Abstract
Background and Purpose
Cannabichromene (CBC) is one of the most abundant phytocannabinoids in Cannabis spp. It has modest antinociceptive and anti‐inflammatory effects and potentiates some effects of Δ9‐tetrahydrocannabinol in vivo. How CBC exerts these effects is poorly defined and there is little information about its efficacy at cannabinoid receptors. We sought to determine the functional activity of CBC at cannabinoid CB1 and CB2 receptors.
Experimental Approach
AtT20 cells stably expressing haemagglutinin‐tagged human CB1 and CB2 receptors were used. Assays of cellular membrane potential and loss of cell surface receptors were performed.
Key Results
CBC activated CB2 but not CB1 receptors to produce hyperpolarization of AtT20 cells. This activation was inhibited by a CB2 receptor antagonist AM630, and sensitive to Pertussis toxin. Application of CBC reduced activation of CB2, but not CB1, receptors by subsequent co‐application of CP55,940, an efficacious CB1 and CB2 receptor agonist. Continuous CBC application induced loss of cell surface CB2 receptors and desensitization of the CB2 receptor‐induced hyperpolarization.
Conclusions and Implications
CBC is a selective CB2 receptor agonist displaying higher efficacy than tetrahydrocannabinol in hyperpolarizing AtT20 cells. CBC can also recruit CB2 receptor regulatory mechanisms. CBC may contribute to the potential therapeutic effectiveness of some cannabis preparations, potentially through CB2 receptor‐mediated modulation of inflammation.
Abbreviations
- AEA
anandamide
- AtT20‐CB1
mouse pituitary tumour cells stably transfected with HA human CB1 cells
- AtT20‐CB2
mouse pituitary tumour cells stably transfected with HA human CB2 cells
- CBC
cannabichromene
- CBD
cannabidiol
- ECS
endocannabinoid system
- GRK
GPCR kinase
- HA
haemagglutinin
- PTX
Pertussis toxin
- THC
tetrahydrocannabinol
What is already known
The phytocannabinoid cannabichromene (CBC) has antinociceptive and anti‐inflammatory effects in vitro and in vivo.
How CBC exerts these effects is largely unknown.
What this study adds
This study shows that CBC is a selective CB2 receptor agonist.
CBC has a higher in vitro efficacy than tetrahydrocannabinol and activates CB2 receptor regulatory pathways.
What is the clinical significance
Cannabis contains a CB2 receptor‐selective compound that could reduce inflammation without producing intoxication.
1. INTRODUCTION
Cannabichromene (CBC) is one of over 100 phytochemicals (collectively referred to as phytocannabinoids) that are found in Cannabis spp (ElSohly & Gul, 2014). CBC was identified in 1966 and is one of the most abundant phytocannabinoids alongside https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2424, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4150, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=740 (Izzo, Borrelli, Capasso, Di Marzo, & Mechoulam, 2009; Turner, ElSohly, & Boeren, 1980). Evaluation of seized cannabis plants in the United States, United Kingdom, and Australia showed CBC concentrations ranging between 0.05 and 0.3% w/w (Mehmedic et al., 2010; Potter, Clark, & Brown, 2008; Swift, Wong, Li, Arnold, & McGregor, 2013). CBC, THC, and CBD are directly synthesized from cannabigerolic acid and share a common 3‐pentylphenol ring (Figure 1; Flores‐Sanchez & Verpoorte, 2008). The therapeutic potential of CBC has been demonstrated in several preclinical studies. For example, CBC decreased carrageenan‐induced and LPS‐induced inflammation in rats and mice, respectively (DeLong, Wolf, Poklis, & Lichtman, 2010; Turner & ElSohly, 1981), and modestly inhibited thermal nociception and potentiated THC antinociception in mice (Davis & Hatoum, 1983; DeLong et al., 2010). While this may be mediated in part through changes in THC distribution in the mice (DeLong et al., 2010), the pharmacological basis for the in vivo actions of CBC remains unclear.
Figure 1.
Chemical structures of some phytocannabinoids
The endocannabinoid system (ECS) comprises the cannabinoid receptors (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=57), endogenous agonists (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2364 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=729), putative endocannabinoid transporters and enzymes involved in the synthesis and metabolism of endocannabinoids (Iannotti, Di Marzo, & Petrosino, 2016). Cannabinoid receptors are differentially distributed in the body. CB1 receptors are the most abundant GPCR in the mammalian brain (Marsicano & Lutz, 1999) and are predominantly expressed in the CNS, while CB2 receptors are expressed abundantly in cells of the immune system and organs such as the spleen. These distributions imply that activation of these receptors will induce different physiological responses. For example, THC causes a distinctive intoxication via stimulation of the CB1 receptors, while stimulation of CB2 receptors does not appear to contribute to the psychoactive effects of cannabis (Deng et al., 2015).
Phytocannabinoids target individual components of the ECS and act on a range of other receptors and ion channels. For example, THC not only activates CB1 and CB2 receptors but also modulates https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=109, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=379 receptors, and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=595 (Barann et al., 2002; Bayewitch et al., 1996; Lauckner et al., 2008; O'Sullivan, Tarling, Bennett, Kendall, & Randall, 2005; Pertwee, 1999; Ryberg et al., 2007). CBD is reported to increase AEA levels by inhibiting the enzyme https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1400; act as a negative allosteric modulator of CB1 receptors; antagonize GPR55 receptors; activate https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=508 channels; and modulate T‐type calcium channels (De Petrocellis et al., 2011; Laprairie, Bagher, Kelly, & Denovan‐Wright, 2015; Ross et al., 2008). The less prevalent phytocannabinoids, such as tetrahydrocannabivarin, are low efficacy CB2 receptor agonists and high potency https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=507 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=485 agonists (De Petrocellis et al., 2011), while cannabinol appears to be an agonist of CB1 and CB2 receptors, and TRPA1 channels (Bolognini et al., 2010; De Petrocellis et al., 2011; Rhee et al., 1997).
CBC has been reported to be a low affinity CB1 and CB2 receptor ligand in binding assays conducted on human receptors expressed in insect cells (Rosenthaler et al., 2014), and it also activates rat TRPA1 channels (De Petrocellis et al., 2011). However, receptor binding does not provide information about ligand efficacy, and whether CBC has efficacy at either receptor remains unresolved. In this study, we sought to characterize the action of CBC at human CB1 and CB2 receptors. To do this, we used an in vitro assay of inwardly rectifying potassium channel activation in intact AtT20 cells that we have used extensively to characterize the activity of cannabinoids at CB1 and CB2 receptors (Banister et al., 2016; Longworth et al., 2017; Redmond et al., 2016; Soethoudt et al., 2017). Using this assay, we find that CBC is an agonist at CB2, but not CB1, receptors.
