Abstract
Stress suppresses pulsatile LH secretion in a variety of species, however the mechanism underlying this inhibition of reproductive function remains unclear. Metabolic stress, particularly hypoglycemia, is a clinically-relevant stress type that is modeled with bolus insulin injection (insulin-induced hypoglycemia). The present study utilized ovariectomized C57BL/6 mice to test the hypothesis that acute hypoglycemia suppresses pulsatile LH secretion via central mechanisms. Pulsatile LH secretion was measured in 90-min sampling periods immediately prior to and following an intraperitoneal (i.p.) injection of saline or insulin. The secretion of LH was not altered over time in fed animals or acute fasted (5 h) animals following an i.p. saline injection. In contrast, insulin elicited a robust suppression of pulsatile LH secretion in fasted animals, preventing LH pulses in 5 of 6 mice. To identify the neuroendocrine site of impairment, a kisspeptin challenge was performed in saline or insulin pre-treated animals in a cross-over design. LH secretion in response to exogenous kisspeptin was not different between animals pre-treated with saline or insulin, indicating normal GnRH cell and pituitary responses during acute hypoglycemia. Based on this finding, the effect of insulin-induced hypoglycemia on arcuate kisspeptin (Kiss1) cell function was determined using c-Fos as a marker of neuronal activation. Insulin caused a significant suppression in the percentage of Kiss1 cells in the arcuate nucleus that contained c-Fos, compared to saline-injected controls. Together these data support the hypothesis that insulin-induced hypoglycemia suppresses pulsatile LH secretion in the female mouse via predominantly central mechanisms, which culminates in suppression of the arcuate Kiss1 population.
Keywords: Metabolic stress, hypoglycemia, LH pulses, arcuate nucleus, GnRH
Introduction:
Acute stress threatens homeostasis and therefore initiates specific physiologic responses to enable survival (1). One common result of the body’s response to acute stress is a disruption of reproductive function mediated, in part, by the suppression of gonadotropin secretion. Luteinizing hormone (LH) is secreted in an episodic or pulsatile pattern in males and in females throughout most of the ovarian cycle, which supports steroidogenesis and gametogenesis from the gonads. Suppression of pulsatile LH secretion has been described during a variety of stress types in both males and females across multiple mammalian species, however the underlying mechanism(s) remain unclear.
Acute hypoglycemia is a type of metabolic stress that has been examined largely using two models, either insulin-induced hypoglycemia (IIH) or administration of glucose anti-metabolites (2-deoxyglucose [2-DG] or 5-thioglucose [5-TG]). During IIH, an acute decrease in blood glucose is typically induced by coupling a brief fast (4–12 hrs) with a bolus insulin injection. IIH has been demonstrated to suppress LH secretion in rats (2), sheep (3), monkeys (4) and humans (5). Administration of 2-DG or 5-TG inhibits glycolysis causing cellular glucoprivation. Similarly, these anti-metabolites have been demonstrated to inhibit pulsatile LH secretion in rats (2), goats (6), and sheep (7).
It is now generally agreed that the GnRH pulse generator is formed by neurons in the arcuate nucleus (ARC) that contain kisspeptin (Kiss1), neurokinin B (NKB), and dynorphin (DYN), and are termed KNDy neurons (8). Indeed, activation of Kiss1 cells in the ARC (ARCKiss1) induces LH secretion (9), and silencing of this cell population inhibits LH pulses in mice (10). These cells cause the release of GnRH from neuron terminals in the median eminence which then elicit the release of LH from gonadotrope cells in the anterior pituitary. Thus, stress-induced impairment of pulsatile LH secretion could be mediated by any of these cell types (i.e. KNDy neurons, GnRH neurons, or gonadotropes). Evidence that multi-unit activity (a correlate of GnRH pulse generator activity) is suppressed during IIH in monkeys (4) and rats (11), and that Kiss1 transcript abundance is reduced following IIH in rats (12), support the hypothesis that metabolic stress suppresses LH secretion via central regulation of the GnRH pulse generator. However, hypoglycemia may directly inhibit GnRH neurons as they have been reported to be glucose sensitive via AMPK, an exquisitely sensitive intracellular energy sensor (13–15). AMPK signaling is also necessary for GnRH-stimulated Lhb gene transcription in primary pituitary cell culture (16), and inhibition of pituitary responsiveness has been reported during other stress types (17), suggesting direct inhibition of gonadotrope function by hypoglycemia. Though, it should be noted that LH responses to exogenous GnRH during IIH were normal in rats (18) and monkeys (19), diminishing support for a direct action upon the gonadotrope. Clearly, identifying which portion(s) of the gonadotropin secretion pathway (i.e. KNDy neurons, GnRH neurons, or gonadotropes) is/are targeted is necessary to understand how metabolic stress impedes gonadotropin secretion.
Our objective in the current study was to identify the endocrine and/or neural pathways by which metabolic stress impairs gonadotropin secretion using acute hypoglycemia induced by IIH as a model. We used the mouse as an experimental model as pulsatile LH secretion can be assessed in this species (20) and complex molecular and transgenic approaches are available that will be useful for future work investigating this neural response. In this study, we tested the hypothesis that metabolic stress suppresses pulsatile LH secretion via suppression of the activity of ARCKiss1 in female mice. We first demonstrated that IIH suppresses pulsatile LH secretion in ovariectomized (OVX) mice. Next, we tested the possibility that metabolic stress impairs the LH response to exogenous kisspeptin (an assessment of GnRH neuron and gonadotrope function). Finally, we examined activation of ARCKiss1 cells during IIH using c-Fos as marker for neuronal activation.
