Abstract
Purpose
Mucinous breast carcinoma (MBC) is a rare type of breast cancer. Although patients with MBC may have a better prognosis than that of patients with invasive ductal carcinoma, many clinicians administer adjuvant chemotherapy regimens similar to those for other breast tumors. Using data from a nationwide clinical database, this study evaluated the significance of adjuvant systemic chemotherapy and whether it can be omitted in MBC patients.
Methods
We included 3,076 patients with a diagnosis of MBC recorded in the Korean Breast Cancer Registry between January 1990 and August 2016. We used the Kaplan-Meier method to analyze breast cancer-specific survival (BCCS) and overall survival (OS). Multivariate analysis was performed using a Cox proportional hazard ratio (HR) model to estimate the adjusted HR for each prognostic factor.
Results
A total of 2,988 MBC patients were enrolled and followed-up for a median of 100 months (range, 2–324 months). Multivariate analysis revealed that axillary lymph node (ALN) metastasis and estrogen receptor (ER) negativity were significant prognostic factors for BCSS. Meanwhile, old age, pathologic tumor stage, and ALN metastasis were significant prognostic factors for OS. Subgroup analysis of ER-positive MBC showed that ALN metastasis was a significant prognostic factor for BCSS. Additionally, old age, pathologic tumor stage, and ALN metastasis were prognostic factors for OS. Ultimately, ALN metastasis was the most statistically significant prognostic factor for MBC. However, chemotherapy had no significant effect on BCSS and OS. The Kaplan-Meier curves of BCSS and OS based on pathologic tumor and nodal stages and age revealed that chemotherapy did not statistically significantly improve prognosis, except for the N3 stage.
Conclusion
Our large retrospective analysis revealed that adjuvant chemotherapy provided little benefit to improve the prognosis of most ER-positive MBC patients. Therefore, chemotherapy can be omitted in the treatment of most ER-positive MBC.
Keywords: Mucinous adenocarcinoma, Breast neoplasm, Chemotherapy, Prognosis
Introduction
Breast cancer is one of the most common cancers in women worldwide and its incidence has been increasing rapidly. Mucinous breast carcinoma (MBC) is a relatively rare type of tumor accounting for only 1%–6% of all breast cancers [1,2,3]. MBCs are associated with a good prognosis and have a better prognosis than that for invasive ductal carcinoma (IDC). They usually occur in women aged above 60 years and in postmenopausal women. MBCs consist of small clusters of tumor cells with large amounts of extracellular and extra-luminal mucus in direct contact with the stroma [3,4,5]. However, the presence of the mucinous carcinoma pattern in a breast mass is not a guarantee of good prognosis. Attentive gross examination and generous histologic sampling are necessary to ensure that the histologic pattern is pure and, thus, of prognostic significance [4]. MBCs are mostly positive for estrogen and/or progesterone receptors (ER/PR-positive) and negative for human epidermal growth factor receptor 2 (HER-2). Additionally, MBCs are less likely to involve the axillary lymph nodes (ALN) than other types of breast cancer. As such, although they are a form of invasive breast cancer, MBCs tend to be less aggressive and respond well to adjuvant treatment and ultimately have a good prognosis [6,7,8]. Different guidelines have been established for adjuvant systemic therapy of MBCs [4,9]. The National Comprehensive Cancer Network (NCCN) guideline recommends treatment according to the hormone receptor status; patients who are hormone receptor-negative are recommended the same treatment as in other breast cancers with poor prognosis, while those who are hormone receptor-positive are recommended to consider chemotherapy only if they have node positivity. Early-stage MBC patients, such as those with N0 or N1mi stage, should receive endocrine therapy, while patients with node positivity (one or more metastases > 2 mm) could be administered adjuvant chemotherapy [10]. However, most treatment guidelines for adjuvant chemotherapy of MBC were derived from IDC treatment experiences. MBC is a relatively rare type of breast cancer; thus, most MBC studies included few patients and limited follow-up periods. Therefore, the treatment guidelines for MBC have not been rigorously validated [11]. Previous studies have shown that many MBC patients receive adjuvant chemotherapy [6,12,13]. However, adjuvant chemotherapy results in increased side effects, including toxicity, and whether adjuvant chemotherapy benefits survival from MBC remains unclear [14]. Therefore, given the good prognosis of MBC, adjuvant chemotherapy may be overtreatment. Using the Korean Breast Cancer Registry (KBCR), this study aimed to verify whether adjuvant chemotherapy has any clinical benefits in ER-positive MBC and whether adjuvant chemotherapy can be omitted in these patients.
Methods
Data source
The KBCR is a prospectively maintained, online-based database of the Korean Breast Cancer Society established in 1996 [15]. More than 100 hospitals throughout the Republic of Korea have voluntarily participated in this program. In 2013, the registry was estimated to include more than 65% of all newly diagnosed breast cancer patients in Korea [16]. Patient data on sex, age, tumor and nodal stage according to the American Joint Committee on Cancer classification, and surgical method are registered as essential data, while pathological findings, laboratory findings, treatment modality (endocrine therapy, radiotherapy, and chemotherapy) are optional data. Data on survival, including dates and causes of death, were obtained from the Korean Central Cancer Registry, Ministry of Health and Welfare, Korea updated on December 31, 2016. The KBCR database does not include the types and dates of tumor recurrence.
Participant selection
Of the 3,076 patients recorded in the KBCR with a pathological diagnosis of MBC between January 1990 and August 2016, we excluded those who had a previous history of breast cancer, had not been treated for breast cancer, or were treated with neoadjuvant and palliative chemotherapy. We collected data on age at diagnosis, method of surgery, pathologic stage, histologic grade, lymphovascular invasion (LVI), ER/PR/HER-2 status, p53, and type of adjuvant treatment. This study protocol was approved by the Institutional Review Board (IRB) of Seoul St. Mary's Hospital (IRB number: KC17RESI0649) and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent from the patients was not required in this study.
Study variables
The primary variables analyzed were breast cancer-specific survival (BCSS) and overall survival (OS) for all MBC and ER-positive MBC patients. The secondary variable was the survival benefit of adjuvant chemotherapy to ER-positive MBC. The BCSS and OS for MBC were calculated using data from the KBCR database. BCSS and OS were defined as the time from the first diagnosis of breast cancer to death from breast cancer and any other causes, respectively. Data were censored on December 31, 2016.