2. METHODS
2.1. Cell culture
Mouse pituitary tumour AtT20 FlpIn cells stably transfected with haemagglutinin (HA)‐tagged human CB1 (AtT20‐CB1) and human CB2 (AtT20‐CB2) receptors (Alexander et al., 2017; Banister et al., 2016) were used. AtT20 FlpIn cells were made in our lab from AtT20 cells obtained from the American Type Culture Collection (RRID:CVCL_4109), using the Flp In System from Thermo Fisher Scientific (#K601001). Tissue culture media and reagents were from Thermo Fisher Scientific (Massachusetts, USA) or Sigma‐Aldrich. Tissue culture wares were sourced from Corning (NY, USA) or Becton Dickinson (North Ryde, Australia). Cells were cultured in T75 flasks using DMEM supplemented with 10% FBS and 1% penicillin–streptomycin (100 U·ml−1) and incubated in a humidified atmosphere with 5% CO2 at 37°C. Zeocin (100 μg·ml−1, Invivogen, California, USA) and hygromycin (80 μg·ml−1, Invivogen) were used to select wild‐type and transfected AtT20 cells respectively. Cells were passaged at 80% confluency and used for assays at above 90% confluency, for up to 15 passages. For experiments, AtT20 cells were resuspended in Leibovitz's L‐15 media containing 1% FBS, 1% P/S, and 15‐mM glucose; 90 μl of the resuspended cells were plated in a black‐walled, 96‐well microplate (Corning) and incubated overnight in humidified air at 37°C. For experiments involving Pertussis toxin (PTX) treatment (Hello Bio, Bristol, UK), 200 ng·ml−1 PTX was added to the L‐15 cell suspension.
2.2. Membrane potential assay
In this assay, a reduction in fluorescence is indicative of cellular hyperpolarization. Changes in the membrane potential of cells were measured in duplicate, using a FLIPR Membrane Potential Assay Kit (blue #R8034) and a FlexStation 3 Microplate Reader (both from Molecular Devices, Sunnyvale, CA). The dye was diluted to 50% of the manufacturers recommended concentration using HBSS. Dye (90 μl) was loaded into each well of the plate and incubated for 1 hr at 37°C prior to testing. The FlexStation 3 recorded fluorescence at 2‐s intervals (ʎexcitation = 530, ʎemission = 565), and drugs were added after an initial 2 min of baseline reading. The volume of each drug addition was 20 μl, and when two drug additions were made, each drug concentration was adjusted to accommodate the change in final volume. The cellular response to the drug is presented as a percentage change in fluorescence from baseline after subtraction of the change produced by vehicle addition. The change in fluorescence was then normalized to the change in fluorescence due to 1‐μM https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=730 (a high efficacy, non‐selective CB1 and CB2 receptor agonist; Banister et al., 2016) to allow more ready comparison across experiments. https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=730 (1‐μM) standard stimuli were obtained in independent experiments in one well of each column of each plate. Concentration‐response curves were fitted to a four‐parameter sigmoidal dose–response curve in Graph Pad Prism (Version 6 GraphPad Software Inc, CA, USA; RRID:SCR_002798) to derive pEC50 and E max.
2.3. Receptor internalization assay
Changes in cell surface CB2 receptors were determined in at least five independent experiments, each performed in triplicate, using whole cell ELISA. Cells in L‐15 media were seeded at 80,000 cells per well in a poly‐d‐lysine (Sigma) coated, black walled, clear bottom 96‐well plate, and incubated for 18 hr at 37°C in humidified air. After incubation, cells were treated with the drug of interest. Reported drug concentrations are final concentrations. For one drug treatment, the volume of cells in L‐15 and compounds were mixed in a 1:1 ratio. For two drug treatments, the volume of cells in L‐15, drug A, and drug B were added in ratio 9:9:2. Following drug treatment, receptor trafficking was inhibited by placing cells on ice. Cells were then fixed with 4% paraformaldehyde for 15 min. Fixed cells were washed three times with 100‐μl PBS and blocked with 1% BSA in PBS for 1 hr at room temperature. Alexa Fluor® 488 anti‐HA Epitope Tag Antibody (Biolegend, UK; RRID:AB_2565072), diluted to 1:250 with blocking solution, was incubated with the cells at 4°C for 18 hr. Cells were then washed three times with 100‐μl PBS followed by the addition of 50‐μl PBS for the quantification of fluorescence intensity using PHERAstar plate reader (BMG Labtech, Germany). Loss of cell surface receptor was calculated as the percentage decrease in fluorescence intensity after the subtraction of background fluorescence (the fluorescence of wild‐type AtT20 cells incubated with the anti‐HA antibody, as above). The background fluorescence was 50 ± 3% of total fluorescence in CB2 expressing cells.
2.4. Data and statistical analysis
Data analysis for the immunohistochemistry was blinded. For the membrane potential assay experiments, blinding is impractical, but very effort was made to vary the location within the plate and the order in which drugs were added to 96‐well plates to minimize the potential confounds of evaporation or unequal exposure time to the MPA dye. All statistical analyses were conducted with GraphPad Prism (Curtis et al., 2015). The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. Data are reported as mean ± SEM. The equation used to fit a four‐parameter sigmoidal dose–response is Y = Bottom + (Top − Bottom)/(1 + 10^((LogEC50‐X)*HillSlope)). Two‐tailed, unpaired t‐tests were used to compare two data groups and one‐way ANOVA for more than two data groups with Tukey post hoc analysis. P values <.05 were considered statistically significant. Unless otherwise stated, five independent replicates were performed for each experiment.
2.5. Materials
CBC was synthesized according to the method of Lee and Wang (2005). Olivetol (1.80 g, 10 mmol) and citral (1.83 g, 12 mmol) were dissolved with stirring in toluene (100 ml), followed by the addition of ethylenediamine (267 μul, 240 mg, 4 mmol) and acetic acid (458 μul, 480 mg, 8 mmol). The mixture was refluxed for 6 hr and concentrated under vacuum. The residue was dissolved in dichloromethane, washed with water and brine, filtered through a plug of silica, and concentrated. Column chromatography was performed multiple times, as separate runs utilizing hexane with dichloromethane (gradient from 5:1 to 1:1) and hexane with ethyl acetate or acetone (preferably acetone; gradient from 67:1 to 50:1) were necessary to remove impurities. Cannabichromene CBC was afforded as a pale orange oil (1.20 g, 3.8 mmol, 38%), which darkened upon exposure to air and light. 1H NMR (CDCl3, 400 MHz) δ 6.66 (1H, d, J = 9.9 Hz), 6.29 (1H, s), 6.14 (1H, s), 5.51 (1H, d, J = 9.9 Hz), 5.37 (1H, s), 5.12 (1H, t, J = 6.9 Hz), 2.44 (2H, t, J = 7.67), 2.22–‐2.07 (2H, m), 1.81–‐1.66 (2H, m), 1.69 (3H, s), 1.60 (3H, s), 1.59–‐1.52 (2H, m), 1.41 (3H, s), 1.37–‐1.25 (4H, m), 0.90 (3H, t, J = 6.6 Hz); 13C NMR (CDCl3, 100 MHz) δ 153.9, 151.1, 144.8, 131.6, 127.2, 124.2, 116.9, 109.1, 107.9, 107.1, 78.3, 41.0, 35.9, 31.5, 30.6, 26.2, 25.7, 22.7, 22.5, 17.6, 14.0. Structure and purity of cannabichromene CBC (>95%) was confirmed using 1H and 13C NMR and LCMS. All physical and spectral properties were consistent with those previously reported (Lee & Wang, 2005).
THC was obtained from THCPharm (Frankfurt, Germany) while CP55,940, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=745, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=750 were purchased from Cayman Chemical (Michigan, USA). Compound 101 was from Hello Bio, Bristol, UK. All drugs were prepared as stock solutions in DMSO and diluted using a 0.01% bovine serum albumin (BSA, Sigma, Castle Hill, Australia) in HEPES‐buffered low potassium Hanks Balanced Salt Solution (HBSS). HBSS comprises (mM) NaCl 145, HEPES 22, Na2HPO4 0.338, NaHCO3 4.17, KH2PO4 0.441, MgSO4 0.407, MgCl2 0.493, glucose 5.56, and CaCl2 1.26; (pH 7.4, osmolarity 315 ± 15 mosmol). Final DMSO concentration was 0.1%.
