Abstract
Currently, neoadjuvant chemotherapy is a standard therapeutic strategy for breast cancer, as it can provide timely and individualized chemo-sensitivity information and is beneficial for custom-designing subsequent treatment strategies. To accurately select candidates for neoadjuvant chemotherapy, the association between various immunohistochemical biomarkers of primary disease and tumor response to neoadjuvant chemotherapy has been investigated, and results have shown that certain pathological indicators evaluated after neoadjuvant chemotherapy are associated with long-term prognosis. The Food and Drug Administration (FDA) has recommended that complete pathological response can be used as a surrogate endpoint for neoadjuvant chemotherapy, which is related to better prognosis. Considering that residual tumor persists in the majority of patients after neoadjuvant chemotherapy, the value of various pathological indicators of residual disease in predicting the long-term outcomes is being extensively investigated. This review summarizes and compares various predictive and prognostic indicators for patients who have received neoadjuvant chemotherapy, and analyzes their efficacy in different breast cancer subtypes.
Keywords: Biomarkers, Breast neoplasms, Neoadjuvant therapy, Pathology, Survival
INTRODUCTION
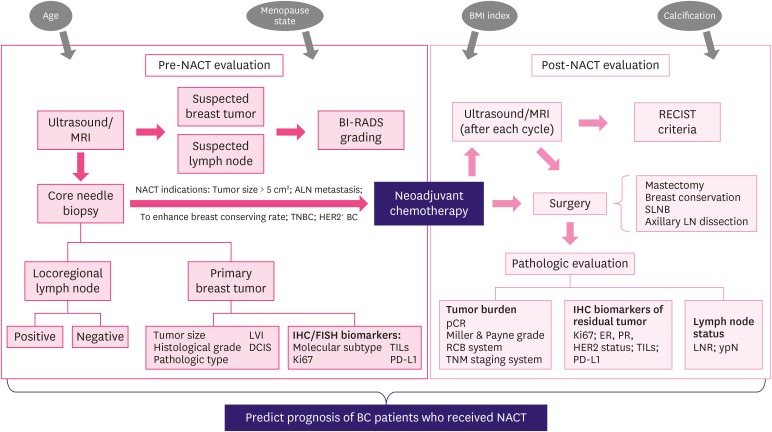
Breast cancer (BC) is one of the most common diagnosed malignant diseases that severely threatens women's life worldwide [1]. Neoadjuvant chemotherapy (NACT) has been accepted as a standard therapeutic strategy for patients with locally advanced BC and inflammatory BC, as it reduces tumor burden prior to surgery, and improves the success rates of operation and the chances of breast conservation surgery [2,3]. Currently, pathological assessment of breast tissue and metastatic lymph nodes after surgery is the main approach for evaluating the treatment response of patients to NACT, which is considered the gold standard. Considering the large number of studies showing that achievement of pathological complete response (pCR) was clearly predictive of improved outcome with a lower probability of recurrence and death [4,5], the Food and Drug Administration (FDA) suggested pCR as a surrogate endpoint for accelerated appraisal of new drugs for NACT in patients with BC. However, some patients still relapse or die even after achieving pCR, whereas some patients without pCR have favorable outcomes; thus, pCR cannot be considered the single standard for predicting long-term survival. Furthermore, all patients do not benefit from NACT, as some patients develop drug resistance and present with stable disease or even disease progression during NACT, thereby missing the best surgical opportunity. Therefore, identification of more biomarkers is important for improving response evaluation and risk stratification before and after NACT, selecting appropriate candidates of NACT, and avoiding unnecessary chemotherapy-related toxicity for patients who respond less to NACT. Furthermore, as a large number of patients with BC who do not achieve pCR usually suffer from higher risk of recurrence and death, it is important to identify more pathological response assessment approaches for evaluating morphological changes and cancer cell regression of tumors in patients with residual disease after NACT. Various approaches have been reported, among which post-treatment pathological grading of Ki-67, hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status, and lymph node status after treatment have been shown to provide prognostic information for patients treated with NACT. In addition, assessment of pretreatment biomarkers, including Ki-67 level, histological grade, and molecular subtype of BC, can also predict the response of patients to NACT (Figure 1).
Figure 1. The general clinical evaluating process of BC patients before and after NACT. Some evaluating results might provide additional prognostic information for patients received NACT (TILs and PD-L1 examination have not been routinely used in current clinical practice).
BC = breast cancer; NACT = neoadjuvant chemotherapy; TILs = tumor-infiltrating lymphocytes; PD-L1 = programmed death ligand 1; MRI = magnetic resonance imaging; BI-RADS = Breast Imaging, Reporting and Data System; LVI = lymphovascular invasion; DCIS = ductal carcinoma in situ; IHC = immunohistochemistry; FISH = fluorescence in situ hybridization; RECIST = Response Evaluation Criteria in Solid Tumors; SLNB = sentinel lymph node biopsy; LN = lymph node; pCR = pathological complete response; RCB = residual cancer burden; TNM = tumor-node-metastasis; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2; LNR = lymph node ratio.
Nevertheless, the results of the previous studies are discordant, indicating that these biomarkers within different study populations might reveal diverse predictive and prognostic efficacy. The discordance might be explained by the heterogeneity of biological features and diversity in the chemo-sensitivity of different BC intrinsic subtypes. Indeed, rational investigation of the predictive and prognostic factors and their analysis in different patient subgroups are beneficial for identifying patients with high risk of relapse or death and require further adjuvant treatment. Therefore, the objective of this review is to systematically overview the predictive and prognostic value of a series of pathological indicators for patients who have received NACT. Apart from the baseline level and post-treatment level of these biomarkers, alterations in the expression of these indicators during NACT and the long-term prognosis have also been reviewed.
pCR
Evaluating the criteria for pCR
Based on the principles of various classification systems, pCR has been defined unanimously as the complete disappearance of invasive cancer cells in primary breast tumor [6,7]. However, whether the ductal carcinoma in situ (DCIS) and residual cancer cells present in axillary lymph nodes should be considered when defining pCR is still debated (Table 1) [6,8,9,10]. Mazouni and colleges [11] from the MD Anderson Cancer Center conducted a retrospective analysis including 2,302 patients treated with NACT and observed similar 5- and 10-year overall survival (OS) and disease-free survival (DFS) between the pCR group (patients achieved pCR both in breast and lymph nodes) and DCIS group (patients without residual disease in primary tumor and lymph nodes, but contained DCIS) after 250 months of follow-up [11]. Concordantly, a meta-analysis of 12 clinical trials conducted by Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC) demonstrated similar event-free survival (EFS) and OS rates irrespective of DCIS [5]. Although several studies have suggested that DCIS is not important for defining pCR, other researchers argue that DCIS might lead to higher local recurrence risk for patients after NACT [12]. Indeed, the individual characteristics of patients must be considered when analyzing the effect of DCIS on survival; thus, patients with DCIS should be evaluated and treated individually. In contrast, CTNeoBC conducted a pooled analysis and demonstrated that it was inappropriate to define patients with isolated cells in axillary lymph nodes as pCR [5]. Therefore, researchers of the Breast International Group-North American Breast Cancer Group (BIG-NABCG) defined pCR as the absence of invasive cancer cells in primary tumor and metastatic lymph nodes, while the presence of DCIS has not been explicitly concluded [13]. Concordantly, Morrow [14] indicated that the presence of residual tumor in axillary lymph nodes definitely increased the risk of relapse and death. Thus, the medical community has accepted that the assessment of axillary lymph node status following NACT is indispensable, as it improves the prognostic efficacy of pCR. A comprehensive review of the results of previous clinical studies showed that pCR can be defined as no residual invasive cancer in the breast and lymph nodes but allows for DCIS (ypT0/isypN0). Patients with residual invasive cancer cells in lymph nodes could not be supposed as achieving pCR, while those who remaining DCIS should be analyzed separately.
Table 1. Definition of pCR.
| Pathologic evaluating system | Definition of pCR |
|---|---|
| BIG-NABCG [13] | No invasive and in situ cancer cell in breast and axillary lymph nodes (ypT0 ypN0) or no invasive cancer cell both in primary breast lesion and metastatic lymph nodes no matter DCIS exists or not (ypT0/is ypN0). |
| JBCS [114]/GEPARDO [8] | No residual cancer cell in surgical specimen, the existence of DCIS should not be included into pCR (ypT0). |
| Miller and Payen system [10] | Absence of primary invasive carcinoma cells in breast tissue, regardless of DCIS (ypT0/is ypN0). |
| NSABP B18 study [6] | The existence of DCIS and positive lymph nodes were allowed (ypT0/is ypN0/+), but it is limited in the patients with clinical complete response. |
| MD Anderson Cancer Center [115,116] | No residual invasive cancer cell both in breast and lymph nodes. |
pCR = pathologic complete response; DCIS = ductal carcinoma in situ; BIG-NABCG = Breast International Group-North American Breast Cancer Group; JBCS = Japanese Breast Cancer Society; GEPARDO = German Preoperative Adriamycin-Docetaxel; NSABP = National Surgical Adjuvant Breast and Bowel Project.
Prognostic efficacy of pCR
pCR represents a satisfying treatment response to NACT. Furthermore, ever since studies of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-18 and B-27 showed significant prolongation of DFS and OS in patients who achieved pCR compared to those who retained residual disease after NACT [15], an increasing number of studies have investigated the prognostic value of pCR. Although BC of young women tend to be more aggressive with a relatively unfavorable prognosis [16], reports show that patients aged ≤ 40 years can also obtain significant survival benefit by achieving pCR after NACT [17]. As a prognostic indicator, pCR has the advantage of reflecting chemo-sensitivity in short time after NACT, which underscores the necessity of subsequent adjuvant treatment following surgery.
However, evidence showing that the increase in pCR rate may translate into corresponding improvement in EFS and OS is lacking, and not all patients who achieved pCR had better survival and lower recurrence rates. The limitation of pCR might be because of the following: 1) some other risk factors of patients might weaken the prognostic benefit of pCR. Although several studies have suggested correlation between pCR and better prognosis, a small group of people show disease relapse and metastasis in the short term, even after achieving pCR after NACT. According to previous studies, factors such as HER2-positivity, axillary lymph nodes metastases, identified lymph nodes < 10, premenopausal status, and clinical stage IIIB–C might enhance the recurrence or metastasis rates for patients who have achieved pCR [18,19,20]; 2) the rates of pCR are discordant among different subtypes and the prognostic effects of pCR do not apply to all molecular subtypes of BC. Studies have shown that pCR rate is higher in patients with triple-negative breast cancer (TNBC) and HER2-positivity than in HR-positive/HER2-negative patients [21,22]. According to the results of CTNeoBC meta-analysis, patients with highly aggressive subtype (such as TNBC or HER2-positive) who achieved pCR showed more prominent improved outcomes than patients with luminal A subtype [5]. von Minckwitz et al. [12] observed a significant association between improved DFS and pCR in luminal B/HER2−, HER2+, and TNBC tumors, but not in the luminal A and luminal B/HER2+ subset [12]. Concordantly, studies have suggested that failure to achieve pCR is related to unfavorable prognosis in TNBC and HER2+ tumors, but not in most of HR-positive patients with BC [12]. Indeed, studies have indicated that HR-positive patients tend to show favorable prognosis although they were less responsive to chemotherapy with relatively lower chances of achieving pCR [5], thereby reflecting the uncertain correlation between pCR and long-term outcomes in patients with HR-positive tumors.
Although the estimated rates of pCR have increased after the addition of new drugs in the routine chemotherapy, a large number of patients fail to achieve pCR following NACT, and not all the patients with pCR show good prognosis. pCR is far from a rationale prognostic factor for HR-positive BC. Therefore, the use of pCR as a replacement of DFS and OS to reflect the long-term prognosis still lacks credibility. The general characteristics of patients should also be considered to comprehensively determine the correlation of pCR with prognosis, thereby facilitating the practice of precision medication.
