Abstract
Background and Purpose
Bacteria producing New Delhi metallo‐β‐lactamase‐1 (NDM‐1) are an increasing clinical threat. NDM‐1 can inactivate almost all β‐lactams and is not sensitive to any existing β‐lactamase inhibitors. To identify effective inhibitors of the NDM‐1 enzyme and clarify the mechanism of action, a “lead compound” for developing more potent NDM‐1 inhibitors needs to be provided.
Experimental Approach
Natural compounds were tested by enzyme inhibition screening to find potential inhibitors. MIC assays, growth curve assays, and time‐kill assays were conducted to evaluate the in vitro antibacterial activity of pterostilbene and the combination of pterostilbene and meropenem. A murine thigh model and a mouse https://academic.oup.com/jac/article-abstract/54/6/1085/856174 model were used to evaluate the in vivo efficacy of combined therapy. Molecular modelling and a mutational analysis were used to clarify the mechanism of action.
Key Results
Pterostilbene significantly inhibited NDM‐1 hydrolysis activity in enzyme inhibition screening assays and effectively restored the effectiveness of meropenem in vitro with NDM‐expressing isolates in antibacterial activity assays. In addition, the combined therapy effectively reduced the bacterial burden in a murine thigh model and protected mice from https://academic.oup.com/jac/article-abstract/54/6/1085/856174 caused by Klebsiella pneumoniae. By means of molecular dynamics simulation, we observed that pterostilbene localized to the catalytic pocket of NDM‐1, hindering substrate binding to NDM‐1 and reducing NDM‐1 activity.
Conclusions and Implications
These findings indicated that pterostilbene combined with meropenem may offer a new safe and potential “lead compound” for the further development of NDM‐1 inhibitors.
Abbreviations
- AMA
aspergillomarasmine A
- FICI
fractional inhibitory concentration index
- MBL
metallo‐β‐lactamase
- MIC, minimal inhibitory concentration MD
molecular dynamics
- NDM‐1
New Delhi metallo‐β‐lactamase‐1
What is already known
New Delhi metallo‐β‐lactamase (NDM) carbapenemases are key resistance enzymes that inactivate almost all β‐lactams.
Inhibitors for this enzyme are urgently needed.
What this study adds
Pterostilbene, located in the catalytic pocket of NDM‐1, inhibits NDM‐1 activity.
Combined therapy with pterostilbene and meropenem inhibits the virulence of resistant bacteria producing NDM‐1.
What is the clinical significance
Pterostilbene may be a potential “lead compound” for NDM‐1 inhibitor.
This study offers novel treatments against NDM‐1‐producing bacteria by combining pterostilbene with meropenem.
1. INTRODUCTION
β‐lactams are the most important and commonly prescribed class of antibiotics for treating infections caused by Gram‐negative bacteria pathogens. However, the selection pressure caused by frequent use of β‐lactams plays a central role in the emergence and spread of antibiotic resistance. One of the most prevalent causes of bacterial resistance to β‐lactam antibiotics is the expression of β‐lactamase enzymes. By DNA sequence similarity, β‐lactamases can be classified into four classes (A to D). Classes A, C, and D enzymes, which employ a nucleophilic serine moiety to hydrolyse β‐lactams, are serine‐β‐lactamases. Class B enzymes are metallo‐β‐lactamases (MBLs; Bush & Jacoby, 2010).
New Delhi metallo‐β‐lactamase 1 (NDM‐1), which is encoded on a readily transferable plasmid, represents the most critical type of MBL and was first identified in Klebsiella pneumoniae in 2008 (Yong et al., 2009). The NDM‐1 active site includes one or two zinc ions for nucleophilic attack on the β‐lactams (Bush & Jacoby, 2010). The first site is tightly coordinated by residues His120, His122, His189, and a bridging water. The second site is coordinated by Asp124, Cys208, His250, and water molecules (Green, Verma, Owens, Phillips, & Carr, 2011; King & Strynadka, 2011). Of particular note is the rapid dissemination of NDM‐1 around the world and that it is readily detected in a number of pathogens, including members of the order Enterobacterales and the genus Acinetobacter, and Pseudomonas (Albu et al., 2016). Most of all, NDM‐1, which is resistant to all clinical existing β‐lactamase inhibitors, can inactivate nearly all known β‐lactam antibiotics, including the “last resort” antibiotics, the carbapenems. Due to the worldwide transmission and high resistance to β‐lactams in clinical use , NDM‐1 is a global threat to public health care. Thus, identification of potent inhibitors to NDM‐1 is urgently needed.
Polyphenols are known for their beneficial effects on health and disease (Casadesus et al., 2004; Joseph, Shukitt‐Hale, & Casadesus, 2005). One popular polyphenol is http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2681 (Figure 1), which was initially isolated from red sandalwood (Pterocarpus santalinus, Seshadri, 1972) and has been found in blueberries, grapes, and other plants. It is a natural dietary antioxidant and is used in the treatment of diabetes in traditional medicine (Schmidlin et al., 2008). Antioxidant, anticancer, anti‐inflammatory, and neuroprotective activities have already been reported for pterostilbene (Joseph, Fisher, Cheng, Rimando, & Shukitt‐Hale, 2008; Pari & Satheesh, 2006; Rimando & Suh, 2008). Here, we have found that pterostilbene exhibits inhibition of NDM‐1 in an enzyme assay. Pterostilbene‐mediated inhibition of NDM‐1 activity exerted a synergistic effect with meropenem, against NDM‐1 expressing Escherichia coli in both in vitro and in vivo experiments.
Figure 1.
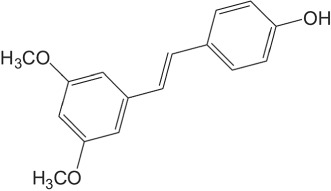
Chemical structure of pterostilbene
2. METHODS
2.1. Protein purification and enzyme inhibition assays
Protein expression and enzyme inhibition assays were performed according to Liao et al. (2016) and Liu et al. (2018), respectively. Assays were read at an absorbance of 492 nm in a 96‐well plate at room temperature by a microplate reader (Tecan Austria GmbH, Grödig, Austria). Plasmids containing the Trp93Ala or Asp124Ala were introduced into pET28a‐NDM‐1 for the expression of these two mutants of NDM‐1. All constructed strains were verified by sequencing.