2.6. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org,, the common portal of data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/2018 (Alexander, Christopoulos et al., 2017; Alexander, Cidlowski et al., 2017; Alexander, Fabbro et al., 2017; Alexander, Striessnig et al., 2017).
3. RESULTS
3.1. CBC evokes cellular hyperpolarization via CB2, but not CB1, receptors
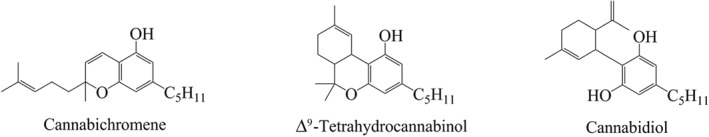
CP55,940, a non‐selective CB1 and CB2 receptor agonist, produced a concentration‐dependent decrease in fluorescence in CB1 cells, with a pEC50 of 7.8 ± 0.1 (Figure 2a). THC also evoked membrane hyperpolarization but with a lower efficacy and potency (pEC50 of 6.6 ± 0.2; E max of 53 ± 3% of CP55,940; Figure 2a). CBC did not hyperpolarize CB1 cells, inducing a negligible change in fluorescence of 2 ± 1% at 30‐μM (Figure 2a–b).
Figure 2.
CBC activates CB2, but not CB1, receptors (a) Concentration‐response curves of CP55,940, THC, and CBC in AtT20‐CB1 cells (n = 5). Results are expressed as mean ± SEM after normalization to 1‐μM CP55,940 hyperpolarization. (b) Representative traces of changes in fluorescence, due to CBC and 1‐μM CP55,940‐induced hyperpolarization in AtT20‐CB1 cells. Drugs were added after 120 s of baseline reading and read over 300 s. (c) Concentration‐response curves of CP55,940, THC, and CBC in AtT20‐CB2 cells (n = 5). Results are expressed as mean ± SEM after normalization to 1‐μM CP55,940 hyperpolarization. (d) Representative traces of changes in fluorescence, due to CBC and 1‐μM CP55,940‐induced hyperpolarization in AtT20‐CB2 cells. Drugs were added after 120 s of baseline reading and read over 300 s
In CB2 cells, CP55,940 produced a concentration‐dependent decrease in fluorescence (pEC50 of 7.1 ± 0.1; Figure 2c). The maximum effect of THC (10‐μM) was 22 ± 3% of CP55,940 (Figure 2c). In contrast to AtT20‐CB1 cells, application of CBC to AtT20‐CB2 cells resulted in a significant hyperpolarization, reaching a maximum of 52 ± 4% of the maximum effect of CP55,940 at the highest concentration of CBC tested (30‐μM, Figure 2c–d); 30‐μM CBC produced a negligible change in fluorescence when applied to wild‐type AtT20 cells (2 ± 0.4%).
3.2. CBC‐induced hyperpolarization is blocked by AM630 and is sensitive to Pertussis toxin
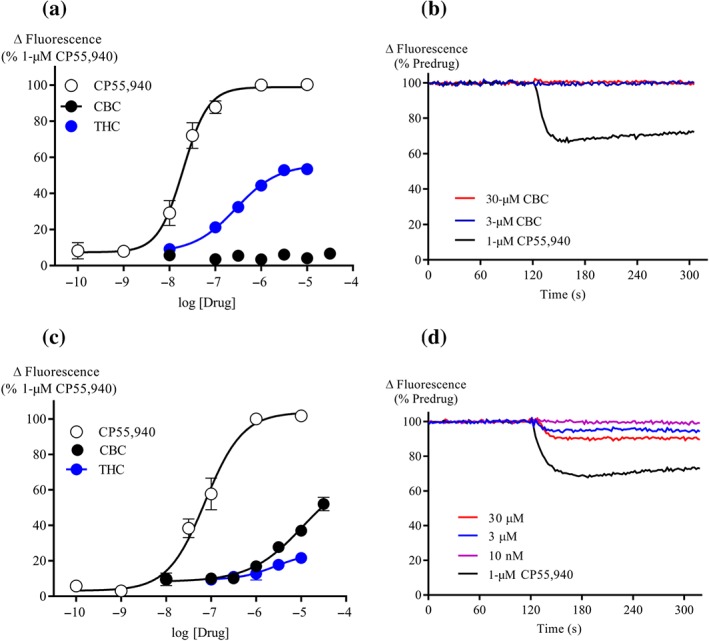
Pretreatment of AtT20‐CB2 cells with AM630 (3‐μM, 5 min), a CB2 receptor selective antagonist (Ignatowska‐Jankowska, Jankowski, & Swiergiel, 2011), inhibited the 10‐μM CBC response by 93 ± 6% compared to vehicle pretreated cells (Figure 3a–b). AM630 similarly inhibited the responses to CP55,940 (300 nM, Figure 3b). Overnight incubation of CB2 cells with 200 ng ml−1 PTX markedly reduced responses to 10‐μM CBC (8 ± 4% of CP55,940) and 1‐μM CP55,940 (11 ± 4%).
Figure 3.
CBC activation of CB2 receptors is blocked by AM630. (a) A representative trace of change in fluorescence of AtT20‐CB2 cells after 5‐min pretreatment with vehicle and 3‐μM AM630, followed by the addition of 10‐μM CBC. (b) Responses to CBC (10‐μM) and CP55,940 (300 nM) in AtT20‐CB2 cells with or without pre‐incubation of AM630 (3‐μM) for 5 min. Results are expressed as mean ± SEM (n = 5). *P < .05, significantly different as indicated; Unpaired Student's t‐test
3.3. CBC inhibits CP55,940 responses at CB2, but not CB1, receptors
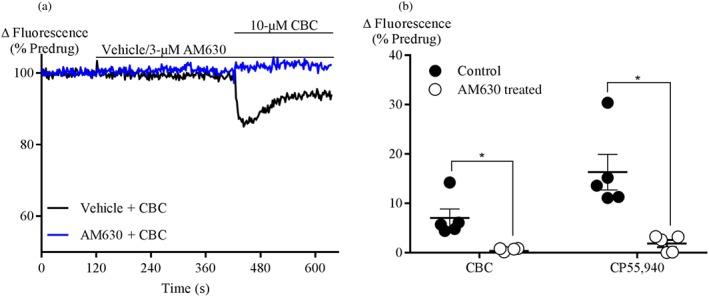
The effect of CBC on responses to CP55,940 and THC was investigated by pre‐incubating cells with CBC (10‐μM, 5 min). In CB1 cells, CBC did not significantly affect the subsequent response to either CP55,940 (100 nM) or THC (10‐μM; Figure 4a–b). In CB2 cells, the CP55,940 (300 nM) response was significantly inhibited by prior application of CBC (10‐μM, 5 min, 44 ± 5%; Figure 4c,d) or CP55,940 (100 nM, 5 min, 23 ± 5%; Figure S1A, B) respectively. Simultaneous application of CP55,940 (300 nM) and CBC (10‐μM) produced a similar change fluorescence to application of CP55,940 (300 nM) alone (112 ± 5%, Figure S1C, D).
Figure 4.
