Abstract
Obstructive Sleep Apnea is a common respiratory disorder characterized by recurrent nocturnal episodes of normal breathing interruption due to upper airway total or partial collapse. Obstructive sleep apnea and cardiovascular diseases has similar risk factors, but the first is also a predisposing factor for cardiovascular pathologies independently of individuals demographic characteristics or risk markers. Heart rate variability is a non-invasive method to evaluate the regulation of autonomic nervous system and its a promising marker for health and disease, such as cardiovascular and respiratory diseases. The aim was to review whether heart rate variability is altered in patients with obstructive sleep apnea. We searched in five databases, including BIREME, Cochrane, Scholar Google, MEDLINE/PubMed and Periodics CAPES, and reference lists were also searched. Only cross-sectional studies comparing the heart rate variability of obstructive sleep patients with controls were included. Two authors independently extracted data and assessed trial quality. Twelve studies (513 participants with obstructive sleep apnea and 340 controls) met the inclusion criteria. This review evidence that adults with obstructive sleep apnea may demonstrate diminished vagal tone and higher sympathetic responsiveness.
Keywords: Autonomic Nervous System, Heart Rate, Sleep Apnea, Obstructive
INTRODUCTION
Obstructive sleep apnea (OSA) is a common respiratory disorder characterized by the presence of 15 or more obstructive episodes per hour of sleep or, at least, five obstructive events added to clinical features as sleep fragmentation, hypoxemia, hypercapnia and sympathetic hyperactivity¹. In adults, OSA prevalence estimates of 3-7%, differing between genders2. Approximately 4% of men would meet the criteria for OSA diagnosis, such as apnea-hyperpnoea index (AHI ≥ 5) and daytime hyper somnolence, while women only 2%³. Recurrent events of normal breathing interruption due to upper airway total or partial collapse during sleep diminish life quality of OSA patients4,5.
Hypoxia and hypercapnia during recurrent obstructive episodes are suggested as main cause of altered autonomic nervous system (ANS) in OSA condition6, once autonomic regulation relies on baroreceptors and quimioreceptors, which can perceive biochemical modifications in PCO2 and pH7. During obstructive events, those hypoxia and hypercapnia episodes can modulate ANS from OSA patients by increasing sympathetic tonus and responsiveness, which can contribute for sympathovagal imbalance and, in consequence, for dysautonomia8.
To evaluate ANS integrity in those patients, heart rate variability (HRV) can be used. HRV is non-invasive method that represents beat-to-beat oscillations and it's considered a measure of neurocardiac function that reflects ANS dynamics9. Lower HRV is observed in several cardiovascular and respiratory diseases such as hypertension and chronic obstructive pulmonary disease10,11, which demonstrates abnormal adaptability of ANS in those patients. Since OSA can altered ANS function by biochemical changes, this study aimed to investigate in the adult population and non-hospital based whether heart rate variability is altered in patients with obstructive sleep apnea.
METHODS
This systematic review was performed according to the PRISMA12 statement to identify all relevant publications reporting patients with obstructive sleep apnea and measures of HRV before 2018. This review was also registered at PROSPERO (International prospective register of systematic review) under CRD42019121413.
Criteria for considering studies for this review
Types of studies: only case-control studies that compared heart rate variability in patients with obstructive sleep apnea and healthy controls at rest, either awake or sleeping.
Types of participants: adult individuals and non-hospital based with obstructive sleep apnea, without any surgical intervention for obstructive sleep apnea.
Types of interventions: there were no interventions, only observational studies were accepted at this review.
Types of outcomes measures: cardiac measures including evaluation of heart rate variability.
Primary outcomes: altered linear and non-linear parameters of heart rate variability indicating autonomic dysfunction/dysautonomia.
Secondary outcomes: altered respiratory measures, such as respiratory frequency.
Search methods for identification of studies
Five electronic databases were searched (last search date: January 19, 2018) including BIREME, MEDLINE, Periódicos CAPES, The Cochrane Library and Scholar Google. To ensure that all potential articles were included, an extensive set of search terms was used for describe the heart rate variability measure (eg, electrocardiography, arterial pulse, heart rate, heart rate variability, hrv, autonomic nervous system, parasympathetic nervous system, sympathetic nervous system, vagus nerve, cardio-autonomic) combined with obstructive sleep apnea terms (eg, apnea, obstructive sleep, osahs, obstructive sleep apnea, sleep apnea syndrome, sleep apnea hypopnea syndrome, sleep apnea syndrome, obstructive syndrome, sleep apnea obstructive syndrome, upper airway resistance sleep apnea, upper airway resistance sleep apnea syndrome).
There were no considerations for date of publication, but the limitation for language was English, Spanish and Portuguese because of lack available resources for translation. The search outputs were managed using EndNote Web. In the search string, the results were limited to all articles published up to January 2018.
Data collection and analysis
Selection of studies
Two authors examined the reference lists of identified articles and selected independently the potentially relevant studies for full text reading. At the full text reading phase, the reviewers also independently evaluate the studies based on eligibility criteria of the population, study design and outcomes. There was no disagreement between the two authors at inclusion or exclusion of studies. Any disagreement would be solved by a third reviewer.
To be included in this review, the studies had to evaluate HRV in adults with obstructive sleep apnea and compare them with healthy controls. Exclusion criteria were:
a. Cardiac measures without heart rate variability;
b. Performed research on unconscious participants (eg, anesthetized participants);
c. Sample size < 10 participants for each group; and
d. HRV measures were performed only after an intervention.