2.2. Bacterial strains and growth conditions
The NDM‐positive isolates originated from our previous study (Wang et al., 2017). LB broth or LB agar was used for the growth of all the bacteria. In growth curve assays, the indicated concentrations of pterostilbene were added to E. coli ZC‐YN3 or K. pneumoniae QD‐KP2 (two NDM‐1 carbapenemase isolates) for co‐culture with constant shaking (180 rpm) at 37°C. The growth curves were measured by spectrophotometers (UNICO UV‐2600, USA) every 30 min at 600 nm.
2.3. Antibacterial activity assays
The MIC values for E. coli and K. pneumoniae isolates in the presence of pterostilbene, meropenem, and combinations of pterostilbene with meropenem were determined following the guidelines of the Clinical and Laboratory Standards Institute by the broth microdilution method (CLSI, Wayne, PA, USA, 2016). The fractional inhibitory concentration index (FICI) values were calculated to evaluate the combination effect. FICI was calculated as follows: FICI = FICPt + FICMep, where FICPt is the MIC of pterostilbene in the combination/MIC of pterostilbene alone and FICMep is the MIC of meropenem in the combination/MIC of meropenem alone. An FICI ≤ 0.5 was used to define synergy (Odds, 2003). The test results were from five independent experiments. In addition, time‐killing assays were conducted according to Wei's method (Kang et al., 2017) to examine the potent synergistic effect of the combination of pterostilbene with meropenem against E. coli ZC‐YN3, K. pneumoniae QD‐KP2 (NDM‐1), E. coli ZC‐YN5 (NDM‐5), and E. coli E2 (NDM‐9).
2.4. Animal studies
All animal care and experimental procedures were in accordance with the guidelines of the Jilin University Institutional Animal Care Committee. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010) and with the recommendations made by the British Journal of Pharmacology. Female BALB/c mice (6‐ to 8‐week‐old, approximately 20 g, IMSR Cat# JAX:000651, RRID:IMSR_JAX:000651) and C57BL/6 mice (6‐ to 8‐week‐old, approximately 18 g, IMSR Cat# JAX:000664, RRID:IMSR_JAX:000664) were obtained from the Jilin University Experimental Animal Centre.
All mice were housed in a specific pathogen‐free (SPF) facility under a 12‐hr light/12‐hr dark cycle at a constant temperature of 23 ± 2°C and humidity (55%). The mice had free access to standard food and water and were acclimated to the laboratory for 1 week prior to the start of the experiments. In animal studies, all infections, treatments, and data collection were blinded. All infections were performed by one experimenter. Subcutaneous injections were performed by a different experimenter. Tissue or survival data collections were performed by two different experimenters excluding the experimenters doing infections and injections. The statistical analysis was performed by two different experimenters excluding all the four experimenters mentioned above. At the end of the experiments, the animals were humanely killed.
To evaluate the in vivo antibacterial effect of pterostilbene plus meropenem, BALB/c mice were divided randomly into four groups (solvent control, meropenem alone, pterostilbene alone, and pterostilbene + meropenem), infected with E. coli ZC‐YN3 and treated with pterostilbene, meropenem, or a combination of both. Fifty microlitres of bacterial suspension (5 × 106 CFU of E. coli ZC‐YN3) were injected intramuscularly into the posterior thigh muscle. The infected BALB/c mice were treated with meropenem (s.c., 10 mg·kg−1), pterostilbene (s.c., 80 mg·kg−1), a combination of both antibiotic and inhibitor, or solvent as control after infection. All groups of mice were treated with three doses at 8‐hr intervals. After 72 hr, mice were killed, and infected thighs were harvested and homogenized. Organ homogenates were diluted and plated for bacteria enumeration.
A mouse https://academic.oup.com/jac/article-abstract/54/6/1085/856174 model caused by K. pneumoniae QD‐KP2 (NDM‐1) was used to evaluate the protective effect of pterostilbene combined with meropenem against lethal infection. The C57BL/6 mice were divided randomly into four groups (solvent control, meropenem alone, pterostilbene alone, and pterostilbene + meropenem) and infected intranasally with a dose of 1 × 109 CFU of the NDM‐1‐producing K. pneumoniae QD‐KP2 isolate for survival experiments. The infected mice were treated as described for E. coli ZC‐YN3 infection. Mice were monitored until Day 5 post‐infection to calculate the survival rate.
2.5. Molecular modelling
In this work, the initial X‐ray crystal structure of NDM‐1 was obtained from the Protein Data Bank (PDB) with PDB code: 4EXS. AutoDock 4 (AutoDock, RRID:SCR_012746) was used to docking the protein with pterostilbene as the initial structure of pterostilbene/NDM‐1 complex during molecular dynamic (MD) simulation (Hu, Barbault, Maurel, Delamar, & Zhang, 2010; Morris et al., 2009). Further, the molecular dynamic simulation of the pterostilbene/NDM‐1 complex systems was performed, and the detailed processes of computational biology method were described in the previous report (Dong et al., 2013; Niu et al., 2013).
2.6. Binding affinity determination of pterostilbene with proteins
According to Bandyopadhyay et al and Jurasekova et al (Bandyopadhyay, Valder, Huynh, Ren, & Allison, 2002; Jurasekova, Marconi, Sanchez‐Cortes, & Torreggiani, 2009), the binding constants (K A) of pterostilbene with NDM‐1 and two mutants of NDM‐1 were measured using the fluorescence‐quenching method.
2.7. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. Data are presented as the mean ± SD from five independent experiments and were analysed using SPSS 19.0 (SPSS, RRID:SCR_002865). The survival curves were estimated by the Kaplan–Meier method, and the generalized Wilcoxon test was used to compare the differences between these survival curves. The data from bacterial enumeration assays were analysed via a one‐way ANOVA with the Neuman–Keuls post hoc test for multiple comparisons among the groups. Other significant differences were analysed by one‐way ANOVA followed by a Dunnett t‐test . P < .05 was considered statistically significant.