Methods:
Animals
Adult female C57BL/6 (Envigo; Experiments 1–2) or Kiss1CreGFP (Cravo et al.(21); Experiment 3) mice were housed under standard conditions with a 12-h light, 12-h dark cycle (lights on at 0600 h) at a University of California, San Diego vivarium. Animals had free access to feed (Harlan irradiated chow #2920X) and water unless noted otherwise. Animals were OVX at approximately 8 wk of age via lumber laparotomy under isoflurane anesthesia with aseptic technique. Mice were acclimatized to tail handling for blood sample collection and intraperitoneal (i.p.) injection for 5 wk before the experimental day, as described previously (22). Frequent blood samples were collected between 1030 h and 1300 h. All animal procedures were performed in accordance with the University of California, San Diego Institutional Animal Care and Use Committee regulations and in accordance with the National Institute of Health guidelines for the care and use of research animals.
Experiment 1: Does IIH inhibit pulsatile LH secretion?
Mice were randomly assigned to receive one of three treatments: normal feed and an i.p. injection of saline (fed + saline; n = 6), 5 h fast and i.p. saline (fast + saline; n = 7), or 5 h fast and an i.p. injection of insulin (0.75mU/g; Humilin R; NDC 0002-8215-01; fast + insulin; n = 6). Blood samples were collected for LH quantification at 6-min intervals from the tail, for 90 min before and after i.p. injection, as described previously (22, 23). Blood glucose concentrations were determined with handheld glucometer (OneTouch Ultra mini; Life Scan) at 0, 45, and 90 min relative to injection.
LH pulses were determined by established criteria (24). Briefly, a pulse was confirmed by three criteria: the peak LH value occurred within 3 samples of the preceding nadir, the difference between peak and preceding nadir was greater than the sensitivity of the assay, and the peak exceed the 95% confidence limit of the variability in the assay. For both of the pre and post injection sampling periods, mean LH (mean of all LH values), pulse frequency (number of pulses per 90 min), and pulse amplitude (mean amplitude of all pulses [peak value minus value of preceding nadir]) was calculated. Two-way analysis of variance (ANOVA) was used to assess differences across time (pre vs. post) and treatment (fed, fast + saline, fast + insulin). Tukey honestly significant difference post-hoc test was applied as appropriate and p < 0.05 was considered statistically significant. All statistics were performed with JMP Version 13 (Cary, NC).
Experiment 2: Does IIH inhibit the LH response to kisspeptin?
The LH response to exogenous kisspeptin was assessed in a cross-over design, such that each animal was administered kisspeptin following insulin and on a separate day kisspeptin following saline, in a randomized order separated by one wk. On the experimental day, mice (n = 8) were fasted for 5 h, administered an i.p. injection of either saline or insulin, and then 45 min later challenged with i.p. kisspeptin as described previously (25) (2 μg/g; rat Kisspeptin-10; Cat: 4243, Tocris). Blood samples were collected at 6-min intervals for 30 min before and after kisspeptin injection for LH assay. Blood glucose was determined at 0 and 75 min relative to insulin or saline injection.
The LH response to kisspeptin was quantified multiple ways. Mean LH was compared across sampling periods (before or after kisspeptin injection) and between treatments (saline vs. insulin) with two-way ANOVA. The amplitude of the response was defined as the difference between the peak LH value following kisspeptin and the LH value immediately before injection. The area under the curve (AUC) for LH values following kisspeptin was calculated by the trapezoidal method. The amplitude, AUC and time from kisspeptin injection to the peak LH value were each compared between treatments with paired t-tests.
Experiment 3: Does IIH inhibit ARC Kiss1 cell activation?
Immunohistochemistry (IHC) for detection of c-Fos, a marker of neural activation (26), was performed to assess ARCKiss1 cell activation. Kiss1CreGFP mice (n = 6 per group) were fasted for 5 h and randomly assigned to receive an i.p injection of saline or insulin. Two h following treatment, mice were deeply anesthetized, the tail was cut for blood glucose measurement, and perfused with heparinized saline and 4% paraformaldehyde in phosphate buffered saline. The brain was removed, stored in 4% paraformaldehyde overnight, then placed in 30% sucrose for 3 d. A cryostat was used to cut 40μm sections in 3 series (tissue sections in each series were 120μm apart). This time point was selected based on maximal suppression of LH observed by 1 h in Experiment 1 and convention that changes in c-Fos protein are observable about 1 h after stimulus (26).
IHC was performed on a series of tissue including the complete ARC nucleus. All steps were performed at room temperature with gentle agitation unless noted otherwise. Tissue was rinsed in phosphate buffer (PB) 12 times, for 10 min each and stored overnight in PB at 4°C. Tissue was rinsed 12 times, for 10 min each in PBS, then incubated in boiling antigen retrieval solution (Citra Buffer, Fisher Scientific) for 10 min, twice. Tissue was rinsed in PBS (4 times for 5 min each; typical rinsing step) and incubated in 1% H2O2 for 10 min. Tissue was rinsed and incubated in blocking solution containing 4% normal goat serum (NGS; Jackson Labs) in PBS with 0.4% TritonX-100 for an hour. Tissue was incubated in rabbit anti-c-Fos antiserum (1:15:000; MilliporeSigma, Cat # ABE45; AB_2631318) for 18 h at 4°C. Tissue was rinsed and incubated in biotinylated goat anti-rabbit antiserum (1:500; BA-1000, Vector Laboratories) for 1 h, ABC amplification kit (1:500; Vector Laboratories, PK-6100) for 1 h, biotinylated tyramide (1:250; Perkin Elmer, SAT70001EA) for 10 min, and streptavidin conjugated to Alexa 647 (1:100; Life, S32357) for 1 h, with intervening rinsing steps. Tissue was then incubated in blocking solution for 1 h and then incubated in blocking solution containing rabbit anti-GFP conjugated to Alexa 488 (1:1000, Life, Cat # A-21311; AB_221477) for 18 h at 4°C. Finally, tissue was rinsed in PB, mounted on SuperFrost slides (Fisher), cover slipped with gelvatol (27), and stored in the dark at 4°C until microscopy. Both antisera have been validated for use in neural tissue (28, 29).