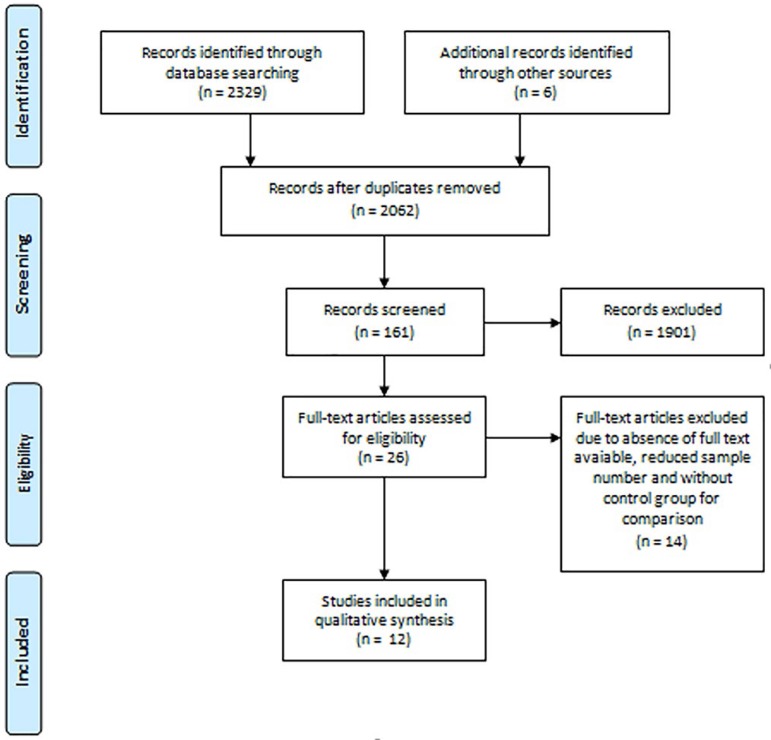
A flowchart of the selection process is included as Figure 1.
Figure 1.
Flowchart of study selection. n=number.
Data extraction and management
Publications reporting survey data at the same location and period were carefully examined to avoid duplicate information. Data was independently extracted from each article. The variables extracted were: (a) country where the study was performed; (b) inclusion criteria for OSA; (c) sample characteristics; (d) HRV measurement data. Data extracted from the studies were presented at Table 1 and Table 2. The first one show the variables extracted from the participants and the last one demonstrate the type of HRV measurement and respective results.
Table 1.
Evidence table for the included studies.
| Study | Country | Inclusion criteria | Sleep Apnea Severity Classification | Associated comorbidities and medications taken |
|---|---|---|---|---|
| Noda et al., 199814 | Japan | Only males with PSG findings of OSA | Obesity and hypertension without cardiovascular and respiratory complications. | |
| Severe OSA: AHI > 20 | ||||
| Wiklund et al., 200017 | Sweden | Adults with snoring and excessive daytime sleepiness who underwent PSG | AHI > 5 in combination with snoring and excessive daytime sleepiness | Hypertension being treated with diuretic and/or ACE inhibitor |
| Aydin et al., 200418 | USA | Newly diagnosed male patients | Free of any other known diseases and receiving no medication | |
| Severe OSA: AHI > 20 | ||||
| Wakai et al., 200416 | Japan | Adults with sleep disturbance who underwent PSG | NR | |
| Severe OSA: AHI > 30 | ||||
| Chrysostomakis et al., 200619 | Greece | Adults with documented moderate or severe OSA | AHI per hour: 58 ± 24 | NR, but all co-morbidities known to affect HRV were excluded and on currently medication with cardioactive drugs, hypnotics or drugs affecting sleep |
| Coruzzi et al., 200620 | Italy | Adults with OSA symptoms for diagnostic PSG | AHI per hour: 18.2 ± 2 | Free of any other known diseases and receiving no medication |
| Aytemir et al., 200721 | Turkey | Consecutive patients referred to laboratory for clinically suspected OSA | Hypertension and use of ACE inhibitor and CCB | |
| OSA: AHI > 5 | ||||
| Zhu et al., 201222 | France | Adults with snoring or any clinical suspicion of OSA who underwent PSG | Free of cardiac arrhythmia or atrioventricular conduction disorder on Holter recording. Medications were not reported. | |
| OSA: AHI > 30/h | ||||
| Chang et al., 201324 | Korea | Untreated male patients with severe OSA | Free of any other known diseases and receiving no psychotropic medications | |
| All patients had severe OSA: > 30 | ||||
| Kim et al., 201523 | Korea | Males with OSA from a retrospective review of patients who undergone PSG | OSA: AHI > 15 | Free of any other known diseases and receiving no medications |
| Palma et al., 201525 | Spain | Consecutive patients recruited from Sleep Unity undergoing diagnostic PSG | Free of any other known diseases and receiving no medications | |
| Moderate OSA, AHI per hour: 26.6 ± 1.8 | ||||
| Xie et al., 201715 | China | Patients with OSA from a retrospective review of those who undergone PSG | Hypertension, diabetes mellitus, hepatopathy, smoking and drinking history and cardiovascular disease manifestations were included | |
| Severe OSA: AHI > 30 |
ACE=angiotensin-converting enzyme; AHI=apnea-hypopnea index; CCB=calcium channel blocker; HRV=heart rate variability; OSA=obstructive sleep apnea; PSG=polysomnography.
Table 2.