Statistical analysis
Univariate and multivariate analyses for BCCS and OS were performed using a Cox proportional hazard ratio (HR) model to estimate adjusted HRs and 95% confidence intervals (CIs) to adjust for prognostic factors. Multivariate analysis was used to investigate variables that were significant in the univariate analysis. The survival analysis was performed using the Kaplan-Meier method and the chemotherapy and no chemotherapy groups were compared using the log-rank test to evaluate the survival difference. The p-values < 0.05 were considered statistically significant. All analyses were performed using the SAS 9.4 (SAS Institute Inc., Cary, USA) and IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, USA).
Results
Patient characteristics
A total of 2,988 MBC patients with available medical records were enrolled in this study. The median follow-up time was 100 months (range, 2–324 months). The mean age of the patients was 49.6 years (range, 20–90 years). Most (63%) patients were aged less than 50 years. Most MBC cases (2,806 patients [93.9%]) had T1 or T2-stage disease. A total of 2,449 (82.0%) patients had ALN negativity. Of the 493 (16.5%) patients with ALN metastasis, 381 (77.3%) and 112 (22.7%) had 1–3 and more than 4 metastases, respectively. In total, 2,581 (86.4%) and 2,143 (71.7%) patients were ER-positive and HER-2 negative, respectively. Of the 2,581 ER-positive patients, 2,135 (82.7%) had no ALN metastasis and 413 (16.0%) had ALN metastasis. Furthermore, 1,416 (47.4%) patients had received chemotherapy, 1,252 (42.0%) patients had not received chemotherapy, and 317 (10.6%) patients had no information about chemotherapy in the database. Among the 1,416 patients who received chemotherapy, 1,182 were ER-positive. Of the 1,182 ER-positive patients who underwent chemotherapy, 348 (29.4%) had ALN metastasis and 826 (70.4%) did not. During the study period, 190 (6.4%) deaths occurred, 57 (1.9%) of which were breast cancer-related. The detailed clinicopathologic characteristics of the patients with MBC are described in Table 1.
Table 1. Clinicopathological characteristics of all MBC, ER(+) MBC, and ER(+) MBC with node(+) patients.
| Variables | Total MBC (n = 2,988) | ER(+) MBC (n = 2,581) | ER(+) MBC with node(+) (n = 413) | |
|---|---|---|---|---|
| Age (yr) | ||||
| ≤ 50 | 1,882 (63.0) | 1,615 (62.6) | 270 (65.4) | |
| > 50 | 1,106 (37.0) | 966 (37.4) | 143 (34.6) | |
| Sex | ||||
| Female | 2,965 (99.2) | 2,560 (99.2) | 412 (99.8) | |
| Male | 23 (0.8) | 21 (0.8) | 1 (0.2) | |
| Breast operation | ||||
| BCS | 1,607 (55.3) | 1,455 (56.4) | 149 (36.1) | |
| Mastectomy | 1,368 (44.3) | 1,118 (43.3) | 264 (63.9) | |
| Unknown | 13 (0.4) | 8 (0.3) | 0 (0 ) | |
| Axillary operation | ||||
| SLNB | 1,149 (38.5) | 1,077 (41.7) | 31 (7.5) | |
| ALND | 1,796 (60.1) | 1,473 (57.1) | 382 (92.5) | |
| Unknown | 43 (1.4) | 31 (1.2) | 0 (0) | |
| pT stage | ||||
| T1 | 1,548 (51.8) | 1,354 (52.5) | 129 (31.2) | |
| T2 | 1,258 (42.1) | 1,084 (42.0) | 232 (56.2) | |
| T3 | 153 (5.1) | 120 (4.6) | 46 (11.1) | |
| T4 | 12 (0.4) | 11 (0.4) | 6 (1.5) | |
| Unknown | 17 (0.6) | 12 (0.5) | 0 (0) | |
| pN stage | ||||
| N0 | 2,449 (82.0) | 2,135 (82.7) | 0 (0) | |
| N1 | 381 (12.8) | 328 (12.7) | 328 (79.4) | |
| N2 | 81 (2.7) | 66 (2.6) | 66 (16.0) | |
| N3 | 31 (1.0) | 19 (0.7) | 19 (4.6) | |
| Unknown | 46 (1.5) | 33 (1.3) | 0 (0) | |
| Histologic grade | ||||
| Grade 1 or 2 | 1,740 (58.3) | 1,629 (63.1) | 233 (56.4) | |
| Grade 3 | 231 (7.7) | 196 (7.6) | 71 (17.2) | |
| Unknown | 1,017 (34.0) | 756 (29.3) | 109 (26.4) | |
| LVI | ||||
| No | 1,662 (55.6) | 1,526 (59.1) | 155 (37.5) | |
| Yes | 726 (24.3) | 660 (25.6) | 208 (50.4) | |
| Unknown | 600 (20.1) | 395 (15.3) | 50 (12.1) | |
| ER status | ||||
| Positive | 2,581 (86.4) | - | - | |
| Negative | 233 (7.8) | - | - | |
| Unknown | 174 (5.8) | - | - | |
| PR status | ||||
| Positive | 2,195 (73.5) | 2,136 (82.7) | 317 (76.8) | |
| Negative | 615 (20.5) | 443 (17.2) | 96 (23.2) | |
| Unknown | 178 (6.0) | 2 (0.1) | 0 (0) | |
| HER-2 status | ||||
| Negative | 2,143 (71.7) | 2,025 (78.5) | 280 (67.8) | |
| Equivocal | 235 (7.9) | 220 (8.5) | 53 (12.8) | |
| Positive | 237 (7.9) | 178 (6.9) | 55 (13.3) | |
| Unknown | 373 (12.5) | 158 (6.1) | 25 (6.1) | |
| p53 | ||||
| Negative | 1,572 (52.6) | 1,391 (53.9) | 210 (50.8) | |
| Positive | 669 (22.4) | 604 (23.4) | 116 (28.1) | |
| Unknown | 833 (27.9) | 586 (22.7) | 87 (21.1) | |
| Radiotherapy | ||||
| Yes | 1,500 (50.2) | 1,369 (53.0) | 198 (47.9) | |
| No | 1,078 (36.1) | 925 (35.8) | 159 (38.5) | |
| Unknown | 410 (13.7) | 287 (11.1) | 56 (13.6) | |
| Chemotherapy | ||||
| Yes | 1,416 (47.4) | 1,181 (45.8) | 348 (84.3) | |
| No | 1,252 (42.0) | 1,173 (45.4) | 36 (8.7) | |
| Unknown | 317 (10.6) | 227 (8.8) | 29 (7.0) | |
| Hormone therapy | ||||
| Yes | 2,328 (77.9) | 2,181 (84.5) | 334 (80.8) | |
| No | 258 (8.6) | 116 (4.5) | 23 (5.6) | |
| Unknown | 402 (13.5) | 284 (11.0) | 56 (13.6) | |
| Death | 190 (6.4) | 125 (4.8) | 43 (10.4) | |
| Breast cancer related | 57 (1.9) | 35 (1.4) | 15 (3.6) | |
| Other cause | 133 (4.5) | 90 (3.4) | 28 (6.8) | |
Values are presented as number (%).