2.8. Materials
Pterostilbene (≥97% pure) was obtained from Sigma‐Aldrich (St. Louis, MO, USA) and dissolved in DMSO (Sigma). Meropenem (≥87% pure) was purchased from the National Institutes for Food and Drug Control (Beijing, China) and was prepared in sterile water. The primers used in site‐directed mutagenesis were Trp93Ala‐F: 5′‐GTCGATACCGCCGCGACCGATGACC‐3′; Trp93Ala‐R: 5′‐GGTCATCGGTCGCGGCGGTATCGAC‐3′; Asp124Ala‐F: 5′‐CTCACGCGCATCAGGCGAAGATGGGCGGTATG‐3′; Asp124Ala‐R: 5′‐CATACCGCCCATCTTCGCCTGATGCGCGTGAG‐3′.
2.9. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org/, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.
3. RESULTS
3.1. Pterostilbene restores susceptibility of E. coli ZC‐YN3 to meropenem
Using an enzyme inhibition assay, we successfully identified a potent inhibitor, pterostilbene. The data showed that pterostilbene exerted a significant dose‐dependent inhibition of the enzyme activity (IC50 = 15.37 μg·ml−1, Figure 2). We further tested the antibacterial activity of pterostilbene alone and the combination of pterostilbene with meropenem against E. coli and K. pneumoniae possessing NDM‐type alleles through the MIC assay. The results of the growth curves indicated that pterostilbene had little effect on E. coli ZC‐YN3 and K. pneumoniae QD‐KP2 growth (Figure 3a,b) but presented synergistic effects with meropenem against NDM‐positive isolates (Table 1). The effects strengthened with the increase in the concentration of pterostilbene (from 32 to 64 μg·ml−1). These results indicated that pterostilbene treatment rescued the antibacterial activity of meropenem against NDM‐producing isolates in vitro in a dose‐dependent manner. In addition, the synergistic activity of this combination was further tested by time‐killing experiments. Figure 4 shows that meropenem with pterostilbene can rapidly reduce viable counts under the lowest detectable limit after 3‐hr incubation, and the bacteriostatic effect lasted for 9 hr (7 hr against E.coli E2 in Figure 4d). In the absence of pterostilbene, obvious regrowth was observed after 3 hr. The results revealed that pterostilbene possesses potential as an NDM‐1 inhibitor and was effective in restoring the antibacterial activity of meropenem.
Figure 2.
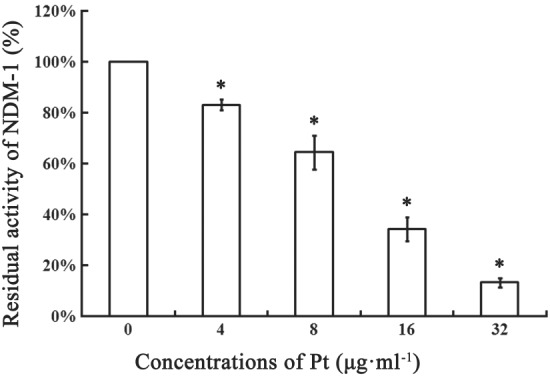
Effect of pterostilbene in the activity of New Delhi metallo‐β‐lactamase‐1 (NDM‐1). Pterostilbene was added to PBS containing 10‐mM ZnCl2 (pH 7.4) in a 96‐well plate to reach the final concentrations of 4, 8, 16, and 32 μg·ml−1 and incubated with the purified NDM‐1 for 10 min. Then 25‐μM nitrocefin (dissolved in sterile water) was added for another incubation at 37°C for 30 min. The absorbance of each sample was read at 492 nm by a microplate reader at room temperature, and residual activity was calculated. The samples without inhibitors (shown as 0 μg·ml−1 pterostilbene) or NDM‐1 served as positive controls or negative controls, respectively. Residual activity = (A − A0) /(A100 − A0) × 100%, where A represents the absorbance of samples at 492 nm and A0 and A100 represent 0% and 100% activity as determined in the negative controls and positive controls, respectively. All data are mean ± SD, n = 5. * P < .05, significantly different from the positive control group; one‐way ANOVA followed by a Dunnett t test. Pt, pterostilbene
Figure 3.
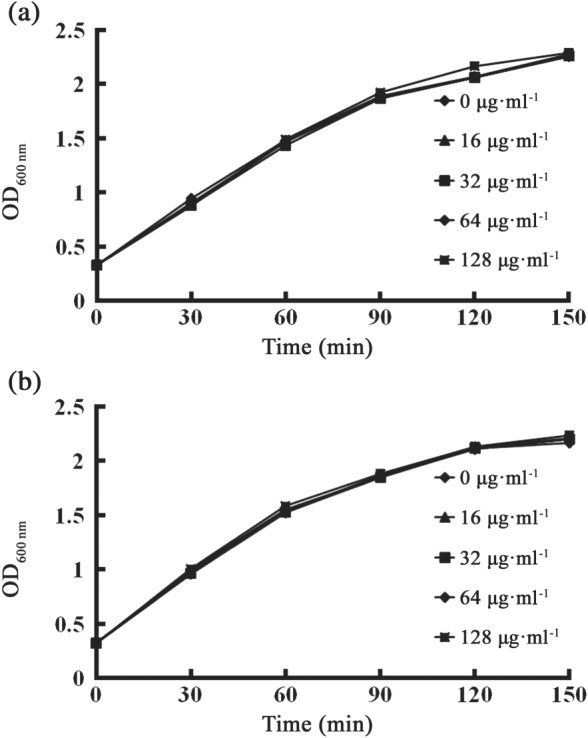
Growth curves for (a) Escherichia coli ZC‐YN3 and (b) Klebsiella pneumoniae QD‐KP2 cultured with pterostilbene. E. coli ZC‐YN3 or K. pneumoniae QD‐KP2 were cultured with various concentrations of pterostilbene (0, 16, 32, 64, or 128 μg·ml−1) with constant shaking (180 rpm) at 37°C. OD600 nm was measured to determine the influence of pterostilbene on the growth of bacteria every 30 min. All data are mean of five independent experiments
Table 1.
