Abstract
Objective:
Insomnia and nightmares are central features of PTSD. However, often they are inadequately assessed and ineffectively resolved following ‘gold-standard’ PTSD treatment. Here, we: 1) evaluate effects of prolonged exposure (PE) on subjectively measured sleep; and 2) present pilot results of an examination of whether adding sleep interventions (Imagery Rehearsal Therapy [IRT] and Cognitive Behavioural Therapy for Insomnia [CBT-I]) to PE improves treatment response, relative to PE-alone, for night- and/or day-time PTSD symptoms among returning U.S. veterans and post-deployment personnel.
Method:
In a parallel-groups randomised controlled trial, participants received 12 sessions of PE followed by IRT (5 weeks) and CBT-I (7 weeks) or PE followed by 12 weeks supportive care therapy (SCT).
Results:
PE did not improve sleep to a clinically meaningful degree, despite significant improvements in both Clinical Administered PTSD Scale (CAPS) and PTSD Checklist. Enhancing treatment with IRT/CBT-I led to greater improvements in insomnia (diary-recorded sleep efficiency) symptoms with large effect size, relative to SCT (p = .068, d = 1.07). There were large improvements in nightmare frequency relative SCT, that did not reach statistical significance (p = .11, d = 0.90). Moreover, there was small improvement in daytime symptoms (CAPS) that did not reach statistical significance (p = .54, d = .31).
Conclusion:
The addition of targeted, validated sleep treatment improves effects of PE and improves night-time symptoms. Thus, evidence-based sleep treatment should be considered in comprehensive PTSD treatment.
Keywords: Sleep, Veterans, Cognitive behavioural therapy for insomnia, Prolonged Exposure, Imagery Rehearsal Therapy
Exposure to trauma during military operations is associated with numerous negative outcomes, including PTSD. Lifetime prevalence of PTSD is estimated at 11 – 17% for recent Iraq and Afghanistan veterans (Hoge et al., 2004). Consequences of PSTD include attempted suicide, functional impairment, alcohol abuse and dependence, reduced health-related quality of life, and significant mental distress (Kessler, 2000). For veterans with PSTD, maximizing treatment efficacy is especially critical post-discharge when trauma survivors must reintegrate into family and community and return to work and/or school. Effective evidence-based treatments for PTSD exist, however, one set of symptoms often unresolved is sleep disturbance.
Sleep disturbances are part of the diagnostic criteria for PTSD, and are recognised as a core feature of PTSD (Spoormaker & Montgomery, 2008). Up to 90% of veterans (Maher, Rego, & Asnis, 2006) with PTSD report sleep disturbances, including formal diagnoses of insomnia and nightmares (Maher et al., 2006), reduced subjective and objective sleep duration and sleep quality (Cox & Olatunji, 2016). In treatment seeking samples, sleep measures almost universally show impairment well within clinical ranges, including on global sleep measures (Galovski et al., 2009; Zayfert & DeViva, 2004), specific sleep symptoms (Pruiskma et al., 2016), and night-to-night variability (Straus, Drummond, Nappi, Jenkins, & Norman, 2015). Sleep disturbances in PTSD are a particular concern because they may impact the overall effectiveness of interventions by increasing distress and daytime impairment above and beyond that related to daytime symptoms (Giosan et al., 2015). Untreated sleep symptoms can persist for years, exacerbate existing PTSD symptoms, and complicate recovery or affect efficacy of first-line PTSD treatment (Koffel, Khawaja, & Germain, 2016). For these reasons, sleep disturbances are considered a modifiable risk factor that, if sufficiently treated, could reduce the overall burden and risk of relapse in PTSD (Germain, 2013).
Existing evidence-based treatments for PTSD, such as prolonged exposure (PE), appear to be less effective in ameliorating sleep disturbance, relative to daytime symptoms, i.e., all other PTSD symptoms apart from nightmares and difficulty sleeping (Galovski et al., 2009; Gutner, Nillni, Suvak, Wiltsey-Stirman, & Resick, 2013; Larsen, Fleming, & Resnick, 2019; Nappi, Drummond, & Hall, 2012; Schnurr & Lunney, 2018). One limitation of these studies, though, is they generally only assessed sleep with limited questions extracted from a larger, non-sleep focused measure (Galovski et al., 2009; Gutner et al., 2013; Larsen et al., 2019; Schnurr & Lunney, 2018), or global sleep measure rather than PTSD-specific sleep issues (Galovski et al., 2009; Gutner et al., 2013). To our knowledge, no study has assessed sleep using well-validated multi-modal tools to understand which sleep problems are most likely to interfere with and least likely to remit through treatment. Therefore, this study’s first aim was to develop a more nuanced understanding of how evidence-based PTSD psychotherapies may improve sleep symptoms.
While it appears daytime PTSD interventions may not sufficiently treat sleep symptoms, evidence-based interventions exist for both insomnia and nightmares (Germain, 2013; Nappi et al., 2012; Talbot et al., 2014). Studies utilizing such interventions within PTSD populations have reported sleep symptoms improved as expected, when they were specifically targeted. Moreover, these studies reported some improvements in daytime symptoms of PTSD (Germain, 2013; Nappi, Drummond, Thorp, & McQuaid, 2010; Talbot et al., 2014). Overall, treatment studies suggest normalization of sleep may have some benefit in reducing daytime PTSD symptoms. However, to our knowledge, no study has tested whether combining traditional PTSD psychotherapy with evidence-based treatments for nightmares (Imagery Rehearsal Therapy [IRT]) and insomnia (Cognitive Behavioural Therapy for Insomnia [CBT-I]) in patients with sleep complaints will increase efficacy of overall treatment. If the addition of sleep treatment to best-practice PTSD therapies improves night-time and/or overall severity of PTSD, this may improve PTSD treatment outcomes. As one of the first pilot studies of its nature, this will also provide key information as to guide implementation of combined protocols in clinics.
