Abstract
Objective:
Inflammation may contribute to brain white matter health in people living with HIV who report cognitive symptoms despite adherence to combination antiretroviral therapy (cART) and viral suppression. We explored relationships between diffusion tensor imaging (DTI) metrics of white matter, plasma biomarkers of immune activation, and cognitive function in the HIV-infected population.
Design:
Retrospective study of older adults living with HIV who are cART adherent, virally suppressed, and self-report cognitive symptoms.
Methods:
MRI, blood draws, and standardized neuropsychological test scores (NPZ) were collected from HIV-infected individuals. DTI metrics (FA, MD, RD, AD) and plasma biomarkers (sCD163, sCD14, neopterin, IP-10, MCP-1) were quantified. Statistical analysis explored associations between biomarker levels or NPZ and DTI metrics using ROI analyses and a voxelwise approach.
Results:
43 participants with median (IQR) age of 64 (62–66 years), CD4 count of 600 (400–760 cell/mm3) who were all virally suppressed (<100 copies/mL) were selected. Higher levels of MCP-1 associated with lower FA and higher MD (p<0.05) across white matter tracts including corpus callosum, corona radiata, and superior longitudinal fasciculus. Higher neopterin associated with higher MD in the genu of corpus callosum, and higher sCD14 associated with lower FA in the bilateral superior corona radiata (p<0.05). Worse global performance and speed domain scores associated with higher MD and lower FA, and worse executive domain scores associated with lower FA (p<0.05).
Conclusions:
Elevated inflammatory plasma biomarkers link to white matter abnormalities among virally suppressed individuals. DTI abnormalities associate to cognitive performance. We conclude that inflammatory processes impact clinically relevant brain health indices despite viral suppression.
Keywords: MRI, Diffusion Tensor Imaging, Neuroimaging, Neurocognitive Disorders, White Matter, Inflammation
1. Introduction
According to the CDC, about 169,000 people living with HIV (PLWH) in the United States were over the age of 60 by the end of 2016, representing 17% of the HIV-infected population. The largest percentage increase in HIV prevalence rates from 2012 to 2016 was in the 65 and older population [1]. Among well-treated older PLWH, HIV-associated non-AIDS comorbidities, including neurological complications, remain common. Among PLWH generally, milder cognitive impairment may affect up to 50% of PLWH [2]. PLWH can experience poor performance across a range of cognitive domains, including executive, attention, and information processing speed [3]. Increasing age is a risk factor for having HIV-associated cognitive dysfunction, motivating analyses in this age group [4–6].
Chronic immune activation and inflammation in response to HIV infection are associated with increased general morbidity and are a commonly proposed mechanism for the persistence of cognitive impairment despite viral suppression [7,8]. PLWH often display evidence of ongoing immune activation in both plasma and cerebrospinal fluid (CSF), even after reaching undetectable plasma viral RNA and among asymptomatic individuals [9–11].
In this study, we examined several promising biomarkers: soluble CD163 (sCD163), soluble CD14 (sCD14), neopterin, interferon gamma-induced protein 10 (IP-10/CXCL10), and monocyte chemoattractant protein 1 (MCP-1/CCL2). Although these biomarkers are not specific to HIV, they have been previously linked to poorer outcomes in HIV. sCD163 and sCD14 are non-specific monocyte/macrophage markers which are shed during activation, and have previously been linked to HIV outcomes, including cognitive impairment [10,12–16]. Neopterin is produced by monocyte/macrophages and thus can also provide information on the activity of these cells [17]. Likewise, neopterin has been linked to poorer brain health in HIV [18]. IP-10 is a chemokine which has been associated with inflammatory diseases; it has been found to stimulate HIV-1 replication through monocyte-derived macrophages and lymphocytes [19,20]. MCP-1 is a chemokine and is a potent monocyte chemoattractant, produced by a variety of cells including monocytes/macrophages [21]. In the context of HIV, it has been implicated in blood-brain barrier disruption and glial activation, as well as brain injury [22,23].
Studies exploring associations among brain imaging metrics and soluble biomarkers often include a study population where some participants do not have viral suppression or are not on cART. Identifying that elevated inflammatory biomarkers in a persistently suppressed and treatment-adherent study group link to brain integrity and cognitive performance would provide valuable insight into the pathophysiology of cognitive impairment.
Diffusion Tensor Imaging (DTI) is a commonly used non-invasive Magnetic Resonance Imaging (MRI) technique based on the diffusion of water, which allows for the characterization of white matter microstructure integrity. Quantified DTI metrics include fractional anisotropy (FA), a measure of the directionality of water diffusion, mean diffusivity (MD), a measure of the magnitude of water diffusion, axial diffusivity (AD), a measure of the water diffusivity in the primary direction, and radial diffusivity (RD), a measure of the water diffusivity in the transverse directions [24,25]. In this study, we took an imaging outcomes-focused approach, with the primary aim being to understand the patterns of relationship between several plasma biomarkers of inflammation in the context of HIV infection and brain integrity measured by DTI metrics of brain white matter. We leveraged a clinically relevant population of treatment-adherent and virally suppressed, but cognitively symptomatic, older adults. We hypothesized that a greater degree of inflammation evidenced by higher biomarker concentrations would be associated with worse brain integrity, represented by lower FA or higher MD. To demonstrate clinical relevance, we also examined the relationship between DTI metrics and neuropsychological test performance in domains often impaired among PLWH, hypothesizing that abnormal DTI metrics would be reflected in worse performance across the executive, attention, and speed domains, and the global averaged score.