MIC and FICI variations of meropenem and meropenem plus pterostilbene against NDM‐carrying isolates
| Strain | MIC (μg·ml−1) | FICI | MIC (μg·ml−1) | FICI | ||
|---|---|---|---|---|---|---|
| Mep | Pt | Mep (+32 μg·ml−1 Pt) | Mep (+64 μg·ml−1 Pt) | |||
| Escherichia coli ATCC‐25922 | 0.032 | >512 | 0.032 | 1.06 | 0.032 | 1.13 |
| E. coli ZC‐YN3 (NDM‐1) | 16 | >512 | 4 | 0.31 | 2 | 0.25 |
| E. coli ZC‐YN5 (NDM‐5) | 32 | >512 | 8 | 0.31 | 4 | 0.25 |
| E. coli ZC‐YN7 (NDM‐9) | 32 | >512 | 8 | 0.31 | 4 | 0.25 |
| Klebsiella pneumoniae QD‐KP2 (NDM‐1) | 16 | >512 | 4 | 0.31 | 2 | 0.25 |
| E. coli D3 (NDM‐1) | 16 | >512 | 4 | 0.31 | 2 | 0.25 |
| E. coli E1 (NDM‐1) | 16 | >512 | 4 | 0.31 | 2 | 0.25 |
| E. coli 2Z49 (NDM‐5) | 16 | >512 | 8 | 0.56 | 4 | 0.38 |
| E. coli 2Z69 (NDM‐5) | 128 | >512 | 64 | 0.56 | 32 | 0.38 |
| E. coli E4 (NDM‐5) | 64 | >512 | 32 | 0.56 | 16 | 0.38 |
| E. coli E2 (NDM‐9) | 64 | >512 | 16 | 0.31 | 8 | 0.25 |
Abbreviations: FICI, fractional inhibitory concentration index; Mep, meropenem; NDM, New Delhi metallo‐β‐lactamase; Pt, pterostilbene.
Note. All data are mean ± SD, n = 5.
Figure 4.

Time‐kill curves of compounds against NDM‐positive isolates. Mid‐logarithmic‐phase bacteria were diluted to 1 × 105 CFU·ml−1 in LB medium and incubated in meropenem with or without 32 μg·ml−1 pterostilbene for 0, 1, 3, 5, 7, 9, and 11 hr. Samples were plated onto agar plates for bacterial counts. After overnight culture, surviving colonies were counted. All data are mean ± SD, n = 5. Mep, meropenem; Pt, pterostilbene
3.2. Pterostilbene combined with meropenem provides protection in vivo
A murine thigh model was used to evaluate the efficacy of pterostilbene plus meropenem in vivo. The results showed that a significant decrease in the bacterial burden in the thigh was observed for mice treated with three doses of the combination therapy compared with mice treated with either agent separately (Figure 5a).
Figure 5.
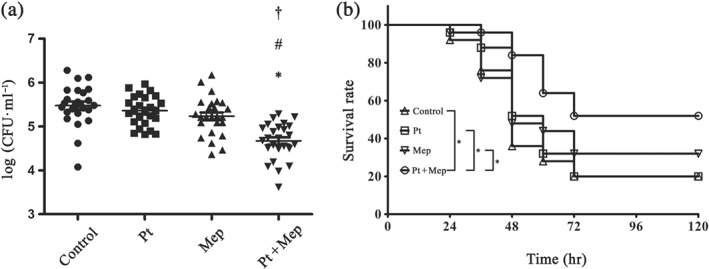
The effect of combined therapy in vivo. (a) Bacterial load in the thigh. BALB/c mice were infected with Escherichia coli ZC‐YN3 and treated with pterostilbene, meropenem, or a combination of both. All groups of mice were treated with three doses at 8‐hr intervals. After 72 hr, mice were killed, and infected thighs were harvested and homogenized. Organ homogenates were diluted and plated for bacteria enumeration. All data are mean ± SD (five samples each group and run in quintuplicate). * P < .05, significantly different from control group; # P < .05, significantly different from Pt group, † P < .05, significantly different from Mep group; one‐way ANOVA with the Neuman–Keuls post hoc test for multiple comparisons. (b) Kaplan–Meier survival analysis in a mouse pneumonia model. C57BL/6 mice were intranasally infected with NDM‐1‐positive Klebsiella pneumoniae QD‐KP2 to induce bacterial pneumonia and treated with pterostilbene, meropenem, a combination of both, or solvent (DMSO) on the same schedule after infection. All groups of mice were treated with three doses at 8‐hr intervals. Mice were monitored until Day 5 post‐infection to evaluate the survival rate. All data are mean ± SD (five samples each group and run in quintuplicate). Significant differences were analysed by the. * P < .05, significantly different as indicated; generalized Wilcoxon test. Mep, meropenem; Pt, pterostilbene
A mouse https://academic.oup.com/jac/article-abstract/54/6/1085/856174 model caused by K. pneumoniae QD‐KP2 (NDM‐1) was used to evaluate the protective effect of pterostilbene combined with meropenem against lethal infection in vivo. As shown in Figure 5b, mice that received a combination therapy were significantly protected from K. pneumoniae QD‐KP2 pneumonia, compared with the control group. Three doses of the combination therapy led to 52% survival at 5 days after infection, while pterostilbene or meropenem alone were unable to improve the survival rate, compared with control group. These findings suggested that pterostilbene in combination with meropenem was effective in vivo.
3.3. Molecular dynamics simulation for NDM‐1‐pterostilbene
To explore the potential binding mode of pterostilbene with NDM‐1 in the active site, molecular dynamics (MD) simulation based on molecular docking was performed in this study. The binding mode of pterostilbene with NDM‐1 is shown in Figure 6. The hydrogen bonding and hydrophobic interactions were clearly observed for the binding of pterostilbene to NDM‐1. During the time course of the MD simulation, pterostilbene could localize to the catalytic pocket of NDM‐1 (residue 110 to 200). In detail, the side chain of Trp93 and Asp124 in NDM‐1 can form a strong interaction with pterostilbene. To explore the energy contributions from the residues of the binding sites in the NDM‐1‐pterostilbene complex, the binding free energy decomposition was calculated. As shown in Figure 7, five residues, Trp93, Gln123, Asp124, Glu152, and His250, made a strong total binding energy contribution (ΔE total ≤ −1.0 kcal·mol−1), suggesting that these five residues are key residues for pterostilbene binding. During complex formation, van der Waals interactions accounted for most of the decomposed energy interaction on residues Trp93, Gln123, and Glu152, while the electrostatic contribution appeared to have a strong contribution on residues Asp124 and His250 for this formation (Figure 7). The interactions between pterostilbene and the residues of the binding sites in NDM‐1 are shown in Figure 8.