The aims of this study were to address the two main gaps highlighted above: 1) to examine the impact of evidence-based PTSD treatment (PE) on well-validated sleep assessments; and 2) to assess the value of adding evidence-based sleep interventions to the end of PTSD treatment, to improve both sleep symptoms and daytime PTSD symptoms. The study recruited OEF/OIF/OND veterans with comorbid diagnoses of PTSD and chronic insomnia, as well as nightmares. All participants received PE and then were randomized to either receive validated sleep interventions (CBT-I + IRT) or control treatment (Supportive Care Therapy; SCT). For the first aim, we hypothesized small improvements in sleep diary-based sleep efficiency (SE) and number of nightmares during PE but that sleep impairment would remain in the clinical range. We also included CAPS total score as a primary outcome, to ensure PE improved overall PTSD symptoms as expected. The second aim focused on changes in the same set of primary outcome variables when sleep interventions were added onto PE in a sequential manner. We hypothesized sleep diary-based SE and number of nightmares would fall within the normal range and overall PTSD symptoms (CAPS total score) would show clinically meaningful improvements following sleep treatment, but not control treatment. Due to final sample size (see below), the second aim must be considered a pilot study. A number of other measures were examined in both aims for exploratory purposes, to provide as full a picture of treatment effects as possible and to address the research gap identified related to limited sleep measures in prior studies. Over the course of the study, participant drop-out was high, therefore, as an exploratory aim, we also explored predictors of treatment completion.
Method
This was a parallel-groups randomised controlled trial comparing a combined PTSD and sleep intervention to a PTSD plus SCT intervention. Participants were recruited throughout a grant period between January 2010 and March 2012 and randomized in equal numbers to receive: 1) 12× 90-minute twice-weekly sessions of PE followed by sleep-specific treatments (5× 60-minute weekly sessions of IRT for nightmares and 7× 60-minute weekly sessions of CBT-I for insomnia); or 2) PE followed by 12× 60-minute weekly sessions of Supportive Care Therapy. The trial was approved by the University of California San Diego, VA San Diego Healthcare System (VASDHS), and Naval Medical Center San Diego IRBs and registered on clinicaltrials.gov ( NCT01009112) prior to first enrollment.
The study was single-blinded, with the outcomes assessor masked to allocation. Randomisation was conducted by primary investigator SPAD. in blocks of 6 using masked allocation. Condition was revealed to the treating therapist only after completion of PE. There were no important changes made to methods or outcomes after trial commencement, nor were there any unintended harms to participants throughout the protocol.
Participants
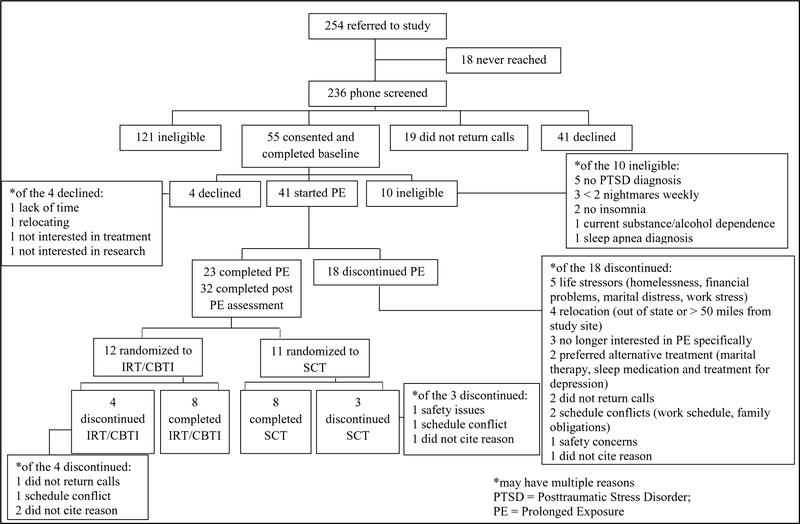
Study participants were 55 U.S. veterans and active duty personnel with PTSD and sleep disturbance from the VASDHS and Naval Medical Center San Diego provided written informed consent. Inclusion criteria included (a) being deployed at least once as part of Operation Iraqi Freedom/Operation Enduring Freedom/Operation New Dawn (OEF/OIF/OND), (b) having a current diagnosis of PTSD from military-related trauma, (c) meeting criteria for comorbid insomnia, and (d) reporting minimum 2 nightmares per week. Exclusion criteria included (a) unmanaged psychosis or manic episodes in the past year, (b) substance/alcohol dependence in the past 6 months, (c) untreated sleep disorders other than insomnia and nightmares, (d) use of hypnotics or prazosin for sleep, (e) history of traumatic brain injury other than mild brain injury, (f) change in dosage and/or type of psychotropic medication in preceding month, and (g) concurrent psychotherapy for PTSD. All screening was conducted by a single licenced and experienced psychiatric nurse. Any questions related to eligibility were resolved through a consensus discussion among the investigators. For details of participant flow through the study, see Figure 1. Briefly, of the 55 veterans consented, 10 did not meet eligibility criteria and so were excluded. The sample at baseline comprised veterans (n = 37), active duty personnel (n = 5), and reserves (n = 3).
Figure 1.
Consort chart.
Treatment Conditions
Prolonged Exposure Therapy.
PE is a manualised treatment (Foa, Hembree, & Rothbaum, 2007). All participants were asked to complete 12× 90-minute twice-weekly sessions. The focus of the treatment is reducing avoidance related to trauma cues and increasing quality of life by having patients take part in in-vivo exposure to trauma reminders and imaginal exposure to the trauma memory. Weekly homework was assigned which included listening to audiotapes of sessions, practising breathing skills, and participating in avoided but safe situations.
Imagery Rehearsal Therapy.
After completing PE, participants randomised to PE+Sleep were invited to attend 5× 60-minute weekly sessions of IRT. IRT is a manualised therapy for nightmares (Forbes et al. 2003). Briefly, the core components of IRT are (1) psychoeducation on sleep, nightmares, and imagery; (2) teaching and homework of 10 minutes daily practise of personalised pleasant imagery scenes; (3) “rescripting” of the nightmare which involves identifying and elaborating upon an alternative, neutral and/or pleasant ending, with 10 minutes daily practise of the “new dream” imagery; (4) problem solving difficulties; and (5) relapse prevention. Daily nightmare logs are kept and reviewed in sessions. Notably, this version of IRT does not include exposure to the nightmare, further differentiating it from PE.
Cognitive Behavioural Therapy for Insomnia.
CBT-I consisted of 7× 60-minute weekly sessions (Perlis, Benson-Jungquist, Smith, & Posner, 2005). The core components are psychoeducation on chronic insomnia; sleep restriction; stimulus control; sleep hygiene; stress reduction and relaxation; “decatastrophisation” of consequences through cognitive restructuring; and relapse prevention.
Supportive Care Therapy Condition.
Participants randomised to PE+SCT received 12× 60-minute weekly sessions of SCT after PE. This controlled non-specific treatment effects associated with therapist contact and homework completion. This non-directive Rogerian therapy includes empathetic listening, clarification of goals, and non-directive questioning. The focus was to help participants better understand their emotional response to PTSD symptoms, and provide supportive, unconditional positive regard to managing their own PTSD symptoms. Components of IRT and CBT-I were strictly avoided.