RESIDUAL CANCER BURDEN (RCB)
Definition of RCB
pCR is related to improved prognosis; however, different degrees of residual disease after NACT persisted in some patients, which prompted the use of other indicators to assess the residual tumor and provide prognostic information. RCB is a pathological assessing system for evaluating residual disease following NACT, which has appealed to an increasing number of researchers in recent years. Compared to pCR, the RCB system can easily quantify post-treatment pathological response and can accurately predict long-term prognosis [23]. Furthermore, the RCB index is a highly reproducible tool for effectively predicting distant recurrence free survival (DRFS) and OS [24]; thus, RCB has the potential to act as a widely-used prognostic indicator in clinical practice. The RCB evaluation system considers both the primary tumor in breast tissue (size, cellularity, and in situ disease) and metastatic lymph nodes (number and size) [25], which can be calculated using a web-based calculator of the MD Anderson Cancer Center. The RCB index is calculated both as a continuous score and a category based on the extent of the residual tumor. RCB-0 is equal to pathological complete response, while the residual disease is divided into RCB-I (minimal residual tumor), RCB-II (moderate residual tumor), and RCB-III (extensive residual tumor) with the cut-off values of 1.36 and 3.28 [24].
Prognostic value of RCB
Several researchers have realized the value of RCB index as an indicator of long-term survival for patients with BC after NACT (Table 2). It is noteworthy that the RCB system has been reported to be a primary or secondary endpoint for NACT due to the prognostic correlation of residual tumors in prospective trials of the Austrian Breast and Colorectal Cancer Study Group (ABCSG) [26]. Peintinger et al. [24] divided RCB into good response class (RCB 0/I) and bad response class (RCBII/III) in terms of the corresponding long-term outcomes. In addition, patients with RCB-I were confirmed to have similar 5-year distant relapse rate as RCB-0 (5.4% and 2.4%, respectively), while patients with RCB-III showed poor prognosis with 5-year distant relapse rate of 53.6% [25]. Symmans et al. [27] conducted a study including five cohorts and reported that RCB index was significantly associated with long-term prognosis in all the molecular subtypes and neoadjuvant treatment regimen cohorts of this study. Furthermore, the RCB index can be integrated with other prognostic biomarkers, which might provide more credible prognostic information for patients with BC. Studies have shown that the prognostic efficacy of tumor-infiltrating lymphocytes (TILs) was limited in patients with TNBC and HER2+ subtypes of BC, but not in those with luminal–HER2 BC [28]. Asano et al. [29] concluded that the integrated indicator ‘TILs-RCB’ is also capable of predicting recurrence rates in HR-positive patients and the prognostic efficacy of ‘TILs-RCB’ is higher than that of TILs or RCB alone. A concordant conclusion was obtained by Luen et al. [30], who showed that residual disease (RD) TILs level can further divide RCB-II patients into relatively higher and lower recurrence-free survival (RFS) groups, reflecting that addition of RD TILs into RCB class improved prognostic efficacy. In addition, RCB was also combined with Ki-67 index to form a prognostic indicator called ‘residual proliferative cancer burden’ (RPCB) to predict long-term prognosis after NACT, and the prognostic value of RPCB was confirmed to be more than that of Ki-67 or RCB alone. Furthermore, the predictive value may be further improved when post-treatment tumor grade and estrogen receptor (ER) status are considered [31].
Table 2. Selected studies assessing the prognostic value of RCB.
| Study (year) | Ref. | Study design | Sample size and NACT regimens | BC subtypes | Biomarker | Prognostic value | ||
|---|---|---|---|---|---|---|---|---|
| Symmans et al. (2017) | [27] | Prospective cohort study | Total (n = 1,158) | All | RCB0 (pCR) vs. RCB1 vs. RCB2 vs. RCB3 | Combined T/FAC cohort: | ||
| - CT only: n = 955 | In combined T/FAC cohort: | TNBC: | ||||||
| - CT + H (H + T/FEC cohort): n = 203 | HER2+ (n = 103) | RFS (10-year): 86% vs. 81% vs. 55% vs. 23%, p < 0.01 | ||||||
| TNBC (n = 219) | HR-positive/HER2-negative: | |||||||
| HR+/HER2− (n = 501) | RFS (10-year): 83% vs. 97% vs. 74% vs. 52%, p < 0.01 | |||||||
| In H+T/FEC cohort: | H + T/FEC cohort: | |||||||
| HR+/HER2+ (n = 108) | RFS (10-year): 95% vs. 77% vs. 47% vs. 21%, p < 0.01 | |||||||
| HR−/HER2+ (n = 95) | Prognostic value were similar for both 5-year RFS and 5/10-year OS (RCB0/1 were significantly better than RCB2 or RCB3 in all treatment cohorts and breast cancer subtypes). | |||||||
| Campbell et al. (2017) | [32] | Prospective cohort study | Total (n = 162) | All | Continuous RCB score | TNBC: RFS: p < 0.0001 | ||
| - CT only | HR+/HER2−: RFS: p = 0.0053 | |||||||
| HER2+: RFS: p = 0.0091 | ||||||||
| Categorical RCB score | pCR vs. RCB1 vs. RCB2 vs. RCB3: | |||||||
| RFS (5-year): 86% vs. 85% vs. 75% vs. 41%, p < 0.0001 | ||||||||
| RCB 3 vs. RCB 0/1/2: | ||||||||
| Hazard ratio, 3.37; 95% CI, 1.96–5.80; p < 0.0001 | ||||||||
| Asano et al. (2017) | [29] | Retrospective analysis | Total (n = 177) | All | RCB-TILs-positive (RCB-I and positive for TILs) versus RCB-TILs-negative | RCB-TILs-positive is better for recurrence in all patients | ||
| - CT only | TNBC (n = 61) | DFS: 51% vs. 22%, hazard ratio, 0.048; 95% CI, 0.012–0.188; p < 0.001 | ||||||
| - CT + H | HER2+ (n = 36) | OS: 51% vs. 25%, p = 0.005 | ||||||
| HRBC (n = 80) | ||||||||
| Sharma et al. (2018) | [117] | Prospective cohorts study | Total (n = 183) | TNBC | RCB classes | RCB0 vs. RCB1: Similar 3-year RFS (90% vs. 93%) and 3-year OS (94% vs. 100%) | ||
| - CT only | RCB2 vs. RCB3: RCB2 better | |||||||
| RFS: hazard ratio, 4.70 (95% CI, 1.97–11.20), p < 0.0001 | ||||||||
| OS: hazard ratio, 4.34 (95% CI, 1.59–11.84), p = 0.002 | ||||||||
| RCB0/1 vs. RCB2/3: RCB0/1 better | ||||||||
| RFS (3-year): 91% vs. 59%, p < 0.0001 | ||||||||
| OS (3-year): 95% vs. 75%, p < 0.0001 | ||||||||
| Luen et al. (2019) | [30] | Prospective study | Total (n = 375) | TNBC with residual disease after NACT | RCB index | Increasing RCB index was significantly associated with worse RFS (p < 0.001) and worse OS (p < 0.001) | ||
| - CT only | RCB1 vs. RCB2 vs. RCB3 | RFS (3-year): 86% vs. 67% vs. 26%, p < 0.001 | ||||||
| Sheri et al. (2014) | [31] | Retrospective analysis | Total (n = 220) | All | Residual proliferative cancer burden (RPCB): | Tertile 1 vs. Tertile 3: | ||
| - CT only | Tertile 1 (score 0–2.8) vs. Tertile 2 (score 2.8–3.72) vs. Tertile 3 (score > 3.72) | RFS: 83% vs. 34% | ||||||
| OS: 93% vs. 46% | ||||||||
| RPCB was significantly more prognostic than either RCB or Ki-67 alone, p < 0.001 | ||||||||
| Addition of post-treatment grade and ER further improved the prediction of outcomes | ||||||||
| Romero et al. (2013) | [118] | Independent prospective cohort study | Total (n = 151) | All | RCB classes and RCB index | RCB0 vs. RCB1 vs. RCB2 vs. RCB3: | ||
| - CT only: n = 105 | OS(5-year): 100% vs. 86.7% vs. 86.7% vs. 54.7% | |||||||
| - CT + H: n = 46 | RFS(5-year): 78.1% vs. 66% vs. 77.5% vs. 32.2% | |||||||
| RCB3 vs. RCB0-2: RCB0-2 better | ||||||||
| OS: hazard ratio, 4.240, p < 0.0001 | ||||||||
| RFS: hazard ratio, 3.859, p < 0.0001 | ||||||||
RCB = residual cancer burden; NACT = neoadjuvant chemotherapy; BC = breast cancer; CT = chemotherapy; H = trastuzumab; T = taxanes; F = 5-fluorouracil; E = epirubicin; C = cyclophosphamide; A= adriamycin; HER2 = human epidermal growth factor receptor 2; TNBC = triple negative breast cancer; HR = hormone receptor; RFS = recurrence free survival; OS = overall survival; CI = confidence interval; HRBC = hormone receptor-positive breast cancer; TILs = tumor infiltrating lymphocytes; ER = estrogen receptor; PR = progesterone receptor.
Although several studies have proved the prognostic effect of RCB in neoadjuvant chemotherapy, its ability to accurately identify high-risk patients is still conflicting [27,32]. The reliability of the RCB evaluation system might be challenged, as it places importance on the presence of residual tumor in post-treatment pathological specimens without considering the baseline data. The crucial problem in the clinical decision-making for patients with RCB-I is the similarity in their prognosis with those who have achieved RCB-0. Furthermore, the reliability of RCB as a surrogate endpoint following NACT should be investigated.
Ki-67 INDEX
Predictive value of pre-treatment Ki-67
Ki-67 is a nuclear antigen present in all phase of the cell cycle except in the G0 phase. It is a tumor proliferation marker and is mainly assessed using immunohistochemistry (IHC). Based on the theory that highly proliferative tumors are highly sensitive to chemotherapy even in HR-positive patients, the pre-treatment Ki-67 level may be a potentially positive predictive factor of NACT response. Indeed, several studies have investigated the function of pre-treatment Ki-67 expression in predicting NACT response of patients across various BC subtypes (Table 3). Results obtained from various patient groups have confirmed that higher baseline Ki-67 expression was significantly associated with better treatment response and improved pCR rate [33,34,35]. Compared to patients with HER2+ and TNBC BC, ER-positive patients with higher pretreatment Ki-67 expression are more likely to show clinical response to NACT [36]. Chen et al. [35] concluded that higher baseline Ki-67 level can be considered a predictive indicator for pCR following NACT only in patients with the luminal subtype, and showed that 25.5% was ideal as the cut-off value for Ki-67 level using receptor operator characteristics (ROC) curve analysis. On the contrary, Alba et al. [37] analyzed the data from four clinical trials and concluded that baseline Ki-67 level > 50% was associated with higher pCR rate, especially in patients with ER−/HER2− and ER−/HER2+ subtypes [37]. In contrast, Kim et al. [38] showed that pretreatment Ki-67 index > 25% was as an independent predictor of pCR, especially in HER2+/ER− patients.
Table 3. Predictive and prognostic value of Ki-67 with different cut-off values.