Figure 6.
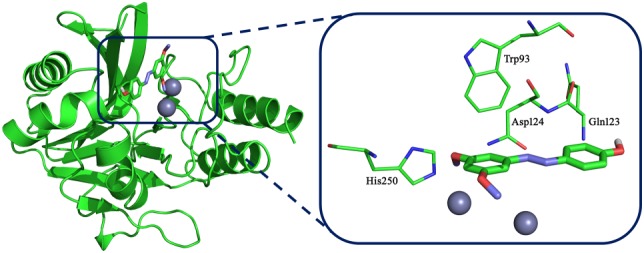
The 3D structure of New Delhi metallo‐β‐lactamase‐1 with pterostilbene. The starting structure of the pterostilbene/New Delhi metallo‐β‐lactamase‐1 complex for molecular dynamic simulation was obtained by a standard docking procedure for a rigid protein and a flexible pterostilbene performed with AutoDock 4
Figure 7.
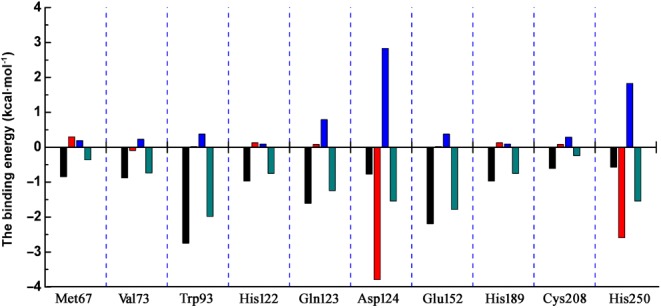
The decomposition of the binding energy on a per‐residue basis in the binding sites of the New Delhi metallo‐β‐lactamase‐1–pterostilbene complex (black histograms: the van der Waals; red histograms: the electrostatic; blue histograms: the solvation; cyan histograms: the total binding energy)
Figure 8.
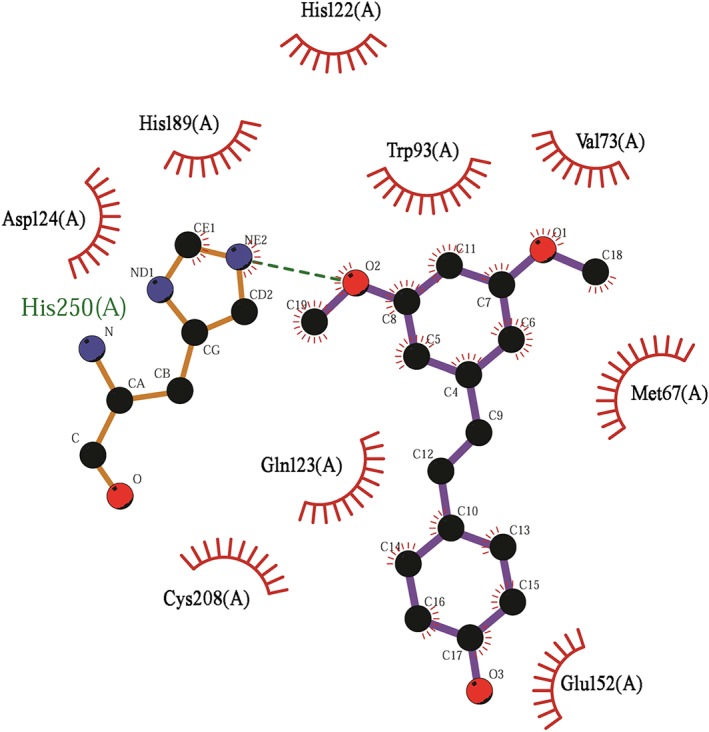
The interactions between pterostilbene and the residues of the binding sites in New Delhi metallo‐β‐lactamase‐1. Interaction between pterostilbene and the residues of the binding sites in New Delhi metallo‐β‐lactamase‐1 are shown using a two‐dimensional diagram by Ligplus
The Molecular Mechanics Generalized Born Surface Area (MM‐GBSA) method was employed to further validate the theoretical results described above (Table 2). Based on the calculation results, the binding free energy, ΔGbind, of the interaction between pterostilbene and wild‐type (WT) NDM‐1 was greater than that of the Trp93Ala and Asp124Ala mutants, indicating that the strongest binding exists in WT–NDM‐1 with pterostilbene. In agreement with these results, the K A of WT–NDM‐1 was greater than that of Trp93Ala and Asp124Ala (Table 2). As shown in Figure 9, the sensitivities of Trp93Ala or Asp124Ala for pterostilbene was less than the WT NDM‐1. Thus, the results from MD simulation for NDM‐1‐pterostilbene complex are reliable. Taken together, our data indicated that the binding of pterostilbene to the activity region (residues of Trp93 and Asp124) inhibits the biological activity fo NDM‐1 9.
Table 2.
The binding free energy (kcal·mol−1) of WT–pterostilbene, Trp93Ala–pterostilbene, and Asp124Ala–pterostilbene systems based on computational method and the values of the binding constants (K A) based on the fluorescence spectroscopy quenching
| WT–NDM‐1 | Trp93Ala | Asp124Ala | |
|---|---|---|---|
| The binding free energy (kcal·mol−1) | −8.58 ± 2.08 | −6.92 ± 1.19 | −7.12 ± 1.61 |
| K A (1 × 104) L·mol−1 | 5.16 ± 1.67 | 4.76 ± 1.14 | 3.52 ± 1.26 |
Abbreviations: NDM‐1, New Delhi metallo‐β‐lactamase‐1; WT, wild type.
Note. All data are mean ± SD, n = 5.
Figure 9.