Treatment Fidelity.
Treatment fidelity was maintained three ways. First, therapists were all licensed or licensed eligible psychologist. They were trained on each intervention through: a) didactics; b) treating two veterans with each intervention prior to conducting therapy for the study (training conducted by authors SBN (PE), SPAD (CBT-I and IRT), and CMN (SCT)); and c) every session included a therapist checklist of key components. Second, therapists had weekly supervision for each treatment with the same individuals who did the training. Third, every session was audio tapped and independent judges (listed in the Acknowledgements) rated treatment fidelity, based on a checklist, for every session of the first two veterans/intervention/therapist and then 20% of sessions for every subsequent veteran/intervention/therapist. Unfortunately, these data are not available, as they were lost during an international relocation by the senior author (SPAD).
Measures
Participants were assessed for daytime and night-time symptoms, medication use, and quality of life pre-treatment (Screening/Week 0) and at weeks 6, 11, and 18. These subsequent assessments coincide with the end of each active treatment. Additionally, participants completed daily sleep diary and nightmare logs throughout the 18 treatment weeks.
Screening Measures.
The following tools assessed eligibility criteria at pre-treatment: (a) SCID for DSM-IV-TR; (b) DUKE Structured Interview for Sleep Disorders (DSISD) which assesses for sleep disorders in both DSM-IV-TR and ICSD (Edinger et al., 2004); (c) Medical History Interview and chart review; (d) Medication Use Interview; and (e) Brief Screening for Traumatic Brain Injury as per VASDHS protocol: if a potential participant screened positive, further questions were asked to determine severity.
Primary Outcome Measurement Tools.
A daily Sleep Diary and Nightmare Log was completed throughout the 18-week treatment and collected primary outcome measures of SE and number of nightmares. This also asked for exploratory subjective measures of bed time, sleep latency, number and duration of awakenings, wake time, total time in bed, total sleep time, and intensity of nightmares. Weekly averages and night-to-night variability in sleep parameters were calculated (Straus et al., 2015).
The Clinician Administered PTSD Scale (CAPS; Blake et al., 1995) is the gold-standard semi-structured interview that corresponds with DSM-IV criteria for PTSD. This interview was used to make a current diagnosis (past month) of PTSD at baseline, and symptoms in the past week at all future assessments. Reliability was good, sample α for subscales = .74-.87, CAPS total score α = .92.
Additional Exploratory Outcome Measurement Tools.
To supplement our primary outcome variables, a number of other measures were gathered at each assessment.
The Insomnia Severity Index (ISI) is a 7-item self-report assessment of severity of insomnia in the past week (Morin, 1993). A higher score indicates more sleep disturbances. Sample α = .80.
The Pittsburgh Sleep Quality Index (PSQI) is a 19-item self-report assessment of sleep quality over the past month (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). Scores ≥5 are considered clinically impaired sleep quality. The PTSD Addendum for the PSQI assessed sleep disruptive behaviours common to PTSD patients and those with chronic nightmares (Germain, Hall, Krakow, Katherine Shear, & Buysse, 2005). Scores ≥4 are in the clinical range. Reliability in this sample was relatively low, PSQI α = .61, PSQI-Addendum α = .68.
Participants wore an actigraph for seven days pre-treatment (week 0), then seven days each preceding all following assessments. Respironics Actiwatch 2 and Actiware software (Respironics) algorithms generated values for objective time in bed, total sleep time, wake after sleep onset, and SE. Rest intervals for analysis were manually set, corresponding to time in bed according to diary entries, although rest intervals could be extended < 60 minutes on either side to account for obvious errors (Straus et al., 2015). Sleep detection settings were set to medium.
The PTSD Checklist-Specific version (PCL-S; Wilkins, Lang, & Norman, 2011) is a self-report measure of PTSD severity. A higher score indicates greater symptom severity. The reliability and validity of the measure has been established (Wilkins et al., 2011), and in this sample α = .91.
The Patient Health Questionnaire-9 (PHQ-9; Kroenke & Spitzer, 2002) assessed severity and frequency of depression symptoms, due to high frequency of comorbid depression in PTSD. It comprises 9 items corresponding to DSM-IV criteria for depression, with higher scores indicating greater symptoms. Sample α = .87.
The Medical Outcome Study Short Form-36-V (Kazis et al., 2006) is a 36-item psychometrically-based index designed to measure general health functioning and quality of life among veterans. Two variables are reported here: Physical and Mental Components. Reliability was low in this sample, α = .33.
Data Analysis
Analysis was conducted using SPSS v. 24. Of the 55 veterans consented for study treatment, 23 (42%) completed PE. Following PE, 12 were randomized to IRT/CBT-I and 11 randomized to SCT. Of these, 8 completed IRT/CBT-I and 8 completed SCT (total study completers: n = 16 [29% of those consented, 70% of those who finished PE]). See Figure 1. All participants were invited to attend all assessment sessions, regardless of whether they completed treatment. This enabled an intent-to-treat analytic framework, where all available data for each time point was analysed (Hollis & Campbell, 1999).
Where there was missing data, a complete case analysis was conducted. Some or all of the pre-treatment assessment was completed by n = 55 participants. At week 6, 32 participants completed some or all post-PE assessment. At week 11 and 18, 15 participants completed some or all assessment. Reasons for missing data included participants declining to complete measures, non-adherence to sleep diary and actigraphy, or technical issues with actigraphy. Exact sample size for each set of measures at each assessment is noted within Tables 1–4.
Table 1.