| Study (year) | Ref. | Sample size and NACT regimens | Subtypes | Cut-off value | Biomarker | Predictive/prognostic value | ||
|---|---|---|---|---|---|---|---|---|
| Penault-Llorca et al. (2008) | [33] | Total (n = 710) | All | 1% | Baseline Ki-67 expression | Positive Ki-67 status was associated with objective clinical response (p = 1.3×10−2) and higher pCR rates (p = 2×10−2). | ||
| - CT only | HR+: n = 363 | Pre-treatment Ki-67 was not prognostic. | ||||||
| HR−: n = 240 | Post-treatment Ki-67 status | Ki-67 was not prognostic with cutoff of 1%, 10% and 20% in this study. | ||||||
| Botero et al. (2016) | [44] | Total (n = 357) | All | 15% | Ki67 expression decrease (from > 15% into ≤ 15%) vs. Ki-67 expression stable > 15% | Ki-67 expression decrease independently predicted LRR (p = 0.03) | ||
| - CT only n = 278 | Ki-67 expression decrease revealed better prognosis: | |||||||
| - CT + H: n = 79 | DFS: p = 0.0001 | |||||||
| OS: p = 0.0016 | ||||||||
| Keam et al. (2011) | [34] | Total (n = 105) | TNBC | 10% | Baseline Ki-67 expression high vs. low | pCR rate: 18.2% vs. 0.0%, p = 0.019 | ||
| - CT only | High Ki-67 expression worse | |||||||
| RFS: p = 0.005 | ||||||||
| OS: p = 0.019 | ||||||||
| RD with high Ki-67 vs. RD with low Ki-67 expression vs. pCR with high Ki-67 | RD with high Ki-67 the worst | |||||||
| RFS: p = 0.0013 | ||||||||
| Sueta et al. (2014) | [36] | Total (n = 121) | All | 35% | Baseline Ki-67 expression | High Ki-67 was significantly related with improved pCR in ER-positive, HER2-negative BC (OR, 6.24; 95% CI, 1.40–27.7; p = 0.016) | ||
| - CT only: n = 91 | Luminal: n = 56 | Median Ki-67 value: 43% vs. 29% (in patients achieved pCR vs. not achieved pCR) | ||||||
| - CT + H: n = 30 | Luminal-HER2: n = 17 | |||||||
| HER2+: n = 22 | ||||||||
| TNBC: n = 26 | ||||||||
| Chen et al. (2018) | [35] | Total (n = 1,010) | All | 14% | Baseline Ki-67 expression | Patients with greater Ki-67 level (≥ 14%) had better clinical and pathological response (p < 0.001) | ||
| - CT only n = 999 | The pretreatment Ki-67 could be used as a predictor of NACT only in luminal subtypes (25.5% is ideal cut-off to differentiate clinical response from non-clinical response) | |||||||
| - CT + H: n = 11 | Ki-67 changes | Statistically significant correlation between Ki-67 decrease and clinical response only existed in luminal (p < 0.001) and luminal-HER2 patients (p = 0.048) | ||||||
| Alba et al. (2016) | [37] | Total (n = 262) | All | 50% | Baseline Ki-67 expression > 50% vs. ≤ 50% | In total: pCR rate: 40% vs. 19%, p = 0.0004 | ||
| - CT only | In ER−/HER2− patients: 42% vs. 15%, p = 0.0337 | |||||||
| - CT + H: most of HER2+ patients | In ER−/HER2+ patients: 64% vs. 45%, p = 0.3238 | |||||||
| Montagna et al. (2014) | [45] | Total (n = 904) | All | 20% | Ki-67 expression decrease (from > 20% into < 20%) versus Ki-67 expression stable > 20% | Ki67 expression decrease revealed better prognosis: | ||
| : All the patients did not achieve pCR | DFS: p < 0.0001 | |||||||
| OS: p < 0.0001 | ||||||||
| Guarner et al. 2009) | [42] | Total (n = 221) | All | 15% | Post-NACT Ki-67 ≥ 15% vs. < 15% | DFS (5-year): 50.2% vs. 77.2%, p = 0.0001 | ||
| - CT only | OS (5-year): 73.1% vs. 87.8%, p = 0.0078 | |||||||
| Baseline Ki67 expression ≥ 15% vs. < 15% | DFS (5-year): 60.5% vs. 83.4%, p = 0.048 | |||||||
| No correlation between baseline Ki67 and OS | ||||||||
| Post-NACT nodes negative + Ki-67 < 15% (low risk) vs. nodes positivity or Ki-67 ≥ 15% (intermediate risk) vs. nodes positive and Ki-67 ≥ 15% (high risk) | Intermediate-risk group vs. Low-risk group: | |||||||
| Hazard ratio for recurrence: 3.1, p = 0.0001 | ||||||||
| Hazard ratio for death: 2.4, p = 0.042 | ||||||||
| High-risk group vs. Low-risk group: | ||||||||
| Hazard ratio for recurrence: 9.3, p = 0.0001 | ||||||||
| Hazard ratio for death: 6.5, p = 0.042 | ||||||||
| von Minckwitz et al. (2013) | [40] | Total (n = 1,151) | All | 0–15%: low-level | post-NACT Ki-67 level | High vs. Intermediate vs. Low: | ||
| - CT only | 15.1–35%: intermediate-level | High-level group showed higher risk (disease relapse: p < 0.0001; death: p < 0.0001) | ||||||
| > 35%: high-level | The prognostic efficacy was more obvious for HR+/HER2-negative and TNBC. | |||||||
| RD with low Ki-67 had comparable outcome to pCR | ||||||||
| Addition of post-treatment Ki-67 to pCR provided better prognostic information than pCR alone in HR+ patients. | ||||||||
NACT = neoadjuvant chemotherapy; CT = chemotherapy (chemotherapy in these studies were based on trastuzumab, taxanes, 5-fluorouracil, epirubicin, cyclophosphamide and adriamycin); HR = hormone receptor; pCR = pathological complete response; H = trastuzumab; LRR = locoregional recurrence; DFS = disease free survival; OS = overall survival; RFS = recurrence-free survival; TNBC = triple-negative breast cancer; RD = residual disease; OR = odds ratio; CI = confidence interval; HER2 = human epidermal growth factor receptor 2; ER = estrogen receptor.
In general, the predictive significance of Ki-67 index varies with studies, which might be because of lack of statistical verification in the cut-off values used in each study. Furthermore, as the ER+ patient groups usually have lower pCR rate irrespective of the baseline level of Ki-67, significant discrepancy in the pCR rate between high and low Ki-67 level patient groups is not easily observed. Nevertheless, it is indubitable that the combination of baseline Ki-67 level based on core needle biopsy with other pre-NACT evaluating biomarkers can provide additional predictive information for treatment response, which can assist in identifying patients who may benefit from NACT.
Prognostic value of Ki-67
Evidently, both changes in Ki-67 level during NACT and post-treatment Ki-67 level in residual disease are effective predictors of long-term survival [31,39]. Previous studies (Table 3) showed that the latter generally tends to have a stronger prognostic impact than the former [40,41]. Keam et al. [34] investigated post-NACT Ki-67 level in 105 patients with TNBC and residual disease, and indicated that > 10% Ki-67 expression in residual disease was significantly associated with poor RFS (p = 0.0013) [34]. Guarneri et al. [42] built a prognostic model based on post-NACT node status, as well as Ki-67 expression level, and concluded that compared to low-risk (nodes negative and Ki-67 < 15%) and intermediate-risk (nodes positivity or Ki-67 ≥ 15%) patients, high-risk patients (nodes positive and Ki-67 ≥ 15%) show significantly higher probability of recurrence and death. It is noteworthy that the prognostic efficacy of the Ki-67 index might differ in various BC subtypes. von Minckwitz et al. [12] concluded that the evaluation of post-treatment Ki-67 provided more prognostic information than evaluation of pCR alone for ER+ patients. The DFS of ER+ patients who had low Ki-67 level (0%–15%) in residual disease was comparable to that of patients who achieved pCR, while post-NACT Ki-67 level > 35% was usually accompanied by higher risk of relapse and required subsequent adjuvant chemotherapy [40]. Furthermore, post-NACT Ki-67 expression has also been confirmed to be an independent prognostic indicator for DFS in HR-negative BC [43]. Similarly, Diaz-Botero et al. [44] and Montagna et al. [45] concluded that Ki-67 expression decreased during NACT correlated statistically with more favorable prognosis than high Ki-67 level retained before and after treatment, although the cut-off values for Ki-67 expression level were different (15% and 20%, respectively).
In conclusion, monitoring of Ki-67 expression level both at pre-treatment and post-treatment stages is necessary to guide subsequent treatment. However, the accuracy of previous conclusions might be affected by errors present in the process of pre-surgical coarse needle biopsy (CNB) and post-surgical pathological specimen slicing [46]; therefore, the pathological evaluation procedure must be further standardized, such that Ki-67 can be used as an effective prognostic indicator more reliably.
Value of Ki-67 in neoadjuvant endocrine therapy (NET)
The Ki-67 expression level reflects the cell proliferative status, while the mechanism of endocrine therapy involves induction of stasis in the cell cycle; therefore Ki-67 analysis has the potential to predict treatment benefits of NET. In a study containing 158 patients who received pre-surgical antiestrogen treatment, Dowsett et al. [47] demonstrated that patients with high Ki-67 level after two weeks of NET revealed an obviously lower RFS than those with low Ki-67 level (p = 0.004), while pre-treatment Ki-67 did not show any prognostic effect. This suggested that short-term change in Ki-67 expression is a valid prognostic indicator of NET. Preoperative endocrine prognostic index (PEPI) score has been accepted as an effective prognostic model that incorporates post-treatment Ki-67 index, tumor histological stage, ER status, post-treatment residual disease size, and metastatic nodal status [48]. PEPI has been shown to be a prognostic marker that can better predict RFS for patients receiving NET, as a result of which some patients can avoid the post-surgical adjuvant treatment [49]. The relevance of PEPI in the context of RFSs has been investigated in a study of 203 patients enrolled in the IMPACT trial. The results showed that patients with pathological stage 1 or 0 and PEPI score 0 had the lowest recurrence rate of 10%, while patients with pathologic stage > 3 and PEPI score 3 showed the highest recurrence rate of 48% [50]. According to the results of The American College of Surgeons Oncology Group (ACOSOG) Z1031A trial (n = 236, ER-positive), patients with PEPI = 0 showed a significantly lower recurrence rate than the patients in the PEPI > 0 group (3.7% vs. 14.4%, p = 0.014). However, 35 patients (2- to 4-week NET, Ki-67 > 10%) who were changed to receive NACT showed lower pCR rate (5.7%) than expected; therefore, this group of patients should urgently follow a new effective alternative treatment strategy [51].
Currently, the Ki-67 index is not being applied in routine clinical practice, as the consensus of rationale cut-off values for defining high versus low Ki-67 level has not been reached. A meta-analysis including 23 studies showed that the pooled HR for OS was significantly higher in the subgroup in which cut-off value of Ki-67 was > 25% than that in which the cut-off value was < 25%, which suggested that selecting a cut-off value > 25% might improve the prognostic prediction ability of Ki-67 [52]. The other disadvantage of using the Ki-67 index might be the lack of standardized measurement technique and the limited reproducibility of the study results. In conclusion, the usefulness of Ki-67 with discordant cut-off values in predicting the prognosis of patients in various molecular subtypes is still uncertain. To address this, more studies should be conducted to determine a standard method of accurately estimating the Ki-67 level.
TILs
Relationship between TILs and treatment response
It is well known that lymphocytes infiltrating tumors modulate the cancer cell killing effect of chemotherapy, and an increasing body of evidence supports the correlation between pre-treatment TIL level and pathological response to NACT (Table 4). High levels of T cells, especially of the CD8+ subtype, have been demonstrated to be predictive of higher pCR rates [53,54,55,56]. A meta-analysis involving 3,251 patients reported that higher pre-treatment TILs correlated well with higher pCR rate in TNBC and HER2+ BC, but not in ER tumors [57]. Tumors that contain more than 50% TILs are defined as lymphocyte-predominant breast cancer (LPBC). Patients with LPBC were confirmed to have higher chance of achieving pCR (p < 0.001) and usually yielded lower RCB class following NACT than non-LPBC patients (p < 0.001) [58]. Furthermore, the extent of elevation in TIL level in the process of NACT can be an indicator of tumor microenvironment response and may influence post-treatment tumor reduction [59].
Table 4. Selected studies assessing TILs and PD-L1 expression for patients achieved NACT.