MBC = mucinous breast cancer; ER = estrogen receptor; LVI = lymphovascular invasion; BCS = breast conserving surgery; SLNB = sentinel lymph node biopsy; ALND = axillary lymph node dissection; PR = progesterone receptor; HER-2 = human epidermal growth factor receptor 2.
Prognostic factors in MBC
Univariate and multivariate analyses were performed to identify the prognostic factors influencing BCSS and OS in all MBC patients (Table 2). In univariate analysis, old age (> 50 years), pathologic tumor stage, pathologic nodal stage, p53, LVI, radiotherapy, hormone therapy, chemotherapy, and hormone receptor (ER and PR) negativity significantly influenced BCSS. In multivariate analysis, pathologic nodal stage and ER negativity were significant prognostic factors for BCSS. Meanwhile, univariate analysis showed that old age (> 50 years), pathologic tumor stage, pathologic nodal stage, LVI, radiotherapy, hormone therapy, HER-2 negativity, and ER and PR negativity significantly influenced OS. Of these, old age (> 50 years), pathologic tumor stage, and pathologic nodal stage were significant prognostic factors for OS in multivariate analysis. However, omission of adjuvant chemotherapy in multivariate analysis did not significantly improve the prognosis for BCSS (HR, 0.577; 95% CI, 0.101–3.286; p = 0.535) or OS (HR, 0.840; 95% CI, 0.602–1.172; p = 0.306).
Table 2. Univariate and multivariate Cox regression analyses for BCSS and OS in all mucinous breast cancer patients.
| Variables | Univariate Cox regression | Multivariate Cox regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value | |||
| BCSS | ||||||||
| Age (yr) | ||||||||
| > 50/≤ 50 | 2.039 | 1.211–3.432 | 0.007 | 1.642 | 0.486–5.549 | 0.425 | ||
| Pathologic tumor stage | ||||||||
| T2, 3, 4/T1 | 3.012 | 1.670–5.432 | < 0.001 | 3.495 | 0.737–16.578 | 0.115 | ||
| Pathologic nodal stage | ||||||||
| N1, 2, 3/N0 | 4.586 | 2.662–7.902 | < 0.001 | 4.049 | 1.179–13.901 | 0.026 | ||
| Histologic grade | ||||||||
| Grade 3/Grade 1, 2 | 2.221 | 0.819–6.021 | 0.117 | - | - | - | ||
| LVI | ||||||||
| Yes/No | 2.125 | 0.999–4.521 | 0.050 | 2.117 | 0.616–7.273 | 0.234 | ||
| ER status | ||||||||
| Negative/Positive | 3.530 | 1.865–6.683 | < 0.001 | 5.676 | 1.464–22.001 | 0.012 | ||
| PR status | ||||||||
| Negative/Positive | 2.089 | 1.170–3.730 | 0.013 | 1.380 | 0.347–5.483 | 0.647 | ||
| p53 | ||||||||
| Positive/Negative | 0.196 | 0.046–0.832 | 0.027 | 0.494 | 0.105–2.332 | 0.373 | ||
| HER-2 status | ||||||||
| Positive/Negative | 1.740 | 0.597–5.069 | 0.310 | - | - | - | ||
| Radiotherapy | ||||||||
| No/Yes | 4.765 | 2.188–10.378 | < 0.001 | 4.025 | 1.057–15.327 | 0.041 | ||
| Hormone therapy | ||||||||
| No/Yes | 3.998 | 2.090–7.645 | < 0.001 | 1.265 | 0.199–8.049 | 0.803 | ||
| Chemotherapy | ||||||||
| No/Yes | 0.385 | 0.183–0.808 | 0.012 | 0.577 | 0.101–3.286 | 0.535 | ||
| OS | ||||||||
| Age (yr) | ||||||||
| > 50/≤ 50 | 2.992 | 2.238–3.999 | < 0.001 | 2.748 | 1.667–4.533 | < 0.001 | ||
| Pathologic tumor stage | ||||||||
| T2, 3, 4/T1 | 2.110 | 1.561–2.850 | < 0.001 | 1.915 | 1.118–3.279 | 0.018 | ||
| Pathologic nodal stage | ||||||||
| N1, 2, 3/N0 | 2.964 | 2.186–4.019 | < 0.001 | 2.787 | 1.617–4.806 | < 0.001 | ||
| Histologic grade | ||||||||
| Grade 3/Grade 1, 2 | 1.250 | 0.708–2.209 | 0.442 | - | - | - | ||
| LVI | ||||||||
| Yes/No | 1.637 | 1.100–2.435 | 0.015 | 1.612 | 0.952–2.731 | 0.076 | ||
| ER status | ||||||||
| Negative/Positive | 2.018 | 1.348–3.019 | 0.001 | 1.111 | 0.389–3.168 | 0.844 | ||
| PR status | ||||||||
| Negative/Positive | 1.503 | 1.074–2.103 | 0.017 | 0.798 | 0.427–1.490 | 0.479 | ||
| p53 | ||||||||
| Positive/Negative | 1.018 | 0.661–1.567 | 0.936 | - | - | - | ||
| HER-2 status | ||||||||
| Positive/Negative | 1.913 | 1.131–3.236 | 0.016 | 1.675 | 0.884–3.177 | 0.114 | ||
| Radiotherapy | ||||||||
| No/Yes | 2.365 | 1.667–3.356 | < 0.001 | 1.426 | 0.860–2.363 | 0.169 | ||
| Hormone therapy | ||||||||
| No/Yes | 1.654 | 1.103–2.481 | 0.015 | 1.515 | 0.727–3.159 | 0.268 | ||
| Chemotherapy | ||||||||
| No/Yes | 0.840 | 0.602–1.172 | 0.306 | - | - | - | ||
BCSS = breast cancer-specific survival; OS = overall survival; HR = hazard ratio; CI = confidence interval; LVI = lymphovascular invasion; ER = estrogen receptor; PR = progesterone receptor; HER-2 = human epidermal growth factor receptor 2.