2. Methods
2.1. Participants
HIV-infected participants over 60 years of age were retrospectively drawn from the UCSF HIV Over 60 Cohort and from pre-intervention assessments from enrollees in a separate ongoing trial underway at the University of California, San Francisco. Participants were recruited between March 2013 and September 2017. Per study protocols, all participants were aged 60 or older, reported persistent viral suppression and were virally suppressed (defined as plasma HIV RNA <100 copies/mL) at the time of examination. All reported adherence to cART for at least 12 months, and endorsed cognitive symptoms, as defined by an endorsement of “almost always,” “very often,” or “fairly often” for at least one cognitive symptom on the Patient Assessment of Own Functioning (PAOF) questionnaire [26]. Baseline CD4 count and viral load were quantified from clinical labs. In two individuals where laboratory measures were not available, we substituted self-reported current CD4 count. Participants self-reported estimated CD4 nadir when known (n=37). Following Frascati guidance at consensus conference attended by a neuroHIV clinician (VV) and at least one neuropsychologist (BM), individuals were categorized using HAND nosology [27]. In accordance with Frascati guidelines for classifying comorbid conditions into secondary, contributing, and confounding conditions, individuals were excluded if they had confounding conditions such as major neurological or psychiatric conditions (e.g. schizophrenia, multiple sclerosis), current brain infection, thyroid abnormality, self-reported current substance use disorder, major stroke, or significant traumatic brain injury. Individuals remained eligible for enrollment if they had potential contributing conditions not considered the primary reason for cognitive impairment, compatible with HAND criteria. Participants were selected for this study based on successful acquisition of baseline structural and diffusion MRI scans, blood draws, and neuropsychological tests.
2.2. Biomarker Acquisition
Cryopreserved plasma aliquots were thawed and prepared following kit manufacturer’s guidelines. MCP-1, sCD163, and IP-10 were measured using a custom multiplex kit (RD Systems, MN, USA), acquired on a Luminex 200™ analyzer (Luminex), and analyzed using Bio-Plex Manager™ software (Bio-Rad). Neopterin and sCD14 were measured by ELISA (Neopterin competitive enzyme immunoassay, ALPCO, NH, USA; Human CD14 Quantikine ELISA kit, RD Systems), optical density read on a microplate spectrophotometer (Bio-Rad), and standard curve interpolation conducted on Prism version 7.0b (Graphpad Software Inc., CA, USA). Samples were run in duplicate and the CV of replicates were less than 10%.
Examination of biomarker distributions revealed two outliers greater than 4 SDs above the biomarker distribution means – one for neopterin and one for MCP-1. Because these values were considered physiologically plausible, we substituted these outlier values with the next most extreme value in the biomarker distribution during statistical analysis, as has been done previously by our group [15]. The biomarker distributions of sCD163 and IP-10 were log10 transformed prior to statistical analysis.
2.3. MRI Acquisition
All participants underwent whole-brain imaging on either a Siemens TIM Trio 3 Tesla MRI scanner with a 12-channel head coil or a Siemens Prisma FIT 3 Tesla MRI scanner with a 64-channel head coil. Diffusion imaging was acquired on the Trio with a 2.2×2.2×2.2mm voxel size, 220×220 FOV, 8,200ms TR, 86ms TE, 180° flip angle, gradients applied in 64 directions with a b-value of 2000 s/mm2 and 0 s/mm2. Diffusion imaging on the Prisma was acquired with 2.0×2.0×2.0mm voxel size, 69 slices, 220×220 FOV, 2,420ms TR, 72.2ms TE, 85°/180° flip/refocusing angle, 3 factor multi-band acceleration, EPI factor 110, gradients applied in 96 directions with b-values of 2500 s/mm2 and 0 s/mm2. All images were visually inspected for quality, excluding any acquisitions which excessive motion or artifacts which would compromise processing.
2.4. Neuropsychological Testing
All participants underwent neuropsychological testing with a median (range) of 39 (0–128) days from the baseline MRI scan. The 90-minute standardized neuropsychological testing battery is designed to capture a wide range of cognitive domains, including episodic memory, language, attention, executive functions, psychomotor speed, motor, and visuo-spatial abilities. As described and utilized in our prior studies, raw scores were standardized and summarized as normalized z-scores (NPZ) in each domain and global averaged scores [28–30].
2.5. Image Processing and Analysis
Image processing was carried out on within scanner model groups to control for minor anatomical and diffusion metric differences between the scanners. Diffusion image processing began with denoising [31]. The FSL MCFLIRT algorithm was utilized to align images to the primary volume of the sequence [32]. Data reflecting absolute displacement parameters beyond 1mm were removed. Background voxels not considered brain tissue were removed using the B0 acquisitions to provide a mask with Otsu thresholding with a 4mm radius and 4 iterations to minimize intra-class variance [33,34]. FSL Eddy was applied using the realigned diffusion images, mask, and b-vectors and b-values to correct for eddy current-induced distortions [35]. The susceptibility distortion corrected diffusion images were then fitted using Dipy with a non-linear least-squares approach to create the DTI tensor maps [34]. The DTI-TK package was used to construct within-scanner group templates, and align and normalize DTI tensor maps to these templates [36].
We performed region of interest (ROI) analysis by warping the Johns Hopkins University (JHU) white matter atlas into the within-scanner group template spaces [37]. We then employed ANTs ImageIntensityStatistics to calculate average FA and MD values in the genu, body, and splenium of the corpus callosum, the right and left anterior, superior, and posterior corona radiata, and the right and left superior longitudinal fasciculus as defined previously by our group [38]. Average FA and MD values per atlas label from both scanner groups were combined into a multi-scanner ROI analysis (n=43) by building a generalized linear model using the statsmodels (statsmodels 0.9.0) package in Python 2.7, regressing each diffusion metric against biomarker levels and neuropsychological testing results, with the inclusion of age and scanner as covariates [39]. To limit multiple comparisons, we examined AD and RD in secondary analyses where significant associations with FA or MD alterations were noted to better characterize the changes. In addition, we performed exploratory analyses by including either duration of infection or the presence of comorbid conditions as covariates in the regression model to examine if these had any effect on the results.