| Study (year) | Ref. | Sample size and NACT regimens | Subtypes | Biomarker | Predictive/prognostic value | |
|---|---|---|---|---|---|---|
| Denkert et al. (2018) | [28] | n=3,771 for predictive value investigation | All | TILs assessed as a continuous parameter | Increased concentration of TILs was linked to increased pCR | |
| n=2,560 for prognostic value investigation | Three groups with different baseline TILs level: low (0%–10%) vs. intermediate (11%–59%) vs. high (≥ 60%) | pCR rate: 20% vs. 27% vs. 44%, p < 0.0001 (all the patients) | ||||
| - CT only: n = 2,518 | 6% vs. 11% vs. 28% (luminal-HER2-negative subtype) | |||||
| - CT + anti-HER2 therapy: n = 1,253 | 32% vs. 39% vs. 48% (HER2-positive subtype) | |||||
| 31% vs. 31% vs. 50% (TNBC subtype) | ||||||
| p < 0.0001 for each subtype | ||||||
| 10% increase in TILs | Was associated with better prognosis in TNBC and HER2+ BC | |||||
| DFS: p = 0.011 (in TNBC); p = 0.017 (in HER2+ BC) | ||||||
| OS: p = 0.032 (in TNBC) | ||||||
| Was associated with adverse prognosis in luminal subtype | ||||||
| OS: p = 0.011 | ||||||
| Miyashita et al. (2015) | [67] | Total (n = 131) | TNBC | High CD8+ TIL group vs. low CD8+ TIL group (cut-off: 100 infiltrating cells prefield) in RD | RFS (5-year): 73% vs. 30%, p < 0.0001 | |
| : 101 of them had RD | BCSS (5-year): 86% vs. 42%, p < 0.0001 | |||||
| - CT only | Higher CD8/FOXP3 ratio vs. lower CD8/FOXP3 ratio (cut-off: 1.6) | RFS (5-year): 72% vs. 40%, p = 0.009 | ||||
| BCSS (5-year): 77% vs. 56%, p = 0.027 | ||||||
| CD8+ TIL and CD8/FOXP3 ratio increased per 1 unit as continuous variable | Had prognostic significance for RFS, p = 0.0249 and p = 0.0631, respectively | |||||
| High vs. low rate of change in CD8+ TIL group | RFS (5-year): 74% vs. 20%, p = 0.011 | |||||
| BCSS (5-year): 81% vs. 52%, p = 0.064 | ||||||
| Higher vs. lower change of the CD8/FOXP3 ratio | RFS (5-year): 68% vs. 41%, p = 0.011 | |||||
| BCSS (5-year): 78% vs. 58%, p = 0.023 | ||||||
| Dieci et al. (2013) | [62] | Total (n = 278) | TNBC | Continuous It-TIL and Str-TIL in RD | Significant prognostic biomarker: higher TILs was related to better prognosis | |
| : TNBC patients without pCR | MFS: hazard ratio, 0.86; 95% CI, 0.77–0.96; p = 0.01 and hazard ratio, 0.85; 95% CI, 0.75–0.98; p = 0.02 for Str-TIL and It-TIL, respectively | |||||
| - CT only | OS: hazard ratio, 0.86; 95% CI, 0.77–0.97, p = 0.01 and hazard ratio, 0.86; 95% CI, 0.75–0.99; p = 0.03 for Str-TIL and It-TIL, respectively | |||||
| High-TIL (It-TIL and/or Str-TIL > 60%) vs. low-TIL (It-TIL and Str-TIL < 60%) | OS (5-year): 91% vs. 55%, p = 0.0017 | |||||
| MFS (5-year): 81.5% vs. 46%, p = 0.0019 | ||||||
| 10% Str-TIL increased | Risk of metastasis and death was reduced by 21%, p < 0.001 | |||||
| 10% It-TIL increased | Risk of metastasis and death was reduced by 22% and 23% respectively, p < 0.001 | |||||
| Hamy et al. (2017) | [60] | Total (n = 175) | HER2-positive BC | Baseline TIL level | Was not significantly associated with pCR | |
| - CT only: n = 5 | The magnitude of TIL level decrease during NACT | Was strongly associated with pCR, p < 10−5 | ||||
| - CT + H: n = 170 | TIL level > 25% in RD | Was significantly associated with an adverse outcome, p = 0.009 | ||||
| Edith et al. (2016) | [61] | Total (n = 2,027) | HER2-positive BC | Patients with LPBC vs. non-LPBC tumor | RFS (10-year): 90.9% vs. 64.3%, p = 0.004 (in arm A); 80% vs. 79.6%, p = 0.79 (in arm C) | |
| - Arm A (CT only): n = 1,081 | ||||||
| - Arm C (CT + H): n = 946 | TILs level as continuous variable | In the multivariable model: was associated with RFS for arm A (p < 0.001), but not for arm C (p = 0.84) | ||||
| Ladoire et al. (2011) | [69] | Cohort 1 (patients HER2+++): n = 111 | All | High CD8/FOXP3 ratio | Was strongly associated with pCR (hazard ratio, 6.28; 95% CI, 2.42–16.27; p < 0.0001) and better RFS (p < 0.0001) and better OS (p = 0.008) | |
| - CT only: n = 48 | Pathological-immunological scoring system | Higher scoring was associated with decreased RFS and OS, p < 0.0001 | ||||
| - CT + H: n = 63 | ||||||
| Cohort 2 (patients HER2−): n = 51 | ||||||
| Chen et al. (2016) | [78] | Total (n = 309) | All | PD-L1 expression in residual tumor among TNBC patients low vs. high | RFS (5-year): 89% vs. 45% | |
| : With RD | OS (5-year): 91% vs. 51% | |||||
| - CT only | Prognostic value of PD-L1 | Was significant in CD8-low patients (p = 0.011 for RFS and p = 0.029 for OS), but not in CD8-high patients | ||||
| - CT + post-operative H therapy for HER2+ patients | PD-L1-high/CD8-low vs. the other 3 groups (PD-L1-high or low/CD8-high and PD-L1-low/CD8-low) | PD-L1-high/CD8-low the worst | ||||
| RFS (5-year): 54% vs. 75%–82% | ||||||
| OS (5-year): 67% vs. 80%–88% | ||||||
| Wimberly et al. (2015) | [73] | Total (n = 94) | All | PD-L1 in epithelium and stoma when measured as a continuous quantitative score | Were correlated with pCR (epithelial: p = 0.0189, stromal: p = 0.0050), especially for HR+ and HER2-amplified BCs | |
| - CT only | Were positively correlates with high TIL component (epithelial p < 0.0001; stromal p = 0.0001) | |||||
| Asano et al. (2018) | [72] | Total (n = 177) | All | PD-1/PD-L1 expression | High PD-1/PD-L1 expression are related to higher rates of non-pCR (p < 0.001) low PD-1/PD-L1 expression were better than high | |
| - CT only: n = 132 | DFS: p = 0.006, p = 0.001, respectively | |||||
| - CT + H: n = 45 | OS: p = 0.048, p = 0.022, respectively the above correlation was significant in TNBC, but not in HER2-positive BC | |||||
| Cerbelli et al. (2017) | [77] | Total (n = 54) | TNBC | Expression of PD-L1 ≥ 25% | Significantly predicted pCR (p = 0.02) | |
| - CT only | Patients with LPBC (pre-treatment TILs level > 50%) | Was significantly associated with higher pCR rate (p < 0.001) | ||||
| Patients with high TILs PD-L1 level ≥ 25% pre-NACT biopsies | 100% achieved pCR | |||||
TILs = tumor-infiltrating lymphocytes; PD-L1 = programmed death ligand 1; NACT = neoadjuvant chemotherapy; CT = chemotherapy (chemotherapy in these studies were based on trastuzumab, taxanes, 5-fluorouracil, epirubicin, cyclophosphamide and adriamycin); HER2 = human epidermal growth factor receptor 2; pCR = pathological complete response; TNBC = triple-negative breast cancer; BC = breast cancer; DFS = disease-free survival; OS = overall survival; RD = residual disease; RFS = recurrence-free survival; BCSS = breast cancer-specific survival; It = intratumoral; Str = stomal; MFS = metastasis-free survival; CI = confidence interval; HR = hormone receptor; LPBC = lymphocyte-predominant breast cancer.
Prognostic value of TILs
With the exception of baseline TIL level, the presence of post-NACT TILs in residual disease can also reflect the response of the tumor immune microenvironment to chemotherapy, which can provide additional prognostic information. A study investigated the prognostic value of TIL level before, during, and after NAC in HER2+ BC; results suggested that stromal TIL level > 25% in residual disease had an adverse prognostic efficacy. However, no association between baseline TIL levels and long-term survival was observed [60]. For HER2-positive patients who received trastuzumab, the prognostic role of TILs has not been definitely ascertained, and further studies should be conducted to determine whether HER2-positive patients with higher TIL level can obtain survival benefit from trastuzumab therapy [56,61]. Dieci et al. [62] observed that the higher post-treatment TIL subgroup showed significantly elevated 5-year metastasis-free survival (MFS) and OS rates for patients with TNBC. Pruneri et al. [63] showed that each 10% increase in TILs correlated significantly with better survival. Denkert et al. [28] confirmed that the predictive value of enhanced TIL level for NACT response was potent across all molecular subtypes. However, patients with luminal subtype BC and increased TIL infiltration showed poor prognosis, whereas patients with HER2 enrichment and TNBC tumor gained survival benefit from the high level of TILs. This supported the hypothesis that the cellular composition of immune infiltration in tumors is different among each molecular subtypes, which determines different clinical outcomes and NACT response [64]. On the other hand, the predictive efficacy of TILs on OS for patients with luminal subtype is opposite for TNBC and HER2+ BC, which may be explained by the resistance of luminal patients to adjuvant endocrine therapy induced by TILs, while TNBC and HER2+ BC patients with higher post-treatment TILs level have more chances of obtaining survival benefit from NACT. Furthermore, infiltrated T cells contain various subpopulations, such as CD8 and Foxp3 cells [65]; the presence of CD8+ T cells was associated with improved outcome, while the presence of Foxp3+ T cells limited anti-tumor immunity [66,67,68]. Ladoire et al. [69] investigated the prognostic role of intratumoral CD8+/FOXP3+ ratio and observed significant association between CD8+/FOXP3+ ratio and RFS/OS, especially in the HER2-positive group. In conclusion, T cells in tumors can modulate the pathological response to NACT and have the potential to act as a surrogate endpoint for long-term outcomes. Therefore, evaluation of detailed tumor immune response will be important for improving prognostic prediction and screening immunotherapy candidates.
PROGRAMMED DEATH LIGAND 1 (PD-L1)
PD-L1 has now been accepted as an immune regulatory molecule that can induce apoptosis and clearance of T-cells. It can weaken the immune response of human cells by combining with the programmed cell death-1 (PD-1) on immune cells. Therefore, PD-L1 plays an important role in the immune evasion mechanism of tumors, facilitating the progression of BC [70]. In addition, studies have reported that PD-L1 was prominently enriched in TNBC, although the clinical significance of PD-L1 for patients with TNBC is still controversial [71,72,73]. Tomioka et al. [74] reported that PD-L1 expression on tumors was significantly associated with the level of stromal TILs infiltrating surgical specimens in TNBC, which was associated with better outcomes. Indeed, many studies have reported the association between PD-L1 expression and TIL level, although the mechanism is still unclear [71,75,76].
The association between PD-L1 expression level and NACT therapeutic response has also been reported (Table 4). Wimberly et al. [73] reported that TILs and PD-L1 expressed in the epithelium or stroma acted as predictive indicators of pCR for patients receiving NACT in univariate and multivariate analysis. Owing to the aggressive features and poor prognosis of TNBC, identification of a predictive biomarker for treatment response is critical for fostering correct therapeutic decisions. Asano et al. [72] demonstrated that high expression of PD-1/PD-L1 was associated with higher non-pCR percentage, and patients with lower PD-1/PD-L1 expression have longer DFS and OS, especially for patients with TNBC. Conversely, Cerbelli et al. [77] concluded that PD-L1 was not able to act as an independent prognostic index in patients with TNBC. The discrepancy might be the result of different cut-off values used and discordant PD-L1 evaluating methods. Chen et al. [78] demonstrated that high expression of PD-L1 in residual disease was an adverse prognostic indicator for RFS and OS, the effect of which was most significant in patients with TNBC. The prognostic role of PD-L1 for RFS and OS was only significant in CD8-low patients (RFS: p = 0.011, OS: p = 0.029), which suggested the necessity of check-point inhibitor therapy for chemo-resistant patients. Recent studies have shown that high PD-L1 expression can attenuate the survival benefit derived from high TIL level following NACT in patients with TNBC [79]. Thus, the combination of the assessment of PD-L1 expression and TIL level might improve the prediction of prognosis for patients with TNBC and assist in guiding TNBC treatment.
PD-L1 evaluation can provide prognostic information and assist in selecting appropriate chemotherapy regimens. The PD-1/PD-L1 pathway in BC is potentially a new target of molecule-based treatments. Thus, it is important to determine the correlation between PD-L1 expression and survival rates of patients in different molecular subtypes to obtain reliable evidence for immune check-point treatment.
HR, HER2, AND INTRINSIC MOLECULAR SUBTYPES
Relationship between molecular subtype and treatment response of NACT
In current clinical practice, BC is divided into diverse intrinsic molecular subtypes using immunohistochemical analysis, including HR-positive subtype (luminal A and luminal B), TNBC, and HER2-positive subtype (HER2-enriched and luminal B/HER2+) [80]. The intrinsic subtype analysis has been demonstrated to significantly affect the prognosis of patients after NACT. Parker et al. [81] established a risk of relapse model combining intrinsic subtype and tumor size, which provides more prognostic and predictive information than other standard parameters. As has been reported previously, the survival benefit derived from NACT varied among different BC molecular subtypes. HR-positive patients have relatively more favorable outcome, although they are less responsive to NACT than TNBC and HER2+ tumors [82]. Indeed, it is widely accepted that patients with TNBC and HER2-enriched tumor showed higher pCR rates than luminal subtype [83,84,85,86]. Prat et al. [83] evaluated the recurrence risk based on BC subtype and confirmed the independent predictive and prognostic value of intrinsic subtype at diagnosis for patients after NACT. The correlation between pCR and survival is significant in partial patients based on molecular subtypes. Díaz-Casas et al. [84] reported longer EFS and OS in patients who achieved pCR, which was statistically significant only in patients with TNBC. In a study involving 13,939 patients, pCR was demonstrated to be significantly associated with 5-year OS in patients with luminal B, HER2+, and TNBC (93.0%, 94.2%, and 90.6%, respectively, HER2+ vs. TNBC, p = 0.016) [87]. Meyers et al. [88] reported high locoregional recurrence (LRR) rate in basal-like subtype patients on NACT, who should be offered stronger local therapy to improve prognosis. Similarly, Yoo et al. [85] and Haque et al. [87] separately concluded that the TNBC subgroup showed poorer survival than other subgroups after NACT, although pCR rate was highest in the group of patients with TNBC. The molecular subtype of breast tumor is an important biomarker in clinical practice for selecting NACT candidates who may benefit more from the treatment.
