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Hayes D., Jr Obstructive sleep apnea syndrome: a potential cause of lower airway obstruction in cystic fibrosis. Sleep Med. 2006;7(1):73–75. doi: 10.1016/j.sleep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald KD, McGinley BM, Brown DJ, Sterni LM, Rosenstein BJ, Mogayzel PJ., Jr Primary snoring and growth failure in a patient with cystic fibrosis. Respir Care. 2009;54(12):1727–1731. [PubMed] [Google Scholar]
- 19.Fauroux B, Pepin JL, Boelle PY, Cracowski C, Murris-Espin M, Nove-Josserand R, et al. Sleep quality and nocturnal hypoxaemia and hypercapnia in children and young adults with cystic fibrosis. Arch Dis Child. 2012;97(11):960–966. doi: 10.1136/archdischild-2011-300440. [DOI] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 21.Shwachman H, Kulczycki LL. Long term study of one hundred five patients with cystic fibrosis. Am J Dis Child. 1958;96(1):6–15. doi: 10.1001/archpedi.1958.02060060008002. [DOI] [PubMed] [Google Scholar]
- 22.Kozlowska WJ, Bush A, Wade A, Aurora P, Carr SB, Castle RA, et al. London Cystic Fibrosis Collaboration Lung function from infancy to the preschool years after clinical diagnosis of cystic fibrosis. Am J Respir Crit Care Med. 2008;178(1):42–49. doi: 10.1164/rccm.200710-1599OC. [DOI] [PubMed] [Google Scholar]
- 23.Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2(1):29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 24.Stanojevic S, Wade A, Cole TJ, Lum S, Custovic A, Silverman M, et al. Asthma UK Spirometry Collaborative Group Spirometry centile charts for young Caucasian children: the Asthma UK Collaborative Initiative. Am J Respir Crit Care Med. 2009;180(6):547–552. doi: 10.1164/rccm.200903-0323OC. [DOI] [PubMed] [Google Scholar]
- 25.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force Standardisation of Spirometry. Eur Resp J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 26.Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax. 2002;57(7):596–601. doi: 10.1136/thorax.57.7.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, et al. American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.3. Darien: American Academy of Sleep Medicine; 2016. [Google Scholar]
- 28.Ramos RT, Salles C, Daltro CH, Santana MA, Gregório PB, Acosta AX. Sleep architecture and polysomnographic respiratory profile of children and adolescents with cystic fibrosis. J Pediatr (Rio J) 2011;87(1):63–69. doi: 10.2223/JPED.2055. [DOI] [PubMed] [Google Scholar]
- 29.Liou TG, Elkin EP, Pasta DJ, Jacobs JR, Konstan MW, Morgan WJ, et al. Year-to-year changes in lung function in individuals with cystic fibrosis. J Cyst Fibros. 2010;9(4):250–256. doi: 10.1016/j.jcf.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urquhart DS, Montgomery H, Jaffé A. Assessment of hypoxia in children with cystic fibrosis. Arch Dis Child. 2005;90(11):1138–1143. doi: 10.1136/adc.2005.071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knudson RJ, Lebowitz MD, Holberg GJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725–724. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 32.Polgar G. Pulmonary function tests in children. J Pediatr. 1979;95(1):168–170. [PubMed] [Google Scholar]
- 33.Aurora RN, Zak RS, Karippot A, Lamm CI, Morgenthaler TI, Auerbach SH, et al. American Academy of Sleep Medicine Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34(3):379–388. doi: 10.1093/sleep/34.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreira G, Haddad F, Bittencourt L. Recomendações para o diagnóstico e tratamento da síndrome da apneia obstrutiva do sono na criança e adolescente. São Paulo: Estação Brasil; 2013. [Google Scholar]
- 35.Ramos RT, Salles C, Gregório PB, Barros AT, Santana A, Araújo-Filho JB, et al. Evaluation of the upper airway in children and adolescents with cystic fibrosis and obstructive sleep apnea syndrome. Int J Pediatr Otorhinolaryngol. 2009;73(12):1780–1785. doi: 10.1016/j.ijporl.2009.09.037. [DOI] [PubMed] [Google Scholar]