Tract-Based Spatial Statistics (TBSS) is a commonly used diffusion imaging analysis method which projects subjects’ FA data onto a mean FA tract skeleton before performing voxelwise statistical analysis on the voxels within that skeleton in order to improve sensitivity, objectivity, and interpretability [40,41]. The statistics are corrected for multiple comparisons by applying 5000 permutations with FSL Randomise and threshold-free cluster enhancement (tfce) [41–43]. We used TBSS to explore the spatial distribution of associations between elevated biomarkers and DTI measurements across the brain white matter without relying on a priori regions. TBSS was performed individually on within-scanner groups in accordance with the suggestion of the developers.
3. Results
3.1. Study Participants’ Characteristics
Study participants were predominantly white (91%), male (86%), and well educated (67% completed at least a bachelor’s degree, Table 1). All were over 60 years of age, with a median (IQR) age of 64 (62–66) years old. According to self-reported date of HIV diagnosis, all but two participants had been known to be infected with HIV for at least a decade, with a median (IQR) duration of infection of 27 (24–31) years. The median (IQR) CD4 count was 600 (400–760) cells. For those that knew their estimated CD4 nadir (n=37, 86%), the median (IQR) was 150 (50–235). The most common potential secondary or contributing risk factors for impairment included treated depression (n=9), followed by past heavy use of alcohol or non-specified substance use and treated sleep apnea (n=6 for each).
Table 1:
Demographic and clinical variables of HIV+ participants.
| Measure | HIV+ Participants (n=43) |
|---|---|
| Age in years, median (IQR) | 64 (62–66) |
| Sex, n (%) male | 39 (90.7%) |
| Race, n (%) white | 37 (86.0%) |
| Years of Education, median (IQR) | 16 (15–18) |
| HAND Diagnosis by Frascati, n (%) | |
| Normal, n (%) | 5 (11.6%) |
| Mild Neurocognitive Disorder (MND), n (%) | 37 (86.0%) |
| HIV-associated Dementia (HAD), n (%) | 1 (2.3%) |
| MRI Scanner Model | |
| 3T Siemens Trio, n (%) | 23 (53.5%) |
| 3T Siemens Prisma, n (%) | 20 (46.5%) |
| CD4 count cells/μL, median (IQR) | 600 (400 – 760) |
| Estimated CD4 nadir cells/μL, median (IQR) | 150 (50–235) |
| Viral Load <100 copies/mL, n (%) | 43 (100%) |
| Self-reported Infection Duration in years, median (IQR) | 27 (24–31) |
| Neuropsychological Testing Z-scores, mean ± standard dev. | |
| Global | −0.55 ± 0.57 |
| Executive Domain | −0.61 ± 1.12 |
| Attention/Working Memory Domain | −0.56 ± 0.74 |
| Speed of Processing Domain | −0.69 ± 1.04 |
| Memory Domain | 0.03 ± 0.87 |
| Verbal Domain | −0.61 ± 0.82 |
| Motor Domain | −1.19 ± 1.20 |
| Plasma biomarker levels, mean ± standard dev. | |
| MCP-1 pg/mL | 64.1 ± 24.6 |
| sCD163 pg/mL | 3.68E5 ± 2.22E5 |
| IP-10 pg/mL | 64.8 ± 50.2 |
| Neopterin nmol/L | 12.0 ± 4.6 |
| sCD14 pg/mL | 2.36E6 ± 9.20E5 |
| Potential Secondary or Contributing Risk Factors for Impairment * (n, %) | |
| Past heavy alcohol use | 6 (14.0%) |
| Marijuana/THC | 4 (9.3%) |
| Past cocaine use | 2 (4.65%) |
| Past methamphetamine use | 3 (7.0%) |
| Past other/unspecified substance use | 6 (14.0%) |
| Treated depression | 9 (20.9%) |
| Treated sleep apnea | 6 (14.0%) |
| Past Hepatitis C infection | 3 (7.0%) |
| Vascular risk factors | 2 (4.65%) |
| Dyslexia | 2 (4.65%) |
| Diabetes | 1 (2.3%) |
| Non-ART Medications, median (IQR) | 8 (4 – 12) |
: Some participants had multiple risk factors. Total participants with at least one risk factor was 29 (67%)
3.2. Neuropsychological Performance
The most impaired domain in our population was motor (−1.19 ± 1.20 standard deviations from normal), and the least impaired domain was memory (0.03 ± 0.87 standard deviations from normal). Among our 43 participants, the majority were categorized as mildly impaired (37, 86% with MND), with one participant (2%) reaching criteria for HIV-associated dementia and the remaining five (12%) not having sufficient abnormality on neuropsychological testing to meet HAND criteria, despite symptoms.
3.3. Diffusion Imaging Associations
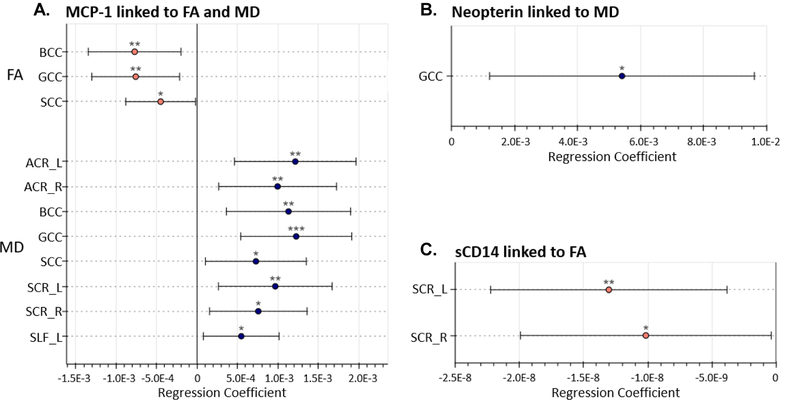
Higher plasma MCP-1, neopterin, and sCD14 levels were associated with worse DTI metrics across multiple regions of the brain (all p-values <0.05, Figure 2). In particular, higher MCP-1 was associated with worse brain integrity in eight out of the eleven ROIs studied (73%, regression coefficients range [5.47E-04 to 1.23E-03], all p<0.05) and was linked to both FA and MD across the corpus callosum. Elevated neopterin was associated with higher MD in the genu of the corpus callosum (regression coefficient 5.42E-03, p=0.012), and elevated sCD14 was associated with lower FA in the bilateral superior corona radiata (regressions coefficients range [−1.30E-08 to −1.02E-08], all p<0.05, Figure 2). Regressing IP-10 and sCD163 levels against either FA or MD did not meet statistical significance at p<0.05 in any ROI studied.