Receptor conversion
HER2 status conversion
Several studies have investigated the switches in HER2 status induced by NACT, and some researchers have confirmed the association between changes in HER2 status and long-term prognosis (Table 5). According to a meta-analysis including 14 studies, the frequency of HER2 receptor conversion was lower than that of ER and progesterone receptor (PR) [89]. The rate of HER2 change has been found to be 10%–30% after receiving cytotoxic chemotherapy alone. However, after combining with the neoadjuvant anti-HER2 target treatment, the frequency of HER2 amplification loss may increase up to 43% [90]. Currently, whether changes in HER2 status affect prognosis is still not clear. Guarneri et al. [91] reported that the loss of HER2 amplification during NACT is related to a tendency toward higher risk of recurrence and death. Furthermore, the cohort that received chemotherapy plus anti-HER2 therapy showed lower rate of HER2 loss (p = 0.019). Nevertheless, other studies have confirmed that the rate of HER2 loss was higher for patients received NACT, while application of trastuzumab did not affect the HER2 loss rate. The discordance might arise from the heterogeneity of agents used and differences in the assessment methods used in different studies. On the contrary, the frequency of change in HER2 status and their effect on survival were investigated by Yoshida et al. [92]. They showed that the incidence of changes in HER2 expression was higher in patients with HER2-positivity before NACT, and that there was no discordance in DFS induced by HER2 status change, irrespective of HER2 status prior to treatment. Thus, HER2 expression might change relatively less during NACT, although the prognostic value of HER2 alteration is not yet clear.
Table 5. The prognostic value of receptor conversion during NACT.
| Study (year) | Ref. | Sample size and NACT regimens | Subtypes | Biomarker | Receptor changes | Prognostic value | ||
|---|---|---|---|---|---|---|---|---|
| Hirata et al. (2009) | [93] | Total (n = 368) | All | ER, PR | Group A (n = 184): patients with consistent HR-positive and received ET | Group A vs. group B vs. group C vs. group D: | ||
| HR+: n = 214 | 3-year DFS: 80.3% vs. 78.4% vs. 36.4% vs. 72.2%, p = 0.008 | |||||||
| HR−: n = 154 | Group B (n = 47): patients with HR status conversion and received ET | Group B and group A was similar: hazard ratio, 1.16; 95% CI, 0.61–2.19 | ||||||
| HER2+: n = 112 | Group C (n = 12): patients with HR status conversion and not received ET T-; Group D (n = 125): patients with consistent HR-negative | Group C was significantly shorter than group A: hazard ratio, 6.88; 95% CI, 3.00–15.80 | ||||||
| HER2−: n = 256 | 5-year OS: 90.3% vs. 86.3% vs. 58.9% vs. 78.2%, p = 0.035 | |||||||
| HR+ to HR−: n = 30 (8.2%) | ||||||||
| HER2+ to HR−: n = 22 (6%) | ||||||||
| HR− to HR+: n = 29 (7.9%) | ||||||||
| HER2− to HER2+: n = 13 (3.5%) | ||||||||
| Lim et al. (2016) | [98] | Total (n = 322) | All | HR, HER2 | HR+/HER2− to TNBC: n = 16 (10.3%) | HR+/HER2− to TNBC vs. consistent HR+/HER2−: | ||
| : 29 received anti-HER2 therapy | HR+/HER2−: n = 165 | TNBC to HR+/HER2−: n = 18 (34.6%) | HR+/HER2− to TNBC group had worse outcomes | |||||
| : Adjuvant ET was offered to all HR-positive patients | HR+/HER2+: n = 64 | HR+/HER2+ to HR+/HER2−: n = 12 (21.4%) | RFS: p < 0.001; hazard ratio, 3.54; 95% CI, 1.60–7.85 | |||||
| HR-HER2+: n = 35 | HR−/HER2+ to HR+/HER2+: n = 10 (38.5%) | OS: p = 0.001; hazard ratio, 3.73; 95% CI, 1.34–10.38 | ||||||
| TNBC: n = 53 | HR+ to HR−: n = 23 (10.8%) | Consistent TNBC vs. TNBC to HR+/HER2−: | ||||||
| HR+ to HR+: n = 189 (89.2%) | Consistent TNBC group had the worst outcomes | |||||||
| HR− to HR+: n = 29 (37.2%) | RFS: p < 0.001; hazard ratio, 3.70; 95% CI, 1.86–7.36 | |||||||
| HR− to HR−: n = 49 (62.8%) | OS: p < 0.001; hazard ratio, 5.85; 95% CI, 2.53–13.51 | |||||||
| HR+ to HR− vs. consistent HR+: HR+ to HR− worse | ||||||||
| RFS: p < 0.001; OS: p < 0.001 | ||||||||
| HR− to HR+ vs. consistent HR−: HR− to HR+ better | ||||||||
| RFS: p < 0.001; OS: p < 0.001 | ||||||||
| Chen et al. (2012) | [99] | Total (n = 224) | HR-positive (ER+: 83.9%, PR+: 84.8%) | HR | HR+ to HR−: 15.2% (more frequently in HER2-positive tumors than negative, p = 0.001) | In the 214 patients, HR+ to HR− was an independent predictive factor for DFS (p = 0.026) and OS (p < 0.001) | ||
| : Patients with HR-positive at diagnosis and had RD) | HER2+ to HER2−: n = 7 (15.2%) | In the 214 patients, HR+ to HR− vs. HR remained+: | ||||||
| n =214 received adjuvant endocrine therapy regardless of post-NACT HR status | HER2− to HER2+: n = 7 (3.9%) | DFS (5-year): 43.5% vs. 67.8%, p = 0.003 | ||||||
| OS (5-year): 59.8% vs. 82.5%, p = 0.001 | ||||||||
| In other 10 patients with HR negative change and without ET: DFS (5-year): 50%; OS (5-year): 60% (similar as those who received ET) | ||||||||
| Tacca et al. (2007) | [100] | Total (n = 420) | All | HR | HR− to HR+: n=61, 42% | HR− to HR+ vs. stable HR−: HR− to HR+ was significantly correlated with better OS (p = 0.045) and DFS (p = 0.039) | ||
| HR−: n = 145 (35%) | HR+ to HR−: n=37, 13% | HR+ to HR− vs. stable HR−: HR+ to HR− was significantly correlated with better OS (p = 0.036), but not DFS. | ||||||
| HR+: n = 275 (65%) | Stable HR+ vs. HR− to HR+: no significant survival difference | |||||||
| HR+ to HR− vs. stable HR+: no significant survival difference | ||||||||
| Increase in Allred score after NACT was significantly associated with better DFS, but not OS post-chemotherapy HR status was a prognostic factor for DFS | ||||||||
| Guarneri et al. (2015) | [91] | Total (n = 107) | HER2+ | HER2 | HER2 loss: 40% of patients with RD in Cohort 1 vs. 14.7% of patients with RD in Cohort 2 (p = 0.019) | Loss of HER2 was significantly associated with higher risk of relapse (hazard ratio, 2.41; p = 0.063) | ||
| Cohort 1 (n = 40): CT only | ||||||||
| Cohort 2 (n = 67): CT + anti-HER2 therapy | ||||||||
| Montagna et al. (2014) | [45] | Total (n = 904) | All | ER, PR, HER2 | HR+ to HR−: 5% | Decrease of PR was associated with better DFS (hazard ratio, 0.73; 95% CI, 0.54–1.00; p = 0.046) and OS (hazard ratio, 0.75; 95% CI, 0.50–1.11; p = 0.149) | ||
| : Patients without pCR | PR > 20% to < 20%: 67% | Patients received ET according to type of ER change had no difference for DFS and OS compared with those without ET | ||||||
| HER2+ to HER2−: 14% | ||||||||
| HER2− to HER2+: 4% | ||||||||
NACT = neoadjuvant chemotherapy; HR = hormone receptor; ER = estrogen receptor; PR = progesterone receptor; ET = endocrine therapy; DFS = disease-free survival; CI, confidence interval; OS = overall survival; HER2 = human epidermal growth factor receptor 2; TNBC = triple-negative breast cancer; RFS = recurrence-free survival; RD = residual disease; CT = chemotherapy (chemotherapy in these studies were based on trastuzumab, taxanes, 5-fluorouracil, epirubicin, cyclophosphamide and adriamycin); pCR = pathological complete response.
HR conversion
A retrospective clinical trial involving 368 cases reported that 16%–23% patients showed HR status change (either positive to negative or negative to positive) following NACT [93]. A review reported that the rates of HR status alteration after NACT ranged between 8% and 33% [94].
Several studies have investigated the effect of receptor changes on long-term prognosis of patients (Table 5). Parinyanitikul et al. [95] observed that receptor changes after NACT were associated with better prognosis. They used 20% as the cut-off value to identify ER and PR expression changes and concluded that patients with absolute ER percentage changes ≥ 20% revealed high 5-year RFS and OS. However, there was no significant difference in the survival rate between patients with ≥ 20% PR changes and those with < 20% PR changes [95]. Montagna and colleagues [45] analyzed the quantitative changes in biological features before and after NACT in 904 patients and concluded that only 5% patients with ER changed from positive to negative, while 67% patients changed from PR > 20% to PR< 20%. Multivariate analysis showed that the decrease in PR correlated significantly with better outcome in terms of DFS than PR < 20% both in biopsy and surgical specimens, although the DFS and OS in patients with ER changes did not differ [45]. Evidently, the DFS and OS decreased significantly in patients with HR changes after NACT who did not receive subsequent ET. Hence, it is necessary to evaluate both pre- and post-NACT HR status. Patients who are positive for HR, either before or after NACT, should accept subsequent endocrine therapy [93].
Furthermore, other studies have suggested that the loss of HRs in residual tumor after NACT was associated with poor prognosis [96,97]. Jin et al. [97] has concluded that switching to TNBC after NACT was an adverse prognostic indicator for poor DFS and OS. The subgroup that lost HR or HER2 expression had relatively higher Ki-67 level. Although all patients with HR+ tumor received endocrine therapy in Lim et al.'s study [98], patients with HR+ to HR− conversion still showed poorer prognosis than those who remained HR+. Similarly, Chen et al. [99] demonstrated shorter 5-year DFS and OS in patients who changed from HR-positive to HR-negative, for whom adjuvant endocrine therapy did not offer any survival benefit. Thus, patients who lose the HR during NACT might show less sensitivity to subsequent endocrine treatment, resulting in relatively higher risk of recurrence and death. Thus, endocrine therapy should be based on clinical information of each patient. On the contrary, patients showing HR-positive changes showed better DFS and OS than patients who consistently showed HR-negative status [98,100]. Tacca et al. [100] retested HR status before and after NACT in 420 patients, among which 98 patients showed HR status conversion. When considering HR-negative as a reference, HR-positive conversion (n = 61) was associated with significantly improved DFS and OS, while those who underwent HR positive-to-negative change showed a survival advantage in terms of OS instead of DFS.
In conclusion, the high percentage of biomarker switch due to NACT necessitates the re-evaluation of receptor expression within post-treatment specimens. According to the results of the post-treatment repeated assessment, patients who are positive for HR or show HER2 amplification in either core-needle biopsy specimen or surgical sample should be administered further adjuvant targeted treatment to improve long-term outcome. The discordance between core needle biopsy and surgical specimen might be the result of regional tumor heterogeneity and receptor re-activation in tumors; furthermore, the technical variability also affects receptor evaluation.