Prognostic factors in ER-positive MBC
We performed a subgroup analysis of 2,581 ER-positive patients. Univariate and multivariate analyses were performed to identify the prognostic factors affecting BCSS and OS in this subgroup (Table 3). In univariate analysis, old age (> 50 years), pathologic nodal stage, radiotherapy, and chemotherapy significantly influenced BCSS, but pathologic nodal stage was the only significant prognostic factor in multivariate analysis. Regarding OS, old age (> 50 years), pathologic tumor stage, pathologic nodal stage, LVI, HER-2 status, and radiotherapy were statistically significant in univariate analysis. However, in multivariate analysis, only old age (> 50 years), pathologic tumor stage, and pathologic nodal stage remained statistically significant prognostic factors for OS. Meanwhile, omission of adjuvant chemotherapy in multivariate analysis did not significantly improve the prognosis for either BCSS (HR, 0.393; 95% CI, 0.106–1.453; p = 0.162) or OS (HR, 0.818; 95% CI, 0.549–1.220; p = 0.326) in ER-positive MBC with ALN metastasis (Supplementary Table 1).
Table 3. Univariate and multivariate Cox regression analyses for BCSS and OS in estrogen receptor-positive mucinous breast cancer.
| Variables | Univariate Cox regression | Multivariate Cox regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted HR | 95% CI | p-value | Adjusted HR | 95% CI | p-value | |||
| BCSS | ||||||||
| Age (yr) | ||||||||
| > 50/≤ 50 | 2.292 | 1.177–4.462 | 0.015 | 1.441 | 0.564–3.682 | 0.445 | ||
| Pathologic tumor stage | ||||||||
| T2, 3, 4/T1 | 1.857 | 0.936–3.687 | 0.077 | - | - | - | ||
| Pathologic nodal stage | ||||||||
| N1, 2, 3/N0 | 4.487 | 2.241–8.985 | < 0.001 | 5.358 | 2.175–13.200 | < 0.001 | ||
| Histologic grade | ||||||||
| Grade 3/Grade 1, 2 | 1.766 | 0.507–6.146 | 0.365 | - | - | - | ||
| LVI | ||||||||
| Yes/No | 2.101 | 0.892–4.946 | 0.089 | - | - | - | ||
| PR status | ||||||||
| Negative/Positive | 1.502 | 0.703–3.210 | 0.293 | - | - | - | ||
| p53 | ||||||||
| Positive/Negative | 0.028 | 0.001–1.452 | 0.076 | - | - | - | ||
| HER-2 status | ||||||||
| Positive/Negative | 1.213 | 0.283–5.208 | 0.795 | - | - | - | ||
| Radiotherapy | ||||||||
| No/Yes | 3.888 | 1.411–10.719 | 0.009 | 3.346 | 1.198–9.293 | 0.021 | ||
| Hormone therapy | ||||||||
| No/Yes | 0.745 | 0.100–5.550 | 0.774 | - | - | - | ||
| Chemotherapy | ||||||||
| No/Yes | 0.208 | 0.061–0.709 | 0.012 | 0.393 | 0.106–1.453 | 0.162 | ||
| OS | ||||||||
| Age (yr) | ||||||||
| > 50/≤ 50 | 3.134 | 2.186–4.492 | < 0.001 | 3.200 | 1.867–5.488 | < 0.001 | ||
| Pathologic tumor stage | ||||||||
| T2, 3, 4/T1 | 2.088 | 1.444–3.020 | < 0.001 | 1.962 | 1.116–3.449 | 0.019 | ||
| Pathologic nodal stage | ||||||||
| N1, 2, 3/N0 | 2.949 | 2.025–4.294 | < 0.001 | 2.876 | 1.665–4.967 | < 0.001 | ||
| Histologic grade | ||||||||
| Grade 3/Grade 1, 2 | 0.849 | 0.390–1.852 | 0.682 | - | - | - | ||
| LVI | ||||||||
| Yes/No | 1.677 | 1.085–2.593 | 0.020 | 1.406 | 0.797–2.480 | 0.240 | ||
| PR status | ||||||||
| Negative/Positive | 1.294 | 0.858–1.954 | 0.219 | - | - | - | ||
| p53 | ||||||||
| Positive/Negative | 0.989 | 0.619–1.578 | 0.962 | - | - | - | ||
| HER-2 status | ||||||||
| Positive/Negative | 1.863 | 1.010–3.439 | 0.046 | 1.495 | 0.698–3.199 | 0.300 | ||
| Radiotherapy | ||||||||
| No/Yes | 2.381 | 1.572–3.606 | < 0.001 | 1.252 | 0.735–2.317 | 0.408 | ||
| Hormone therapy | ||||||||
| No/Yes | 0.890 | 0.389–2.034 | 0.782 | - | - | - | ||
| Chemotherapy | ||||||||
| No/Yes | 0.818 | 0.549–1.220 | 0.326 | - | - | - | ||
BCSS = breast cancer-specific survival; OS = overall survival; HR = hazard ratio; CI = confidence interval; LVI = lymphovascular invasion; PR = progesterone receptor; HER-2 = human epidermal growth factor receptor 2.