CBC antagonizes CP55,940 and THC response in CB2 cells. Representative traces of the effect of CBC (10‐μM) on (a) CP55,940 (100 nM) on fluorescence in AtT20‐CB1 cells loaded with a membrane potential‐sensitive dye. (b) THC (10‐μM) hyperpolarization in AtT20‐CB1. (c) CP55,940 (300 nM) hyperpolarization in AtT20‐CB2 cells. After 2‐min baseline reading, cells were pretreated with vehicle or 10‐μM CBC for 5 min, followed by the addition CP55,940. (d) Summary data of the effect of 10‐μM CBC on 300‐nM CP55,940 in AtT20‐CB2 cells. Results are expressed as mean ± SEM after normalization to 1‐μM CP55,940 hyperpolarization (n = 5. *P < .05, significantly different as indicated; Unpaired Student's t‐test. Note: truncated axes)
3.4. CBC treatment causes cell surface loss of CB2 receptors
CB2 receptors undergo agonist‐induced loss of surface receptors following prolonged stimulation (Grimsey, Goodfellow, Dragunow, & Glass, 2011). We found that 1‐μM CP55,940 internalized CB2 surface receptors to 59 ± 3% of basal surface level (BSL) after 30‐min treatment. CBC also internalized CB2 surface receptors (10‐μM, 77 ± 5%; 30‐μM, 71 ± 3%). When CB2 cells were pretreated with AM630 (3‐μM, 5 min), 10‐μM CBC did not produce significant loss of surface CB2 receptors after 30‐min treatment (105 ± 4% of BSL; Figure 5a). The amount of cell surface receptors did not change when the cells were exposed to AM630 (3‐μM), followed by vehicle for 30 min (97 ± 8% of BSL). To assess the possible role of G protein receptor kinases in CBC‐mediated receptor internalization, we pretreated cells with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=8437 (10‐μM), a https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=507 inhibitor (Lowe et al., 2015), for 1 hr and then challenged them with CBC. There was no significant change in 10‐μM CBC internalization of CB2 surface receptors following Compound 101 pretreatment (Figure 5b).
Figure 5.
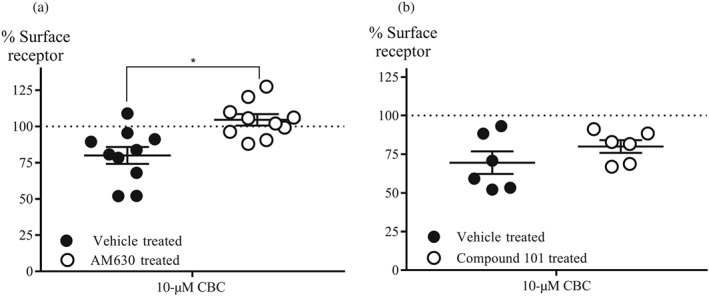
Effect of AM630 and Compound 101 on CBC internalization of CB2 cell surface receptors. (a) Summary data of the effect of AM630 on CBC internationalization of surface receptors. Cells were pretreated with AM630 (3‐μM, 5 min) followed by CBC (10‐μM, 30 min) in the continuous presence of antagonist (n = 10). *P < .05, significantly different as indicated; One‐way ANOVA followed by Tukey post‐hoc test. (b) Summary data of the effect of Compound 101 on CBC internationalization of surface receptors. Cells were pretreated with Compound 101 (10‐μM, 60 min), followed by CBC (10‐μM, 30 min) in the continuous presence of the GRK2/3 inhibitor (n = 5). All results are expressed as mean percentage of the basal surface receptor level (BSL) ± SEM, which is the percentage of vehicle‐treated AtT20‐CB2 cells, after subtraction of background signal
3.5. Effect of CBC on desensitization of CB2 receptor signalling
In the membrane potential assay, prolonged stimulation of AtT20 cells expressing cannabinoid receptors results in the slow reversal of cellular hyperpolarization (Cawston et al., 2013). Continuous stimulation of CB2 receptors for 30 min by 1‐μM CP55,940 or 10‐μM CBC resulted in a reversal of the cellular hyperpolarization by 88 ± 3% (Figure 6a) and 73 ± 6% (Figure 6b–c) respectively. The desensitization did not change significantly when cells were pretreated with Compound 101 (10‐μM, 60 min; Figure 6a–c).
Figure 6.
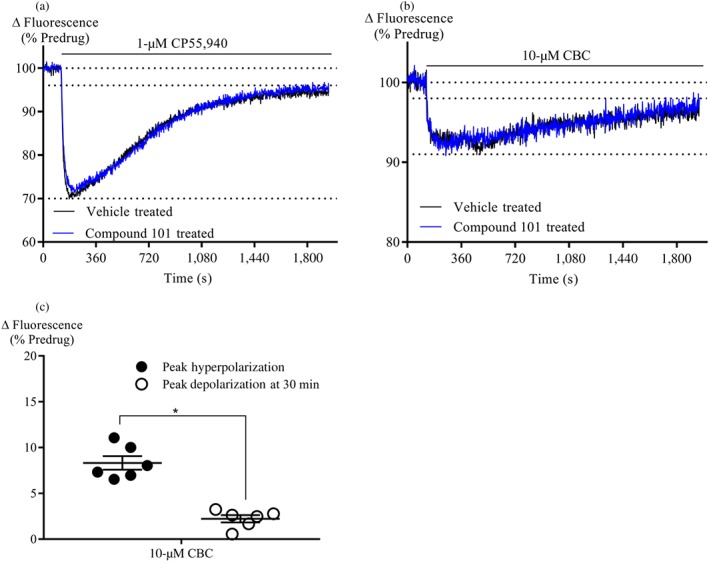
Desensitization of AtT20‐CB2 cells signalling. (a) A representative trace of 1‐μM CP55,940 desensitization of AtT20‐CB2 cell signalling in the presence of vehicle or Compound 101. (b) A representative trace of 10‐μM CBC desensitization of AtT20‐CB2 cell signalling in the presence of vehicle or Compound 101. Cells were pre‐incubated with Compound 101 (10‐μM, 60 min) before CP55,940 or CBC addition. CP55,940 or CBC were added after 2 min of baseline reading and read for 30 min. (c) Summary data of CBC (10‐μM, 30 min) desensitization of AtT20‐CB2 receptor signalling. Peak hyperpolarization was determined within 5 min of drug addition, and peak depolarization was determined at 30 min of drug addition. All data are expressed as mean change in fluorescence due to cellular hyperpolarization ± SEM, after subtraction of baseline (n = 6). *P < .05, significantly different as indicated; Unpaired Student's t‐test. Note: truncated axes
4. DISCUSSION
In this study, we have found that CBC is a phytocannabinoid with selective CB2 receptor agonist actions. We have also provided evidence that it signals through the Gi/o type G proteins and induces CB2 receptor internalization and signalling desensitization that is independent of GRK2/3 kinases.