Sample characteristics from included studies.
| Study | Obstructive sleep apnea | Controls | ||||
|---|---|---|---|---|---|---|
| Number | Age | Gender | Number | Age | Gender | |
| Noda et al., 199814 ╡ | 18 | 55.9 (43 - 76) | 18 ♂ | 10 | 53.2 (45 - 65) | 10 ♂ |
| Wiklund et al., 200017 | 51 | 52 (30 - 75) | 12 ♀, 39 ♂ | 66 | 52 (30 - 76) | 32 ♀, 34 ♂ |
| Aydin et al., 200418┼ | 36 | NA | 36 ♂ | 24 | 43.58 + 7.6 | 24 ♂ |
| Wakai et al., 200416¥ | 36 | NA | NA | 19 | 53.2 + 11.6 | 10 ♀, 9 ♂ |
| Chrysostomakis et al., 200619 ╧ | 31 | 49.2 ± 7.6 | 8 ♀, 18 ♂ | 19 | 51.6 + 9.6 | 8 ♀,11 ♂ |
| Coruzzi et al., 200620 | 10 | 48 ± 10 | 4 ♀, 6 ♂ | 10 | 42 + 8 | 5 ♀, 5 ♂ |
| Aytemir et al., 200721 | 45 | 51 ± 9 | 11 ♀, 34 ♂ | 24 | 50 + 9 | 5 ♀, 19 ♂ |
| Zhu et al., 201222 | 23 | 45 ± 8 | 8 ♀, 15 ♂ | 23 | 45 + 15 | 9 ♀, 14 ♂ |
| Chang et al., 201324 | 13 | 49.8 ± 7 | 13 ♂ | 13 | 46 + 9.4 | 13 ♂ |
| Kim et al., 201523 | 83 | 40.43 ± 9.92 | 83 ♂ | 81 | 38.69 + 10.03 | 81 ♂ |
| Palma et al., 201525╫ | 30 | NA | NA | 20 | 51.2 + 11.8 | 5 ♀, 15 ♂ |
| Xie et al., 201715╤ | 137 | NA | NA | 31 | 48 + 13.24 | 4 ♀, 27 ♂ |
Data are reported as mean + SD (range).
Recruited patients allocated in two groups: severe OSAS (n=10) and mild OSAS (n=8).
Recruited patients allocated in two groups: severe OSAS (n=19; age 44.15 ± 8.3) and mild OSAS (n=17, age 47.17 ± 9.4).
OSA patients divided in two groups: severe OSAS (n=19; age 47.3 ± 13.3, gender 1 ♀, 18 ♂) and mild OSAS (n=17, age 54.8 ± 8.8, gender 2 ♀, 15 ♂).
OSA patients had different severity OSA classification: severe OSA (n=21) and mild OSA (n=5).
Recruited patients allocated in two groups: moderate OSA (n=16; age 51.8 ± 13.4, gender 3 ♀, 13 ♂) and severe OSA (n=14; age 52.3 ± 11.9, gender 3 ♀, 11 ♂).
Recruited patients allocated in two groups: severe OSA (n=82; age 50.6 ± 12.28, gender 14 ♀, 68 ♂) and mild-moderate OSA (n=55; age 50.57 ± 12.02, gender 12 ♀, 43 ♂).
♀, female; ♂, male; NA, not applicable; NR, not reported.
Assessment of risk of bias in included studies
The Newcastle-Ottawa Quality Assessment Scale for Cross-sectional studies13 was used for the assessment of bias. This scale includes three domains: selection, comparability and exposure. The assessment strategy of cross-sectional studies relies on the number of stars at each topic, so that selection can obtain 5 stars at maximum; comparability, 2 stars; and outcome, 3 stars.
RESULTS
Description of studies
First, the research identified a total of 2,335 studies. Of this 2,335, 273 studies were removed as duplicates and after title and abstract reading, 26 studies were selected, as shown at Figure 1. After full text reading, 12 studies were included at this review. Excluded studies were due to absence of full text available, control group for comparison and reduced sample number.
Studies characteristics
From literature research, twelve cross-sectional studies were included, including a sample size of 513 OSA patients with different severity levels and 340 controls for comparison. The earliest study was from 199814 and the latest, 201715. Those studies were from several different countries, such as Japan14,16, Sweden17, USA18, Greece19, Italy20, Turkey21, France22, Korea23,24, Spain25 and China15. Six of them had controlled for additional factors such as medications intake and comorbidities. A summary of the included studies is provided in Table 1. Among OSA patients and controls, the majority was male, aging over than 30 years old. Sample characteristics are shown in Table 2. Most of them selected 24-h Holter as method of recording14,16,18,18,21,22, but electrocardiogram (ECG)17,20,24 and polysomnography (PSG)15,23,25 recording were also used. The majority of studies used only linear methods including time and frequency domain14-23,25, but one included non-linear measures24. No secondary expected outcomes were evaluated. Methodological features and HRV findings are shown in Table 3.
Table 3.
Methods of HRV measurement and obstructive sleep apnea findings.