Images were collected with a Nikon Ti2-E inverted microscope with DS-Qi2 monochrome CMOS camera controlled by NIS-elements. For each animal, 2–6 hemi-sections encompassing each of the rostral ARC (rARC), middle ARC (mARC) or caudal ARC (cARC) were imaged and analyzed by an observer blinded to treatments using ImageJ software (30) following minor adjustments to brightness and contrast. Images from the cARC of 2 saline-treated mice were not analyzed, due to poor tissue quality. The total number of GFP-labeled Kiss1 cells and the portion of GFP-labeled Kiss1 cells that contained c-Fos were counted and averaged per animal for each region of the ARC and compared between treatments using t-tests.
Ultrasensitive mouse LH enzyme-linked immunosorbent assay
Whole blood (3μL) samples collected from the tail-tip were immediately mixed into assay buffer and placed on ice until frozen (−20°C). LH was measured in singleton at the University of Virginia Ligand Assay Core based on the protocol by Steyn et al. (20). The functional sensitivity (i.e. lowest concentration that demonstrates accuracy within 20% of expected values and intraassay coefficient of variation <20%) was 0.32 ng/mL. The intra- and interassay coefficient of variation was 2.2% and 7.6%, respectively.
Results:
Experiment 1: Does IIH inhibit pulsatile LH secretion?
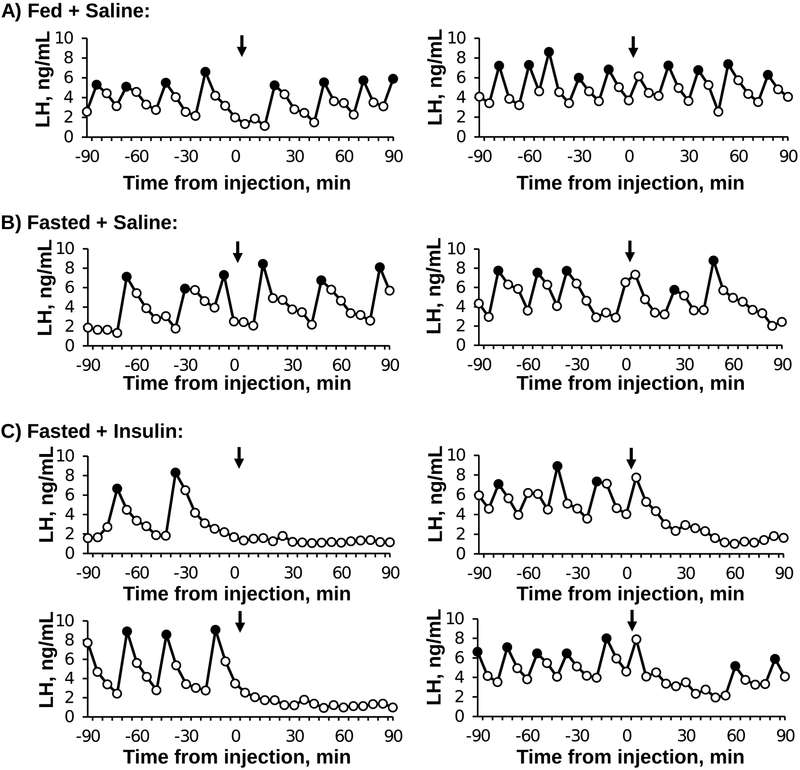
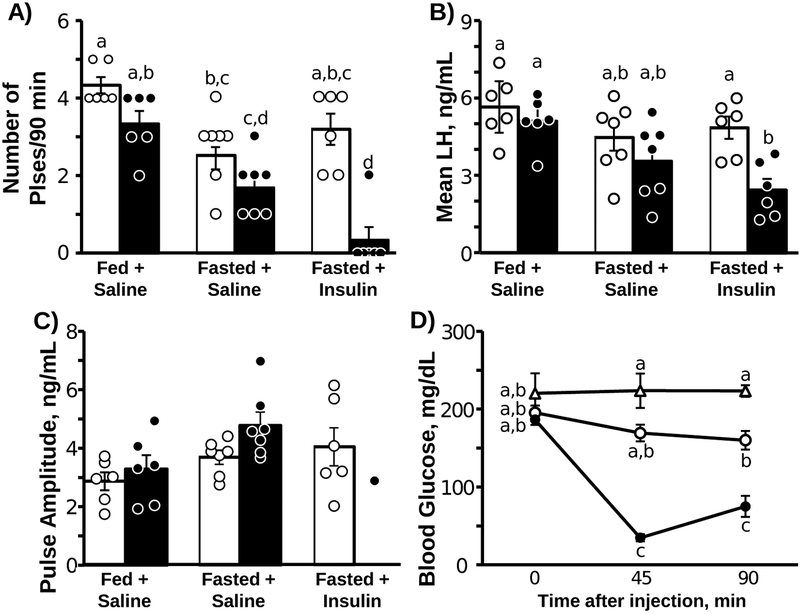
Representative LH profiles are depicted for two fed + saline, two fasted + saline, and four fasted + insulin mice in Figure 1. Mean values for all animals and the results of the statistical analysis are presented in Figure 2. LH pulses were clearly evident in all animals during the pre-injection sampling period. By two-way ANOVA, there was a significant sampling period by treatment interaction effect for the number of pulses per 90 min (F2,16 = 10.19, P = 0.0014; Fig 2A) and mean LH (F2,16 = 7.60, P = 0.0048; Fig 2B). Of interest, fasted animals tended to have fewer LH pulses in the pre-injection period compared to the fed animals; a significant reduction was identified only in saline-treated animals (pre period: fed + saline vs. fasted + saline). In animals injected with saline (either fed or fasted), LH pulses were clearly evident post-injection and neither the frequency nor mean LH concentration differed across sampling periods in either group (Fig 2A and 2B). In contrast, insulin induced a rapid and robust suppression in the number of LH pulses as well as a significant reduction in mean LH during the post-injection period (Fig 2A; and 2B). No detectable LH pulses were identified in five out of six mice treated with insulin. Two pulses were identified toward the end of the sampling period in the remaining insulin-treated animal (Fig 1C); the amplitude of these pulses (2.9 ± 3.2 ng/mL) was comparable to those of both groups of saline-injected animals, which did not differ across sampling periods or treatments (F2,11= 1.97, P = 0.18, Fig 2C).