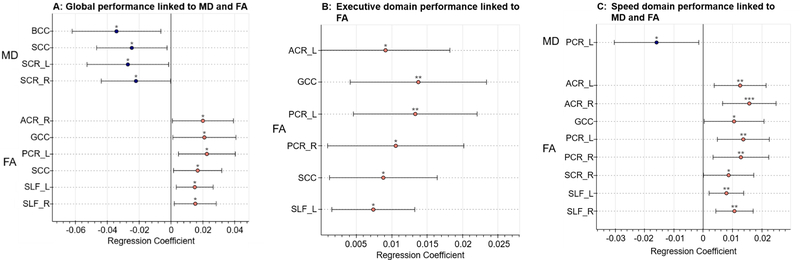
Figure 2: Results of neurocognitive test performance regressed against DTI metrics.
Regression coefficients of neurocognitive test performance in standardized z scores presented with 95% confidence intervals. Only associations where p<0.05 are reported. (A) Better performance globally is linked to lower MD and higher FA values in widespread regions. (B) Better executive domain performance is linked to higher FA values in widespread regions. (C) Better speed of processing domain performance is linked to higher FA in widespread regions and to lower MD in the left posterior corona radiata.
*: p<0.05; **: p<0.01; ***: p<0.001
ACR: anterior corona radiata. BCC: body of corpus callosum. GCC: genu of corpus callosum. PCR: Posterior corona radiata. SCC: splenium of corpus callosum. SLF: superior longitudinal fasciculus.
L: Left. R: Right.
AD and RD were run in secondary analyses to further characterize the regions in which biomarkers significantly associated to the MD or FA levels (Table 2). In the regions where MCP-1 and neopterin were significantly associated with MD, AD and RD also significantly associated (p<0.05, with the exception of the direct association between MD in the splenium of corpus callosum with MCP-1, p=0.056). All significant biomarker associations were directionally consistent with each other, including a direct relationship with MD, RD, and AD, and an indirect relationship with FA.
Table 2:
Results of secondary and exploratory regression analyses
| Biomarker | ROI | Metric | Regression Coefficient | p-value | Adjusted Regression Coefficient | Adjusted p-value |
|---|---|---|---|---|---|---|
| MCP-1 | ACR_L | AD | 1.37E-03 | <0.001 *** | 1.04E-03 | 0.005 ** |
| MD | 1.21E-03 | 0.002 ** | 9.53E-04 | 0.012 * | ||
| RD | 1.14E-03 | 0.005 ** | 9.08E-04 | 0.025 * | ||
| ACR_R | AD | 1.01E-03 | 0.008 ** | 6.98E-04 | 0.055 * | |
| MD | 9.96E-04 | 0.007 ** | 7.68E-04 | 0.039 * | ||
| RD | 9.87E-04 | 0.012 * | 8.02E-04 | 0.046 * | ||
| BCC | AD | 8.79E-04 | 0.028 * | 6.49E-04 | 0.109 | |
| FA | −7.72E-04 | 0.008 ** | −6.70E-04 | 0.028 * | ||
| MD | 1.13E-03 | 0.004 ** | 9.28E-04 | 0.020 * | ||
| RD | 1.26E-03 | 0.002 ** | 1.07E-03 | 0.012 * | ||
| GCC | AD | 1.16E-03 | 0.005 ** | 6.72E-04 | 0.049 * | |
| FA | −7.59E-04 | 0.006 ** | −6.54E-04 | 0.023 * | ||
| MD | 1.23E-03 | <0.001 *** | 8.52E-04 | 0.005 ** | ||
| RD | 1.26E-03 | <0.001 *** | 9.43E-04 | 0.004 ** | ||
| SCC | FA | −4.51E-04 | 0.041 * | −3.35E-04 | 0.135 | |
| MD | 7.27E-04 | 0.022 * | 5.42E-04 | 0.091 | ||
| RD | 7.20E-04 | 0.021 * | 5.48E-04 | 0.083 | ||
| SCR_L | AD | 1.25E-03 | 0.019 * | 9.29E-04 | 0.083 | |
| MD | 9.67E-04 | 0.007 ** | 8.14E-04 | 0.028 * | ||
| RD | 8.23E-04 | 0.008 ** | 7.56E-04 | 0.020 * | ||
| SCR_R | AD | 1.00E-03 | 0.038 * | 7.10E-04 | 0.144 | |
| MD | 7.57E-04 | 0.014 * | 6.24E-04 | 0.049 * | ||
| RD | 6.34E-04 | 0.014 * | 5.82E-04 | 0.033 * | ||
| SLF_L | AD | 5.30E-04 | 0.030 * | 4.65E-04 | 0.070 | |
| MD | 5.47E-04 | 0.022 * | 4.96E-04 | 0.048 * | ||
| RD | 5.55E-04 | 0.029 * | 5.12E-04 | 0.056 | ||
| Neopterin | GCC | AD | 6.41E-03 | 0.007 ** | 2.83E-03 | 0.172 |
| MD | 5.41E-03 | 0.012 * | 2.50E-03 | 0.201 | ||
| RD | 4.91E-03 | 0.026 * | 2.33E-03 | 0.273 | ||
| sCD14 | SCR_L | FA | −1.30E-08 | 0.006 ** | −1.43E-08 | 0.002 ** |
| SCR_R | FA | −1.02E-08 | 0.041 * | −1.15E-08 | 0.018 * | |
Only associations where unadjusted p-value<0.05 are reported. Inclusion of AD and RD in the models demonstrates that links between inflammatory biomarkers levels and MD are also indicative of links between inflammatory biomarker levels and both AD and RD. Adjusting the model to include the presence of comorbid conditions as a covariate demonstrates that despite the loss of power in the model, many ROIs remain significantly associated at p<0.05, and others retain a pattern suggestive of a trend.