CBC produced a dose‐dependent cell activation indicated by cellular hyperpolarization in CB2 cells but with no analogous hyperpolarization in CB1 cells. This is consistent with a previous finding that CBC apparently does not stimulate [35S]GTPγS binding via CB1 receptors expressed in CHO cells (Romano et al., 2013) or inhibit AC activity in N18 cells natively expressing mouse CB1 receptors (Howlett, 1987). Although no cannabinoid‐antagonist dependent effects have been elucidated in other assays, CBC has been reported to weakly inhibit cellular AEA uptake and the 2‐arachidonoyl‐glycerol hydrolyzing enzyme https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1399, both of which may conceivably lead to an indirect activation of cannabinoid receptors through increase in extracellular endocannabinoids (De Petrocellis et al., 2011; Ligresti et al., 2006). However, the rapid onset of cellular hyperpolarization in CB2 cells upon addition of CBC suggests a direct receptor activation. Our findings are also consistent with previous studies which concluded that CBC does not significantly affect the CB1 receptor‐mediated psychoactive effects of cannabis in vivo (DeLong et al., 2010; Ilan, Gevins, Coleman, ElSohly, & de Wit, 2005).
Cannabinoid receptors mediate downstream signalling predominantly through the Gi/o protein family (Mallipeddi, Janero, Zvonok, & Makriyannis, 2017), but CB1 receptors can couple Gs proteins when there is no functional Gi/o coupling (Bonhaus, Chang, Kwan, & Martin, 1998; Glass & Felder, 1997) and affect Gq in some environments (Lauckner, Hille, & Mackie, 2005). The loss of CBC signalling upon PTX treatment confirms Gi/o‐protein coupling in the hyperpolarization assay, consistent with previous findings with these cells (Banister et al., 2016).
CBC‐induced hyperpolarization in CB2 cells was absent in wild‐type AtT20 cells and blocked by the selective CB2 receptor antagonist AM630. This blockade is likely due to competitive binding at the CB2 receptor site, supporting the hypothesis that CBC effects are mediated through the CB2 receptor orthosteric site. It is noteworthy that SR144,528, a CB2 receptor antagonist, does not block the anti‐inflammatory effects of CBC either in vitro (inhibition of nitrite formation in peritoneal macrophages) or in vivo (LPS‐induced paw oedema) assays (DeLong et al., 2010; Romano et al., 2013). The receptor mechanisms underlying these anti‐inflammatory effects are not yet fully defined.
THC is a low efficacy agonist in many assays of CB1 and CB2 receptor function (Bayewitch et al., 1996; Soethoudt et al., 2017). Therefore, we investigated whether CBC could be acting as an antagonist at the CB1 receptor, as it had been previously reported to bind at the CB1 receptors, albeit with lower affinity than CB2 receptors (Rosenthaler et al., 2014). Using sub‐maximal concentrations of a high efficacy agonist (300‐nM CP55,940) and maximum concentration of a lower efficacy agonist (10‐μM THC), we found that CBC did not alter the onset, and extent, of cellular hyperpolarization in cells expressing CB1 receptors. These suggest that CBC does not significantly interact with the CB1 receptor site. However, CBC significantly reduced the extent of CP55,940‐induced hyperpolarization in CB2 cells after 5‐min treatment. This is likely due to receptor desensitization, as CBC (10‐μM) and CP55940 (300 nM) added at the same time produced a similar effect to CP55940 (300 nM) alone. We showed that lower concentration of CP55940 (100 nM) also produced a modest degree of desensitization to subsequent addition of a higher concentration (300 nM) of same drug.
Stimulation of both CB1 and CB2 receptors has been implicated in antinociception (Bisogno et al., 2009; Guindon, Desroches, & Beaulieu, 2007; Kinsey, Long, Cravatt, & Lichtman, 2010; La Rana et al., 2006; Lichtman, Shelton, Advani, & Cravatt, 2004). CB1 receptors are involved in the attenuation of synaptic transmission of nociception in the brain and primary afferent neurons, while CB2 receptors contribute to antinociception by inhibiting the release of proinflammatory factors around nociceptive neuron terminals (Manzanares, Julian, & Carrascosa, 2006). As THC analgesia is at least partly mediated through CB1 receptors (Mao, Price, Lu, Keniston, & Mayer, 2000) and CBC is a ligand for CB2 receptors, it is possible that the potentiation of THC analgesia by CBC, in addition to pharmacokinetic interaction (Davis & Hatoum, 1983; DeLong et al., 2010), may be a result of CBC stimulation of CB2 receptor‐mediated inhibition of the release of proinflammatory factors. Apart from CB2 receptor‐related anti‐inflammatory activities, CBC may act directly or indirectly on proteins such as TRPA1 or https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=18 (De Petrocellis et al., 2008; Maione et al., 2011; Shinjyo & Di Marzo, 2013).
Upon sustained exposure to agonists, CB2 receptors undergo receptor internalization, resulting in signalling desensitization (Bouaboula, Dussossoy, & Casellas, 1999; Shoemaker, Joseph, Ruckle, Mayeux, & Prather, 2005). Our results show that CBC caused both loss of surface receptors and signalling desensitization of CB2 receptors . However, the loss of cell surface receptors was less than that observed with CP55,940. This may be due to lower efficacy of CBC in comparison to CP55,940, which is among the most efficacious cannabinoids for internalization (Atwood, Wager‐Miller, Haskins, Straiker, & Mackie, 2012). CBC‐induced loss of surface CB2 receptors was antagonized by AM630, an effect that further underlines that the agonist effect of CBC is CB2 receptor mediated. AM630 is an inverse agonist at CB2 receptors (Ross et al., 1999) and has previously been reported to increase (Grimsey et al., 2011), or have no effect (Atwood et al., 2012), on CB2 surface receptor levels. Under our experimental conditions, AM630 did not have any appreciable effect on the cell surface receptors.
In the canonical view, GPCR signal desensitization is usually mediated by GRK‐mediated phosphorylation of GPCRs with phosphorylated receptors interacting with arrestins to prevent further downstream signalling (Gainetdinov, Premont, Bohn, Lefkowitz, & Caron, 2004). Information about the mechanisms of desensitization of CB2 receptor signalling is sparse, and the GRK involved in CBC‐induced CB2 surface receptor internalization and desensitization have not been identified. Our results suggest that the GRK2/3 kinases are likely not to be involved in these processes, consistent with previous findings suggesting that GRK2/3 were probably not involved in CB2 receptor internalization (Bouaboula et al., 1999).
Beta‐caryophyllene, which is a terpenoid found in relative abundance within cannabis and food plants, is a naturally occurring, CB2 receptor‐selective agonist (Gertsch et al., 2008). It has CB2 receptor‐mediated anti‐inflammatory activities both in vitro and in vivo. Here, we have shown that CBC, a phytocannabinoid, is also a CB2 receptor‐selective agonist. This selectivity implies that CBC and/or its derivatives may be further investigated as potential therapeutic agents influencing the non‐psychotropic CB2 receptor pathways of the ECS. Understanding its mechanism of anti‐inflammatory activity in vitro and in vivo, as well as activity at other targets, would be valuable in developing its therapeutic potential. Notably, the combination of CBC with THC produces enhanced antinociception and anti‐inflammatory responses in vivo (Davis & Hatoum, 1983; DeLong et al., 2010). This may reflect pharmacokinetic interactions with THC but also the pharmacodynamic effects of CBC itself on inflammatory processes. Further research might investigate CBC in combination with THC and CBD to formulate an optimal analgesic cannabis‐based medicine, with minor psychotropic effects and potentiated analgesia.