Figure 1:
Representative LH pulse profiles in OVX female mice from each group: Fed + Saline (A), Fasted + Saline (B), and Fasted + Insulin (C). Filled data points represent pulses. Time of injection with saline or insulin is designated by the arrow (0 min).
Figure 2:
Mean (±SEM) number of LH pulses (A), mean LH concentrations (B), LH pulse amplitude (C), blood glucose concentrations (D). A-C) Open bars and data points indicate pre-injection sampling period, filled bars and data points indicate post-injection period (n=6 or 7/group). D) Δ Fed + Saline, ○ Fasted + Saline, ● Fasted + Insulin. Unique letters signify significant differences between values (P < 0.05).
As expected, blood glucose values were not altered by saline injection in either fasted or fed animals. In contrast, insulin caused an 81.2% and 59.7% reduction in blood glucose 45 and 90 min after injection, respectively (Fig 2D; F4,35 =18.76, P < 0.0001).
Experiment 2: Does IIH inhibit GnRH cell or gonadotrope secretion?
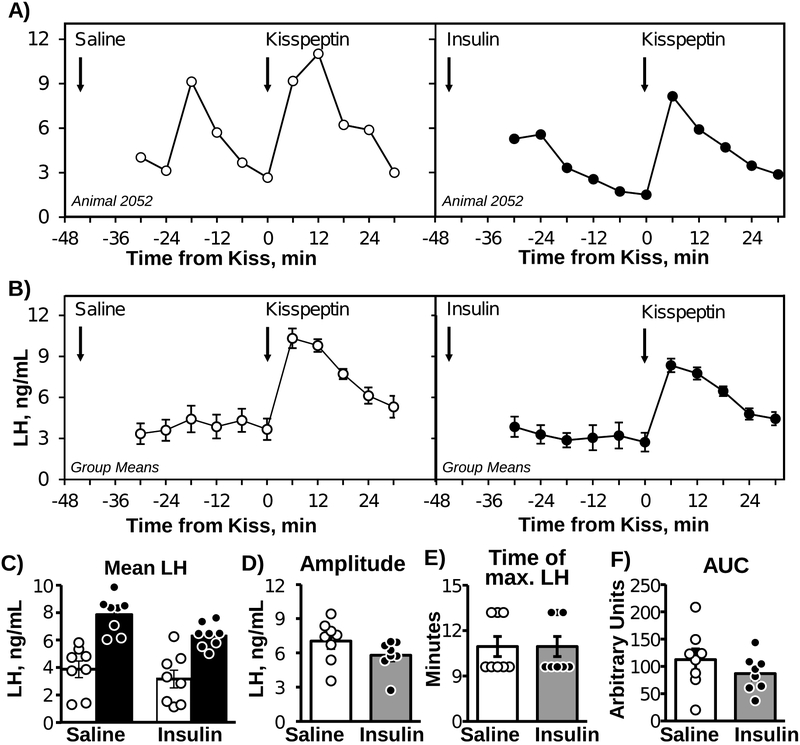
Representative LH profiles and mean LH concentrations before and after kisspeptin injection, either following saline or insulin pre-treatment, are displayed in Figures 3A (individual cross-over profile) and 3B (group means). Following saline pre-treatment, mean LH increased from 3.87 ± 0.6 ng/mL to 7.86 ± 0.47 ng/mL in response to kisspeptin (Fig 3B); there was a significant (F1,21 = 58.71, P < 0.001) effect of time, but no time x treatment interaction (F1,21= 0.40, P > 0.40). Similarly, following pre-treatment with insulin, kisspeptin increased mean LH from 3.17 ± 0.65 ng/mL to 6.36 ± 0.31 ng/mL. The amplitude of the response (peak LH value minus time 0 LH value) was similar between saline and insulin pre-treatment (Fig 3C). Neither the time of the maximum LH value (Fig 3D), nor the AUC for LH values following kisspeptin injection (Fig 3E), significantly differed between saline and insulin pre-treatment.
Figure 3:
Representative LH concentration profile from one OVX mouse challenged with kisspeptin, following saline (left) or insulin (right) pretreatment in a cross-over design (A). Mean (±SEM) LH concentrations prior to and in response to kisspeptin challenge following saline (left) or insulin (right) pre-treatment (B) in OVX mice (n=8; cross-over design). Arrows indicate the time of saline or insulin pre-treatment (−45 min) or the time of kisspeptin challenge (0 min). Mean LH before (white bars) and after (black bars) kisspeptin injection (C), change in LH concentration (D), time of maximal LH concentration after kisspeptin injection (E), and area under the curve (AUC) following kisspeptin injection (F).
Experiment 3: Does IIH inhibit ARC Kiss1 cell activity?