: p<0.05.
: p<0.01.
: p<0.001.
ACR: anterior corona radiata. BCC: body of corpus callosum. GCC: genu of corpus callosum. PCR: Posterior corona radiata. SCC: splenium of corpus callosum. SLF: superior longitudinal fasciculus. L: Left. R: Right.
L: Left. R: Right.
Exploratory analyses including duration of infection and presence of comorbid conditions as covariates in the regression models were run to test the effect of inclusion. Duration of infection did not affect any results – the same regions associated with the same diffusion measures, and duration of infection did not significantly associate with any DTI metrics. Adjusting for the presence of comorbid conditions in regression models retained most results as when run without their inclusion (Table 2). With the added covariate, neopterin no longer linked to MD in the genu of corpus callosum, and MCP1 no longer linked to AD in the body of corpus callosum, bilateral superior corona radiata, and left superior longitudinal fasciculus, and no longer linked to any diffusion metric in the splenium of corpus callosum at p<0.05, although the direction of the regression coefficient was retained.
Furthermore, there were strong links between neuropsychological performance and worse DTI metrics (Figure 2). Decreased FA across the corpus callosum, corona radiata, and superior longitudinal fasciculus associated with poorer performance on the global averaged score (regression coefficients range [1.50E-02 to 2.26E-02], all p<0.05), as well as the executive and speed domains (regression coefficients range [7.40E-03 to 1.38E-02] and [7.92E-03 to 1.57E-02] respectively, all p<0.05). Poorer global performance also associated with higher MD in the corpus callosum and superior corona radiata (regression coefficients range [−3.43E-02 to −2.21E-02], all p<0.05), and poorer speed domain performance was associated with higher MD in the left posterior corona radiata (regression coefficient −1.59E-02, p=0.031). Correlations between the attention domain and neuropsychological test performance did not reach significance at p<0.05 in any of the studied ROIs.
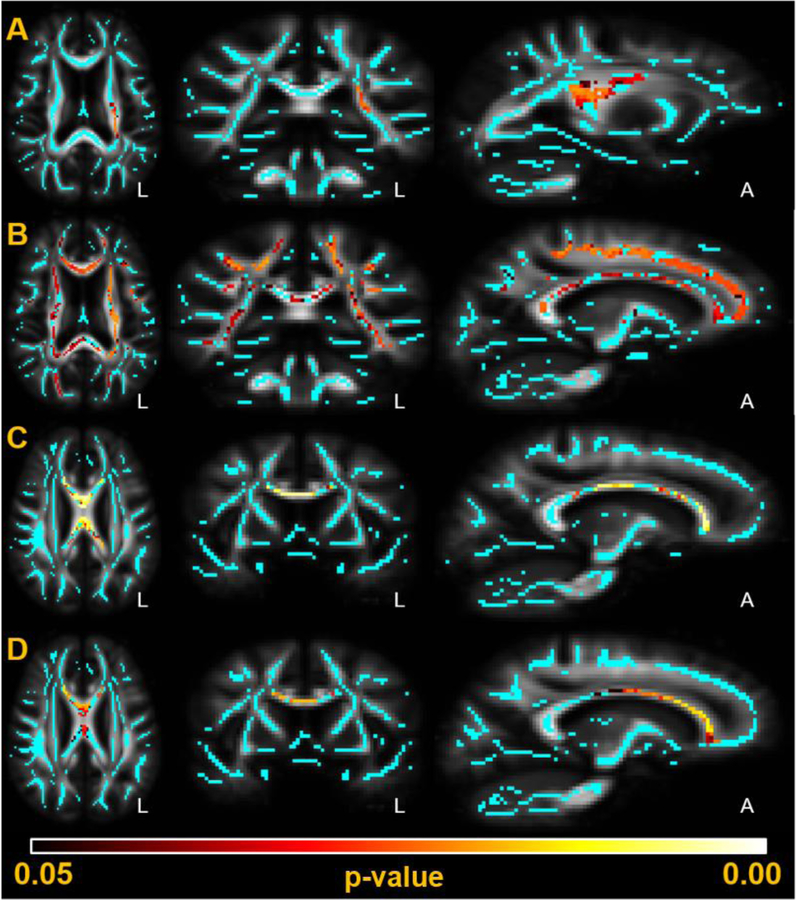
TBSS analysis on the Siemens Prisma scanner images (n=20) revealed correlations at p<0.05 between elevated sCD14 and decreased FA in the left corona radiata, and correlations at p<0.05 between elevated MCP-1 and higher MD in widespread regions of the brain (Figure 3A, Figure 3B). TBSS analysis on the Siemens Trio scanner images (n=23) revealed correlations at p<0.05 between elevated MCP-1 and both lower FA and higher MD in the corpus callosum (Figure 3C, Figure 3D). Neopterin, IP-10, and sCD163 did not reach significance in any voxels in the skeleton, and sCD14 did not reach significance in any voxel on the Siemens Trio images.