AUTHOR CONTRIBUTIONS
M.U., M.S., and M.C. designed and analysed experiments. S.D. synthesized CBC, and M.U. conducted all other experiments. M.U., S.D., M.S., I.M., and M.C. prepared the manuscript. All authors have seen the final paper.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1‐S2. CBC does not significantly influence CP55,940 activation of CB2 receptors, when co‐administered. S1. Representative traces of changes in fluorescence, due to CBC (10‐μM), CP55,940 (300 nM) and their mixture induced hyperpolarisation in AtT20 CB2 cells. Drugs were added after 120 sec of baseline reading and read over 300 sec. S2: Summary data of the mixture of CBC (10‐μM), CP55,940 (300 nM) and their mixture in AtT20 CB2 cells. Results are expressed as mean ± SEM after normalisation to 1‐μM CP55,940 hyperpolarisation.
Figure S3‐S4. CB2 receptors are desensitised at 5 min of agonist stimulation. S3 Representative traces of changes in fluorescence, due to 300 nM CP55,940 hyperpolarisation after pre‐treatment with 100 nM CP55940 in AtT20 CB2 cells. After 2mins baseline reading, cells were pre‐treated with vehicle or 100 nM CP55,940 for 5 min, followed by addition of 300 nM CP55,940. S4 Summary data of the effect of 100 nM CP55,940 on 300 nM CP55,940 in AtT20‐CB2 cells. Results are expressed as mean ± SEM after normalization to 1‐μM CP55,940 hyperpolarisation.
ACKNOWLEDGEMENTS
M.U. is a recipient of the Macquarie University Postgraduate Research Scholarships for International Students. We thank Dr Samuel Banister for his helpful comments on this study.
Udoh M, Santiago M, Devenish S, McGregor IS, Connor M. Cannabichromene is a cannabinoid CB2 receptor agonist. Br J Pharmacol. 2019;176:4537–4547. 10.1111/bph.14815
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Marrion, N. V. , Peters, J. A. , … CGTP Collaborators (2017). The concise guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. British Journal of Pharmacology, 174, S17–S129. 10.1111/bph.13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174, S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood, B. K. , Wager‐Miller, J. , Haskins, C. , Straiker, A. , & Mackie, K. (2012). Functional selectivity in CB2 cannabinoid receptor signaling and regulation: Implications for the therapeutic potential of CB2 ligands. Molecular Pharmacology, 81(2), 250–263. 10.1124/mol.111.074013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banister, S. D. , Longworth, M. , Kevin, R. , Sachdev, S. , Santiago, M. , Stuart, J. , … Kassiou, M. (2016). Pharmacology of valinate and tert‐leucinate synthetic cannabinoids 5F‐AMBICA, 5F‐AMB, 5F‐ADB, AMB‐FUBINACA, MDMB‐FUBINACA, MDMB‐CHMICA, and their analogues. ACS Chemical Neuroscience, 7(9), 1241–1254. 10.1021/acschemneuro.6b00137 [DOI] [PubMed] [Google Scholar]
- Barann, M. , Molderings, G. , Brüss, M. , Bönisch, H. , Urban, B. W. , & Göthert, M. (2002). Direct inhibition by cannabinoids of human 5‐HT3A receptors: Probable involvement of an allosteric modulatory site. British Journal of Pharmacology, 137(5), 589–596. 10.1038/sj.bjp.0704829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayewitch, M. , Rhee, M. H. , Avidor‐Reiss, T. , Breuer, A. , Mechoulam, R. , & Vogel, Z. (1996). (‐)‐Δ9‐tetrahydrocannabinol antagonizes the peripheral cannabinoid receptor‐mediated inhibition of adenylyl cyclase. The Journal of Biological Chemistry, 271(17), 9902–9905. 10.1074/jbc.271.17.9902 [DOI] [PubMed] [Google Scholar]
- Bisogno, T. , Ortar, G. , Petrosino, S. , Morera, E. , Palazzo, E. , Nalli, M. , … Endocannabinoid Research Group (2009). Development of a potent inhibitor of 2‐arachidonoylglycerol hydrolysis with antinociceptive activity in vivo. Biochimica et Biophysica Acta (BBA) ‐ Molecular and Cell Biology of Lipids, 1791(1), 53–60. 10.1016/j.bbalip.2008.10.007 [DOI] [PubMed] [Google Scholar]
- Bolognini, D. , Costa, B. , Maione, S. , Comelli, F. , Marini, P. , Di Marzo, V. , … Pertwee, R. G. (2010). The plant cannabinoid Δ9‐tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. British Journal of Pharmacology, 160(3), 677–687. 10.1111/j.1476-5381.2010.00756.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhaus, D. W. , Chang, L. K. , Kwan, J. , & Martin, G. R. (1998). Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: Evidence for agonist‐specific trafficking of intracellular responses. The Journal of Pharmacology and Experimental Therapeutics, 287, 884–888. [PubMed] [Google Scholar]
- Bouaboula, M. , Dussossoy, D. , & Casellas, P. (1999). Regulation of peripheral cannabinoid receptor CB2 phosphorylation by the inverse agonist SR 144528. Implications for receptor biological responses. The Journal of Biological Chemistry, 274(29), 20397–20405. 10.1074/JBC.274.29.20397 [DOI] [PubMed] [Google Scholar]
- Cawston, E. E. , Redmond, W. J. , Breen, C. M. , Grimsey, N. L. , Connor, M. , & Glass, M. (2013). Real‐time characterization of cannabinoid receptor 1 (CB1) allosteric modulators reveals novel mechanism of action. British Journal of Pharmacology, 170(4), 893–907. 10.1111/bph.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Bond, R. A. , Spina, D. , Ahluwalia, A. , Alexander, S. P. A. , Giembycz, M. A. , … McGrath, J. C. (2015). Experimental design and analysis and their reporting: New guidance for publication in BJP. British Journal of Pharmacology, 172(14), 3461–3471. 10.1111/bph.12856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, W. M. , & Hatoum, N. S. (1983). Neurobehavioral actions of cannabichromene and interactions with Δ9‐tetrahydrocannabinol. General Pharmacology, 14(2), 247–252. 10.1016/0306-3623(83)90004-6 [DOI] [PubMed] [Google Scholar]
- De Petrocellis, L. , Ligresti, A. , Moriello, A. S. , Allarà, M. , Bisogno, T. , Petrosino, S. , … Di Marzo, V. (2011). Effects of cannabinoids and cannabinoid‐enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. British Journal of Pharmacology, 163(7), 1479–1494. 10.1111/j.1476-5381.2010.01166.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis, L. , Vellani, V. , Schiano‐Moriello, A. , Marini, P. , Magherini, P. C. , Orlando, P. , & Di Marzo, V. (2008). Plant‐derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type‐1 and melastatin type‐8. Journal of Pharmacology and Experimental Therapeutics, 325(3), 1007–1015. 10.1124/jpet.107.134809 [DOI] [PubMed] [Google Scholar]
- DeLong, G. T. , Wolf, C. E. , Poklis, A. , & Lichtman, A. H. (2010). Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Δ9‐tetrahydrocannabinol. Drug and Alcohol Dependence, 112(1–2), 126–133. 10.1016/J.DRUGALCDEP.2010.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, L. , Guindon, J. , Cornett, B. L. , Makriyannis, A. , Mackie, K. , & Hohmann, A. G. (2015). Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1‐dependent withdrawal. Biological Psychiatry, 77(5), 475–487. 10.1016/j.biopsych.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly, M. , & Gul, W. (2014). Constituents of cannabis sativa In Pertwee R. G. (Ed.), Handbook of cannabis (pp. 3–22). Oxford, UK: Oxford University Press; ISBN: 978‐0‐19‐966268‐5. 10.1093/acprof:oso/9780199662685.003.0001 [DOI] [Google Scholar]