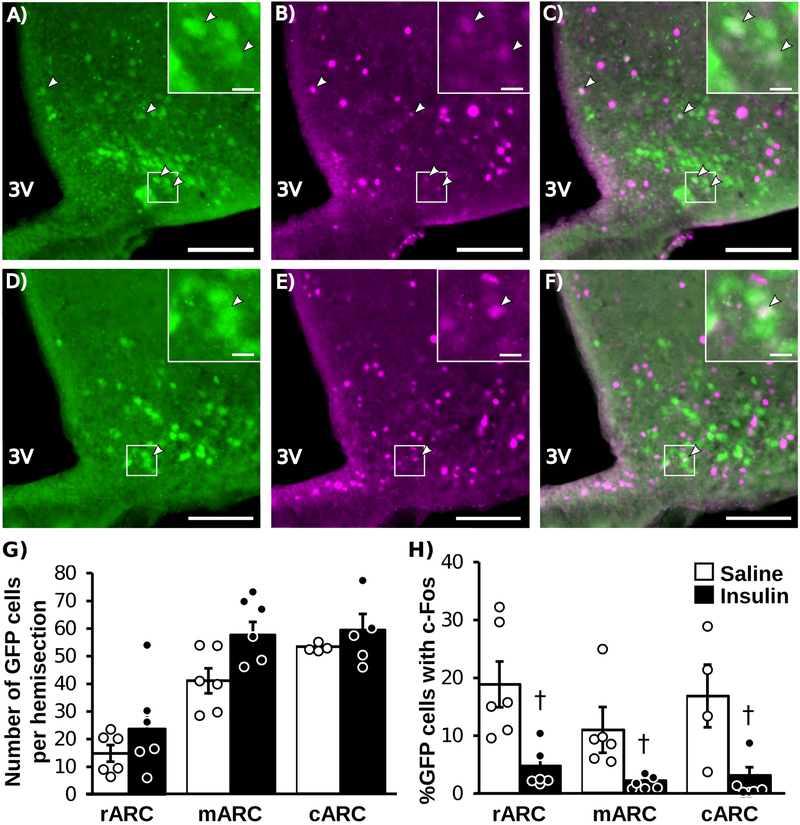
Representative images of IHC for Kiss1 (GFP) and c-Fos in an animal treated with saline (A-C) or insulin (D-F) are shown in Figure 4. In saline-treated animals, 11–18% of GFP-labeled Kiss1 cells contained c-Fos. Insulin caused a significant reduction in the percentage of GFP-labeled Kiss1 cells that contained c-Fos in each of the rostral (T(10) = 3.48, P = 0.012), middle (T(10)= 3.00, P = 0.028), and caudal (T(8) = 2.68, P = 0.0328) aspects of the ARC (Fig 4H). As expected with this animal model in which GFP is permanently expressed in Kiss1 cells under the CAG promoter, the total number of Kiss1 cells was not different between treatments in each region of the ARC. At the time of euthanasia, blood glucose values were lower in animals treated with insulin compared to values in control animals (P < 0.05; saline: 207.6 ± 11.2 mg/dL, insulin: 127 ± 12.5 mg/dL).
Figure 4:
Representative photomicrographs of dual-labeled Kiss1CreGFP and c-Fos cells in the rostral ARC of an OVX female mice treated with saline (A-C) or insulin (D-F). GFP-labeled Kiss1 cells (A,D), c-Fos (B, E), and merged images (C, F). White boxes indicate location of zoomed panels. White arrows indicate dual labeled cells, main panel scale bar = 50μm, inset panel scale bar = 10μm. Mean (±SEM) Total number of KissCreGFP cells (G) and percentage of KissCreGFP cells that contain c-Fos (H); n=6/group except for saline-treated cARC (n=4). White bars indicate saline-treated animals, black bars indicate insulin-treated animals. Dagger (†) indicates significant difference between treatments (P < 0.05).
Discussion:
The objective of the present study was to test the hypothesis that acute metabolic stress suppresses pulsatile LH secretion via impairment of KNDy cell activity in female mice. First, we demonstrated that acute metabolic stress diminishes LH secretion in OVX females via a robust suppression in detectable LH pulses following IIH. Next, we determined that IIH does not alter the LH response to kisspeptin. Further supporting the hypothesis that IIH suppresses KNDy cell activity, we showed that IIH reduces the percentage of ARCKiss1 cells that contain c-Fos. Taken together, these data support the hypothesis that acute hypoglycemia suppresses LH via central mechanisms in female mice.
One unresolved point regarding our study as well as reports in humans (5), monkeys (4), sheep (3) and rats (2), is whether hypoglycemia or hyperinsulinemia is the causal agent during IIH-induced suppression of LH. Several lines of evidence support the hypothesis that this suppressive effect is mediated by hypoglycemia per se, as opposed to direct actions of insulin. First, in men, insulin infusion during a euglycemic clamp did not alter LH pulses (5). Second, in rats (11) and sheep (3), glucose infusion following IIH almost immediately restores LH pulses, and in monkeys, also restores corresponding MUA volleys (31). Finally, glucose anti-metabolites, 2-DG and 5-TG (i.e. no insulin administration) similarly suppress pulsatile LH secretion in numerous species (2, 6, 7). Thus, it is likely that hypoglycemia, as opposed to hyperinsulinemia inhibits LH pulses in mice, but euglycemic clamp studies will be necessary to test this hypothesis.
Conceptually, the suppression of pulsatile LH secretion that we and others have documented in response to acute metabolic stress could be mediated by inhibition of either KNDy cells, GnRH cells, or gonadotrope cells of the pituitary, or some combination of these neuroendocrine sites. We measured LH concentrations following kisspeptin administration to determine if GnRH cell or gonadotrope function is impaired during IIH. We found no significant difference in the magnitude of the LH response, the timing of the increment in LH, nor the area under the curve following kisspeptin during IIH, as compared to control conditions. Therefore, we conclude that impairment of GnRH cells or gonadotrope cells during IIH has only a minor role in the suppression of pulsatile LH secretion. There are two caveats to this interpretation. First, endogenous pulsatility may cause a reduced response to kisspeptin, and second is the possibility that the high dose of kisspeptin used here stimulated maximal GnRH release that masked a subtle reduction in responsiveness to physiological doses of kisspeptin. Despite these possibilities, the likely cause of suppression of GnRH secretion is impaired kisspeptin secretion from KNDy cells. Our finding that IIH reduces the percentage of ARCKiss1 cells expressing c-Fos supports the hypothesis that acute hypoglycemia impairs KNDy cell activity. Although it is unknown whether the insulin-treated animals resumed LH pulses at the time of neural tissue collection (2 h post injection), the reduction in c-Fos co-expression provides evidence for a suppression in KNDy cell activation as a result of insulin treatment. These findings are in line with reports from monkeys (4) and rats (11) which showed IIH inhibits MUA volleys recorded from the mediobasal hypothalamus, a corelate of the GnRH pulse generator, now presumed to arise from KNDy neurons. Thus, our data extend the current literature to support the hypothesis that IIH suppresses pulsatile LH secretion primarily by inhibition of KNDy cells in mice.