Figure 3: DTI metrics significantly associate with plasma biomarker levels of MCP-1 and sCD14 in TBSS analysis.
Significant correlations (p<0.05, red-yellow) are overlaid against the mean FA template and mean FA skeleton (cyan) created during TBSS analysis. Analysis was run on the images acquired from the two MRI scanners independently. (A): sCD14 indirectly associates with FA on the Siemens Prisma MRI scanner in the left corona radiata. (B): MCP-1 directly associates with MD on the Siemens Prisma MRI scanner across the white matter skeleton, including the corpus callosum and corona radiata. (C): MCP-1 indirectly associates with FA on the Siemens Trio MRI scanner in the corpus callosum. (D): MCP-1 directly associates with MD on the Siemens Trio MRI scanner in the corpus callosum.
4. Discussion
We demonstrate a link between elevated non-specific plasma biomarkers of inflammation and physiological changes in brain integrity among predominately older men living with chronic HIV infection and suppressed plasma HIV RNA. On ROI analysis, higher plasma MCP-1, neopterin, and sCD14 levels each associated with either higher MD, lower FA, or both, which is consistent with the direction of change expected with brain white matter injury. Both ROI and voxel-wise TBSS analysis highlight that elevated plasma MCP-1 is prominently associated with lower FA and higher MD across widespread areas in the brain. We performed secondary statistical analyses on AD and RD metrics in the regions that MD and FA were significantly associated to elevated biomarkers to better characterize the white matter alterations, where we found that the increased diffusivity detected by MD was reflected by significantly higher metrics in both RD and AD.
Diffusion MRI is a sensitive but non-specific method of characterizing white matter, and these associations may be explained by the potential interaction of a variety of different physiological and pathological conditions in the brain. Higher MD and lower FA, as we linked to higher inflammatory biomarker levels, are generally interpreted as reflecting worse integrity, though areas of crossing white matter fibers complicate interpretation. Alterations in MD and RD are consistent with previous findings in HIV literature, and were proposed to indicate inflammatory demyelination, though definitive interpretation in the context of multiple potentially underlying processes including inflammation, demyelination, axonal injury, or cell infiltration may be difficult [11,44–47]. AD can be interpreted as reflective of axonal health, and ongoing axonal injury measured by elevated CSF neurofilament protein (NFL) was identified in both HIV-associated dementia and cognitively asymptomatic HIV patients [48,49]. In the context of multiple sclerosis, a disease characterized by inflammatory demyelination and axonal injury, both AD increases and decreases have been found; potential explanations include an unclear directional effect of chronic inflammation on AD over long periods of time or that AD increases may reflect compensatory mechanisms in the presence of white matter damage [50]. The associations with higher AD we find in our study were not previously reported and warrant further investigation. Widespread involvement of MCP-1 implicates monocyte-associated inflammation in peripheral blood associated with central nervous system alterations and could include blood-brain barrier disruption as contributors to white matter integrity as detected by DTI analysis [51,52]. This is supported by the associations of neopterin, an immune activation marker, and sCD14, a monocyte activation marker [17,53,54]. Overall, these results are in-line with the hypothesis that in PLWH, persistent inflammation and immune activation impact brain health despite persistent viral suppression.
Connections between DTI metrics and neuropsychological test scores in the global, executive, and psychomotor speed domains add further evidence that the physiological changes we identify have clinical relevance, and are consistent with our previously published data [55]. In our initial analysis, we failed to find significant associations between plasma biomarkers and neuropsychological testing. Previous results with larger study populations (n=253) and others (n=68) connected inflammatory biomarkers with neuropsychological test scores, suggesting that we had insufficient power in the current study to identify these associations, though our samples may have also differed in the degree of impairment or in other demographic variables [10,15].
Our study population was carefully selected to consist of older individuals under stable and successful antiretroviral treatment. This approach strengthens the clinical relevance of our conclusions as these findings demonstrate that despite optimal adherence to cART and suppression of viral RNA to unquantifiable levels, white matter damage remains linked to elevated inflammatory biomarkers. This suggests that cART may not be enough to fully eliminate the physiological changes in the brain we detect by DTI, in turn correlating to neuropsychological performance. The selection of individuals who are cognitively symptomatic similarly strengthens the clinical relevance of our finding, as this is the population likely to seek care for cognitive issues. However, broad generalizability of these data to individuals with substantial cognitive confounding conditions and to those who are asymptomatic or inadequately treated is not possible. The presence of potential risk factors classified as secondary or contributing does not preclude HAND diagnosis but may play a non-negligible role in white matter health. It was not possible for us to exclude all comorbidities as the majority of these older participants present at least one (n=29, 67%). Inclusion of comorbidities as a covariate in our models did not significantly alter our results linking FA, MD, and RD to MCP-1, although many AD associations no longer met significance and neopterin was no longer linked to MD. These may reflect lower sensitivity and low power due to smaller sample size. Our results suggest that within the HIV-infected population, the contributions of elevated MCP-1 and sCD14 to white matter health are likely to be significantly associated independent of other comorbidities.
Most of our participants have a long history of infection over two decades (n=41, 95%). Thus, the white matter injury we detect may be a legacy of the pre-cART era that is unable to be fully recovered by cART introduction. In our models, we did not detect any associations between duration of infection and DTI metrics. The associations with current levels of inflammatory biomarkers indicate that, although the initial trigger could have been historical, the effects are enduring. These findings are complementary to our prior work demonstrating an increased atrophy rate associated with HIV infection in a similarly well-controlled study population, though there exists conflicting literature reporting stable atrophy rates [56,57].