ANALYSIS OF POST-NACT LYMPH NODE RATIO (LNR), TUMOR-NODE-METASTASIS (TNM) STAGING, AND LYMPHOVASCULAR INVASION (LVI)
LNR is determined by the proportion of positive lymph nodes in the total nodes excised after surgery. Tsai et al. [101] demonstrated that the HR-positive subgroup accounted for the major percentage of node-positive BC after NACT, and that the percentage of HR-positive patients increased with increased LNR level. LNR has been widely demonstrated to be an important prognostic indicator for survival of patients with BC [102]. Tsai et al. [101] and Keam et al. [103] used different cut-off values for LNR (0.15 and 0.25) to show that lower LNR was significantly associated with better outcomes. According to subgroup analysis by Tsai et al. [101], lower LNR value was significantly associated with longer DFS only in HR-positive patients with BC and TNBC. Concordantly, Liao et al. [104] reported that the lymph node status provided significant prognostic information for OS and RFS, and that the predictive value was better for OS of luminal A, B, and HER2 BC, and for RFS in luminal B and HER2 BC [104]. Currently, TNM staging divides surgically excised lymph nodes into 4 ypN stages (ypN0–3), which is based on the number of involved lymph nodes [105]. The survival difference between ypN+ and ypN0 has been reported in various studies [101,106]. Therefore, LNR is valuable for predicting the prognosis for BC patients who received NACT and may act as an indicator for identifying patients at high risk of recurrence in neoadjuvant settings (Table 6).
Table 6. The prognostic value of traditional pathologic indicators.
| Study (year) | Ref. | Sample size | Subtypes | Biomarker | Predictive/prognostic value | |
|---|---|---|---|---|---|---|
| Tsai et al. (2016) | [101] | Total (n = 428) | All | Nodal stage: ypN0 vs. ypN1 vs. ypN2 vs. ypN3 | 5-year DFS: 91.5% vs. 74.5% vs. 49.8% vs. 50.7% difference was only significant between ypN0 and ypN+, p < 0.001 | |
| : 263 were node negative and 165 was node positive | In node-positive group | LNR categories: low (≤ 0.2) vs. imtermediate (0.21–0.65) vs. high (> 0.65) | DFS: 69.1% vs. 71.4% vs. 49.3%, p < 0.001 | |||
| HR+: n=92 (59.2%) | In subgroup analysis: significant in HR-positive BC (p = 0.02) and TNBC (p = 0.003) | |||||
| HER2+: n = 33 (18.7%) | LNR value ≤ 0.15 vs. > 0.15 | DFS: 73.2% vs. 61.4%, p = 0.08 | ||||
| TNBC: n = 40 (21.5%) | In subgroup analysis: significant in HR-positive BC (94.1% vs. 67.7%, p = 0.04) and TNBC (94.1% vs. 47.8%, p = 0.001) | |||||
| Keam et al. (2008) | [103] | Total (n = 205) (stage II/III) | All | LNR ≤ 0.25 vs. > 0.25 | RFS: patients with LNR > 0.25 was significantly shoter (hazard ratio, 2.701; p = 0.001) | |
| OS: patients with LNR > 0.25 was signifiantly shorter (hazard ratio, 4.109; p = 0.006) | ||||||
| pCR rate: 10.7% vs. 1.2%, p = 0.009 | ||||||
| Carey et al. (2005) | [107] | Total (n = 132) | ER+: n = 64 (48%) | Revised AJCC TNM grade: stage 0 vs. 1 vs. 2 vs. 3 | DDFS (5-year): 95% vs. 84% vs. 72% vs. 47%, p < 0.001 | |
| : Nonmetastasis patients with RD after NACT | ER−: n = 54 (41%) | OS (5-year): 95% vs. 90% vs. 71% vs. 61%, p = 0.006 | ||||
| Gabani et al. (2019) | [111] | Total (n = 153) | TNBC | LVI: with LVI vs. without LVI | LRR: hazard ratio, 3.92; 95% CI, 1.64–9.38 | |
| 4-year rate of locoregional control: 61.2% vs. 85%, p < 0.001 | ||||||
| ENE: with ENE vs. without ENE | LRR: hazard ratio, 3.32; 95% CI, 1.35–8.15 | |||||
| 4-year rate of locoregional control: 51.9% vs. 83.9%, p = 0.002 | ||||||
| Liu et al. (2016) | [110] | Total (n = 166) | All | The presence of post-NACT LVI | Was associated with worse PFS (hazard ratio, 3.37; 95% CI, 1.87–6.06; p < 0.01) and OS (hazard ratio, 4.35; 95% CI, 1.61–11.79; p < 0.01) | |
| In subtype analysis: HR+ and HER2+ BC patients without LVI had significantly better PFS (p < 0.01) and OS (p < 0.01); TNBC patients with LVI had the worst PFS (p < 0.01) and OS (p < 0.01). | ||||||
ER = estrogen receptor; PR = progesterone receptor; HR = hormone receptor; HER2 = human epidermal growth factor receptor 2; TNBC = triple-negative breast cancer; LNR = lymph node ratio; DFS = disease-free survival; BC = breast cancer; RFS = recurrence-free survival; OS = overall survival; RD = residual disease; NACT = neoadjuvant chemotherapy; TNM = tumor-node-metastasis; DDFS = distant disease-free survival; LVI = lymphovascular invasion; LRR = locoregional recurrence; CI = confidence interval; ENE = extranodal extension; PFS = progression-free survival.
The American Joint Committee on Cancer (AJCC) TNM staging system can function as a practical and reproducible pathological evaluation approach for BC patients undergoing NACT. The efficacy of the revised AJCC TNM staging system in predicting the prognosis of patients treated with NACT has been demonstrated (Table 6). Carey et al. [107] performed post-NACT pathological evaluation based on the revised AJCC system in the residual disease of 132 patients and observed an association between higher stage and lower distant disease-free survival (DDFS) rate. Campbell et al. [32] used the I-SPY 1 trial dataset to compare the prognostic value of pCR, RCB, and American Joint Committee on Cancer post-neoadjuvant therapy staging system (yAJCC) system and showed both the RCB and yAJCC systems identified patients with higher recurrence risk, especially when considering molecular subtype.
LVI refers to the presence of tumor cells in lymphatic or blood vessels of patients with BC. The presence of LVI has been found to promote axillary lymph node metastasis or even distant metastasis [108]. Studies have indicated that post-NACT LVI is associated with adverse prognostic effect for patients with BC (Table 6). Hamy et al. [109] analyzed 1,033 patients with invasive BC who received NACT and concluded that the presence of LVI after NACT correlated with poor DFS, OS, RFS, and MFS in all subtypes. Liu et al. [110] reported an association between post-NACT LVI and poor PFS (progression-free survival) as well as OS (p < 0.91, respectively). Evidently, TNBC patients with LVI harbored residual cancer after NACT and were at significantly higher risk of LRR (p < 0.001) [111]. The LVI situation is associated with many other clinical and pathological characteristics, including HR status, number of involved lymph nodes, and residual disease burden [112,113]. Thus, LVI may act as a post-NACT indicator that may provide additional prognostic information in combination with other pathological biomarkers.
CONCLUSION
As suggested by the FDA, the most widely-agreed definition of pCR is the absence of invasive cancer cells both in primary breast tumor and axillary nodes (ypT0/Tis ypN0). Patients with pCR are significantly associated with prolonged DFS and OS, although achievement of pCR cannot be definitely translated to favorable prognosis. Both pCR and RCB are indicators of pathological response; however, the former is a dichotomous index that divides tumors into complete and incomplete regression; the latter is a continuous index, which quantifies the residual disease more systematically. Based on previous studies, the IHC biomarkers (including Ki-67, TILs, ER, PR, HER2, and PD-L1) correlated with chemo-sensitivity and long-term prognosis of patients who received NACT, and the combination of some biomarkers might provide higher clinical relevance. For example, RPCB has been demonstrated to be a better prognostic predictor than RCB or Ki67 alone, as it incorporates the size as well as the proliferation potential of post-treatment residual disease. Studies have reported that RCB-TIL is possibly an indicator that can predict survival in all the molecular subtypes, suggesting that the combination of RCB and TILs may be used as prognostic indicators for a wider population. Thus, the integration of various indicators might improve the accuracy of pathological evaluation and prognostic prediction. Nevertheless, most of the current consensus is based on retrospective clinical studies, which support the clinical application of these pathological indicators, although more prospective studies with larger sample size should be conducted. Different studies use the cut-off values empirically without using widely accepted statistical methods, which result in discordance among their conclusions. Establishment of qualified pathological evaluation systems by further studying the current pathological indicators or investigation of new biomarkers is warranted in the future. An ideal pathological indicator should meet the following requirements: 1) it should be amenable for complete evaluation using a unified technical approach that facilitates clinical application; 2) it should be amenable for definition using a grading criterion to identify patients with high risk after NACT; 3) it should possesses specific prognostic value in various patient subgroups. Furthermore, the wide application of a pathological evaluation system depends on the professional training of pathologists, further indicating the necessity of interdisciplinary cooperation.
Footnotes
Funding: This work was supported by National Natural Science Foundation of China (81773083, 81702881, 81702593 and 81773163) and Liaoning Science and Technology Project (2013225585).
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Wang M2, Guo J.
- Supervision: Zhang Q, Xu Y.
- Writing - original draft: Li X, Wang M1.
- Writing - review & editing: Wang M2, Yu X, Guo J, Sun T, Yao L.