The molecular mechanism for suppression of KNDy cell activity is unknown, but one intriguing possibility includes dysregulation of NKB or dynorphin signaling. Though these molecules have not been studied in the context of acute hypoglycemia, there are some limited data in a more chronic starvation model that cast doubt on their involvement. For example, administration of either a tachykinin receptor agonist or kappa opioid receptor antagonist stimulate LH secretion during starvation as they do in fed animals (32, 33), indicating that effects of these signaling molecules is not altered during starvation. Moreover, starvation does not alter Tac2 or pDyn transcript abundance in the hypothalamus (32, 33), suggesting that alteration in KNDy peptides gene expression is not involved in the suppression of LH during chronic metabolic stress. With regard to our study, the rapid suppression of LH secretion observed during acute hypoglycemia (<30 min) is unlikely to be is mediated by changes in gene expression, within KNDy cells or elsewhere, as these changes typically occur over the course of hours.
The mechanism by which acute hypoglycemia (or any other acute stress type) inhibits KNDy cell function is a major outstanding question. Though KNDy cells themselves could be directly glucose sensitive as reported for some other ARC populations (34), the more likely possibility is that hypoglycemia is detected in the area postrema of the brainstem. The area postrema contains glucose sensitive cells (35) and rats with surgical ablation of the area postrema display completely normal LH pulses during IIH (36). These data indicate a critical role for the area postrema during LH suppression in response to IIH, and also suggest that the pulse generator, GnRH cells and gonadotropes can function normally during hypoglycemia, and are therefore not directly impaired by low blood glucose. Whether the area postrema directly or indirectly innervates the ARC remains an open question. A recent tracing study revealed neural connections between hindbrain epyndomocytes (including the cells of the area postrema) and ARCKiss1 cells (37). However, cells in the brainstem were not labeled by a mono-synaptic anterograde tracer delivered to ARCKiss1 neurons (38), implicating a multi-synaptic pathway. Of interest, several signaling molecules including corticotropin releasing hormone receptor agonists (31, 39), CGRP (40) and RFRP-3 (41), have each been implicated in this neurocircuit. Clearly, considerable work is necessary to elucidate the neural pathway underlying the reproductive impairment by acute hypoglycemia, as well as to determine whether this pathway is unique to metabolic stress or a common response to different stress types.
Though our data complement and extend the findings of many published studies, by indicating impairment of pulsatile LH secretion via suppression of KNDy cells, our data raise several questions. First, fasting has been reported to attenuate the testosterone response to kisspeptin in male monkeys (42), which would be unexpected based on our finding that IIH does not reduce the LH response to kisspeptin. Although our study differed in numerous ways, including species, sex, gonad status, and response variable, the metabolic stress model employed may be the critical difference. In our kisspeptin challenge study (Exp 2), animals were fasted for 5 h before they received bolus injection of insulin, as compared with the monkey study in which animals were fasted for 48 h. Thus, these models are substantially different in that our control animals were briefly fasted so that we could asses the effect of the acute decrement in blood glucose (induced by insulin injection) as opposed to a starvation model which would include a myriad of physiological responses (43). Second, Fergani et al. reported an increase in the percentage of ARCKiss1 cells that contained c-Fos following insulin injection in mid-follicular phase ewes (44), a treatment previously demonstrated to blunt and delay the LH surge (45). An intriguing possibility for these differences may concern the seemingly contradictory actions of the norepinephrine neurons in the A2 region of the brainstem which have been implicated in both the inhibition of LH during metabolic stress (46, 47) and the stimulation of surge-type LH secretion (48, 49). Much is still to be learned about these brainstem neurons in the control of LH. One final question raised by our study is the observation that brief fasting (5 h) decreased LH pulse frequency, which was significant in saline-injected fasted controls. We were surprised to see such an effect in mice because our experiment was conducted during lights-on when nocturnal species typically consume little feed (43). Whether this effect is mediated by nutritional deficiency, as reported in monkeys during a one-day fast (50), or the psychological effects of missing feed (i.e. psychological stress) remains to be determined. Ultimately, these findings underscore the importance of utilizing defined stress models with careful attention to potential species differences and the modulating role of ovarian cycle stage or gonadal steroid status.
In summary, we demonstrate that acute metabolic stress suppresses pulsatile LH secretion in female mice. Moreover, this impairment in LH secretion is likely mediated by inhibition of ARCKiss1 neuron function. These data provide strong rational for the investigation of neural populations afferent to ARCKiss1 cells that may be responsible for the suppression of pulsatile LH secretion during acute metabolic stress.
Acknowledgements:
This work was supported by NIH grant R01 HD086100, R01 HD095412, P50 HD012303 and NSF grant IOS-1457226 and the UCSD Health Sciences Senate. R.B.M. was supported by NIH grant F32 HD096811 and T32 HD007203 and K.T. was partially supported by a UCSD Eureka! Undergraduate Research Scholarship and an Endocrine Summer Research Fellowship. Serum hormone assays were performed by The University of Virginia Ligand Assay Core Laboratory, which is supported through National Institute of Child Health and Human Development Grant P50 HD028934.
The authors wish to thank Dr. Carol Elias for her generous gift of the Kiss1CreGFP mouse line. We thank the Nikon Imaging Center at UC San Diego for access to microscopes and technical assistance with imaging.