It is important to consider the setting of this study to understand external validity. The participant population was gathered retrospectively and consists only of self-reported cognitively symptomatic PLWH. This process increased the likelihood that testing abnormalities were true changes from baseline, but also excludes impaired individuals with asymptomatic neurocognitive impairment (ANI), which may make up nearly 70% of HAND cases [2]. Likewise, our population consists of aging, predominantly white male individuals, so the results cannot be generalized to a younger age range, or to populations of different ethnic backgrounds or women.
Due to technical changes that occurred at our center over the course of this study, images were acquired on an MRI machine before (Siemens TIM Trio) and after (Siemens Prisma FIT) a software and hardware upgrade, as reported in the methods, which may confound the ROI results where we examined them as one group. Due to the differences in pulse sequence and hardware, the DTI metrics between the scanners differed slightly in both magnitude and variance (Supplemental Table 1). However, we controlled for this in our statistical analysis, and within-scanner ROI analyses demonstrated similar trends (Supplemental Figure 1). Thus, we are confident that the scanner change does not significantly bias the findings. Because we lack a matched control sample population of either HIV-infected asymptomatic or HIV-uninfected individuals to compare to, we can only discuss the links between relative biomarker elevation and diffusion metrics within the HIV-infected, virally suppressed, and symptomatic population. Our study is cross-sectional, which restricts our ability to assign causal direction to the association between inflammatory biomarkers and white matter alterations. As the biomarkers we examined are non-specific, the associations to HIV are inferred but cannot be definitely confirmed. A longitudinal study including seronegative individuals would better capture the impact of time on the progression of both the biomarkers and the DTI metrics. Future work is planned to examine the impact of higher baseline inflammatory and immune activation markers on quantitative imaging measures of the brain over time.
In conclusion, we demonstrate that in virally suppressed HIV-infected older adults who are optimally adherent to cART with persistently suppressed plasma HIV RNA, elevated inflammatory and immune activation plasma biomarkers MCP-1, neopterin, and sCD14 associate with higher MD and lower FA, pointing to brain white matter microstructure abnormality and further evidencing the importance of myeloid origin cells in the pathogenesis of brain abnormalities in older PLWH. Poorer cognitive performance also links to these metrics, supporting the hypothesis that persistent immune activation and inflammation despite viral suppression impacts brain integrity and may contribute to cognitive impairment in the cART era.
Supplementary Material
Figure 1: Results of biomarkers regressed against DTI metrics.
Regression coefficients of biomarkers presented with 95% confidence intervals. Only associations where p<0.05 are reported. (A) Higher MCP-1 levels linked to lower FA in the corpus callosum and higher MD values in widespread regions including the corpus callosum and corona radiata. (B) Higher neopterin levels linked to higher MD in the genu of corpus callosum. (C) Higher sCD14 levels linked to lower FA in the bilateral superior corona radiata.
*: p<0.05. **: p<0.01. ***: p<0.001.
ACR: anterior corona radiata. BCC: body of corpus callosum. GCC: genu of corpus callosum. PCR: Posterior corona radiata. SCC: splenium of corpus callosum. SLF: superior longitudinal fasciculus.
L: Left. R: Right.
5. Acknowledgements
We thank the study participants and the support of the Memory and Aging Center at UCSF.
Funding: This study was supported by the National Institute of Health grants K24MH098759, P30MH075673, R01NR015223, and R01MH113406.
6. References
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report, 2017. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (accessed 28 Feb2019).
- 2.Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 2009; 19:152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, et al. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology 2004; 63:822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhaskaran K, Mussini C, Antinori A, Walker AS, Dorrucci M, Sabin C, et al. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol 2008; 63:213–221. [DOI] [PubMed] [Google Scholar]
- 6.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, et al. HIV-associated neurocognitive disorder-pathogenesis and prospects for treatment HHS Public Access. Nat Rev Neurol 2016; 12:234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao VR, Ruiz AP, Prasad VR. Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND). AIDS Res Ther 2014; 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booiman T, Wit FW, Maurer I, De Francesco D, Sabin CA, Harskamp AM, et al. High cellular monocyte activation in people living with human immunodeficiency virus on combination antiretroviral therapy and lifestyle-matched controls is associated with greater inflammation in cerebrospinal fluid. Open Forum Infect Dis 2017; 4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. Aids 2013; 27:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, et al. Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. American Academy of Neurology; 2016. [DOI] [PMC free article] [PubMed]
- 12.Rahimian P, He JJ. HIV/neuroAIDS biomarkers. Prog Neurobiol 2017; 157:117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burdo TH, Lackner A, Williams KC. Monocyte/Macrophages And Their Role In Hiv Neuropathogenesis. Immunol Rev 2013; 254:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr 2011; 57:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imp BM, Rubin LH, Tien PC, Plankey MW, Golub ET, French AL, et al. Monocyte Activation Is Associated With Worse Cognitive Performance in HIV-Infected Women With Virologic Suppression. J Infect Dis 2017; 215:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after antiretroviral therapy. J Infect Dis 2011; 204:154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab 2002; 3:175–87. [DOI] [PubMed] [Google Scholar]
- 18.Fleischman DA, Arfanakis K, Leurgans S, Keating SM, Lamar M, Bennett DA, et al. Neopterin is associated with hippocampal subfield volumes and cognition in HIV. Neurol Neuroimmunol neuroinflammation 2018; 5:e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gisslén M, Chiodi F, Fuchs D, Norkrans G, Svennerholm B, Wachter H, et al. Markers of immune stimulation in the cerebrospinal fluid during HIV infection: a longitudinal study. Scand J Infect Dis 1994; 26:523–33. [DOI] [PubMed] [Google Scholar]