Efficacy of chemotherapy for MBC
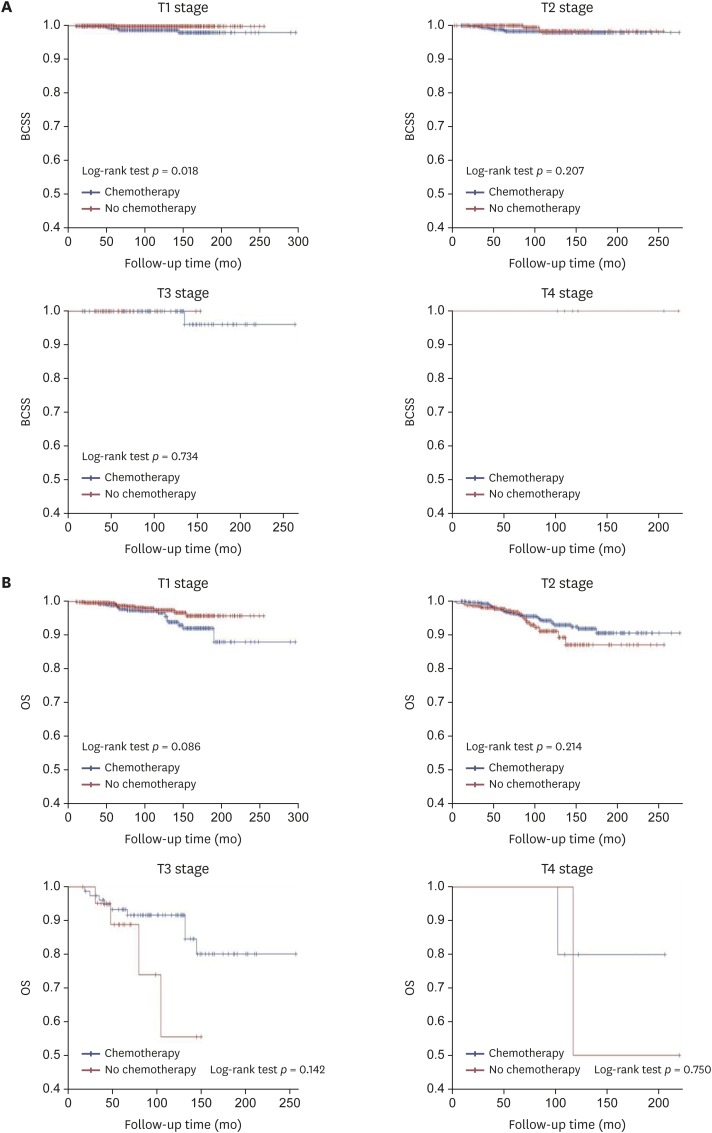
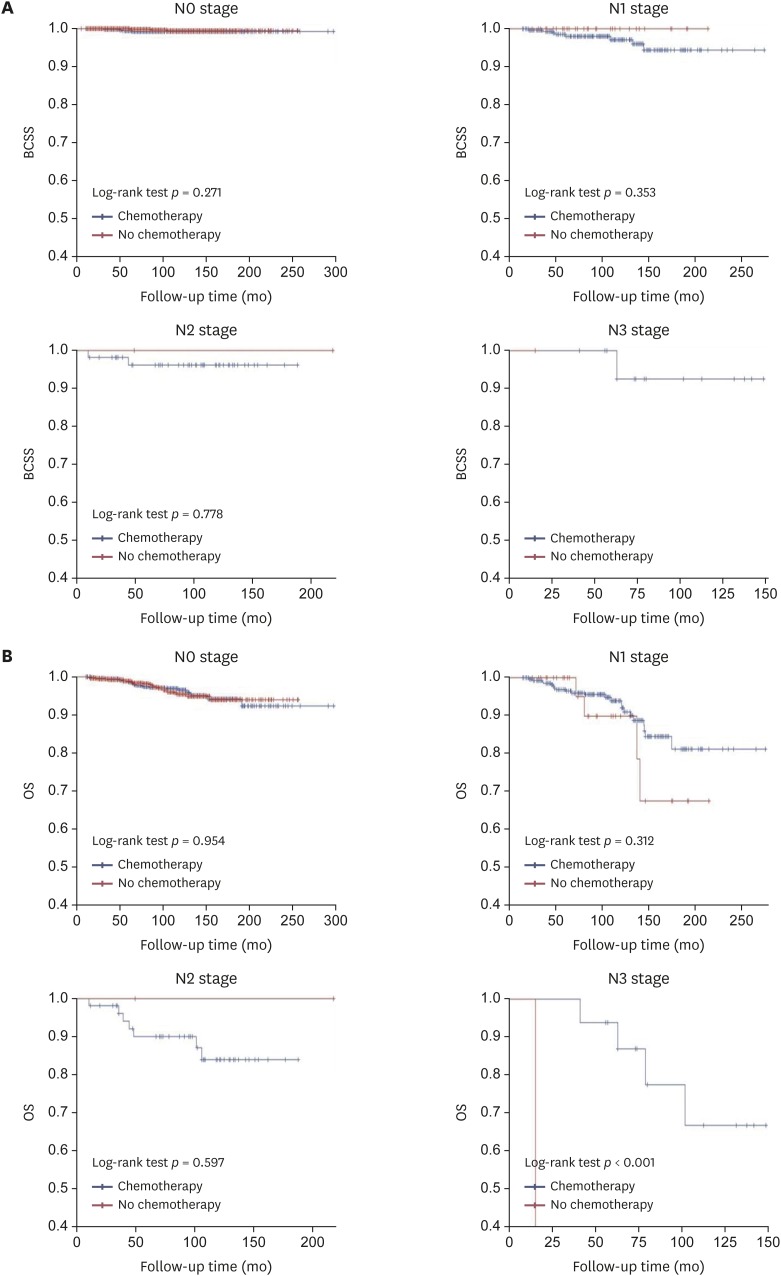
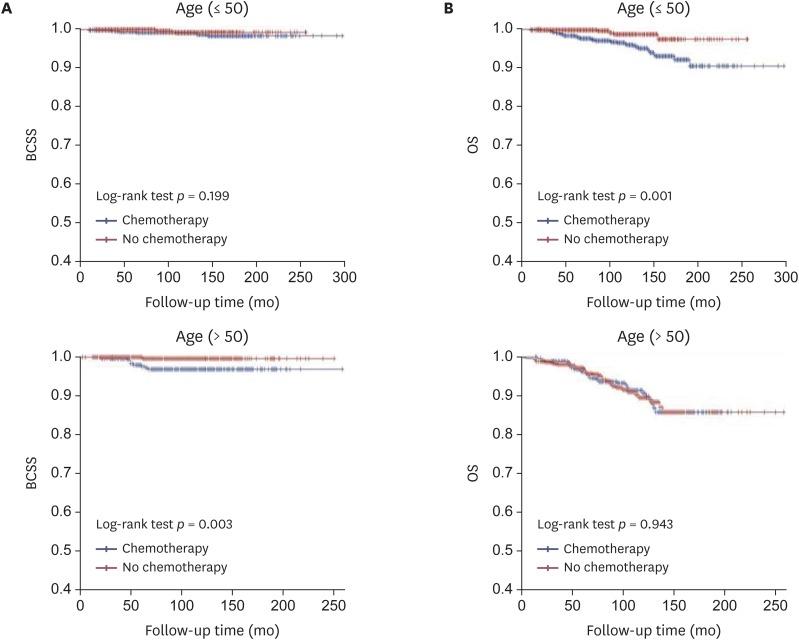
The efficacy of adjuvant chemotherapy for the 2,581 ER-positive MBC patients was analyzed according to pathologic tumor and nodal stage and age. The 5-year and 10-year BCSS of ER-positive MBC patients were 99.2% and 98.3%, respectively, while the 5-year and 10-year OS were 97.8% and 94.3%, respectively. The 5-year and 10-year BCSS of ER-positive MBC with ALN metastasis were 97.9% and 95.9%, respectively, while the 5-year and 10-year OS were 95.5% and 88.7%, respectively. The Kaplan-Meier curves for BCSS and OS based on pathologic tumor stage revealed that chemotherapy did not statistically significantly improve the prognosis (Figure 1). There were significant differences in BCSS in T1 stage disease depending on whether or not chemotherapy was performed, and the BCSS was longer in patients who did not receive chemotherapy. In the analysis for OS among patients with T3 disease, the adjuvant chemotherapy group showed a better OS than that of the group without adjuvant chemotherapy, but the difference was not statistically significant. Comparison of the chemotherapy efficacy was difficult in the analysis of BCSS and OS in patients with T3 and T4 stage disease due to the small number of patients in these groups. When stratified by pathologic nodal stage, the Kaplan-Meier curves for BCSS and OS also showed no statistically significant difference based on chemotherapy except for the N3 stage (Figure 2). It was difficult to obtain meaningful results for pathologic N2 and N3 stage disease because of the small number of patients. When stratified by age, the Kaplan-Meier curves of BCSS and OS showed no significant difference between younger (≤ 50 years) and older (> 50 years) patients. However, BCSS and OS differed significantly between older (> 50 years) and younger (≤ 50 years) patients. The results showed better survival when chemotherapy was omitted (Figure 3).
Figure 1. Kaplan-Meier curve of (A) BCSS and (B) OS rates according to chemotherapy in estrogen-receptor-positive mucinous breast cancer patients, stratified by pathologic tumor stage.
BCSS = breast cancer-specific survival; OS = overall survival.
Figure 2. Kaplan-Meier curve of (A) BCSS and (B) OS rates according to chemotherapy in estrogen-receptor-positive mucinous breast cancer patients, stratified by pathologic nodal stage.
BCSS = breast cancer-specific survival; OS = overall survival.
Figure 3. Kaplan-Meier curve of (A) BCSS and (B) OS rates according to chemotherapy in estrogen-receptor-positive mucinous breast cancer, stratified by age.
BCSS = breast cancer-specific survival; OS = overall survival.
Discussion
