Periodontal disease (PD), an inflammatory condition impacting the supporting structures of teeth, is associated with a higher risk of cardiovascular disease (CVD). PD associates with atherosclerotic inflammation (1), which in-turn associates with increased CVD risk (2). However, the mechanisms by which PD may cause atherosclerotic inflammation is not established; greater insights into those mechanisms are needed.
Several mechanisms linking PD to atherosclerosis have been hypothesized. Bacterial by-products released from inflamed periodontal tissues prompt cytokine release from inflammatory cells, which in-turn can stimulate atherosclerotic disease via heightened atherosclerotic inflammation. Indirect mechanisms are also suspected. In that regard, the bone marrow is increasingly recognized as a key participant in atherosclerosis, representing a critical source of inflammatory cells that potentiates atherosclerotic inflammation via a “hematopoietic-arterial axis” (2,3). Because cytokines and bacterial by-products increase marrow myelopoiesis and glycolysis (4), we hypothesized that PD may potentiate this hematopoietic-arterial axis.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) imaging can non-invasively measure PDinflammation (1,5). Additionally, 18F-FDG-PET/CT provides a validated measure of hematopoietic tissue activity, and arterial inflammation (1,2), thereby making it an ideal tool for investigating a possible biological relationship that may exist between these tissues. Accordingly, we used 18F-FDG PET/CT imaging in 2 cohorts to simultaneously quantify these tissue activities (1,2). The first cohort was a larger retrospective cohort composed of 304 individuals (median age 54.0 [Q1 to Q3: 44.0 to 63.8] years, 41.6% males) who underwent clinical 18F-FDG-PET/CT imaging, largely for cancer screening (12%) or surveillance of prior cancer (88%), and were confirmed not to have active cancer. None were receiving cancer therapeutics nor had a prior history of clinical atherosclerosis. The second cohort was a smaller validation cohort composed of 29 healthy subjects (median age 53.0 [Q1 to Q3: 48.5 to 59.0] years, 100% males) in whom 18F-FDG PET/CT imaging was performed to study relationships between tissue inflammation and circulating inflammatory markers.
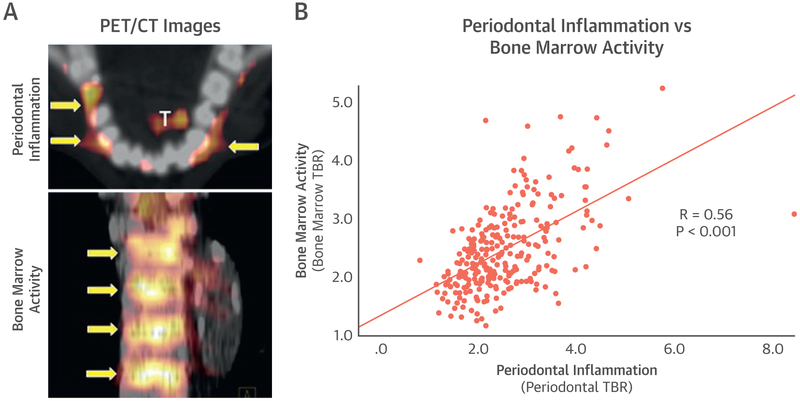
In the retrospective cohort, PDinflammation significantly associated with hematopoietic tissue activity (as bone marrow activity, r = 0.56, p < 0.001) and arterial inflammation (r = 0.56, p < 0.001) (Figure 1). Further, bone marrow activity also associated with arterial inflammation (r = 0.57, p < 0.001). Notably, mediation (path) analysis demonstrated that bone marrow activity significantly mediated the relationship between PDinflammation and arterial inflammation (standardized beta-coefficient: 0.23 [95% confidence interval: 0.15 to 0.33], p < 0.05), accounting for 44% of the total relationship between PDinflammation and arterial inflammation.
FIGURE 1. Periodontal Inflammation and Bone Marrow Activity.
(A) Representative periodontal (top) and bone marrow (bottom) fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) images. Yellow arrows show locations of increased activity. “T” indicates activity in the tongue. (B) The relationship between periodontal inflammation and bone marrow activity (r = 0.56, p < 0.001), as quantified using 18F-FDG-PET/CT. TBR = target to background ratio.
In the separate validation cohort, PDinflammation similarly associated with both bone marrow activity (r = 0.51, p = 0.01) and arterial inflammation (r = 0.49, p = 0.015). Furthermore, PDinflammation associated with C-reactive protein (r = 0.56, p = 0.028). Additionally, bone marrow activity associated with C-reactive protein (r = 0.43, p = 0.030) and several leukocyte measures, including white blood cell count (r = 0.54, p = 0.018) and absolute monocyte count (r = 0.79, p = 0.001), but not red blood cell measures (including the hematocrit [r = 0.20, p = 0.41] or hemoglobin concentration [r = 0.23, p = 0.34]).
To our knowledge, this is the first study to demonstrate a link between PDinflammation, hematopoietic activity, and arterial inflammation. Additionally, we observed strong relationships between hematopoietic activity and inflammatory biomarkers as well as circulating monocytes. Furthermore, mediation analysis provided supportive evidence that the link between PDinflammation and arterial inflammation is substantially mediated by heightened hematopoietic activity.
Hematopoietic tissues have become increasingly recognized as key participants in atherogenic mechanisms (3). Prior human studies have shown a link between bone marrow activity, arterial inflammation, and CVD events (2). Herein, we extend this concept by showing PDinflammation may act at the front-end of this pathway: ↑periodontal inflammation→↑bone marrow activity →↑arterial inflammation →↑CVD risk (Figure 1). Because these findings are based on imaging measures rather than clinical evaluation of PD, future studies should evaluate whether aggressive treatment of clinical PD may reduce activity of the hematopoietic-arterial axis and whether this may attenuate the burden of consequent CVD.
Acknowledgments
The following National Institutes of Health grants provided support for this study: R01HL122177 (Dr. Tawakol), R01HL137913 (Dr. Tawakol), R01HL129856 (Dr. Hsue), T32HL076136 (Dr. Osborne), R01DE02520 (Dr. Van Dyke), and KL2TR002542 (Dr. Osborne). Harvard Catalyst provided statistical support. Outside the submitted work, Dr. Tawakol has received consulting fees for Actelion; and institutional research grants from Actelion and Genentech. Dr. Hsue has received research grants from Merck and Gilead. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Fifer KM, Qadir S, Subramanian S, et al. Positron emission tomography measurement of periodontal 18F-fluorodeoxyglucose uptake is associated with histologically determined carotid plaque inflammation. J Am Coll Cardiol 2011;57:971–6. [DOI] [PubMed] [Google Scholar]
- 2.Tawakol A, Ishai A, Takx RA, et al. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet 2017;389:834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dutta P, Courties G, Wei Y, et al. Myocardial infarction accelerates atherosclerosis. Nature 2012;487:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitroulis I, Ruppova K, Wang B, et al. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 2018;172: 147–161 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kito S, Koga H, Kodama M, et al. Reflection of (1)(8)F-FDG accumulation in the evaluation of the extent of periapical or periodontal inflammation. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;114:e62–9. [DOI] [PubMed] [Google Scholar]