- 20.Lane BR, King SR, Bock PJ, Strieter RM, Coffey MJ, Markovitz DM. The C-X-C chemokine IP-10 stimulates HIV-1 replication. Virology 2003; 307:122–34. [DOI] [PubMed] [Google Scholar]
- 21.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J Interf Cytokine Res 2009; 29:313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Bales RA, Ethisham A, et al. Inflammatory changes and breakdown of microvascular integrity in early human immunodeficiency virus dementia. J Neurovirol 2004; 10:223–232. [DOI] [PubMed] [Google Scholar]
- 23.Chang L, Ernst T, St Hillaire C, Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir Ther 2004; 9:431–40. [DOI] [PubMed] [Google Scholar]
- 24.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996; 111:209–219. [DOI] [PubMed] [Google Scholar]
- 25.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics 2007; 4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chelune GJ, Heaton RK, Lehman RAW. Neuropsychological and Personality Correlates of Patients’ Complaints of Disability In: Advances in Clinical Neuropsychology. Boston, MA: Springer US; 1986. pp. 95–126. [Google Scholar]
- 27.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69:1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milanini B, Catella S, Perkovich B, Esmaeili-Firidouni P, Wendelken L, Paul R, et al. Psychiatric symptom burden in older people living with HIV with and without cognitive impairment: the UCSF HIV over 60 cohort study. AIDS Care 2017; 29:1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiao S, Rosen HJ, Nicolas K, Wendelken LA, Alcantar O, Rankin KP, et al. Deficits in self-awareness impact the diagnosis of asymptomatic neurocognitive impairment in HIV. AIDS Res Hum Retroviruses 2013; 29:949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milanini B, Wendelken LA, Esmaeili-Firidouni P, Chartier M, Crouch P-C, Valcour V. The Montreal Cognitive Assessment (MoCA) to screen for cognitive impairment in HIV over age 60. J Acquir Immune Defic Syndr 2014; 67:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veraart J, Novikov DS, Christiaens D, Ades-aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage 2016; 142:394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002; 17:825–841. [DOI] [PubMed] [Google Scholar]
- 33.Otsu N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans Syst Man Cybern 1979; 9:62–66. [Google Scholar]
- 34.Garyfallidis E, Brett M, Amirbekian B, Rokem A, van der Walt S, Descoteaux M, et al. Dipy, a library for the analysis of diffusion MRI data. Front Neuroinform 2014; 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016; 125:1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Yushkevich PA, Rueckert D, Gee JC. Unbiased white matter atlas construction using diffusion tensor images. Med Image Comput Comput Assist Interv 2007; 10:211–8. [DOI] [PubMed] [Google Scholar]
- 37.Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008; 40:570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samboju V, Philippi CL, Chan P, Cobigo Y, Fletcher JLK, Robb M, et al. Structural and functional brain imaging in acute HIV. NeuroImage Clin 2018; 20:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seabold S, Perktold J. Statsmodels: Econometric and Statistical Modeling with Python. ; 2010. http://statsmodels.sourceforge.net/ (accessed 8 Oct2018).
- 40.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006; 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 41.Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Klein JC, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc 2007; 2:499–503. [DOI] [PubMed] [Google Scholar]
- 42.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- 43.Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage 2014; 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh SW, Shin N-Y, Choi JY, Lee S-K, Bang MR. Altered White Matter Integrity in Human Immunodeficiency Virus-Associated Neurocognitive Disorder: A Tract-Based Spatial Statistics Study. Korean J Radiol 2018; 19:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su T, Caan MWA, Wit FWNM, Schouten J, Geurtsen GJ, Cole JH, et al. White matter structure alterations in HIV-1-infected men with sustained suppression of viraemia on treatment. Aids 2016; 30:311–322. [DOI] [PubMed] [Google Scholar]
- 46.Underwood J, Cole JH, Caan M, De Francesco D, Leech R, Van Zoest RA, et al. Gray and White Matter Abnormalities in Treated Human Immunodeficiency Virus Disease and Their Relationship to Cognitive Function. Clin Infect Dis 2017; 65:422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002; 17:1429–1436. [DOI] [PubMed] [Google Scholar]
- 48.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - A technical review. NMR Biomed 2002; 15:435–455. [DOI] [PubMed] [Google Scholar]
- 49.Jessen Krut J, Mellberg T, Price RW, Hagberg L, Fuchs D, Rosengren L, et al. Biomarker Evidence of Axonal Injury in Neuroasymptomatic HIV-1 Patients. PLoS One 2014; 9:e88591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fink F, Klein J, Lanz M, Mitrovics T, Lentschig M, Hahn HK, et al. Comparison of diffusion tensor-based tractography and quantified brain atrophy for analyzing demyelination and axonal loss in MS. J Neuroimaging 2010; 20:334–344. [DOI] [PubMed] [Google Scholar]
- 51.Yao Y, Tsirka SE. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci 2014; 71:683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Semple BD, Kossmann T, Morganti-Kossmann MC. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J Cereb Blood Flow Metab 2010; 30:459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagberg L, Cinque P, Gisslen M, Brew BJ, Spudich S, Bestetti A, et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther 2010; 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. Published Online First: 2015. doi: 10.1097/QAD.0000000000000735 [DOI] [PMC free article] [PubMed]
- 55.Nir TM, Jahanshad N, Busovaca E, Wendelken L, Nicolas K, Thompson PM, et al. Mapping white matter integrity in elderly people with HIV. Hum Brain Mapp 2014; 35:975–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanford R, Fellows LK, Ances BM, Collins DL. Association of Brain Structure Changes and Cognitive Function With Combination Antiretroviral Therapy in HIV-Positive Individuals. JAMA Neurol 2018; 75:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clifford KM, Samboju V, Cobigo Y, Milanini B, Marx GA, Hellmuth JM, et al. Progressive Brain Atrophy Despite Persistent Viral Suppression in HIV Patients Older Than 60 Years. J Acquir Immune Defic Syndr 2017; 76:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.