- Flores‐Sanchez, I. J. , & Verpoorte, R. (2008). Secondary metabolism in cannabis. Phytochemistry Reviews, 7(3), 615–639. 10.1007/s11101-008-9094-4 [DOI] [Google Scholar]
- Gainetdinov, R. R. , Premont, R. T. , Bohn, L. M. , Lefkowitz, R. J. , & Caron, M. G. (2004). Desensitization of G protein–coupled receptors and neuronal functions. Annual Review of Neuroscience, 27, 107–144. 10.1146/annurev.neuro.27.070203.144206 [DOI] [PubMed] [Google Scholar]
- Gertsch, J. , Leonti, M. , Raduner, S. , Racz, I. , Chen, J.‐Z. , Xie, X.‐Q. , … Zimmer, A. (2008). Beta‐caryophyllene is a dietary cannabinoid. Proceedings of the National Academy of Sciences, 105(26), 9099–9104. 10.1073/pnas.0803601105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, M. , & Felder, C. C. (1997). Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: Evidence for a Gs linkage to the CB1 receptor. The Journal of Neuroscience, 17(14), 5327–5333. 10.1523/JNEUROSCI.17-14-05327.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsey, N. L. , Goodfellow, C. E. , Dragunow, M. , & Glass, M. (2011). Cannabinoid receptor 2 undergoes Rab5‐mediated internalization and recycles via a Rab11‐dependent pathway. Biochimica et Biophysica Acta (BBA) ‐ Molecular Cell Research, 1813(8), 1554–1560. 10.1016/J.BBAMCR.2011.05.010 [DOI] [PubMed] [Google Scholar]
- Guindon, J. , Desroches, J. , & Beaulieu, P. (2007). The antinociceptive effects of intraplantar injections of 2‐arachidonoyl glycerol are mediated by cannabinoid CB2 receptors. British Journal of Pharmacology, 150(6), 693–701. 10.1038/sj.bjp.0706990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett, A. C. (1987). Cannabinoid inhibition of adenylate cyclase: Relative activity of constituents and metabolites of Marihuana. Neuropharmacology, 26(5), 507–512. 10.1016/0028-3908(87)90035-9 [DOI] [PubMed] [Google Scholar]
- Iannotti, F. A. , Di Marzo, V. , & Petrosino, S. (2016). Endocannabinoids and endocannabinoid‐related mediators: Targets, metabolism and role in neurological disorders. Progress in Lipid Research, 62, 107–128. 10.1016/J.PLIPRES.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Ignatowska‐Jankowska, B. , Jankowski, M. M. , & Swiergiel, A. H. (2011). Cannabidiol decreases body weight gain in rats: Involvement of CB2 receptors. Neuroscience Letters, 490(1), 82–84. 10.1016/J.NEULET.2010.12.031 [DOI] [PubMed] [Google Scholar]
- Ilan, A. B. , Gevins, A. , Coleman, M. , ElSohly, M. A. , & de Wit, H. (2005). Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behavioural Pharmacology, 16(5–6), 487–496. 10.1097/00008877-200509000-00023 [DOI] [PubMed] [Google Scholar]
- Izzo, A. A. , Borrelli, F. , Capasso, R. , Di Marzo, V. , & Mechoulam, R. (2009). Non‐psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends in Pharmacological Sciences, 30(10), 515–527. 10.1016/J.TIPS.2009.07.006 [DOI] [PubMed] [Google Scholar]
- Kinsey, S. G. , Long, J. Z. , Cravatt, B. F. , & Lichtman, A. H. (2010). Fatty acid amide hydrolase and monoacylglycerol lipase inhibitors produce anti‐allodynic effects in mice through distinct cannabinoid receptor mechanisms. The Journal of Pain, 11(12), 1420–1428. 10.1016/j.jpain.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rana, G. , Russo, R. , Campolongo, P. , Bortolato, M. , Mangieri, R. A. , Cuomo, V. , … Calignano, A. (2006). Modulation of neuropathic and inflammatory pain by the endocannabinoid transport inhibitor AM404 [N‐(4‐hydroxyphenyl)‐eicosa‐5,8,11,14‐tetraenamide]. Journal of Pharmacology and Experimental Therapeutics, 317(3), 1365–1371. 10.1124/jpet.105.100792 [DOI] [PubMed] [Google Scholar]
- Laprairie, R. B. , Bagher, A. M. , Kelly, M. E. M. , & Denovan‐Wright, E. M. (2015). Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. British Journal of Pharmacology, 172(20), 4790–4805. 10.1111/bph.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner, J. E. , Hille, B. , & Mackie, K. (2005). The cannabinoid agonist WIN55,212‐2 increases intracellular calcium via CB 1 receptor coupling to Gq/11 G proteins. Proceedings of the National Academy of Sciences of the United States of America, 102(52), 19144–19149. 10.1073/pnas.0509588102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner, J. E. , Jensen, J. B. , Chen, H.‐Y. , Lu, H.‐C. , Hille, B. , & Mackie, K. (2008). GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proceedings of the National Academy of Sciences of the United States of America, 105(7), 2699–2704. 10.1073/pnas.0711278105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.‐R. , & Wang, X. (2005). Concise synthesis of biologically interesting (±)‐cannabichromene, (±)‐cannabichromenic acid, and (±)‐daurichromenic acid. Bulletin of the Korean Chemical Society, 26, 1933–1936. 10.5012/bkcs.2005.26.12.1933 [DOI] [Google Scholar]
- Lichtman, A. H. , Shelton, C. C. , Advani, T. , & Cravatt, B. F. (2004). Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor‐mediated phenotypic hypoalgesia. Pain, 109(3), 319–327. 10.1016/j.pain.2004.01.022 [DOI] [PubMed] [Google Scholar]
- Ligresti, A. , Moriello, A. S. , Starowicz, K. , Matias, I. , Pisanti, S. , De Petrocellis, L. , … Di Marzo, V. (2006). Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. Journal of Pharmacology and Experimental Therapeutics, 318(3), 1375–1387. 10.1124/jpet.106.105247 [DOI] [PubMed] [Google Scholar]
- Longworth, M. , Banister, S. D. , Boyd, R. , Kevin, R. C. , Connor, M. , McGregor, I. S. , & Kassiou, M. (2017). Pharmacology of cumyl‐carboxamide synthetic cannabinoid new psychoactive substances (NPS) CUMYL‐BICA, CUMYL‐PICA, CUMYL‐5F‐PICA, CUMYL‐5F‐PINACA, and their analogues. ACS Chemical Neuroscience, 8(10), 2159–2167. 10.1021/acschemneuro.7b00267 [DOI] [PubMed] [Google Scholar]