Wang M1, Mozhi Wang; Wang M2, Mengshen Wang
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Popat S, Smith IE. Re: Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:858. doi: 10.1093/jnci/dji147. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–1516. doi: 10.1245/s10434-011-2108-2. [DOI] [PubMed] [Google Scholar]
- 4.Viale G. Characterization and clinical impact of residual disease after neoadjuvant chemotherapy. Breast. 2013;22(Suppl 2):S88–S91. doi: 10.1016/j.breast.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 6.Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681–695. doi: 10.1002/cncr.10741. [DOI] [PubMed] [Google Scholar]
- 7.Bear HD, Anderson S, Smith RE, Geyer CE, Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–2027. doi: 10.1200/JCO.2005.04.1665. [DOI] [PubMed] [Google Scholar]
- 8.von Minckwitz G, Costa SD, Raab G, Blohmer JU, Eidtmann H, Hilfrich J, et al. Dose-dense doxorubicin, docetaxel, and granulocyte colony-stimulating factor support with or without tamoxifen as preoperative therapy in patients with operable carcinoma of the breast: a randomized, controlled, open phase IIb study. J Clin Oncol. 2001;19:3506–3515. doi: 10.1200/JCO.2001.19.15.3506. [DOI] [PubMed] [Google Scholar]
- 9.Kurosumi M, Akashi-Tanaka S, Akiyama F, Komoike Y, Mukai H, Nakamura S, et al. Histopathological criteria for assessment of therapeutic response in breast cancer (2007 version) Breast Cancer. 2008;15:5–7. doi: 10.1007/s12282-007-0016-x. [DOI] [PubMed] [Google Scholar]
- 10.Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12:320–327. doi: 10.1016/s0960-9776(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 11.Mazouni C, Peintinger F, Wan-Kau S, Andre F, Gonzalez-Angulo AM, Symmans WF, et al. Residual ductal carcinoma in situ in patients with complete eradication of invasive breast cancer after neoadjuvant chemotherapy does not adversely affect patient outcome. J Clin Oncol. 2007;25:2650–2655. doi: 10.1200/JCO.2006.08.2271. [DOI] [PubMed] [Google Scholar]
- 12.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 13.Bossuyt V, Provenzano E, Symmans WF, Boughey JC, Coles C, Curigliano G, et al. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann Oncol. 2015;26:1280–1291. doi: 10.1093/annonc/mdv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow M. Parsing pathologic complete response in patients receiving neoadjuvant chemotherapy for breast cancer. JAMA Oncol. 2016;2:516–517. doi: 10.1001/jamaoncol.2015.4919. [DOI] [PubMed] [Google Scholar]
- 15.Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE, Jr, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30:3960–3966. doi: 10.1200/JCO.2011.40.8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuire A, Brown JA, Malone C, McLaughlin R, Kerin MJ. Effects of age on the detection and management of breast cancer. Cancers (Basel) 2015;7:908–929. doi: 10.3390/cancers7020815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spring L, Greenup R, Niemierko A, Schapira L, Haddad S, Jimenez R, et al. Pathologic complete response after neoadjuvant chemotherapy and long-term outcomes among young women with breast cancer. J Natl Compr Canc Netw. 2017;15:1216–1223. doi: 10.6004/jnccn.2017.0158. [DOI] [PubMed] [Google Scholar]
- 18.Tanioka M, Shimizu C, Yonemori K, Yoshimura K, Tamura K, Kouno T, et al. Predictors of recurrence in breast cancer patients with a pathologic complete response after neoadjuvant chemotherapy. Br J Cancer. 2010;103:297–302. doi: 10.1038/sj.bjc.6605769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez-Angulo AM, McGuire SE, Buchholz TA, Tucker SL, Kuerer HM, Rouzier R, et al. Factors predictive of distant metastases in patients with breast cancer who have a pathologic complete response after neoadjuvant chemotherapy. J Clin Oncol. 2005;23:7098–7104. doi: 10.1200/JCO.2005.11.124. [DOI] [PubMed] [Google Scholar]
- 20.Chaudry M, Lei X, Gonzalez-Angulo AM, Mittendorf EA, Valero V, Tripathy D, et al. Recurrence and survival among breast cancer patients achieving a pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2015;153:417–423. doi: 10.1007/s10549-015-3533-x. [DOI] [PubMed] [Google Scholar]
- 21.Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48:3342–3354. doi: 10.1016/j.ejca.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahleh Z, Sivasubramaniam D, Dhaliwal S, Sundarajan V, Komrokji R. Residual cancer burden in locally advanced breast cancer: a superior tool. Curr Oncol. 2008;15:271–278. doi: 10.3747/co.v15i6.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peintinger F, Sinn B, Hatzis C, Albarracin C, Downs-Kelly E, Morkowski J, et al. Reproducibility of residual cancer burden for prognostic assessment of breast cancer after neoadjuvant chemotherapy. Mod Pathol. 2015;28:913–920. doi: 10.1038/modpathol.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–4422. doi: 10.1200/JCO.2007.10.6823. [DOI] [PubMed] [Google Scholar]
- 26.Symmans WF, Wei C, Gould R, Zhang Y, Hunt KK, Buchholz TA, et al. Long-term prognostic value of residual cancer burden (RCB) classification following neoadjuvant chemotherapy. Cancer Res. 2013;73:S6-02. [Google Scholar]
- 27.Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35:1049–1060. doi: 10.1200/JCO.2015.63.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 29.Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Hatano T, et al. Prediction of survival after neoadjuvant chemotherapy for breast cancer by evaluation of tumor-infiltrating lymphocytes and residual cancer burden. BMC Cancer. 2017;17:888. doi: 10.1186/s12885-017-3927-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luen SJ, Salgado R, Dieci MV, Vingiani A, Curigliano G, Gould RE, et al. Prognostic implications of residual disease tumor-infiltrating lymphocytes and residual cancer burden in triple-negative breast cancer patients after neoadjuvant chemotherapy. Ann Oncol. 2019;30:236–242. doi: 10.1093/annonc/mdy547. [DOI] [PubMed] [Google Scholar]
- 31.Sheri A, Smith IE, Johnston SR, A'Hern R, Nerurkar A, Jones RL, et al. Residual proliferative cancer burden to predict long-term outcome following neoadjuvant chemotherapy. Ann Oncol. 2015;26:75–80. doi: 10.1093/annonc/mdu508. [DOI] [PubMed] [Google Scholar]
- 32.Campbell JI, Yau C, Krass P, Moore D, Carey LA, Au A, et al. Comparison of residual cancer burden, American Joint Committee on Cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657) Breast Cancer Res Treat. 2017;165:181–191. doi: 10.1007/s10549-017-4303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penault-Llorca F, Abrial C, Raoelfils I, Chollet P, Cayre A, Mouret-Reynier MA, et al. Changes and predictive and prognostic value of the mitotic index, Ki-67, cyclin D1, and cyclo-oxygenase-2 in 710 operable breast cancer patients treated with neoadjuvant chemotherapy. Oncologist. 2008;13:1235–1245. doi: 10.1634/theoncologist.2008-0073. [DOI] [PubMed] [Google Scholar]
- 34.Keam B, Im SA, Lee KH, Han SW, Oh DY, Kim JH, et al. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res. 2011;13:R22. doi: 10.1186/bcr2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen R, Ye Y, Yang C, Peng Y, Zong B, Qu F, et al. Assessment of the predictive role of pretreatment Ki-67 and Ki-67 changes in breast cancer patients receiving neoadjuvant chemotherapy according to the molecular classification: a retrospective study of 1010 patients. Breast Cancer Res Treat. 2018;170:35–43. doi: 10.1007/s10549-018-4730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sueta A, Yamamoto Y, Hayashi M, Yamamoto S, Inao T, Ibusuki M, et al. Clinical significance of pretherapeutic Ki67 as a predictive parameter for response to neoadjuvant chemotherapy in breast cancer: is it equally useful across tumor subtypes? Surgery. 2014;155:927–935. doi: 10.1016/j.surg.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 37.Alba E, Lluch A, Ribelles N, Anton-Torres A, Sanchez-Rovira P, Albanell J, et al. High proliferation predicts pathological complete response to neoadjuvant chemotherapy in early breast cancer. Oncologist. 2016;21:778. doi: 10.1634/theoncologist.2015-0312erratum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim KI, Lee KH, Kim TR, Chun YS, Lee TH, Park HK. Ki-67 as a predictor of response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2014;17:40–46. doi: 10.4048/jbc.2014.17.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsubara N, Mukai H, Masumoto M, Sasaki M, Naito Y, Fujii S, et al. Survival outcome and reduction rate of Ki-67 between pre- and post-neoadjuvant chemotherapy in breast cancer patients with non-pCR. Breast Cancer Res Treat. 2014;147:95–102. doi: 10.1007/s10549-014-3084-6. [DOI] [PubMed] [Google Scholar]
- 40.von Minckwitz G, Schmitt WD, Loibl S, Müller BM, Blohmer JU, Sinn BV, et al. Ki67 measured after neoadjuvant chemotherapy for primary breast cancer. Clin Cancer Res. 2013;19:4521–4531. doi: 10.1158/1078-0432.CCR-12-3628. [DOI] [PubMed] [Google Scholar]
- 41.Jones RL, Salter J, A'Hern R, Nerurkar A, Parton M, Reis-Filho JS, et al. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2009;116:53–68. doi: 10.1007/s10549-008-0081-7. [DOI] [PubMed] [Google Scholar]
- 42.Guarneri V, Piacentini F, Ficarra G, Frassoldati A, D'Amico R, Giovannelli S, et al. A prognostic model based on nodal status and Ki-67 predicts the risk of recurrence and death in breast cancer patients with residual disease after preoperative chemotherapy. Ann Oncol. 2009;20:1193–1198. doi: 10.1093/annonc/mdn761. [DOI] [PubMed] [Google Scholar]
- 43.Tan QX, Qin QH, Yang WP, Mo QG, Wei CY. Prognostic value of Ki67 expression in HR-negative breast cancer before and after neoadjuvant chemotherapy. Int J Clin Exp Pathol. 2014;7:6862–6870. [PMC free article] [PubMed] [Google Scholar]
- 44.Diaz-Botero S, Espinosa-Bravo M, Gonçalves VR, Esgueva-Colmenarejo A, Peg V, Perez J, et al. Different prognostic implications of residual disease after neoadjuvant treatment: impact of Ki 67 and site of response. Ann Surg Oncol. 2016;23:3831–3837. doi: 10.1245/s10434-016-5339-4. [DOI] [PubMed] [Google Scholar]
- 45.Montagna E, Bagnardi V, Viale G, Rotmensz N, Sporchia A, Cancello G, et al. Changes in PgR and Ki-67 in residual tumour and outcome of breast cancer patients treated with neoadjuvant chemotherapy. Ann Oncol. 2015;26:307–313. doi: 10.1093/annonc/mdu528. [DOI] [PubMed] [Google Scholar]
- 46.Bossuyt V, Symmans WF. Standardizing of Pathology in Patients Receiving Neoadjuvant Chemotherapy. Ann Surg Oncol. 2016;23:3153–3161. doi: 10.1245/s10434-016-5317-x. [DOI] [PubMed] [Google Scholar]
- 47.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A'Hern R, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167–170. doi: 10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]
- 48.Kurozumi S, Matsumoto H, Inoue K, Tozuka K, Hayashi Y, Kurosumi M, et al. Impact of combining the progesterone receptor and preoperative endocrine prognostic index (PEPI) as a prognostic factor after neoadjuvant endocrine therapy using aromatase inhibitors in postmenopausal ER positive and HER2 negative breast cancer. PLoS One. 2018;13:e0201846. doi: 10.1371/journal.pone.0201846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma CX, Bose R, Ellis MJ. Prognostic and predictive biomarkers of endocrine responsiveness for estrogen receptor positive breast cancer. Adv Exp Med Biol. 2016;882:125–154. doi: 10.1007/978-3-319-22909-6_5. [DOI] [PubMed] [Google Scholar]
- 50.Ellis MJ, Tao Y, Luo J, A'Hern R, Evans DB, Bhatnagar AS, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380–1388. doi: 10.1093/jnci/djn309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ, et al. Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance) J Clin Oncol. 2017;35:1061–1069. doi: 10.1200/JCO.2016.69.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrelli F, Viale G, Cabiddu M, Barni S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153:477–491. doi: 10.1007/s10549-015-3559-0. [DOI] [PubMed] [Google Scholar]
- 53.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 54.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi R, Tanaka M, Yano A, Tse GM, Yamaguchi M, Koura K, et al. Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum Pathol. 2012;43:1688–1694. doi: 10.1016/j.humpath.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 56.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 57.Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K. The value of tumor infiltrating lymphocytes (TILs) for predicting response to neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e115103. doi: 10.1371/journal.pone.0115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang HW, Jung H, Hyeon J, Park YH, Ahn JS, Im YH, et al. A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients. Breast Cancer Res Treat. 2019;173:255–266. doi: 10.1007/s10549-018-4981-x. [DOI] [PubMed] [Google Scholar]
- 59.Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–3030. [PubMed] [Google Scholar]
- 60.Hamy AS, Pierga JY, Sabaila A, Laas E, Bonsang-Kitzis H, Laurent C, et al. Stromal lymphocyte infiltration after neoadjuvant chemotherapy is associated with aggressive residual disease and lower disease-free survival in HER2-positive breast cancer. Ann Oncol. 2017;28:2233–2240. doi: 10.1093/annonc/mdx309. [DOI] [PubMed] [Google Scholar]
- 61.Perez EA, Ballman KV, Tenner KS, Thompson EA, Badve SS, Bailey H, et al. Association of stromal tumor-infiltrating lymphocytes with recurrence-free survival in the N9831 adjuvant trial in patients with early-stage HER2-positive breast cancer. JAMA Oncol. 2016;2:56–64. doi: 10.1001/jamaoncol.2015.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2014;25:611–618. doi: 10.1093/annonc/mdt556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pruneri G, Vingiani A, Bagnardi V, Rotmensz N, De Rose A, Palazzo A, et al. Clinical validity of tumor-infiltrating lymphocytes analysis in patients with triple-negative breast cancer. Ann Oncol. 2016;27:249–256. doi: 10.1093/annonc/mdv571. [DOI] [PubMed] [Google Scholar]