| Study | Condition of recording and data lengh for analysis | Derived HRV measures | HRV and OSA findings |
|---|---|---|---|
| Noda et al., 199814 | 24-h Holter recordings | LF (0.04-0.15 Hz), HF (0.15-0.40 Hz), LF/HF | LF/HF ratio was elevated during sleep and daytime in patients with severe OSAS compared with patients with mild OSA and controls |
| Wiklund et al., 200017 | 10 min ECG recording at supine position a day after the sleep recording | PMF (0.04-0.15 Hz), PHF(0.15-0.40 Hz), PTOT | Decreased high-frequency component in supine position in OSAS |
| Aydin et al., 200418 | 24-h Holter recordings | Total power (0-0.4 Hz), ULF (0-0.0033 Hz), VLF (0.0033-0.04 Hz), LF (0.04-0.15 Hz), HF (0.15-0.40 Hz), LF/HF, SDNN, SDANN, RMSSD | SDNN and SDANN were lower in both mild and severe OSAS while RMSSD values were lower only in severe OSAS in comparison to controls. Total power, ULF, VLF, LF and LF/HF values of both groups of OSAS were higher than controls, but HF values were lower |
| Wakai et al., 200416 | 24-h Holter recordings | ULF (0.0001-0.003 Hz), VLF (0.003-0.04 Hz), LF (0.04-0.15 Hz), HF (0.15-0.40 Hz) | VLF and LF during sleep was higher in severe OSA patients than mild OSAS and controls |
| Chrysostomakis et al., 200619 | 24-h Holter recordings | NN, SDNN, SD, PNN50, RMSSD, SDANN | PNN50 and RMSSD were higher at night in patients with severe and moderate OSA |
| Coruzzi et al., 200620 | 20 min ECG recording at supine position | RRI, LF (0.04-0.14 Hz), HF (0.15-0.50 Hz) | RRI, RRI variation and HF were higher in controls than OSA patients but LF and LF/HF ration were lower |
| Aytemir et al., 200721 | 24-h Holter recordings | Total power, VLF (0-0.04 Hz), LF (0.04-0.15 Hz), HF (0.15-0.40 Hz), LF/HF, SDNN, RMSSD, pNN50 | At day time, SDNN was higher in control than OSA patients. During night time, RMSSD and 24h HFnu were higher, while 24h LF and LF/HF ratio were lower in controls than OSA patients |
| Zhu et al., 201222 | 24-h Holter recordings | VLF (0.003-0.04 Hz), LF (0.04-0.15 Hz), LFnu, HF (0.15-0.40 Hz), HFnu, LF/HF, SDNN, RMSSD, pNN50 | Patients with severe OSA exhibited a shorter mean NN overnight |
| Chang et al., 201324 | 15 min ECG recording in sitting position | LF (0.04-0.15 Hz), HF (0.15-0.40 Hz), LF/HF, RMSSD, SampEnRR | HF and SampEnRR were higher among controls, while LF and LF/HF were higher in OSA patients |
| Kim et al., 201523 | PSG recordings | TP, VLF (<0.04 Hz), LF (0.04-0.15 Hz), LFnu, HF (0.15-0.40 Hz), HFnu, LF/HF, SDNN, SDNNi, RMSSD, pNN50, TINN, HRVtri | All frequency domain parameters, except HF which was decreased, were increased in OSA patients. Among time domain parameters, all parameters were also increased in OSA group |
| Palma et al., 201525 | PSG recordings | LF (0.04-0.15 Hz), HF (0.15-0.40 Hz), LF/HF | OSA group during sleep exhibit higher LF and lower HF modulations |
| Xie et al., 201715 | PSG recordings | LF (0.04-0.15 Hz), HF (0.15-0.40 Hz), SDNN, RMSSD, pNN50 | SDNN and HF were higher in controls while LF/HF was lower in comparison to OSA group |
ECG=electrocardiography; HRV=heart rate variability; HRVtri=heart rate variability triangular index; HF=high frequency; HFnu=normalized units of high frequency component; LF=low frequency; LFnu=normalized units of low frequency component; LF/HF=ratio of low frequency and high frequency; OSA=obstructive sleep apnea; PHF=spectral power of the high-frequency component; PMF=spectral power of the mid-frequency component; pNN50=percentage of adjacent NN intervals; PTOT=variance of total spectral power; RMSSD=root mean squared successive difference; RRI=mean R-R interval; SampEnRR=sample entropy of R-R intervals; SDANN=stardard deviation of 5-min average NN intervals; SDNN=standard deviation of NN interval; TINN=triangular interpolation of NN intervals; T p = total power; ULF=ultralow frequency; VLF=very low frequency.
Risk of bias analysis
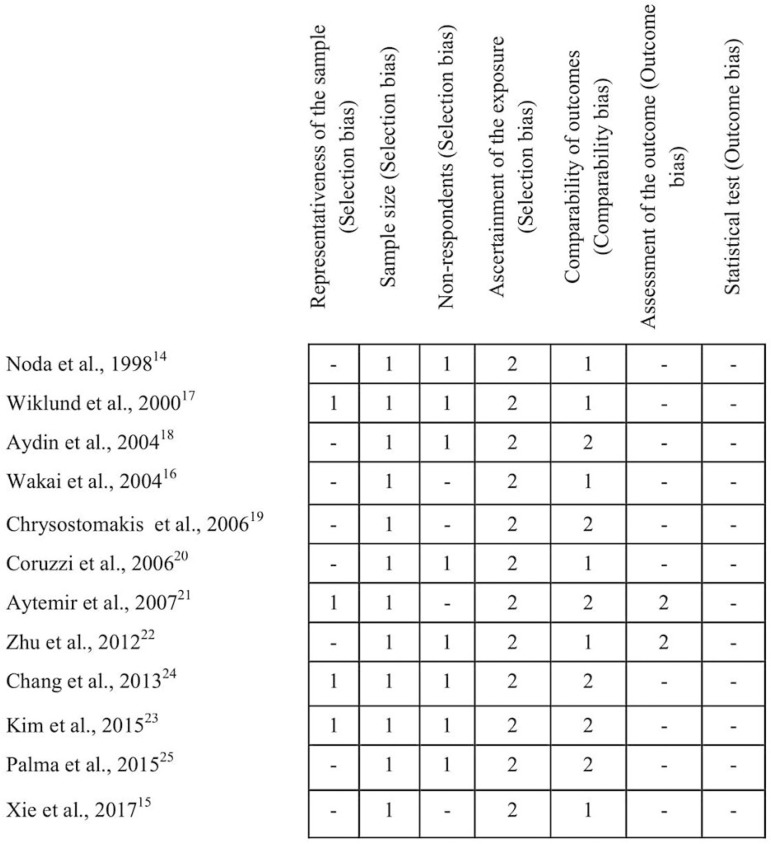
At selection criteria, ten studies were adequate14,17-25 while two had higher risk of bias15,16 (Table 4). At comparability score, six articles received the maximum score18,19,21,23-25, which corresponds to control for the most important factor and also any additional factor, whereas the others didn't control for any additional factor14-17,20,22. Outcome assessment score evidenced that only two studies had an independent blind assessment protocol21,22, but all studies researched had issues with the statistical analysis. The description of quality appraisal of the selected studies is shown in Figure 2.
Table 4.