- Lowe, J. D. , Sanderson, H. S. , Cooke, A. E. , Ostovar, M. , Tsisanova, E. , Withey, S. L. , … Bailey, C. P. (2015). Role of G protein‐coupled receptor kinases 2 and 3 in μ‐opioid receptor desensitization and internalization. Molecular Pharmacology, 88(2), 347–356. 10.1124/mol.115.098293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione, S. , Piscitelli, F. , Gatta, L. , Vita, D. , De Petrocellis, L. , Palazzo, E. , … Di Marzo, V. (2011). Non‐psychoactive cannabinoids modulate the descending pathway of antinociception in anaesthetized rats through several mechanisms of action. British Journal of Pharmacology, 162(3), 584–596. 10.1111/j.1476-5381.2010.01063.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallipeddi, S. , Janero, D. R. , Zvonok, N. , & Makriyannis, A. (2017). Functional selectivity at G‐protein coupled receptors: Advancing cannabinoid receptors as drug targets. Biochemical Pharmacology, 128, 1–11. 10.1016/j.bcp.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares, J. , Julian, M. , & Carrascosa, A. (2006). Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Current Neuropharmacology, 4(3), 239–257. 10.2174/157015906778019527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, J. , Price, D. D. , Lu, J. , Keniston, L. , & Mayer, D. J. (2000). Two distinctive antinociceptive systems in rats with pathological pain. Neuroscience Letters, 280(1), 13–16. 10.1016/S0304-3940(99)00998-2 [DOI] [PubMed] [Google Scholar]
- Marsicano, G. , & Lutz, B. (1999). Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. European Journal of Neuroscience, 11(12), 4213–4225. 10.1046/j.1460-9568.1999.00847.x [DOI] [PubMed] [Google Scholar]
- Mehmedic, Z. , Chandra, S. , Slade, D. , Denham, H. , Foster, S. , Patel, A. S. , … ElSohly, M. A. (2010). Potency trends of Δ9‐THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. Journal of Forensic Sciences, 55(5), 1209–1217. 10.1111/j.1556-4029.2010.01441.x [DOI] [PubMed] [Google Scholar]
- O'Sullivan, S. E. , Tarling, E. J. , Bennett, A. J. , Kendall, D. A. , & Randall, M. D. (2005). Novel time‐dependent vascular actions of Δ9‐tetrahydrocannabinol mediated by peroxisome proliferator‐activated receptor γ. Biochemical and Biophysical Research Communications, 337(3), 824–831. 10.1016/j.bbrc.2005.09.121 [DOI] [PubMed] [Google Scholar]
- Pertwee, R. G. (1999). Pharmacology of cannabinoid receptor ligands. Current Medicinal Chemistry, 6(8), 635–664. [PubMed] [Google Scholar]
- Potter, D. J. , Clark, P. , & Brown, M. B. (2008). Potency of Δ9–THC and other cannabinoids in cannabis in England in 2005: Implications for psychoactivity and pharmacology. Journal of Forensic Sciences, 53(1), 90–94. 10.1111/j.1556-4029.2007.00603.x [DOI] [PubMed] [Google Scholar]
- Redmond, W. J. , Cawston, E. E. , Grimsey, N. L. , Stuart, J. , Edington, A. R. , Glass, M. , & Connor, M. (2016). Identification of N‐arachidonoyl dopamine as a highly biased ligand at cannabinoid CB1 receptors. British Journal of Pharmacology, 173(1), 115–127. 10.1111/bph.13341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, M. H. , Vogel, Z. , Barg, J. , Bayewitch, M. , Levy, R. , Hanus, L. , … Mechoulam, R. (1997). Cannabinol derivatives: Binding to cannabinoid receptors and inhibition of adenylylcyclase. Journal of Medicinal Chemistry, 40(20), 3228–3233. 10.1021/jm970126f [DOI] [PubMed] [Google Scholar]
- Romano, B. , Borrelli, F. , Fasolino, I. , Capasso, R. , Piscitelli, F. , Cascio, M. , … Izzo, A. (2013). The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. British Journal of Pharmacology, 169(1), 213–229. 10.1111/bph.12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthaler, S. , Pöhn, B. , Kolmanz, C. , Huu, C. N. , Krewenka, C. , Huber, A. , … Moldzio, R. (2014). Differences in receptor binding affinity of several phytocannabinoids do not explain their effects on neural cell cultures. Neurotoxicology and Teratology, 46, 49–56. 10.1016/j.ntt.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Ross, R. A. , Brockie, H. C. , Stevenson, L. A. , Murphy, V. L. , Templeton, F. , Makriyannis, A. , & Pertwee, R. G. (1999). Agonist‐inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. British Journal of Pharmacology, 126(3), 665–672. 10.1038/sj.bjp.0702351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, H. R. , Napier, I. , Connor, M. (2008). Inhibition of recombinant human T‐type calcium channels by delta9‐tetrahydrocannabinol and cannabidiol. Journal of Biological Chemistry, 283, 16124–16134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg, E. , Larsson, N. , Sjögren, S. , Hjorth, S. , Hermansson, N.‐O. , Leonova, J. , … Greasley, P. J. (2007). The orphan receptor GPR55 is a novel cannabinoid receptor. British Journal of Pharmacology, 152(7), 1092–1101. 10.1038/sj.bjp.0707460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinjyo, N. , & Di Marzo, V. (2013). The effect of cannabichromene on adult neural stem/progenitor cells. Neurochemistry International, 63(5), 432–437. 10.1016/j.neuint.2013.08.002 [DOI] [PubMed] [Google Scholar]
- Shoemaker, J. L. , Joseph, B. K. , Ruckle, M. B. , Mayeux, P. R. , & Prather, P. L. (2005). The endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. The Journal of Pharmacology and Experimental Therapeutics, 314(2), 868–875. 10.1124/jpet.105.085282 [DOI] [PubMed] [Google Scholar]
- Soethoudt, M. , Grether, U. , Fingerle, J. , Grim, T. W. , Fezza, F. , de Petrocellis, L. , … van der Stelt, M. (2017). Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off‐target activity. Nature Communications, 8, 13958 10.1038/ncomms13958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift, W. , Wong, A. , Li, K. M. , Arnold, J. C. , & McGregor, I. S. (2013). Analysis of cannabis seizures in NSW, Australia: Cannabis potency and cannabinoid profile. PLoS ONE, 8(7), e70052 10.1371/journal.pone.0070052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, C. E. , & ElSohly, M. A. (1981). Biological activity of cannabichromene, its homologs and isomers. Journal of Clinical Pharmacology, 21(S1), 283S–291S. 10.1002/j.1552-4604.1981.tb02606.x [DOI] [PubMed] [Google Scholar]
- Turner, C. E. , ElSohly, M. A. , & Boeren, E. G. (1980). Constituents of Cannabis sativa L. XVII. A review of the natural constituents. Journal of Natural Products, 43(2), 169–234. 10.1021/np50008a001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1‐S2. CBC does not significantly influence CP55,940 activation of CB2 receptors, when co‐administered. S1. Representative traces of changes in fluorescence, due to CBC (10‐μM), CP55,940 (300 nM) and their mixture induced hyperpolarisation in AtT20 CB2 cells. Drugs were added after 120 sec of baseline reading and read over 300 sec. S2: Summary data of the mixture of CBC (10‐μM), CP55,940 (300 nM) and their mixture in AtT20 CB2 cells. Results are expressed as mean ± SEM after normalisation to 1‐μM CP55,940 hyperpolarisation.
Figure S3‐S4. CB2 receptors are desensitised at 5 min of agonist stimulation. S3 Representative traces of changes in fluorescence, due to 300 nM CP55,940 hyperpolarisation after pre‐treatment with 100 nM CP55940 in AtT20 CB2 cells. After 2mins baseline reading, cells were pre‐treated with vehicle or 100 nM CP55,940 for 5 min, followed by addition of 300 nM CP55,940. S4 Summary data of the effect of 100 nM CP55,940 on 300 nM CP55,940 in AtT20‐CB2 cells. Results are expressed as mean ± SEM after normalization to 1‐μM CP55,940 hyperpolarisation.