This study investigated the need for chemotherapy in the treatment of ER-positive MBC. Our findings showed that adjuvant chemotherapy did not significantly improve BCSS and OS in most MBC patients. We also found a good prognosis of MBC, similar to previous reports [6,9]. Among patients with ER-positive MBC, ALN metastasis was a significant prognostic factor for BCSS, while old age, pathologic tumor, and ALN metastasis were significant prognostic factors for OS.
MBC is a relatively rare histologic type of tumor that accounts for only 1%–6% of all breast cancers. MBC is characterized by a large amount of extracellular mucin [1,6]. Previous studies have shown that MBC has characteristics different from those of other invasive breast cancers, including high ER/PR expression, less ALN metastasis, and lower HER-2 expression [9,17,18], which were also observed in our study. Due to these characteristics, MBC has a better prognosis than those of other invasive breast cancers. MBC can be divided into pure MBC (pMBC) and mixed MBC (mMBC) depending on the quantification of cellularity [19]. pMBC consists exclusively of tumor tissues with mucinous components above 90% while mMBC contains mucinous areas covering more than 50% but less than 90% of the total area, as well as admixing, usually with an infiltrating ductal epithelial component [20]. Recent studies have evaluated the differences in clinicopathological features and survival between pMBC and mMBC [13,21,22]. Nodal positivity in pMBC ranges from 2%–14% [1,21,23], compared to 46%–64% in mMBC [2,7,21]. However, the KBCR does not provide data to distinguish between the histologic subtypes of MBC. Most patients in our study were thought to have pMBC because 16.5% had axillary nodal positivity.
The NCCN guidelines consider hormone receptors to play an important role in the treatment of MBC. Hormone receptor-positive patients are recommended to consider chemotherapy if they have ALN metastasis, whereas adjuvant chemotherapy is not recommended for patients without metastatic ALN. Meanwhile, because ER-negative MBC has a poor prognosis, the recommended treatment strategy is similar to that for other breast cancers [10]. Our results revealed that ER-negativity had a significant effect on BCSS. Due to the low prevalence, limited follow-up period, and good prognosis, few studies on MBC have been conducted, and most of the previous studies had a small sample size. The clinicopathologic factors affecting the prognosis of MBC are unclear. Furthermore, most treatment guidelines for adjuvant chemotherapy of MBC were derived from IDC treatment experiences [14] and have not been rigorously validated [11]. Therefore, the treatment guidelines should be validated to establish more appropriate guidelines for MBC.