Footnotes
Conflict of Interest:
The Authors have nothing to disclose.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References:
- 1.Hart BL. Biological basis of the behavior of sick animals. Neuroscience and biobehavioral reviews. 1988;12(2):123–37. [DOI] [PubMed] [Google Scholar]
- 2.Goubillon ML, Thalabard JC. Insulin-induced hypoglycemia decreases luteinizing hormone secretion in the castrated male rat: involvement of opiate peptides. Neuroendocrinology. 1996;64(1):49–56. [DOI] [PubMed] [Google Scholar]
- 3.Clarke IJ, Horton RJ, Doughton BW. Investigation of the mechanism by which insulin-induced hypoglycemia decreases luteinizing hormone secretion in ovariectomized ewes. Endocrinology. 1990;127(3):1470–6. [DOI] [PubMed] [Google Scholar]
- 4.Chen MD, O’Byrne KT, Chiappini SE, Hotchkiss J, Knobil E. Hypoglycemic ‘stress’ and gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: role of the ovary. Neuroendocrinology. 1992;56(5):666–73. [DOI] [PubMed] [Google Scholar]
- 5.Oltmanns KM, Fruehwald-Schultes B, Kern W, Born J, Fehm HL, Peters A. Hypoglycemia, but not insulin, acutely decreases LH and T secretion in men. The Journal of clinical endocrinology and metabolism. 2001;86(10):4913–9. [DOI] [PubMed] [Google Scholar]
- 6.Ohkura S, Ichimaru T, Itoh F, Matsuyama S, Okamura H. Further evidence for the role of glucose as a metabolic regulator of hypothalamic gonadotropin-releasing hormone pulse generator activity in goats. Endocrinology. 2004;145(7):3239–46. [DOI] [PubMed] [Google Scholar]
- 7.Bucholtz DC, Vidwans NM, Herbosa CG, Schillo KK, Foster DL. Metabolic interfaces between growth and reproduction. V. Pulsatile luteinizing hormone secretion is dependent on glucose availability. Endocrinology. 1996;137(2):601–7. [DOI] [PubMed] [Google Scholar]
- 8.Moore AM, Coolen LM, Porter DT, Goodman RL, Lehman MN. KNDy Cells Revisited. Endocrinology. 2018;159(9):3219–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(42):13109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(47):E10216–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He D, Funabashi T, Sano A, Uemura T, Minaguchi H, Kimura F. Effects of glucose and related substrates on the recovery of the electrical activity of gonadotropin-releasing hormone pulse generator which is decreased by insulin-induced hypoglycemia in the estrogen-primed ovariectomized rat. Brain research. 1999;820(1–2):71–6. [DOI] [PubMed] [Google Scholar]
- 12.Kinsey-Jones JS, Li XF, Knox AM, Wilkinson ES, Zhu XL, Chaudhary AA, et al. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. Journal of neuroendocrinology. 2009;21(1):20–9. [DOI] [PubMed] [Google Scholar]
- 13.Lin SC, Hardie DG. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell metabolism. 2018;27(2):299–313. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Bosch MA, Levine JE, Ronnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(38):10153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roland AV, Moenter SM. Glucosensing by GnRH neurons: inhibition by androgens and involvement of AMP-activated protein kinase. Molecular endocrinology (Baltimore, Md). 2011;25(5):847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AMP-Activated Protein Kinase Is a Key Intermediary in GnRH-Stimulated LH Gene Transcription, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams CY, Harris TG, Battaglia DF, Viguie C, Karsch FJ. Endotoxin inhibits pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology. 2001;142(5):1915–22. [DOI] [PubMed] [Google Scholar]
- 18.Howland BE. Effect of glucoprivation induced by 2-deoxy-D-glucose on serum gonadotropin levels, pituitary response to GnRH and progesterone-induced release of luteinizing hormone in rats. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 1980;12(10):520–3. [DOI] [PubMed] [Google Scholar]
- 19.Lujan ME, Krzemien AA, Van Vugt DA. Hypoglycemia does not affect gonadotroph responsiveness to gonadotropin-releasing hormone in rhesus monkeys. Endocrine. 2003;21(2):109–14. [DOI] [PubMed] [Google Scholar]
- 20.Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J Jr., Atkin S, Bookout AL, et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCosh RB, Kreisman MJ, Breen KM. Frequent Tail-tip Blood Sampling in Mice for the Assessment of Pulsatile Luteinizing Hormone Secretion. Journal of visualized experiments : JoVE. 2018(137). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang JA, Song CI, Hughes JK, Kreisman MJ, Parra RA, Haisenleder DJ, et al. Acute Psychosocial Stress Inhibits LH Pulsatility and Kiss1 Neuronal Activation in Female Mice. Endocrinology. 2017;158(11):3716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman RL, Karsch FJ. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology. 1980;107(5):1286–90. [DOI] [PubMed] [Google Scholar]
- 25.Tonsfeldt KJ, Schoeller EL, Brusman LE, Cui LJ, Lee J, Mellon PL. The Contribution of the Circadian Gene Bmal1 to Female Fertility and the Generation of the Preovulatory Luteinizing Hormone Surge. Journal of the Endocrine Society. 2019;3(4):716–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. The Journal of comparative neurology. 1990;296(4):517–30. [DOI] [PubMed] [Google Scholar]
- 27.Harlow E, Lane D. Mounting samples in gelvatol or mowiol. CSH protocols. 2006;2006(1). [DOI] [PubMed] [Google Scholar]