Demographic information and trauma characteristics by treatment condition
| Measure | All Consented (n = 55) | Completed PE (n = 23) | IRT/CBTI (n = 12) | SCT (n = 11) | p value (IRT/CBTI vs SCT) | |
|---|---|---|---|---|---|---|
| Sex | Female | 9 | 4 | 4 | 0 | .035 |
| Age (years) | M ± SD | 35.0 ± 1.4 | 36.54 ± 9.8 | 36.8 ± 10.4 | 36.0 ± 9.5 | .86 |
| Race | Black | 10 | 7 | 3 | 4 | .70 |
| Caucasian | 28 | 12 | 1 | 6 | ||
| Pacific | 4 | 3 | 2 | 1 | ||
| Islander | 1 | 1 | 1 | 0 | ||
| Asian | 2 | 0 | 0 | 0 | ||
| Biracial | ||||||
| Ethnicity | Non-Hispanic | 19 | 18 | 10 | 8 | .54 |
| Hispanic | 26 | 5 | 2 | 3 | ||
| Minority | Minority | 19 | 7 | 4 | 8 | .75 |
| Non-minority | 26 | 16 | 8 | 3 | ||
| Marital Status | Single | 11 | 5 | 2 | 3 | .26 |
| Married | 19 | 8 | 3 | 5 | ||
| Divorced | 10 | 6 | 4 | 2 | ||
| Separated | 4 | 3 | 3 | 0 | ||
| Widowed | 1 | 1 | 0 | 1 | ||
| Number of Children | M ± SD | 1.40 ± 1.71 | 1.61 ± 1.97 | 1.58 ± 2.31 | 1.64 ± 1.63 | .95 |
| Number of Deployments | 1 | 22 | 11 | 7 | 4 | .53 |
| 2 | 17 | 8 | 3 | 5 | ||
| 3 | 6 | 4 | 2 | 2 | ||
| Months Since Deployment | M ± SD | 59.4 ± 4.5 | 60.1 ± 35.12 | 52.9 ± 39.1 | 67.9 ± 30.0 | .32 |
| Months since index trauma | M ± SD | 78.0 ± 8.4 | 84.0 ± 64.8 | 78.4 ± 68.1 | 90.2 ± 63.7 | .67 |
| Months since symptoms | M ± SD | 63.6 ± 8.7 | 67.0 ± 65.5 | 34.5 ± 36.6 | 92.7 ± 81.1 | .07 |
Table 4.
Means and standard deviations at each assessment point, separated into the Evidence-Based Sleep Treatment and Supportive Care Treatment groups.
| Supportive Care Therapy | Sleep Treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre Treatment Week 0 M ± SD |
PE Completed Week 6 M ± SD |
PE + 5 weeks SCT Week 11 M ± SD |
PE + 12 weeks SCT Week 18 M ± SD |
Pre Treatment Week 0 M ± SD |
PE Completed Week 6 M ± SD |
PE + IRT Week 11 M ± SD |
PE + IRT+CBTI Week 18 M ± SD |
|
| PTSD, Depression and Quality of Life | n = 11 | n = 11 | n = 8 | n = 8 | n = 12 | n = 12 | n = 6 | n = 7 |
| CAPS Total score | 70.09 ± 15.56 | 61.36 ± 19.50 A | 55.13 ± 23.44 | 52.38 ± 18.52 A | 74.08 ± 13.70 | 53.33 ± 19.38 A | 44.33 ± 19.05 A | 37.57 ± 20.97 AB |
| Re-experiencing | 19.45 ± 4.78 | 20.27 ± 5.62 | 17.38 ± 6.85 | 16.75 ± 6.78 | 19.42 ± 5.90 | 16.92 ± 6.13 | 12.83 ± 9.89 | 13.17 ± 8.13 |
| Avoidance/numbing | 25.82 ± 9.09 | 18.54 ± 11.30 A | 14.88 ± 12.63 A | 14.00 ± 8.38 A | 30.42 ± 6.14 | 17.83 ± 9.45 A | 14.67 ± 7.12 A | 12.00 ± 8.32 A |
| Hyperarousal | 24.82 ± 4.40 | 22.54 ± 4.68 | 22.88 ± 5.84 | 21.63 ± 5.43 D | 24.25 ± 4.81 | 18.58 ± 6.13 A | 16.83 ± 7.22 A | 12.00 ± 9.14 ABCD |
| CAPS without Sleep Items | 56.91 ± 15.59 | 49.45 ± 19.00 | 42.63 ± 22.50 | 40.63 ± 17.39 A | 61.67 ± 12.99 | 42.17 ± 18.66 A | 35.17 ± 16.03 | 31.17 ± 19.26 A |
| PCL Total score | 58.27 ± 9.73 | 45.80 ± 16.73^ | 40.75 ± 14.54 A | 40.38 ± 14.17 A | 58.92 ± 9.27 | 39.75 ± 14.58 A | 33.71 ± 12.43 A | 34.43 ± 16.15 A |
| PHQ | 14.91 ± 5.56 | 11.64 ± 6.67 | 10.25 ± 5.97 | 11.75 ± 6.98 | 17.08 ± 5.57 | 9.42 ± 5.37 A | 6.33 ± 4.59 A | 7.29 ± 5.77 A |
| VR-36 Physical Component | 43.71 ± 11.21 | 43.58 ± 10.88 | 44.32 ± 7.96 | 43.20 ± 9.93 | 46.07 ± 11.24† | 45.35 ± 10.28 | 47.23 ± 7.79# | 49.24 ± 7.54 |
| VR-36 Mental Component | 31.70 ± 13.26 | 38.28 ± 12.72 | 40.33 ± 11.61 | 42.27 ± 11.66 | 30.52 ± 8.20† | 40.04 ± 11.28 A | 45.83 ± 10.75#A | 42.63 ± 11.26 A |
| Sleep Diary | n = 11 | n = 10 | n = 8 | n = 8 | n = 12 | n = 11 | n = 6 | n = 7 |
| Sleep Efficiency* | 76.51 ± 10.07 | 81.74 ± 10.55 | 74.70 ± 17.75 | 74.61 ± 11.59 D | 73.33 ± 12.11 | 78.77 ± 9.79 | 82.82 ± 8.29 | 91.29 ± 4.89 CDY |
| Total number of nightmares* | 6.64 ± 4.20 | 4.10 ± 3.84 A | 5.88 ± 6.88 | 4.50 ± 6.78 | 6.50 ± 3.94 | 4.00 ± 3.77 A | 3.67 ± 3.27 | 2.14 ± 2.73 |
| Total Sleep Time (minutes) | 357.36 ± 99.82 | 361.57 ± 106.82 | 332.45 ± 135.32 | 304.12 ± 103.10 | 321.42 ± 57.85 | 324.45 ± 70.40 | 365.26 ± 65.68 A | 326.29 ± 91.15 |
| Sleep Latency (minutes)* | 55.93 ± 37.80 | 32.24 ± 24.12 | 38.02 ± 17.01 | 39.54 ± 18.66 | 35.76 ± 29.83 | 30.33 ± 17.57 | 31.79 ± 18.38 | 21.37 ± 19.52 C |
| Wake After Sleep Onset (minutes)* | 52.24 ± 16.60 | 42.70 ± 24.00 | 50.58 ± 29.01 | 51.31 ± 23.86 | 85.54 ± 57.10 | 59.27 ± 44.22 | 42.15 ± 32.09 AB | 31.12 ± 39.01 BCYX |
| Highest nightmare rating* | 8.00 ± 1.34 | 5.30 ± 2.98 A | 6.63 ± 2.39 | 5.50 ± 3.42 | 7.17 ± 2.48 | 6.27 ± 3.29 | 5.33 ± 3.08X | 4.57 ± 3.36 |
| Night-to-night variability: SL* | 40.15 ± 25.02 | 18.95 ± 14.02 A | 20.89 ± 13.75 A | 29.84 ± 20.18 | 30.19 ± 38.54 | 15.90 ± 13.36 | 15.88 ± 13.11 | 14.61 ± 27.23 |
| Night-to-night variability: WASO* | 41.21 ± 24.16 | 34.92 ± 31.64 | 40.15 ± 25.41 | 34.29 ± 19.15 | 72.21 ± 47.43 | 46.26 ± 27.06 | 25.71 ± 16.91 | 20.67 ± 12.35 AB |
| Night-to-night variability: TST* | 91.72 ± 36.32 | 102.49 ± 51.03 | 111.45 ± 69.03 | 115.91 ± 84.97 | 133.55 ± 64.37 | 82.06 ± 37.55 A | 65.42 ± 29.66 | 57.54 ± 43.14 A |
| Night-to-night variability: SE* | 12.26 ± 7.67 | 10.28 ± 10.11 | 14.43 ± 14.52 | 14.88 ± 4.98 | 16.95 ± 6.75 | 12.45 ± 5.23 | 7.86 ± 5.20 A | 8.02 ± 8.40 |