Methodological quality of included studies determined by Newcastle-Ottawa scale.
| Study | Selection | Comparability | Outcomes | Total |
|---|---|---|---|---|
| Noda et al., 199814 | 4 | 1 | - | 5 |
| Wiklund et al., 200017 | 5 | 1 | - | 6 |
| Aydin et al., 200418 | 4 | 2 | - | 6 |
| Wakai et al., 200416 | 3 | 1 | - | 4 |
| Chrysostomakis et al., 200619 | 3 | 2 | - | 5 |
| Coruzzi et al., 200620 | 4 | 1 | - | 5 |
| Aytemir et al., 200721 | 4 | 2 | 2 | 8 |
| Zhu et al., 201222 | 4 | 1 | 2 | 7 |
| Chang et al., 201324 | 5 | 2 | - | 7 |
| Kim et al., 201523 | 5 | 2 | - | 7 |
| Palma et al., 201525 | 4 | 2 | - | 6 |
| Xie et al., 201715 | 3 | 1 | - | 4 |
Assessment strategy: selection (max. 5 stars), comparability (max. 2 stars) and outcome (max. 3 stars). Range 0-10 stars.
Figure 2.
Summary of risk of bias assessment: authors judgments of each item of Newcastle-Ottawa scale for the included studies.
Heart rate variability parameters
HRV analysis comprehends linear methods and non-linear methods. The linear methods are divided in two domains: time and frequency. Variables of time domain provide information of time distribution over R-R intervals and frequency domain about power distribution26. Non-linear methods of HRV analysis are less explored than the linear methods, but they can provide entropy information, which is relevant in complex organisms. Six studies choose only one linear method: time domain19 and frequency domain14,16,17,20,25. Both time and frequency domain measures were used in five studies15,18,21,22,23. The last one study used time and frequency domain and also non-linear analysis24.
Frequency domain variables
Ultra low frequency and very low frequency
ULF and VLF components are less common in scientific literature due to lack of information in which mechanisms are involved in this phenomenon. Yet, two studies evidenced that OSA patients (582.7718; 17415.34 ± 14838.2823) have higher VLF component than controls (510.2118; 11827.12 ± 9464.0623; p < 0.05). One of them also demonstrates that ULF is lower in controls (119.05) than OSA (165.24; p < 0.05)18.
Low-frequency HRV
LF values are derived from both sympathetic and vagal activity on the heart, but with sympathetic predominance27. Six studies demonstrated that controls had significant (p < 0.05) lower LF than OSA individuals 16,18,21,23-25 while four14,15,20,22 didn't have any significant results. However, one study17 showed different results, evidencing that OSA group (2.58 ± 0.65) had significant (p < 0.01) lower LF values than control (2.96 ± 0.53). Two studies16,25 had no numerical data, only result description.
High-frequency HRV
HF component in HRV analysis represents vagal cardiac modulation27, when increased is a healthy predictor. In five studies17,18,20,24,25, OSA patients demonstrates lower HF values than controls, which indicates lesser parasympathetic predominance and a risk condition for cardiovascular events. In contrary, only one study21 indicate that OSA patients had higher HF values (13 ± 5) than controls (8 ± 4, p = 0.001) on 24h analysis. Two studies divided OSA patients in two groups mild and severe OSA for comparison with controls, but had discrepant results14,18. Both studies corroborate that severe OSA patients had significant (p < 0.0514; p < 0.0118) lower HF (3.80 ± 0.5714; 332.5018) values than control group (4.50 ± 0.5814; 351.4718). Yet, in comparison to mild OSA and controls, one study18 evidenced that patients also had lower HF than controls (341.12; p < 0.01), while the other14 showed the opposite (4.51 ± 0.84; p < 0.05). One study25 did not present any numerical data, only p-values, but affirmed that OSA patients had lower values of HF components. Four15,16,22,23 other studies had non-significant results for this variable.
Low-frequency to high-frequency ratio heart rate variability
LF/HF ratio are suggested as a sympathovagal balance index, in which higher values indicates sympathetic predominance and ANS imbalance27. Seven studies evidenced that HF/LF was higher in OSA patients and controls14,15,18,21,23-25. Two studies divided OSA patients in two groups mild and severe OSA for comparison with controls14,18. In mild OSA, one study14 evidenced that control group (1.30 ± 0.09) have higher LF/HF ratio than patients (1.28 ± 0.11, p < 0.05), which differ from the other study18 that also shows a significant (p < 0.01) lower LF/HF ratio in control group (1:3) compared to OSA (1:4). Similarly, severe OSA patients had significantly (p < 0.05) higher LF/HF ratio (1.40 ± 0.10) than controls in one study14, while the other study18 results demonstrates lower LF/HF ratio in OSA group (1:4; p < 0.01). One study25 didn't show any absolute values, only p-values, and affirmed that OSA participants had higher HF/LF ratio. One study22 had non-significant results at frequency domain analysis.
Time domain variables
SDNN, SDANN and SDNNi
All of those variables are used to determine global HRV and higher values indicate an adaptable ANS. Seven studies15,18,19,21-24 applied at least one of them for HRV analysis but three19,22,24 of them didn't have any significant result. One study18 divided OSA patients in two groups due to disease severity: mild and severe. Mild OSA patients had both SDNN (124.94) and SDANN (112.29) significantly lower than controls (respectively, 131.05; 126.95, p < 0.05). Identically, severe patients also had diminished SDNN (120.38) and SDANN (108.63) values than control group (p < 0.05). Two studies also corroborates with those results. One study21 showed that, during day time, OSA individuals (89 ± 24) had lower SDNN than controls (110 ± 26, p < 0.01). Xie et al. (2017) also presented evidence in which control individuals (139.7 ± 25.1) had an increased SDNN values than OSA group (125.0 ± 28.4, p = 0.011). As an discrepant result, one study 23 indicated that OSA participants had higher SDNN (99.54 ± 34.34) and SDNNi (74.78 ± 33.78) than controls (respectively, 88.37 ± 31.34, p = 0.031; 62.40 ± 29.11, p = 0.013).