Effects of pterostilbene in the activity of wild type (WT)–New Delhi metallo‐β‐lactamase‐1 (NDM‐1) and its mutants. Pterostilbene was added to assay buffer in a 96‐well plate to reach the final concentrations of 4, 8, 16, and 32 μg·ml−1 and pre‐incubated with the purified WT–NDM‐1 enzyme and its mutants Trp93Ala and Asp124Ala for 10 min. Then 25‐μM nitrocefin was added for another incubation at 37°C for 30 min. The absorbance of each sample was read at 492 nm by a microplate reader at room temperature, and residual activity was calculated as described in Figure 2. All data are mean ± SD, n = 5. * P < .05, significantly different from the positive control group; one‐way ANOVA followed by a Dunnett t test
4. DISCUSSION
The spread of antibiotic resistance caused by MBLs is identified as a global health care threat (Nordmann, Poirel, Walsh, & Livermore, 2011). Currently, the current options to treat NDM infections are tigecycline and colistin, which are stable towards NDM‐type β‐lactamases. However, their use is not recommended due to their toxicity (Dixit, Madduri, & Sharma, 2014; Ordooei Javan, Shokouhi, & Sahraei, 2015). Designing inhibitors that specifically target antibiotic resistance genes is a workable strategy to combat antibiotic resistance. Previous studies with inhibitors targeted to Ser‐β‐lactamases have shown that antibiotic and inhibitor combinations are highly successful in the clinic (Shlaes, 2013). In view of the pivotal role of NDM in carbapenem‐resistant Gram‐negative pathogens, NDM‐1 is the main target for development of inhibitors. NDM‐1‐targeting inhibitors can disrupt the ability of carbapenemases to cleave the β‐lactam ring, restoring the antibacterial activity of carbapenem against NDM‐1‐positive pathogens. Given the emergence and spread of MBLs and the lack of effective antibiotics targeting these resistance genes, developing inhibitors for MBLs is an urgent medical need. Although several compounds have been reported to inhibit NDM‐1 and had good in vitro and in vivo activity, most of them are based on the ability to chelate zinc ions (Rotondo & Wright, 2017). For instance, aspergillomarasmine A (AMA) is a natural product that can effectively rescue meropenem activity both in vitro and in vivo. However, as a strong metal ion chelator, AMA displayed toxicity in mice (LD50 = 159.8 mg·kg−1, i.v.). In a similar manner based on metal chelation, captopril and many other thiol‐containing derivatives have been reported as inhibitors of NDM‐1 (Guo et al., 2011; King, Worrall, Gruninger, & Strynadka, 2012). However, the adverse effects associated with captopril and its derivatives may limit their clinical applications (Kitamura, Aihara, Osawa, Naito, & Ikezawa, 1990). To date, no NDM‐1 inhibitors have been tested in human clinical trials due to practical and technical limitations (Ning et al., 2018).
In this study, we identified pterostilbene, a well‐known phytochemical, as an effective agent in inhibiting NDM‐1 activity. Pterostilbene possessed little antibacterial activity but induced a strong dose‐dependent inhibition against NDM‐1 in vitro. Notably, in the presence of 64 μg·ml−1 pterostilbene, the reduction in the MIC of the isolates resistant to meropenem (E. coli ZC‐YN3, K. pneumoniae QD‐KP2, E. coli D3, and E. coli E1) reached the susceptibility category in vitro (MIC for susceptibility of meropenem are ≤2 μg·ml−1 according to EUCAST) and the intermediate category (MIC = 2 μg·ml−1 according to CLSI). Furthermore, in vivo evidence was obtained in a murine thigh infection model caused by a clinical NDM‐1‐producing isolate of E. coli and a mouse https://academic.oup.com/jac/article-abstract/54/6/1085/856174 model caused by K. pneumoniae QD‐KP2 (NDM‐1), with pterostilbene disrupting the activity of MBL and restoring the antibiotic activity of meropenem. These findings suggested that pterostilbene in combination with meropenem was effective in vivo and that pterostilbene may have the potential to extend the utility of meropenem.
To explore the mechanism of the interaction between pterostilbene and NDM‐1, the MD simulation for the NDM‐1–pterostilbene complex system was carried out. We found that pterostilbene could localize to the catalytic pocket of NDM‐1, which is very close to the binding site of the substrate. Due to the binding of pterostilbene with NDM‐1, the binding of substrate with NDM‐1 was blocked, resulting in the loss of biological activity of NDM‐1. Pterostilbene, which is reported to have excellent bioavailability (80% to 95%), a good cellular uptake, and a long half‐life (105 min; Cichocki et al., 2008; Moon, McCormack, McDonald, & McFadden, 2013), exhibits promise in treating local and systemic infections. Additionally, Riche et al. reported that 100–250 mg daily of pterostilbene in adults with hyperlipidaemia did not produce significant adverse drug events (Riche et al., 2013). In another study conducted by Hougee et al., treatment with 450 mg daily of Pterocarpus marsupium extract in healthy volunteers did not produce signs of toxicity (Hougee et al., 2005). Therefore, pterostilbene may have low toxicity for administration to humans.
In conclusion, pterostilbene restores the activity of meropenem by inhibiting NDM‐1 activity. Pterostilbene in combination with meropenem or other known carbapenems could be useful clinically to address the challenge of MBL‐positive pathogens. These data indicate that pterostilbene, a natural compound in blueberries, combined with meropenem is a promising and a potent therapeutic strategy for the treatment of MBL‐mediated infections. Hence, pterostilbene may become a safe and potential “lead compound” for the development of more powerful NDM‐1 inhibitors.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
S.L., X.D., and J.W. conceived and designed the experiments. S.L., J.Z., Y.Z., N.H., and J.L. performed the experiments. X.N. and Y.W. conceived, designed, and performed the computational analyses. X.N. and Y.W. analysed the data. S.L., X.D., and J.W. wrote the paper.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
ACKNOWLEDGEMENTS
This work was supported by the National Key Research and Development Program of China (2018YFD0500300 and 2016YFD05013).