| Actigraphy | n = 9 | n = 8 | n = 6 | n = 6 | n = 9 | n = 10 | n = 7 | n = 7 |
| Total Sleep Time (minutes) | 330.65 ± 39.70 | 344.56 ± 49.74 | 339.89 ± 25.63 | 350.19 ± 37.90 | 365.21 ± 62.08 | 334.06 ± 44.66 | 354.94 ± 65.24 | 283.75 ± 65.20 |
| Wake After Sleep Onset | 55.18 ± 29.12 | 55.00 ± 25.78 | 47.31 ± 21.92 | 57.94 ± 30.46 | 44.22 ± 9.07 | 39.58 ± 16.44 | 43.32 ± 18.61 | 53.76 ± 30.02 |
| Sleep Efficiency | 68.03 ± 8.69 | 85.89 ± 6.67 A | 75.68 ± 5.99 B | 75.45 ± 7.11 B | 75.48 ± 11.63 | 89.54 ± 3.90 A | 76.35 ± 9.49 B | 69.57 ± 15.96 B |
| Time in Bed | 385.83 ± 58.43 | 402.77 ± 60.43 | 387.20 ± 36.18 | 408.13 ± 61.47 D | 410.30 ± 60.59 | 373.64 ± 51.55 | 398.26 ± 62.03 | 337.56 ± 48.37 D |
| Retrospective Sleep Measures | n = 11 | n = 11 | n = 8 | n = 8 | n = 12 | n = 12 | n = 5 | n = 7 |
| Insomnia Severity Index | 18.73 ± 4.10 | 16.36 ± 5.54 | 18.50 ± 5.35 D | 16.75 ± 6.02 | 18.75 ± 3.98 | 14.42 ± 5.84 A | 9.20 ± 6.26 DX | 9.71 ± 7.13 ABC |
| Pittsburgh Sleep Quality Index | 15.09 ± 3.73 | 13.36 ± 2.06 | 14.75 ± 1.67 D | 13.13 ± 3.31 D | 14.75 ± 3.05 | 11.50 ± 2.58 A | 9.60 ± 4.04 DX | 7.71 ± 4.50 ABCD |
| Pittsburgh Sleep Quality Index-Addendum | 10.82 ± 2.86 | 8.00 ± 3.66 A | 8.75 ± 4.40 | 14.38 ± 5.76 ABC | 8.42 ± 3.92 | 7.92 ± 4.19 | 4.60 ± 4.39B | 8.29 ± 5.59 |
Primary outcome variables are presented in boldface.
used non-parametric, Mann-Whitney U and Wilcoxon signed-rank test;
different from week 0 of same condition;
different from week 6 measure of same condition;
different from week 11 of same condition;
different from same week of different condition;
change from week 6 is significantly greater than SCT;
change from week 11 is significantly greater than SCT;
n = 11;
n = 6;
n = 10;
all significance designation based on p<.05.
For completeness of presentation of data, results presented include primary outcome measures of diary-derived SE, frequency of nightmares, and CAPS score, as well as other exploratory data including retrospective sleep measures, other diary-derived variables, actigraphy, and self-reported PTSD, depression, and quality of life measures. We will present these measures for completeness, without discussion or interpretation.
To address aim 1 and test whether sleep improved over PE, repeated-measures t-tests compared results at Week 0 (pre-treatment) and Week 6 (immediately post-treatment). Due to significant skew in variable distributions, non-parametric Wilcoxon signed-rank tests compared groups for all diary-derived variables except total sleep time.
To address aim 2, which is the pilot study component, analysis used t-tests for change scores from the prior assessment for each variable. Our a priori hypothesis was that there would be a time × treatment interaction and change score t-tests provide a direct test of that specific effect. While repeated-measures ANOVA would also be appropriate, small sample size lacks sufficient power for an ANOVA. Where significant skewness in distributions of sleep diary variables indicated non-parametric analysis, Mann-Whitney U tests were conducted.
Because attrition was high, we decided to explore predictors of treatment completion as an exploratory analysis, to inform future research and treatment design. In order to assess differences between study completers and non-completers, we considered a “PE completer” any participant who received all components of the protocol (Foa et al., 2007) in a minimum of 10 sessions. We used a broad range of variables to explore possible individual-level predictors. We considered general and military-related predisposing determinants (individual characteristics existing before onset of illness), enabling factors (factors allowing access to healthcare services), and psychiatric and sleep-related factors (perception of illness and its severity). In addition, we examined variables related to duration since symptom onset and trauma exposure. For each set of variables, we conducted a standard logistic regression to determine which predictors uniquely contributed to treatment completion. We then tested an overall model containing all significant predictors from the initial regressions, again using standard logistic regression. Standard regression was selected because of lack of theory to generate hypotheses about relative importance needed for hierarchical regression.
To assess whether there were differences in individuals who completed the sleep portion of the protocol or not, the same predictors as those assessing PE completion were assessed, as well as PTSD night-time and daytime symptoms post-PE completion. Post-PE diary-based SE was dropped due to collinearity (r > .7) with other sleep variables.
To assist interpretation of effects for the pilot component of this study, effect sizes were calculated and reported.
Results
Table 1 provides a detailed characterization of the sample. Table 2 presents data demonstrating the efficacy of PE on global PTSD symptoms (clinical and self-rated), depression, and quality of life, with medium to large effect sizes.