Some studies have identified ALN metastasis as the strongest predictor of disease-specific survival because most patients presenting with LN metastases develop distant metastasis [13,22]. In our study, ALN metastasis was also the most significant prognostic factor for BCSS and OS. Old age (> 50 years) and pathologic tumor stage were significant factors for OS. Previous studies revealed local or distant recurrence in some MBC patients. As such, many clinicians administer adjuvant chemotherapy similar to those for other breast tumors despite the good prognosis of MBC [6,11,12]. This trend was consistent with our findings. In our study, 1,415 (47.4%) patients received adjuvant chemotherapy; of them, 1,181 patients were ER-positive. However, in contrast to the guidelines, 826 of 1,945 MBC (42.5%) patients with positive ER expression but without ALN metastasis received adjuvant chemotherapy. Current guidelines recommend adjuvant chemotherapy in MBC patients with ER positivity and ALN metastasis [10]. In our study, 348 of 384 (90.6%) patients were administered chemotherapy. These results show that, contrary to guideline recommendations, a large number of patients with MBC undergo chemotherapy in the real world.
Recent studies have reported that luminal breast cancer patients do not equally benefit from adjuvant chemotherapy [24,25,26]. Furthermore, clinical trials have shown that adjuvant chemotherapy may not be necessary even in cases with ALN metastasis or large tumor size in luminal breast cancer [27]. Gene expression profiles have been developed to identify the individual risk of recurrence to avoid unnecessary adjuvant cytotoxic chemotherapy [28]. Chemotherapy is also less effective in MBC because of the higher ER and PR and lower HER-2 expression. These observations suggest that adjuvant chemotherapy can be omitted in MBC, which generally has a good prognosis. However, gene expression profiles cannot be used to predict recurrence risk in MBC due to the abundant mucinous content [11]. Therefore, we investigated the benefit of adjuvant chemotherapy in ER-positive MBC. Several clinical studies have already shown a good prognosis in MBC patients, even those with high-risk factors [7,13,22].
When stratified by pathologic tumor stage, pathologic nodal stage, and age, our Kaplan–Meier curve analysis revealed that, except for N3 stage, adjuvant chemotherapy did not significantly improve BCSS and OS. We did not obtain significant results in pathologic T stage analysis for BCSS and OS due to the sample sizes of T3 and T4 patients. In our study, 94.5% of patients with ER-positive MBC had T1 and T2 stage disease. Pathologic N stage analysis showed differences in BCSS and OS between the chemotherapy and no chemotherapy groups for N2 and N3-stage disease. Only N3 stage showed a significant difference. However, the sample size was too small to interpret these results. N2 and N3-stage disease is also extremely rare among ER-positive MBC patients, accounting for only 3.3% of cases. Further studies are warranted to determine the efficacy of chemotherapy in a larger number of patients with other stages of disease. Our study found that most ER-positive MBC patients had T1 or T2 or N1-stage disease and that chemotherapy did not improve survival in most of these patients. The results of our multivariate analysis also indicated that the omission of adjuvant chemotherapy did not have a significant effect on BCCS and OS, despite the presence of ALN metastasis. Analysis of BCSS and OS for N1 stage showed a lower OS than BCSS in the no chemotherapy group, but the difference was not statistically significant. These results suggest that the patients who did not receive chemotherapy had a higher rate of other-cause deaths because of underlying disease or old age.
Our study had several limitations. First, the KBCR does not record the type of MBC as pure or mixed and provides no detailed information about recurrence. Second, because of the retrospective and voluntary nature of the KBCR, a large portion of data were missing, particularly regarding pathologic details and treatment. These missing data may have affected our results. Furthermore, it is difficult to reach a definitive conclusion due to the retrospective study design. Despite these limitations, our study is valuable in that, to the best of our knowledge, it is the first to investigate the efficacy of adjuvant chemotherapy in ER-positive MBC patients. Our study was also among the few large-scale MBC studies with a relatively long-term follow-up. Further, the KBCR is a nationwide database that prospectively collects data from more than 65% of patients undergoing breast cancer surgery in Korea; thus, our findings may be representative of all women with breast cancer in Korea.
In conclusion, the results of this large retrospective analysis revealed that adjuvant chemotherapy does not improve the prognosis in most ER-positive MBC patients, regardless of ALN metastasis. The good prognosis of patients with MBC was also confirmed. Furthermore, the pathologic nodal stage was the most important factor influencing patient prognosis. Ultimately, adjuvant chemotherapy can be omitted in the treatment of most ER-positive MBC.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
- Conceptualization: Kim HS, Lee JU, Son D, Kim YJ.
- Formal analysis: Kim HS.
- Investigation: Kim HS.
- Methodology: Kim HS.
- Supervision: Lee JU, Yoo TK, Chae BJ, Park WC.
- Writing - original draft: Kim HS.
- Writing - review & editing: Kim HS, Park WC.