RMSSD and pNN50
Both RMSSD and pNN50 are vagal related variables and higher values correlates with parasympathetic predominance27. Although seven studies15,18,19,21-24 used those variables, only three18,19,21 had significant results in, at least, one of them. During night time recordings, one study21 indicated that RMSSD is significantly ( p = 0.036) higher in controls participants (41 ± 18) than OSA group (32 ± 16). On severe OSA patients, other study18 also corroborates with those findings, exhibiting that OSA (31.54) had lower RMSSD values than controls (44.16, p < 0.05). Yet, the last study had discrepant results. RMSSD was increased in OSA group (54.7 ± 23.1) than healthy participants (44.0 ± 15.9), while pNN50 was diminished in controls (13.8 ± 9.7) than patients (19.5 ± 12.5).
Geometrical measures
RRtri and TINN are calculated from a density histogram construction of normal R-R intervals and express overall variability of RR intervals28. Only one study23 included these geometrical variables, but TINN results had no statistical relevance. Yet, RRtri values indicated that OSA participants (18.46 ± 6.68) was lower than controls (16.28 ± 5.64; p = 0.026), which corroborates with other time domain findings that OSA patients have diminished global HRV and vagal predominance of heart control.
Non-linear method
Entropy variables include approximate entropy (ApEn) and sample entropy (SampEn). ApEn detect the changes occurring in an experimental time series and provides a non-negative number for the series by measuring the degree of irregularity and complexity of a signal while SampEn is a measure of existing disorder in some series29,30. Higher values at ApEn and SampEn demonstrates that this evaluated system is more complex and, consequently, greater ANS adaptability. Only one study included entropy measures for HRV analysis24. This study evidenced that OSA patients (1.32 ± 0.07) have a lower mean value of SampEn than controls (1.59 ± 0.11, p = 0.001), which corroborates with ANS abnormality in those individuals.
DISCUSSION
To our knowledge this is the first systematic review involving HRV and OSA patients. This study aimed to investigate whether OSA patients have autonomic impairment evaluated by HRV analysis, including linear and non-linear methods. Although sympathetic responsiveness is well-known as disease pathophysiology, only a few studies used HRV as ANS evaluation prior to an intervention and also compared to healthy individuals. From those twelve studies included in our review, only two had higher risk of bias that could compromise this review results. Yet, these results seams reliable in both awake and sleep patients.
OSA is a major modifiable risk factor for cardiovascular diseases (CVD)31 and also associated with cardiovascular morbidity and mortality, being a predisposing factor for CVD independently of individual's demographic characteristics or risk markers32. Although, etiopathogenic mechanisms underlying OSA and cardiovascular risk remain unclear, ANS impairment is mostly studied8,33.
Several physiological factors derived from recurrent upper airway obstruction can lead to autonomic dysfunction and OSA cardiovascular comorbidities. Obstructive events increased negative intrathoracic pressure and added to hypoxia cause deleterious hemodynamic consequences, such as sympathetic overactivity, augmented reactive oxygen species (ROS) and vasoactive compensatory mechanisms31-35. Also, duration of obstructive episode and level of oxygen desaturation determine sympathetic activity and blood pressure35.
The majority of studies demonstrate by both time and frequency domain that OSA patients have higher sympathetic components and lower parasympathetic predominance than healthy controls. These results confirm autonomic dysfunction in those patients, but also give another therapeutical perspective. HRV assessment can be used as tool to evaluate efficacy at OSA patients' treatment due its association to autonomic impairment. Furthermore, HRV can be associated with respiratory methods to modulate ANS, such as cardiorespiratory biofeedback36, which may positively benefit those patients.
At frequency domain analysis, VLF and ULF is mainly influenced by circadian HR differences, and cannot be estimated by short term analysis, only by 24-h Holter37. Increased VLF power may be associated with increased sympathetic activity9, which was observed in OSA patients at our review18,23. Both components represent 95% of total power and literature suggests that those carry highest predictive value38, being more strongly associated with overall health status than HF component9. Although physiological correlates of ULF and VLF is still unclear, studies with OSA patients should add those variables for analysis to identify whether those components plays a role in OSA pathophysiology.
The strengths of this systematic review include sample size number, the use of strict methodological inclusion criteria, a quality assessment of included studies and consistent results. However, some studies limitations should be pointed, including reduced number of female participants. From 848 participants, OSA and controls, 692 are male, and some studies only included male subjects. In HRV research, females are less studied for the influence of menstrual cycle on autonomic cardiac modulation39. Although OSA is more frequent in men than women40, further investigation between HRV gender differences in those patients may clear if hormones are also involved in this respiratory obstructive disorder.
Several studies also include participants with associated comorbidities and with medications taken, which can affect and compromise HRV results41, once they can influence ANS integrity. Another limitation includes different data length, device and condition of recording. These differences between studies affect the accuracy of HRV analysis41 and, mainly, the comparison of their results. For example, long 24-hour data collection favors HRV assessment as it involves metabolism and circadian cycle, for example, and it's considered a "gold standard"9.
This analysis will be different from shorter 20 minutes, because all those metabolic factors will not be considered. The inclusion of only observational studies can also be a limiting factor, once intervention studies add more information about cause and effect being helpful to elucidate the relationship between OSA and sympathetic responsiveness. Also, all those selected studies used AHI as sleep apnea severity classification but this index does not correlate completely with cardiovascular risk in adults.
Two cohort studies42,43 evidenced that prediction of cardiovascular outcomes may be independent of AHI. AHI only represents the number of both apnea and hypopnea events in every hour during sleep15, which documents the frequency of those events but not, for example, duration or magnitude of each event. Although some of those selected studies cross-data between AHI and HRV, more studies should be done to clarify if the current hypopnea definition and sleep apnea severity classification still applies as a predictor of cardiovascular risk in OSA patients42.