Table 2.
Baseline and post-Prolonged Exposure treatment means for PTSD, Depression and Quality of Life
| Pre-treatment | Post-treatment | Paired sample t test | ||||
|---|---|---|---|---|---|---|
| Measures | n | M ± SD | M ± SD | t or z value | p | ES (d) |
| CAPS Total score | 30 | 75.27 ± 14.91 | 62.30 ± 21.56 | 3.97 | <.001 | .70 |
| Re-experiencing | 30 | 20.77 ± 5.99 | 19.47 ± 6.52 | 1.21 | .24 | .21 |
| Avoidance/numbing | 30 | 29.27 ± 7.43 | 21.47 ± 11.13 | 4.12 | <.001 | .82 |
| Hyperarousal | 30 | 25.20 ± 4.64 | 21.37 ± 6.98 | 3.60 | .001 | .65 |
| PCL Total score | 32 | 60.47 ± 8.78 | 46.84 ± 16.74 | 5.33 | <.001 | 1.02 |
| PHQ-9 | 32 | 16.28 ± 5.23 | 11.84 ± 6.07 | 3.57 | .001 | .78 |
| VR-36 Physical Component | 28 | 44.93 ± 11.00 | 43.68 ± 10.75 | 0.76 | .45 | .12 |
| VR-36 Mental Component | 28 | 29.52 ± 10.29 | 37.66 ± 11.81 | −4.79 | <.001 | .74 |
Aim 1: Effect of PE on Sleep (Table 3)
Table 3.
Baseline and post-Prolonged Exposure treatment means for prospective and retrospective sleep measures
| Pre-treatment | Post- PE treatment | Paired sample t tests | |||
|---|---|---|---|---|---|
| Measures | M ± SD | M ± SD | t or Z | p | ES (d) |
| Sleep Diary (n = 25) | |||||
| Sleep Efficiency* | 71.97 ± 15.05 | 77.34 ± 12.64 | −1.66 | .098 | .39 |
| Total number of nightmares* | 6.72 ± 4.30 | 4.36 ± 3.57 | −2.76 | .006 | .60 |
| Total Sleep Time (minutes) | 336.03 ± 94.32 | 339.94 ± 88.05 | −0.22 | .83 | .04 |
| Sleep Latency (minutes)* | 57.35 ± 55.07 | 42.54 ± 35.59 | −1.06 | .29 | .32 |
| Wake After Sleep Onset (minutes)* | 75.50 ± 51.80 | 56.01 ± 45.20 | −2.46 | .014 | .40 |
| Highest nightmare rating* | 7.56 ± 2.02 | 6.04 ± 2.94 | −1.73 | .083 | .60 |
| Night-to-night variability: SL* | 35.54 ± 30.81 | 26.90 ± 28.42 | −1.84 | .065 | .29 |
| Night-to-night variability: WASO* | 55.02 ± 38.27 | 45.93 ± 30.51 | −0.44 | .66 | .26 |
| Night-to-night variability: TST* | 123.56 ± 58.95 | 107.52 ± 68.75 | −1.19 | .23 | .25 |
| Night-to-night variability: SE* | 15.16 ± 7.72 | 13.20 ± 8.87 | −1.09 | .28 | .24 |
| Retrospective Sleep Measures | |||||
| Insomnia Severity Index (n = 32) | 18.97 ± 4.01 | 16.22 ± 6.29 | 2.31 | .028 | .52 |
| Pittsburgh Sleep Quality Index (n = 29) | 14.76 ± 3.30 | 13.07 ± 3.22 | 2.07 | .048 | .52 |
| Pittsburgh Sleep Quality Index-Addendum (n = 30) | 9.67 ± 3.62 | 8.47 ± 3.88 | 1.58 | .13 | .32 |
Primary outcome variables are presented in boldface.
used non-parametric, Wilcoxon test
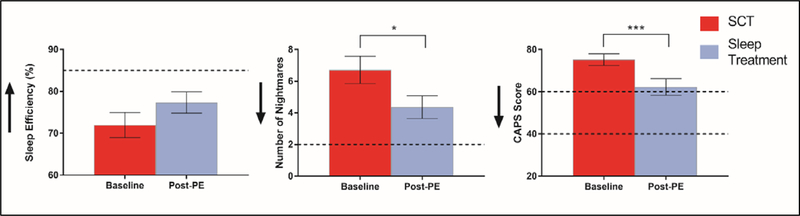
There was no significant effect of PE on SE (mean change score = 5.37%, z = −1.66, p = .098, d = .39). Participants did report significantly fewer weekly nightmares post-PE (mean decrease = 2.36 nightmares, z = −2.76, p = .006, d = .60). However, each of these measures remained in the clinical range for the majority of participants. After PE, 80% (n = 20) continued to experience minimum 2 nightmares per week and 76% (n = 19) had SE < 85%. Figure 2 displays results of paired-samples t tests before and after PE.
Figure 2.
M ± SD scores at week 0 (baseline) and 6 (post-PE). Arrows indicate direction of improvement. Dotted lines indicate clinical thresholds used: for CAPS, Moderately severe (60) and Probably PTSD (40); Sleep Efficiency 85%; Number of Nightmares 2/week. *p < .05, ***p<.001.
Aim 2: Effect of Evidence-Based Sleep Treatments on Primary Outcomes
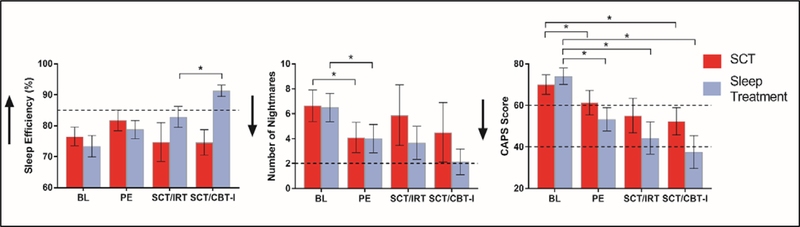
Table 4 displays primary and exploratory outcome variables for each group at week 6 (all participants had completed PE), week 11 (post SCT or IRT), and week 18 (post SCT or CBT-I). Following PE treatment, participants in SCT and IRT/CBT-I groups did not significantly differ on any primary outcome variable, all p > .05. Figure 3 displays results of independent samples t-tests between sleep and control conditions.
Figure 3.