- 28.Kreisman M, McCosh R, Tian K, Song C, Breen K. Estradiol enables chronic corticosterone to inhibit pulsatile LH secretion and suppress Kiss1 neuronal activation in female mice. Neuroendocrinology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scharner S, Prinz P, Goebel-Stengel M, Kobelt P, Hofmann T, Rose M, et al. Activity-Based Anorexia Reduces Body Weight without Inducing a Separate Food Intake Microstructure or Activity Phenotype in Female Rats-Mediation via an Activation of Distinct Brain Nuclei. Frontiers in neuroscience. 2016;10:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9(7):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen MD, Ordog T, O’Byrne KT, Goldsmith JR, Connaughton MA, Knobil E. The insulin hypoglycemia-induced inhibition of gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: roles of vasopressin and corticotropin-releasing factor. Endocrinology. 1996;137(5):2012–21. [DOI] [PubMed] [Google Scholar]
- 32.Matsuzaki T, Tungalagsuvd A, Munkhzaya M, Iwasa T, Yano K, Mayila Y, et al. Neurokinin B receptor agonist and Dynorphin receptor antagonist stimulated luteinizing hormone secretion in fasted male rodents. Endocrine journal. 2018;65(4):485–92. [DOI] [PubMed] [Google Scholar]
- 33.Navarro VM, Ruiz-Pino F, Sanchez-Garrido MA, Garcia-Galiano D, Hobbs SJ, Manfredi-Lozano M, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32(7):2388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muroya S, Yada T, Shioda S, Takigawa M. Glucose-sensitive neurons in the rat arcuate nucleus contain neuropeptide Y. Neuroscience letters. 1999;264(1–3):113–6. [DOI] [PubMed] [Google Scholar]
- 35.Funahashi M, Adachi A. Glucose-responsive neurons exist within the area postrema of the rat: in vitro study on the isolated slice preparation. Brain research bulletin. 1993;32(5):531–5. [DOI] [PubMed] [Google Scholar]
- 36.Cates PS, O’Byrne KT. The area postrema mediates insulin hypoglycaemia-induced suppression of pulsatile LH secretion in the female rat. Brain research. 2000;853(1):151–5. [DOI] [PubMed] [Google Scholar]
- 37.Deura C, Minabe S, Ikegami K, Inoue N, Uenoyama Y, Maeda KI, et al. Morphological analysis for neuronal pathway from the hindbrain ependymocytes to the hypothalamic kisspeptin neurons. The Journal of reproduction and development. 2019;65(2):129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeo SH, Kyle V, Blouet C, Jones S, Colledge WH. Mapping neuronal inputs to Kiss1 neurons in the arcuate nucleus of the mouse. PloS one. 2019;14(3):e0213927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li XF, Bowe JE, Kinsey-Jones JS, Brain SD, Lightman SL, O’Byrne KT. Differential role of corticotrophin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. Journal of neuroendocrinology. 2006;18(8):602–10. [DOI] [PubMed] [Google Scholar]
- 40.Li XF, Bowe JE, Mitchell JC, Brain SD, Lightman SL, O’Byrne KT. Stress-induced suppression of the gonadotropin-releasing hormone pulse generator in the female rat: a novel neural action for calcitonin gene-related peptide. Endocrinology. 2004;145(4):1556–63. [DOI] [PubMed] [Google Scholar]
- 41.Poling MC, Shieh MP, Munaganuru N, Luo E, Kauffman AS. Examination of the influence of leptin and acute metabolic challenge on RFRP-3 neurons of mice in development and adulthood. Neuroendocrinology. 2014;100(4):317–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wahab F, Aziz F, Irfan S, Zaman WU, Shahab M. Short-term fasting attenuates the response of the HPG axis to kisspeptin challenge in the adult male rhesus monkey (Macaca mulatta). Life sciences. 2008;83(19–20):633–7. [DOI] [PubMed] [Google Scholar]
- 43.Jensen TL, Kiersgaard MK, Sorensen DB, Mikkelsen LF. Fasting of mice: a review. Laboratory animals. 2013;47(4):225–40. [DOI] [PubMed] [Google Scholar]
- 44.Fergani C, Routly JE, Jones DN, Pickavance LC, Smith RF, Dobson H. Kisspeptin, c-Fos and CRFR type 2 co-expression in the hypothalamus after insulin-induced hypoglycaemia. Reproduction in domestic animals = Zuchthygiene. 2014;49(3):433–40. [DOI] [PubMed] [Google Scholar]
- 45.Fergani C, Saifullizam AK, Routly JE, Smith RF, Dobson H. Estrous behavior, luteinizing hormone and estradiol profiles of intact ewes treated with insulin or endotoxin. Physiology & behavior. 2012;105(3):757–65. [DOI] [PubMed] [Google Scholar]
- 46.Paranjape SA, Briski KP. Recurrent insulin-induced hypoglycemia causes site-specific patterns of habituation or amplification of CNS neuronal genomic activation. Neuroscience. 2005;130(4):957–70. [DOI] [PubMed] [Google Scholar]
- 47.I’Anson H, Sundling LA, Roland SM, Ritter S. Immunotoxic destruction of distinct catecholaminergic neuron populations disrupts the reproductive response to glucoprivation in female rats. Endocrinology. 2003;144(10):4325–31. [DOI] [PubMed] [Google Scholar]
- 48.Scott CJ, Rawson JA, Pereira AM, Clarke IJ. Oestrogen receptors in the brainstem of the female sheep: relationship to noradrenergic cells and cells projecting to the medial preoptic area. Journal of neuroendocrinology. 1999;11(10):745–55. [DOI] [PubMed] [Google Scholar]
- 49.Conde GL, Bicknell RJ, Herbison AE. Changing patterns of Fos expression in brainstem catecholaminergic neurons during the rat oestrous cycle. Brain research. 1995;672(1–2):68–76. [DOI] [PubMed] [Google Scholar]
- 50.Schreihofer DA, Amico JA, Cameron JL. Reversal of fasting-induced suppression of luteinizing hormone (LH) secretion in male rhesus monkeys by intragastric nutrient infusion: evidence for rapid stimulation of LH by nutritional signals. Endocrinology. 1993;132(5):1890–7. [DOI] [PubMed] [Google Scholar]