On another hand, HRV can be used as a morbidity and mortality predictor, especially SDNN that is considered "gold standard" for medical stratification in 24h period of recording44. One review study suggests that HRV can be used to assess clinical progress and also performance gain in athletes, in a pre/post treatment protocol44. In OSA patients, the evaluation of dysautonomia with HRV may be a non-invasive and easy tool for prognostic overview. Finally, Periódicos CAPES database only allows a few search terms, which may influence directly the search, since not all search terms were used.
In summary, this review evidence that adults with OSA may demonstrate diminished vagal tone and higher sympathetic responsiveness, which represents a sympathetic-parasympathetic imbalance and can corroborate with dysautonomia hypothesis for OSA correlation with CVD. Alternative interventions such as cardiovascular biofeedback should be investigated in those patients to improve autonomic balance and, in consequence, ANS associated symptoms. Yet, more studies should be done to evaluate this population, especially women.
REFERENCES
- 1.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, et al. Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 2.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis. 2009;51(4):285–293. doi: 10.1016/j.pcad.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnea. Cochrane Database Syst Rev. 2004;(4):CD004435–CD004435. doi: 10.1002/14651858.CD004435.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Pereira A. Síndrome da apneia obstrutiva do sono: fisiopatologia, epidemiologia, consequências, diagnóstico e tratamento. Arq Med. 2007;21(6):159–173. [Google Scholar]
- 6.Gozal D, Hakim F, Kheirandish-Gozal L. Chemoreceptors, baroreceptors, and autonomic deregulation in children with obstructive sleep apnea. Respir Physiol Neurobiol. 2013;185(1):177–185. doi: 10.1016/j.resp.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper HE, Clutton-Brock TH, Parkes MJ. Contribution of the respiratory rhythm to sinus arrhythmia in normal unanesthetized subjects during positive-pressure mechanical hyperventilation. Am J Physiol Heart Circ Physiol. 2004;286(1):H402–H411. doi: 10.1152/ajpheart.00504.2003. [DOI] [PubMed] [Google Scholar]
- 8.Kheirandish-Gozal L, Bhattacharjee R, Gozal D. Autonomic alterations and endothelial dysfunction in pediatric obstructive sleep apnea. Sleep Med. 2010;11(7):714–720. doi: 10.1016/j.sleep.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front Psychol. 2014;5:1040–1040. doi: 10.3389/fpsyg.2014.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel PA, Diwan JS, Shah CJ, Mehta HB. Study of heart rate variability in hypertensive subjects. Natl J Integr Res Med. 2015;6(1):1–6. [Google Scholar]
- 11.Roque AL, Valenti VE, Massetti T, da Silva TD, Monteiro CB, Oliveira FR, et al. Chronic obstructive pulmonary disease and heart rate variability: a literature update. Int Arch Med. 2014;7:43–43. doi: 10.1186/1755-7682-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. ESH Working Group on CV Risk in Low Resource Settings Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(1):e0147601. doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noda A, Yasuma F, Okada T, Yokota M. Circadian rhythm of autonomic activity in patients with obstructive sleep apnea syndrome. Clin Cardiol. 1998;21(4):271–276. doi: 10.1002/clc.4960210408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie J, Yu W, Wan Z, Han F, Wang Q, Chen R. Correlation Analysis between Obstructive Sleep Apnea Syndrome (OSAS) and Heart Rate Variability. Iran J Public Health. 2017;46(11):1502–1511. [PMC free article] [PubMed] [Google Scholar]
- 16.Wakai M, Samejima Y, Goshima K, Yamamoto J. Altered heart rate variability in severe sleep apnea syndrome. Sleep Biol Rhythms. 2004;2(1):87–88. [Google Scholar]
- 17.Wiklund U, Olofsson BO, Franklin K, Blom H, Bjerle P, Niklasson U. Autonomic cardiovascular regulation in patients with obstructive sleep apnoea: a study based on spectral analysis of heart rate variability. Clin Physiol. 2000;20(3):234–241. doi: 10.1046/j.1365-2281.2000.00251.x. [DOI] [PubMed] [Google Scholar]
- 18.Aydin M, Altin R, Ozeren A, Kart L, Bilge M, Unalacak M. Cardiac autonomic activity in obstructive sleep apnea. Tex Heart Inst J. 2004;31(2):132–136. [PMC free article] [PubMed] [Google Scholar]
- 19.Chrysostomakis SI, Simantirakis EN, Schiza SE, Karalis IK, Klapsinos NC, Siafakas NM, et al. Continuous positive airway pressure therapy lowers vagal tone in patients with obstructive sleep apnoea-hypopnoea syndrome. Hellenic J Cardiol. 2006;47(1):13–20. [PubMed] [Google Scholar]
- 20.Coruzzi P, Gualerzi M, Bernkopf E, Brambilla L, Brambilla V, Broia V, et al. Autonomic cardiac modulation in obstructive sleep apnea: effect of an oral jaw-positioning appliance. Chest. 2006;130(5):1362–1368. doi: 10.1378/chest.130.5.1362. [DOI] [PubMed] [Google Scholar]
- 21.Aytemir K, Deniz A, Yavuz B, Ugur Demir A, Sahiner L, Ciftci O, et al. Increased myocardial vulnerability and autonomic nervous system imbalance in obstructive sleep apnea syndrome. Respir Med. 2007;101(6):1277–1282. doi: 10.1016/j.rmed.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Zhu K, Chemla D, Roisman G, Mao W, Bazizi S, Lefevre A, et al. Overnight heart rate variability in patients with obstructive sleep apnoea: a time and frequency domain study. Clin Exp Pharmacol Physiol. 2012;39(11):901–908. doi: 10.1111/1440-1681.12012. [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Kim SY, Park DY, Wu HW, Hwang GS, Kim HJ. Clinical Implication of Heart Rate Variability in Obstructive Sleep Apnea Syndrome Patients. J Craniofac Surg. 2015;26(5):1592–1595. doi: 10.1097/SCS.0000000000001782. [DOI] [PubMed] [Google Scholar]