SUPPLEMENTARY MATERIAL
Univariate and Multivariate Cox regression analyses for BCSS and OS in ER-positive mucinous breast cancer with axillary lymph node metastasis
References
- 1.Azzopardi JG, Ahmed A, Millis RR. Problems in breast pathology. Major Probl Pathol. 1979;11:i–xvi. [PubMed] [Google Scholar]
- 2.Fentiman IS, Millis RR, Smith P, Ellul JP, Lampejo O. Mucoid breast carcinomas: histology and prognosis. Br J Cancer. 1997;75:1061–1065. doi: 10.1038/bjc.1997.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komaki K, Sakamoto G, Sugano H, Morimoto T, Monden Y. Mucinous carcinoma of the breast in Japan. A prognostic analysis based on morphologic features. Cancer. 1988;61:989–996. doi: 10.1002/1097-0142(19880301)61:5<989::aid-cncr2820610522>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Gallager HS. Pathologic types of breast cancer: their prognoses. Cancer. 1984;53(Suppl):623–629. doi: 10.1002/1097-0142(19840201)53:3+<623::aid-cncr2820531307>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Lacroix-Triki M, Suarez PH, MacKay A, Lambros MB, Natrajan R, Savage K, et al. Mucinous carcinoma of the breast is genomically distinct from invasive ductal carcinomas of no special type. J Pathol. 2010;222:282–298. doi: 10.1002/path.2763. [DOI] [PubMed] [Google Scholar]
- 6.Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011;14:308–313. doi: 10.4048/jbc.2011.14.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen BB, Rose C, Christensen IB. Prognostic factors in primary mucinous breast carcinoma. Am J Clin Pathol. 1987;87:155–160. doi: 10.1093/ajcp/87.2.155. [DOI] [PubMed] [Google Scholar]
- 8.Avisar E, Khan MA, Axelrod D, Oza K. Pure mucinous carcinoma of the breast: a clinicopathologic correlation study. Ann Surg Oncol. 1998;5:447–451. doi: 10.1007/BF02303864. [DOI] [PubMed] [Google Scholar]
- 9.Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008;111:541–547. doi: 10.1007/s10549-007-9809-z. [DOI] [PubMed] [Google Scholar]
- 10.Goetz MP, Gradishar WJ, Anderson BO, Abraham J, Aft R, Allison KH, et al. NCCN guidelines insights: breast cancer, version 3.2018. J Natl Compr Canc Netw. 2019;17:118–126. doi: 10.6004/jnccn.2019.0009. [DOI] [PubMed] [Google Scholar]
- 11.Fu J, Wu L, Jiang M, Li D, Jiang T, Hong Z, et al. Clinical Nomogram for predicting survival outcomes in early mucinous breast cancer. PLoS One. 2016;11:e0164921. doi: 10.1371/journal.pone.0164921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan B, Yao R, Shi J, Xu QQ, Zhou YD, Mao F, et al. Prognosis of subtypes of the mucinous breast carcinoma in Chinese women: a population-based study of 32-year experience (1983–2014) Oncotarget. 2016;7:38864–38875. doi: 10.18632/oncotarget.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Teng XD, Guo XX, Zhao JS, Li ZG. Clinicopathological characteristics and prognosis of mucinous breast carcinoma. J Cancer Res Clin Oncol. 2014;140:265–269. doi: 10.1007/s00432-013-1559-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao AY, He M, Liu ZB, Di GH, Wu J, Lu JS, et al. Outcome of pure mucinous breast carcinoma compared to infiltrating ductal carcinoma: a population-based study from China. Ann Surg Oncol. 2012;19:3019–3027. doi: 10.1245/s10434-012-2322-6. [DOI] [PubMed] [Google Scholar]
- 15.Park EH, Min SY, Kim Z, Yoon CS, Jung KW, Nam SJ, et al. Basic facts of breast cancer in Korea in 2014: the 10-year overall survival progress. J Breast Cancer. 2017;20:1–11. doi: 10.4048/jbc.2017.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang SY, Kim YS, Kim Z, Kim HY, Lee SK, Jung KW, et al. Basic findings regarding breast cancer in Korea in 2015: data from a breast cancer registry. J Breast Cancer. 2018;21:1–10. doi: 10.4048/jbc.2018.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diab SG, Clark GM, Osborne CK, Libby A, Allred DC, Elledge RM. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol. 1999;17:1442–1448. doi: 10.1200/JCO.1999.17.5.1442. [DOI] [PubMed] [Google Scholar]
- 18.Northridge ME, Rhoads GG, Wartenberg D, Koffman D. The importance of histologic type on breast cancer survival. J Clin Epidemiol. 1997;50:283–290. doi: 10.1016/s0895-4356(96)00366-6. [DOI] [PubMed] [Google Scholar]
- 19.Silverberg SG, Kay S, Chitale AR, Levitt SH. Colloid carcinoma of the breast. Am J Clin Pathol. 1971;55:355–363. doi: 10.1093/ajcp/55.3.355. [DOI] [PubMed] [Google Scholar]
- 20.Azzopardi JG, Chepick OF, Hartmann WH, Jafarey NA, Llombart-Bosch A, Ozzello L, et al. The World Health Organization histological typing of breast tumors--second edition. Am J Clin Pathol. 1982;78:806–816. doi: 10.1093/ajcp/78.6.806. [DOI] [PubMed] [Google Scholar]
- 21.Skotnicki P, Sas-Korczynska B, Strzepek L, Jakubowicz J, Blecharz P, Reinfuss M, et al. Pure and mixed mucinous carcinoma of the breast: a comparison of clinical outcomes and treatment results. Breast J. 2016;22:529–534. doi: 10.1111/tbj.12621. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen BB. Human mucinous breast carcinomas and their lymph node metastases. A histological review of 247 cases. Pathol Res Pract. 1985;180:377–382. doi: 10.1016/S0344-0338(85)80110-2. [DOI] [PubMed] [Google Scholar]
- 23.Memis A, Ozdemir N, Parildar M, Ustun EE, Erhan Y. Mucinous (colloid) breast cancer: mammographic and US features with histologic correlation. Eur J Radiol. 2000;35:39–43. doi: 10.1016/s0720-048x(99)00124-2. [DOI] [PubMed] [Google Scholar]
- 24.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piccart M, Rutgers E, van't Veer L, Sleets L, Delaloge S, Viale G, et al. Primary analysis of the EORTC 10041/ BIG 3-04 MINDACT study: a prospective, randomized study evaluating the clinical utility of the 70-gene signature (MammaPrint) combined with common clinical-pathological criteria for selection of patients for adjuvant chemotherapy in breast cancer with 0 to 3 positive nodes. Cancer Res. 2016;76(Suppl 14):CT039. [Google Scholar]
- 26.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen TO, Jensen MB, Burugu S, Gao D, Jørgensen CL, Balslev E, et al. High-risk premenopausal luminal a breast cancer patients derive no benefit from adjuvant cyclophosphamide-based chemotherapy: results from the DBCG77B clinical trial. Clin Cancer Res. 2017;23:946–953. doi: 10.1158/1078-0432.CCR-16-1278. [DOI] [PubMed] [Google Scholar]
- 28.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360:790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Univariate and Multivariate Cox regression analyses for BCSS and OS in ER-positive mucinous breast cancer with axillary lymph node metastasis