Liu S, Zhang J, Zhou Y, et al. Pterostilbene restores carbapenem susceptibility in New Delhi metallo‐β‐lactamase‐producing isolates by inhibiting the activity of New Delhi metallo‐β‐lactamases. Br J Pharmacol. 2019;176:4548–4557. 10.1111/bph.14818
Shui Liu and Jian Zhang contributed equally to this work.
Contributor Information
Xuming Deng, Email: dengxm@jlu.edu.cn.
Jianfeng Wang, Email: wjf927@jlu.edu.cn.
REFERENCES
- Albu, S. A. , Koteva, K. , King, A. M. , Al‐Karmi, S. , Wright, G. D. , & Capretta, A. (2016). Total synthesis of aspergillomarasmine A and related compounds: A sulfamidate approach enables exploration of structure–activity relationships. Angewandte Chemie, 55(42), 13259–13262. 10.1002/anie.201606657 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay, S. , Valder, C. R. , Huynh, H. G. , Ren, H. , & Allison, W. S. (2002). The βG156C substitution in the F1‐ATPase from the thermophilic Bacillus PS3 affects catalytic site cooperativity by destabilizing the closed conformation of the catalytic site. Biochemistry, 41(48), 14421–14429. 10.1021/bi026243g [DOI] [PubMed] [Google Scholar]
- Bush, K. , & Jacoby, G. A. (2010). Updated functional classification of β‐lactamases. Antimicrobial Agents and Chemotherapy, 54(3), 969–976. 10.1128/AAC.01009-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadesus, G. , Shukitt‐Hale, B. , Stellwagen, H. M. , Zhu, X. , Lee, H. G. , Smith, M. A. , & Joseph, J. A. (2004). Modulation of hippocampal plasticity and cognitive behavior by short‐term blueberry supplementation in aged rats. Nutritional Neuroscience, 7(5–6), 309–316. 10.1080/10284150400020482 [DOI] [PubMed] [Google Scholar]
- Cichocki, M. , Paluszczak, J. , Szaefer, H. , Piechowiak, A. , Rimando, A. M. , & Baer‐Dubowska, W. (2008). Pterostilbene is equally potent as resveratrol in inhibiting 12‐O‐tetradecanoylphorbol‐13‐acetate activated NFκB, AP‐1, COX‐2, and iNOS in mouse epidermis. Molecular Nutrition & Food Research, 52(Suppl 1), S62–S70. [DOI] [PubMed] [Google Scholar]
- CLSI, Wayne, PA, USA (2016). Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: Twenty‐sixth informational supplement M100S‐S26.
- Dixit, D. , Madduri, R. P. , & Sharma, R. (2014). The role of tigecycline in the treatment of infections in light of the new black box warning. Expert Review of Anti‐Infective Therapy, 12(4), 397–400. 10.1586/14787210.2014.894882 [DOI] [PubMed] [Google Scholar]
- Dong, J. , Qiu, J. , Zhang, Y. , Lu, C. , Dai, X. , Wang, J. , … Deng, X. (2013). Oroxylin A inhibits hemolysis via hindering the self‐assembly of α‐hemolysin heptameric transmembrane pore. PLoS Computational Biology, 9(1), e1002869 10.1371/journal.pcbi.1002869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, V. L. , Verma, A. , Owens, R. J. , Phillips, S. E. , & Carr, S. B. (2011). Structure of New Delhi metallo‐β‐lactamase 1 (NDM‐1). Acta Crystallographica. Section F, Structural Biology and Crystallization Communications, 67(Pt 10), 1160–1164. 10.1107/S1744309111029654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y. , Wang, J. , Niu, G. , Shui, W. , Sun, Y. , Zhou, H. , … Rao, Z. (2011). A structural view of the antibiotic degradation enzyme NDM‐1 from a superbug. Protein & Cell, 2(5), 384–394. 10.1007/s13238-011-1055-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougee, S. , Faber, J. , Sanders, A. , de Jong, R. B. , van den Berg, W. B. , Garssen, J. , … Smit, H. F. (2005). Selective COX‐2 inhibition by a Pterocarpus marsupium extract characterized by pterostilbene, and its activity in healthy human volunteers. Planta Medica, 71(5), 387–392. 10.1055/s-2005-864130 [DOI] [PubMed] [Google Scholar]
- Hu, R. , Barbault, F. , Maurel, F. , Delamar, M. , & Zhang, R. (2010). Molecular dynamics simulations of 2‐amino‐6‐arylsulphonylbenzonitriles analogues as HIV inhibitors: Interaction modes and binding free energies. Chemical Biology & Drug Design, 76(6), 518–526. 10.1111/j.1747-0285.2010.01028.x [DOI] [PubMed] [Google Scholar]
- Joseph, J. A. , Fisher, D. R. , Cheng, V. , Rimando, A. M. , & Shukitt‐Hale, B. (2008). Cellular and behavioral effects of stilbene resveratrol analogues: Implications for reducing the deleterious effects of aging. Journal of Agricultural and Food Chemistry, 56(22), 10544–10551. 10.1021/jf802279h [DOI] [PubMed] [Google Scholar]
- Joseph, J. A. , Shukitt‐Hale, B. , & Casadesus, G. (2005). Reversing the deleterious effects of aging on neuronal communication and behavior: Beneficial properties of fruit polyphenolic compounds. The American Journal of Clinical Nutrition, 81(Suppl 1), 313S–316S. 10.1093/ajcn/81.1.313S [DOI] [PubMed] [Google Scholar]
- Jurasekova, Z. , Marconi, G. , Sanchez‐Cortes, S. , & Torreggiani, A. (2009). Spectroscopic and molecular modeling studies on the binding of the flavonoid luteolin and human serum albumin. Biopolymers, 91(11), 917–927. 10.1002/bip.21278 [DOI] [PubMed] [Google Scholar]