M ± SD scores at each assessment – week 0 (pre-treatment), 6 (post-PE), 11 (post-IRT or 5 weeks SCT) and 18 (post-IRT/CBTI or 12 weeks SCT) for the Evidence-Based Sleep Treatment (blue/light grey) and SCT (red/dark grey) groups. For SCT, n = 11 (weeks 0 and 6) and n = 8 (weeks 11 and 18). For Evidence-Based Sleep Treatments, n = 12 (weeks 0 and 6), n = 6 (week 11) and n = 7 (week 18). Arrows indicate direction of improvement. Dotted lines indicate clinical thresholds used: for CAPS, Moderately severe (60) and Probably PTSD (40); Sleep Efficiency, 85%; Number of Nightmares, 2/week. * p < .05.
Relative to the end of PE (week 6), IRT increased diary-derived SE with non-significant but medium-large effect size (U = 10.00, p = .11, d = .64). Nightmare frequency decreased with large effect size but also did not meet statistical significance (t(11) = 1.76, p = .10, d = .96). CAPS scores did not change over IRT differently according to condition (t(12) = 0.15, p = .88, d = .08).
Relative to week 11 (end of IRT or 5 weeks of SCT), CBT-I significantly increased SE compared to SCT (t(12) = −2.21, p = .04, d = 1.25). There was no significant change in nightmare frequency according to treatment condition (U = 21.00, p = .68, d = .01). Compared with SCT, CBT-I showed a small treatment effect on CAPS Total scores (t(12) = 0.66, p = .51, d = .36).
In examining the combined effects of both sleep treatments, relative to SCT, (i.e., week 6 vs week 18), IRT/CBT-I trended towards improved SE with large effect size (t(12) = −2.00, p = .068, d = 1.07). Nightmare frequency showed large treatment effects but did not reach statistical significance (t(12) = 1.68, p = .11, d = .90). CAPS total scores showed small treatment effects (t(13) = 0.61, p = .54, d = .31).
Exploratory Analysis: Predictors of Treatment Completion
There were no demographic differences between those who started and those who did not start treatment. Reasons for reported discontinuation and are presented in Figure 1. A majority of participants who discontinued PE (n = 12; 66%) did so in the first half of treatment (before session 7). Logistic regression analyses revealed baseline PHQ-9 total score and night-to-night variability of total sleep time uniquely predicted PE treatment completion; Χ2(5) = 22.61, p < .001. For each 1-point increase in PHQ-9, the odds of completing PE treatment increased by 31.8%. The more consistent one’s total sleep time was night to night, the more likely one was to complete PE treatment. All participants who completed PE went on to attend a minimum of one session of the sleep/supportive care treatment. Analyses assessing post-PE predictors of full treatment completion revealed that in combination, a suite of sleep predictors (diary-based total sleep time, night-to-night variability of total sleep time, night-to-night variability of SE, and ISI) predicted full treatment completion; Χ2(5) = 19.31, p = .001. Night-to-night variability in total sleep time uniquely predicted completion such that the more consistent one’s total sleep time, the more likely one was to complete all phases of treatment.
Discussion
The present study examined the impact of PE on well-validated subjective measures of sleep, and then piloted the potential benefit of adding sleep-specific treatments (i.e., Imagery Rehearsal Therapy [IRT] and Cognitive Behavioural Therapy for Insomnia [CBT-I]) following PE to explore if sleep interventions would enhance night-time and/or daytime treatment outcomes. As expected, PE was efficacious for improving day but not night-time PTSD symptoms, whereas a combination of PE and sleep-specific treatment showed preliminary evidence of being efficacious at improving both outcomes. Thus, this study adds to the evidence that a comprehensive approach to PTSD treatment should address both night-time and daytime symptoms of the disorder.
Prolonged exposure improves day but not night-time symptoms of PTSD
PE was, as expected, effective for treating daytime symptoms, specifically global PTSD severity, quality of life, and depression (Table 2, Figure 2). However, PE did not significantly improve SE, and participants still experienced clinically significant sleep impairment in both SE and nightmare frequency. This replicates previous findings which showed sleep symptoms were residual post-PE (Gutner et al., 2013; Pruiskma et al., 2016; Zayfert & DeViva, 2004). Despite previous literature consistently demonstrating improved global PTSD symptoms with similar large effect sizes as the present data, sleep difficulties (self-reported duration or quality), were consistently maintained in the clinical range after behavioural treatment. Our findings of 75–80% of individuals falling within clinical insomnia/nightmare ranges echoed a recent report by Schnurr and Lunney (2018) showing over half their sample still reported sleep difficulties post-PE. Overall, the conclusion drawn is while PE is clearly effective for treating daytime symptoms of PTSD, it is insufficient for treating sleep-specific night-time symptoms.
Importantly, this finding also supports the notion of impaired sleep being a core feature of PTSD (Germain, 2013; Spoormaker & Montgomery, 2008). Historically, sleep disruption was considered a secondary symptom of the underlying disorder. If this were the case, sleep-related symptoms should be alleviated once the underlying disorder is treated. The fact this does not occur is concerning for two reasons: 1) sleep impairment is highly prevalent in patients diagnosed with PTSD (Germain, 2013); and 2) it suggests the gold standard PTSD treatments need to be augmented to provide maximal relief of the 24-hour symptom profile in PTSD.
PE with sleep-specific treatment improves night-time and daytime symptoms of PTSD
A critical finding of the second part of this study, representing a pilot study into efficacy of adding evidence-based sleep treatments onto PE, is that night-time and to a more modest extent, daytime symptoms of PTSD continued to improve among participants who received a sleep-specific therapy following PE (i.e., IRT + CBT-I; Figure 3). Specifically, the first sleep treatment, IRT, led to large improvements in nightmare frequency, as well as daily sleep diary sleep efficiency . This is consistent with other literature showing the efficacy of IRT (Forbes et al., 2003; Nappi et al., 2010). Following completion of CBT-I, participants demonstrated further improvement in night-time PTSD symptoms. Indeed, at the conclusion of all treatments, participants who received CBT-I did not, on average, meet clinical insomnia thresholds (i.e., SE > 85%). Participants who received SCT, on the other hand, had final scores well into the clinical range (SE = 74.6%). This demonstrates sleep disturbances do not resolve naturally with time. Additionally, effect sizes observed for SE (d = 1.07) and nightmare frequency (d = .90) are substantially larger than moderately sized sleep improvements previously recorded both in our study as well as previous research, over the course of PE or other gold-standard PTSD treatment (Gutner et al., 2013; Schnurr & Lunney, 2018). Moreover, CBT-I did not lead to further improvements in nightmare symptoms. Thus, these preliminary findings support including a combination of nightmare specific treatment with CBT-I in treatment of patients with PTSD (Germain, 2013).