- 24.Chang JS, Lee SD, Ju G, Kim JW, Ha K, Yoon IY. Enhanced cardiorespiratory coupling in patients with obstructive sleep apnea following continuous positive airway pressure treatment. Sleep Med. 2013;14(11):1132–1138. doi: 10.1016/j.sleep.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Palma JA, Iriarte J, Fernandez S, Alegre M, Valencia M, Artieda J, et al. Long-term continuous positive airway pressure therapy improves cardiac autonomic tone during sleep in patients with obstructive sleep apnea. Clin Auton Res. 2015;25(4):225–232. doi: 10.1007/s10286-015-0297-7. [DOI] [PubMed] [Google Scholar]
- 26.Tracy LM, Ioannou L, Baker KS, Gibson SJ, Georgiou-Karistianis N, Giummarra MJ. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain. 2016;157(1):7–29. doi: 10.1097/j.pain.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 27.Vanderlei LC, Pastre CM, Hoshi RA, Carvalho TD, Godoy MF. Basic notions of heart rate variability and its clinical applicability. Rev Bras Cir Cardiovasc. 2009;24(2):205–217. doi: 10.1590/s0102-76382009000200018. [DOI] [PubMed] [Google Scholar]
- 28.Dias de Carvalho T, Marcelo Pastre C, Claudino Rossi R, de Abreu LC, Valenti VE, Marques Vanderlei LC. Geometric index of heart rate variability in chronic obstructive pulmonary disease. Rev Port Pneumol. 2011;17(6):260–265. doi: 10.1016/j.rppneu.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Ferreira MT, Messias M, Vanderlei LCM, Pastre CM. Caracterização do Comportamento Caótico da Variabilidade da Frequência Cardíaca (VFC) em Jovens Saudáveis. Tend Mat Apl Comput. 2010;11(2):141–150. [Google Scholar]
- 30.Madeiro JPV, Seisdedos CRV, Cortez PC, Marques JAL. Análise de desempenho da entropia aproximada (ApEn) na análise da variabilidade da frequência cardíaca (VFC); V Latin American Congress on Biomedical Engineering CLAIB; 2011; Habana, Cuba: May-21. 2011. pp. 1182–1185. [Google Scholar]
- 31.Khayat R, Pleister A. Consequences of Obstructive Sleep Apnea: Cardiovascular Risk of Obstructive Sleep Apnea and Whether Continuous Positive Airway Pressure Reduces that Risk. Sleep Med Clin. 2016;11(3):273–286. doi: 10.1016/j.jsmc.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Jean-Louis G, Zizi F, Clark LT, Brown CD, McFarlane SI. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med. 2008;4(3):261–272. [PMC free article] [PubMed] [Google Scholar]
- 33.Kufoy E, Palma JA, Lopez J, Alegre M, Urrestarazu E, Artieda J, et al. Changes in the heart rate variability in patients with obstructive sleep apnea and its response to acute CPAP treatment. PLoS One. 2012;7(3):e33769. doi: 10.1371/journal.pone.0033769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton GS, Solin P, Naughton MT. Obstructive sleep apnoea and cardiovascular disease. Intern Med J. 2004;34(7):420–426. doi: 10.1111/j.1445-5994.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 35.Narkiewicz K, Somers VK. Cardiovascular variability characteristics in obstructive sleep apnea. Auton Neurosci. 2001;90(1-2):89–94. doi: 10.1016/S1566-0702(01)00272-7. [DOI] [PubMed] [Google Scholar]
- 36.Gomes JS, Coghi MF, Coghi PF. Cardiovascular biofeedback and its applications: review of literature. Av Psicol Latinoam. 2014;32(2):199–216. [Google Scholar]
- 37.Bauer A, Camm AJ, Cerutti S, Guzik P, Huikuri H, Lombardi F, et al. Reference values of heart rate variability. Heart Rhythm. 2017;14(2):302–303. doi: 10.1016/j.hrthm.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 38.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 39.Tada Y, Yoshizaki T, Tomata Y, Yokoyama Y, Sunami A, Hida A, et al. The Impact of Menstrual Cycle Phases on Cardiac Autonomic Nervous System Activity: An Observational Study Considering Lifestyle (Diet, Physical Activity, and Sleep) among Female College Students. J Nutr Sci Vitaminol (Tokyo) 2017;63(4):249–255. doi: 10.3177/jnsv.63.249. [DOI] [PubMed] [Google Scholar]
- 40.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev. 2008;12(6):481–496. doi: 10.1016/j.smrv.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taralov ZZ, Terziyski KV, Kostianev SS. Heart Rate Variability as a Method for Assessment of the Autonomic Nervous System and the Adaptations to Different Physiological and Pathological Conditions. Folia Med (Plovdiv) 2015;57(3-4):173–180. doi: 10.1515/folmed-2015-0036. [DOI] [PubMed] [Google Scholar]
- 42.Campos-Rodriguez F, Martínez-García MA, Reyes-Nuñez N, Selma-Ferrer MJ, Punjabi NM, Farre R. Impact of different hypopnea definitions on obstructive sleep apnea severity and cardiovascular mortality risk in women and elderly individuals. Sleep Med. 2016;27(28):54–58. doi: 10.1016/j.sleep.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Sankari A, Finn LA, Maresh S, Hamdon MS, Al-kubaisi G, Badr MS, et al. A new marker of cardiovascular disease in patients with sleep-disordered breathing: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2016;193:A2523–A2523. [Google Scholar]
- 44.Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health. 2017;5:258–258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]