- Kang, W. , Liu, H. , Ma, L. , Wang, M. , Wei, S. , Sun, P. , … Dou, J. (2017). Effective antimicrobial activity of a peptide mutant Cbf‐14‐2 against penicillin‐resistant bacteria based on its unnatural amino acids. European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences, 105, 169–177. 10.1016/j.ejps.2017.05.030 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, D. , & Strynadka, N. (2011). Crystal structure of New Delhi metallo‐β‐lactamase reveals molecular basis for antibiotic resistance. Protein Science: A Publication of the Protein Society, 20(9), 1484–1491. 10.1002/pro.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, D. T. , Worrall, L. J. , Gruninger, R. , & Strynadka, N. C. (2012). New Delhi metallo‐β‐lactamase: Structural insights into β‐lactam recognition and inhibition. Journal of the American Chemical Society, 134(28), 11362–11365. 10.1021/ja303579d [DOI] [PubMed] [Google Scholar]
- Kitamura, K. , Aihara, M. , Osawa, J. , Naito, S. , & Ikezawa, Z. (1990). Sulfhydryl drug‐induced eruption: A clinical and histological study. The Journal of Dermatology, 17(1), 44–51. 10.1111/j.1346-8138.1990.tb01608.x [DOI] [PubMed] [Google Scholar]
- Liao, D. , Yang, S. , Wang, J. , Zhang, J. , Hong, B. , Wu, F. , & Lei, X. (2016). Total synthesis and structural reassignment of aspergillomarasmine A. Angewandte Chemie, 55(13), 4291–4295. 10.1002/anie.201509960 [DOI] [PubMed] [Google Scholar]
- Liu, S. , Zhou, Y. , Niu, X. , Wang, T. , Li, J. , Liu, Z. , … Deng, X. (2018). Magnolol restores the activity of meropenem against NDM‐1‐producing Escherichia coli by inhibiting the activity of metallo‐β‐lactamase. Cell Death Discovery, 4, 28 10.1038/s41420-018-0029-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, D. , McCormack, D. , McDonald, D. , & McFadden, D. (2013). Pterostilbene induces mitochondrially derived apoptosis in breast cancer cells in vitro. The Journal of Surgical Research, 180(2), 208–215. 10.1016/j.jss.2012.04.027 [DOI] [PubMed] [Google Scholar]
- Morris, G. M. , Huey, R. , Lindstrom, W. , Sanner, M. F. , Belew, R. K. , Goodsell, D. S. , & Olson, A. J. (2009). AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. Journal of Computational Chemistry, 30(16), 2785–2791. 10.1002/jcc.21256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning, N. Z. , Liu, X. , Chen, F. , Zhou, P. , Hu, L. , Huang, J. , … Wang, H. (2018). Embelin restores carbapenem efficacy against NDM‐1‐positive pathogens. Frontiers in Microbiology, 9, 71 10.3389/fmicb.2018.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu, X. , Qiu, J. , Wang, X. , Gao, X. , Dong, J. , Wang, J. , … Deng, X. (2013). Molecular insight into the inhibition mechanism of cyrtominetin to α‐hemolysin by molecular dynamics simulation. European Journal of Medicinal Chemistry, 62, 320–328. 10.1016/j.ejmech.2013.01.008 [DOI] [PubMed] [Google Scholar]
- Nordmann, P. , Poirel, L. , Walsh, T. R. , & Livermore, D. M. (2011). The emerging NDM carbapenemases. Trends in Microbiology, 19(12), 588–595. 10.1016/j.tim.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Odds, F. C. (2003). Synergy, antagonism, and what the chequerboard puts between them. The Journal of Antimicrobial Chemotherapy, 52(1), 1 10.1093/jac/dkg301 [DOI] [PubMed] [Google Scholar]
- Ordooei Javan, A. , Shokouhi, S. , & Sahraei, Z. (2015). A review on colistin nephrotoxicity. European Journal of Clinical Pharmacology, 71(7), 801–810. 10.1007/s00228-015-1865-4 [DOI] [PubMed] [Google Scholar]
- Pari, L. , & Satheesh, M. A. (2006). Effect of pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin‐ and nicotinamide‐induced diabetic rats. Life Sciences, 79(7), 641–645. 10.1016/j.lfs.2006.02.036 [DOI] [PubMed] [Google Scholar]
- Riche, D. M. , McEwen, C. L. , Riche, K. D. , Sherman, J. J. , Wofford, M. R. , Deschamp, D. , & Griswold, M. (2013). Analysis of safety from a human clinical trial with pterostilbene. Journal of Toxicology, 2013, 463595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimando, A. M. , & Suh, N. (2008). Biological/chemopreventive activity of stilbenes and their effect on colon cancer. Planta Medica, 74(13), 1635–1643. 10.1055/s-0028-1088301 [DOI] [PubMed] [Google Scholar]
- Rotondo, C. M. , & Wright, G. D. (2017). Inhibitors of metallo‐β‐lactamases. Current Opinion in Microbiology, 39, 96–105. 10.1016/j.mib.2017.10.026 [DOI] [PubMed] [Google Scholar]
- Schmidlin, L. , Poutaraud, A. , Claudel, P. , Mestre, P. , Prado, E. , Santos‐Rosa, M. , … Hugueney, P. (2008). A stress‐inducible resveratrol O‐methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiology, 148(3), 1630–1639. 10.1104/pp.108.126003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri, T. R. (1972). Polyphenols of Pterocarpus and Dalbergia woods. Phytochemistry, 11(3), 881–898. 10.1016/S0031-9422(00)88430-7 [DOI] [Google Scholar]
- Shlaes, D. M. (2013). New β‐lactam‐β‐lactamase inhibitor combinations in clinical development. Annals of the new York Academy of Sciences, 1277, 105–114. 10.1111/nyas.12010 [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Zhang, R. , Li, J. , Wu, Z. , Yin, W. , Schwarz, S. , … Shen, J. (2017). Comprehensive resistome analysis reveals the prevalence of NDM and MCR‐1 in Chinese poultry production. Nature Microbiology, 2, 16260 10.1038/nmicrobiol.2016.260 [DOI] [PubMed] [Google Scholar]
- Yong, D. , Toleman, M. A. , Giske, C. G. , Cho, H. S. , Sundman, K. , Lee, K. , & Walsh, T. R. (2009). Characterization of a new metallo‐β‐lactamase gene, bla NDM‐1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrobial Agents and Chemotherapy, 53(12), 5046–5054. 10.1128/AAC.00774-09 [DOI] [PMC free article] [PubMed] [Google Scholar]