- 64.Ali HR, Chlon L, Pharoah PD, Markowetz F, Caldas C. Patterns of immune infiltration in breast cancer and their clinical implications: a gene-expression-based retrospective study. PLoS Med. 2016;13:e1002194. doi: 10.1371/journal.pmed.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating Foxp3+ regulatory T cells. Clin Cancer Res. 2008;14:2413–2420. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 66.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 67.Miyashita M, Sasano H, Tamaki K, Hirakawa H, Takahashi Y, Nakagawa S, et al. Prognostic significance of tumor-infiltrating CD8+ and FOXP3+ lymphocytes in residual tumors and alterations in these parameters after neoadjuvant chemotherapy in triple-negative breast cancer: a retrospective multicenter study. Breast Cancer Res. 2015;17:124. doi: 10.1186/s13058-015-0632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang D, Gao Z, Cai Z, Wang M, He J. Clinicopathological and prognostic significance of FOXP3+ tumor infiltrating lymphocytes in patients with breast cancer: a meta-analysis. BMC Cancer. 2015;15:727. doi: 10.1186/s12885-015-1742-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ladoire S, Mignot G, Dabakuyo S, Arnould L, Apetoh L, Rébé C, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol. 2011;224:389–400. doi: 10.1002/path.2866. [DOI] [PubMed] [Google Scholar]
- 70.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 71.Ali HR, Glont SE, Blows FM, Provenzano E, Dawson SJ, Liu B, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol. 2015;26:1488–1493. doi: 10.1093/annonc/mdv192. [DOI] [PubMed] [Google Scholar]
- 72.Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Morisaki T, et al. Prediction of treatment responses to neoadjuvant chemotherapy in triple-negative breast cancer by analysis of immune checkpoint protein expression. J Transl Med. 2018;16:87. doi: 10.1186/s12967-018-1458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015;3:326–332. doi: 10.1158/2326-6066.CIR-14-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tomioka N, Azuma M, Ikarashi M, Yamamoto M, Sato M, Watanabe KI, et al. The therapeutic candidate for immune checkpoint inhibitors elucidated by the status of tumor-infiltrating lymphocytes (TILs) and programmed death ligand 1 (PD-L1) expression in triple negative breast cancer (TNBC) Breast Cancer. 2018;25:34–42. doi: 10.1007/s12282-017-0781-0. [DOI] [PubMed] [Google Scholar]
- 75.Park IH, Kong SY, Ro JY, Kwon Y, Kang JH, Mo HJ, et al. Prognostic implications of tumor-infiltrating lymphocytes in association with programmed death ligand 1 expression in early-stage breast cancer. Clin Breast Cancer. 2016;16:51–58. doi: 10.1016/j.clbc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 76.Mori H, Kubo M, Yamaguchi R, Nishimura R, Osako T, Arima N, et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017;8:15584–15592. doi: 10.18632/oncotarget.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cerbelli B, Pernazza A, Botticelli A, Fortunato L, Monti M, Sciattella P, et al. PD-L1 expression in TNBC: a predictive biomarker of response to neoadjuvant chemotherapy? BioMed Res Int. 2017;2017:1750925. doi: 10.1155/2017/1750925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen S, Wang RX, Liu Y, Yang WT, Shao ZM. PD-L1 expression of the residual tumor serves as a prognostic marker in local advanced breast cancer after neoadjuvant chemotherapy. Int J Cancer. 2017;140:1384–1395. doi: 10.1002/ijc.30552. [DOI] [PubMed] [Google Scholar]
- 79.Zhu X, Zhang Q, Wang D, Liu C, Han B, Yang JM. Expression of PD-L1 attenuates the positive impacts of high-level tumor-infiltrating lymphocytes on prognosis of triple-negative breast cancer. Cancer Biol Ther. 2019;20:1105–1112. doi: 10.1080/15384047.2019.1595282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oh DS, Troester MA, Usary J, Hu Z, He X, Fan C, et al. Estrogen-regulated genes predict survival in hormone receptor-positive breast cancers. J Clin Oncol. 2006;24:1656–1664. doi: 10.1200/JCO.2005.03.2755. [DOI] [PubMed] [Google Scholar]
- 83.Prat A, Fan C, Fernández A, Hoadley KA, Martinello R, Vidal M, et al. Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy. BMC Med. 2015;13:303. doi: 10.1186/s12916-015-0540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Díaz-Casas SE, Castilla-Tarra JA, Pena-Torres E, Orozco-Ospino M, Mendoza-Diaz S, Nuñez-Lemus M, et al. Pathological response to neoadjuvant chemotherapy and the molecular classification of locally advanced breast cancer in a Latin American cohort. Oncologist. 2019 doi: 10.1634/theoncologist.2019-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoo C, Ahn JH, Jung KH, Kim SB, Kim HH, Shin HJ, et al. Impact of immunohistochemistry-based molecular subtype on chemosensitivity and survival in patients with breast cancer following neoadjuvant chemotherapy. J Breast Cancer. 2012;15:203–210. doi: 10.4048/jbc.2012.15.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao Y, Dong X, Li R, Ma X, Song J, Li Y, et al. Evaluation of the pathological response and prognosis following neoadjuvant chemotherapy in molecular subtypes of breast cancer. Onco Targets Ther. 2015;8:1511–1521. doi: 10.2147/OTT.S83243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haque W, Verma V, Hatch S, Suzanne Klimberg V, Brian Butler E, Teh BS. Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res Treat. 2018;170:559–567. doi: 10.1007/s10549-018-4801-3. [DOI] [PubMed] [Google Scholar]
- 88.Meyers MO, Klauber-Demore N, Ollila DW, Amos KD, Moore DT, Drobish AA, et al. Impact of breast cancer molecular subtypes on locoregional recurrence in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. Ann Surg Oncol. 2011;18:2851–2857. doi: 10.1245/s10434-011-1665-8. [DOI] [PubMed] [Google Scholar]
- 89.Zhang N, Moran MS, Huo Q, Haffty BG, Yang Q. The hormonal receptor status in breast cancer can be altered by neoadjuvant chemotherapy: a meta-analysis. Cancer Invest. 2011;29:594–598. doi: 10.3109/07357907.2011.621913. [DOI] [PubMed] [Google Scholar]
- 90.Mittendorf EA, Wu Y, Scaltriti M, Meric-Bernstam F, Hunt KK, Dawood S, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15:7381–7388. doi: 10.1158/1078-0432.CCR-09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guarneri V, Dieci MV, Barbieri E, Piacentini F, Omarini C, Ficarra G, et al. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol. 2013;24:2990–2994. doi: 10.1093/annonc/mdt364. [DOI] [PubMed] [Google Scholar]
- 92.Yoshida A, Hayashi N, Suzuki K, Takimoto M, Nakamura S, Yamauchi H. Change in HER2 status after neoadjuvant chemotherapy and the prognostic impact in patients with primary breast cancer. J Surg Oncol. 2017;116:1021–1028. doi: 10.1002/jso.24762. [DOI] [PubMed] [Google Scholar]
- 93.Hirata T, Shimizu C, Yonemori K, Hirakawa A, Kouno T, Tamura K, et al. Change in the hormone receptor status following administration of neoadjuvant chemotherapy and its impact on the long-term outcome in patients with primary breast cancer. Br J Cancer. 2009;101:1529–1536. doi: 10.1038/sj.bjc.6605360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van de Ven S, Smit VT, Dekker TJ, Nortier JW, Kroep JR. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev. 2011;37:422–430. doi: 10.1016/j.ctrv.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 95.Parinyanitikul N, Lei X, Chavez-MacGregor M, Liu S, Mittendorf EA, Litton JK, et al. Receptor status change from primary to residual breast cancer after neoadjuvant chemotherapy and analysis of survival outcomes. Clin Breast Cancer. 2015;15:153–160. doi: 10.1016/j.clbc.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 96.Chen S, Huang L, Chen CM, Shao ZM. Progesterone receptor loss identifies luminal-type local advanced breast cancer with poor survival in patients who fail to achieve a pathological complete response to neoadjuvant chemotherapy. Oncotarget. 2015;6:18174–18182. doi: 10.18632/oncotarget.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jin X, Jiang YZ, Chen S, Yu KD, Shao ZM, Di GH. Prognostic value of receptor conversion after neoadjuvant chemotherapy in breast cancer patients: a prospective observational study. Oncotarget. 2015;6:9600–9611. doi: 10.18632/oncotarget.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lim SK, Lee MH, Park IH, You JY, Nam BH, Kim BN, et al. Impact of molecular subtype conversion of breast cancers after neoadjuvant chemotherapy on clinical outcome. Cancer Res Treat. 2016;48:133–141. doi: 10.4143/crt.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen S, Chen CM, Yu KD, Zhou RJ, Shao ZM. Prognostic value of a positive-to-negative change in hormone receptor status after neoadjuvant chemotherapy in patients with hormone receptor-positive breast cancer. Ann Surg Oncol. 2012;19:3002–3011. doi: 10.1245/s10434-012-2318-2. [DOI] [PubMed] [Google Scholar]
- 100.Tacca O, Penault-Llorca F, Abrial C, Mouret-Reynier MA, Raoelfils I, Durando X, et al. Changes in and prognostic value of hormone receptor status in a series of operable breast cancer patients treated with neoadjuvant chemotherapy. Oncologist. 2007;12:636–643. doi: 10.1634/theoncologist.12-6-636. [DOI] [PubMed] [Google Scholar]
- 101.Tsai J, Bertoni D, Hernandez-Boussard T, Telli ML, Wapnir IL. Lymph node ratio analysis after neoadjuvant chemotherapy is prognostic in hormone receptor-positive and triple-negative breast cancer. Ann Surg Oncol. 2016;23:3310–3316. doi: 10.1245/s10434-016-5319-8. [DOI] [PubMed] [Google Scholar]
- 102.Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 103.Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, et al. Clinical significance of axillary nodal ratio in stage II/III breast cancer treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2009;116:153–160. doi: 10.1007/s10549-008-0160-9. [DOI] [PubMed] [Google Scholar]
- 104.Liao GS, Chou YC, Hsu HM, Dai MS, Yu JC. The prognostic value of lymph node status among breast cancer subtypes. Am J Surg. 2015;209:717–724. doi: 10.1016/j.amjsurg.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 105.Connolly JL. Changes and problematic areas in interpretation of the AJCC Cancer Staging Manual, 6th Edition, for breast cancer. Arch Pathol Lab Med. 2006;130:287–291. doi: 10.5858/2006-130-287-CAPAII. [DOI] [PubMed] [Google Scholar]
- 106.Schiffman SC, McMasters KM, Scoggins CR, Martin RC, Chagpar AB. Lymph node ratio: a proposed refinement of current axillary staging in breast cancer patients. J Am Coll Surg. 2011;213:45–52. doi: 10.1016/j.jamcollsurg.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 107.Carey LA, Metzger R, Dees EC, Collichio F, Sartor CI, Ollila DW, et al. American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst. 2005;97:1137–1142. doi: 10.1093/jnci/dji206. [DOI] [PubMed] [Google Scholar]
- 108.Lee AH, Pinder SE, Macmillan RD, Mitchell M, Ellis IO, Elston CW, et al. Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer. 2006;42:357–362. doi: 10.1016/j.ejca.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 109.Hamy AS, Lam GT, Laas E, Darrigues L, Balezeau T, Guerin J, et al. Lymphovascular invasion after neoadjuvant chemotherapy is strongly associated with poor prognosis in breast carcinoma. Breast Cancer Res Treat. 2018;169:295–304. doi: 10.1007/s10549-017-4610-0. [DOI] [PubMed] [Google Scholar]
- 110.Liu YL, Saraf A, Lee SM, Zhong X, Hibshoosh H, Kalinsky K, et al. Lymphovascular invasion is an independent predictor of survival in breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat. 2016;157:555–564. doi: 10.1007/s10549-016-3837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gabani P, Merfeld E, Srivastava AJ, Weiner AA, Ochoa LL, Mullen D, et al. Predictors of locoregional recurrence after failure to achieve pathologic complete response to neoadjuvant chemotherapy in triple-negative breast cancer. J Natl Compr Canc Netw. 2019;17:348–356. doi: 10.6004/jnccn.2018.7103. [DOI] [PubMed] [Google Scholar]
- 112.Debled M, de Mascarel I, Brouste V, Mauriac L, MacGrogan G. Re: Population-based study of peritumoral lymphovascular invasion and outcome among patients with operable breast cancer. J Natl Cancer Inst. 2010;102:275–276. doi: 10.1093/jnci/djp490. [DOI] [PubMed] [Google Scholar]
- 113.Ugras S, Stempel M, Patil S, Morrow M. Estrogen receptor, progesterone receptor, and HER2 status predict lymphovascular invasion and lymph node involvement. Ann Surg Oncol. 2014;21:3780–3786. doi: 10.1245/s10434-014-3851-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kurosumi M. Significance and problems in evaluations of pathological responses to neoadjuvant therapy for breast cancer. Breast Cancer. 2006;13:254–259. doi: 10.2325/jbcs.13.254. [DOI] [PubMed] [Google Scholar]
- 115.Green MC, Buzdar AU, Smith T, Ibrahim NK, Valero V, Rosales MF, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23:5983–5992. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 116.Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 117.Sharma P, López-Tarruella S, García-Saenz JA, Khan QJ, Gómez HL, Prat A, et al. Pathological response and survival in triple-negative breast cancer following neoadjuvant carboplatin plus docetaxel. Clin Cancer Res. 2018;24:5820–5829. doi: 10.1158/1078-0432.CCR-18-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Romero A, García-Sáenz JA, Fuentes-Ferrer M, López Garcia-Asenjo JA, Furió V, Román JM, et al. Correlation between response to neoadjuvant chemotherapy and survival in locally advanced breast cancer patients. Ann Oncol. 2013;24:655–661. doi: 10.1093/annonc/mds493. [DOI] [PubMed] [Google Scholar]