Interestingly, the present data also reveal improvement in daytime symptoms following the combined sleep interventions. Specifically, we saw improvement in CAPS scores following sleep treatment, relative to SCT, when compared to scores post-PE (Table 3, Figure 3). The final assessment for the sleep treatment group was the only timepoint participants scored below the CAPS clinical cut-off. This finding illustrates the critical role sleep likely plays in maintenance of and recovery from PTSD. Sleep has a restorative function, is vital for emotional processing (Walker & van Der Helm, 2009), and relates to fear processes known to be abnormal in PTSD (Marshall, Acheson, Risbrough, Straus, & Drummond, 2014; Straus, Acheson, Risbrough, & Drummond, 2017). Given this, individuals with nightmares, insomnia and PTSD could be more vulnerable to trauma cues and negative affect, and thus more likely to engage in avoidance behaviours, compared with an equivalent well-rested individual with PTSD. Therefore, it is possible improvements in daytime symptoms of PTSD could be due to enhanced coping or improved extinction processes resulting from improved sleep. The relationship between disrupted sleep and impaired emotion regulation and fear processes also argues for studies examining whether treating sleep prior to daytime PTSD symptoms (i.e., reversing the order of treatment presentation from this study design) might also be valuable. While we elected to trial a protocol that most closely resembles a typical PTSD treatment pathway, it is certainly important to examine benefits of receiving sleep treatments first, followed by PE.
This study exclusively enrolled veterans of OEF/OIF/OND operations and generalization beyond that cohort should be done with caution, especially given study limitation described below. Speculatively, though, one would expect the results related to residual sleep symptoms after PE to apply to veterans of other eras and non-veterans, given the consistency of this finding in the literature . Results from the sleep interventions could be more mixed, though. CBT-I has proven efficacious in all age groups, as well as specifically in PTSD samples (Talbot et al., 2014), suggesting those findings would likely generalize widely. On the other hand, while IRT appears to be effective compared to no treatment or very non-specific controls in mixed eras of veterans (Nappi et al., 2010) and in civilians (Ellis, Rufino, & Nadorff, 2019), IRT was not at all effective in a large (n = 124) sample of Vietnam veterans, when compared to an active control condition (Cook et al., 2010). Thus, it is unclear to what extent those specific findings would generalize.
It is important to acknowledge limitations within this study. As is common in PTSD treatment studies, retention was challenging and attrition high: our drop-out from PE was 43% (n = 18). Attrition continued within this protocol after PE, throughout the sleep treatments and SCT. Our dropout during the second phase of this protocol was 30% (4 discontinued sleep treatments, 3 SCT). A primary reason for attrition of this sample was time commitment: this was a lengthy 18-week protocol. As life events arose throughout, participation waned. This resulted in the second aim of this study presenting a small sample size that must be considered only pilot data for this type of protocol. The study was designed to present each intervention in one of its most common, complete forms. In clinics, many may not choose to engage in an 18-week treatment and of those who do, many more may not make it through. The potential benefits we demonstrated however, highlight need to test ways to shorten the combined intervention. For example, Colvonen, Drummond, Angkaw, and Norman (2018) recently piloted a protocol where CBT-I and PE was delivered mostly simultaneously over 15 weeks. While this does not reduce the length much, it is a step in the right direction. Additionally, the final sample size led to limited statistical power for our analysis in Aim 2. A post hoc power analysis based on effect sizes observed in Aim 2 (change from Week 6 to Week 18), revealed we would have needed the following sample sizes to obtain statistical power at the .80 level: SE, n = 18, frequency of nightmares, n = 48. Current sample size affects generalisability of these findings, as does a lack of follow-up data. Finally, a key limitation of this study was data loss. A large amount of actigraphy data was lost due to technical problems and participant adherence. As a result, actigraphy sample size was lower than ideal and data not analysed as a primary outcome. Similarly, treatment fidelity ratings were lost, and thus we cannot confirm the extent to which treatments protocols were strictly followed. While we took several steps to maintain good fidelity (see Methods), poor fidelity would have reduced treatment effects and/or reduced differences between sleep interventions and SCT.
Overall, this study showed sleep did not improve in a clinically meaningful manner throughout PE. Treating night-time symptoms of PTSD with validated, targeted treatments improved symptoms more than SCT. Current findings coupled with other evidence suggest IRT and CBT-I lead to significant sleep improvement in patients with insomnia and PTSD, and argue that insomnia and nightmare symptoms should be specifically assessed following completion of PE. For those who experience residual sleep symptoms after PE, sleep treatments may be effective for improving both night-time and daytime symptoms.
Clinical Impact Statement.
Sleep problems, namely insomnia and nightmares, are a key part of Post-Traumatic Stress Disorder (PTSD). However current “gold standard” exposure treatments for PTSD do not fully resolve sleep problems. Here, we demonstrate that Prolonged Exposure reduced global PTSD symptoms but did not improve sleep. Subsequent evidence-based sleep interventions, Imagery Rehearsal Therapy and Cognitive Behavioural Therapy for Insomnia, improved night-time, with modest impact on daytime, symptoms. Results show need to specifically target both daytime and night-time PTSD symptoms for optimal clinical outcomes.
Acknowledgements
We would like to express our deep thanks to Jennifer Salamat and Kathy Resovsky for their work on recruitment, screening, and assessment on the project, as well as the veterans for their time and their service. We would like to thank the following individuals for serving as treatment fidelity rater for this study: Dr. Steven R. Thorp (PE), Dr. Michael L. Perlis (CBT-I), Dr. Richard J. Ross (IRT), Dr. John R. McQuaid (SCT).
Funding
This work was supported by the National Institute of Nursing Research 1-RC1-NR011728–01. EM Walters, J Clark and J Lies receive financial support from the Australian Government through Research Training Program (RTP) Scholarships. Funders played no role in design or implementation of the project, nor analysis or interpretation of the data.
Footnotes
Trial Registry
www.clinicaltrials.gov; Identifier: NCT01009112
Conflicts of Interest
The authors declare no conflicts of interest associated with this publication.
References
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, & Keane TM (1995). The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress, 8(1), 75–90. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Colvonen PJ, Drummond SPA, Angkaw AC, & Norman SB (2018). Piloting Cognitive-Behavioral Therapy for Insomnia Integrated With Prolonged Exposure. Psychological Trauma: Theory, Research, Practice, and Policy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JM, Harb GC, Gehrman PR, Cary MS, Gamble GM, Forbes D, & Ross RJ (2010). Imagery rehearsal for posttraumatic nightmares: a randomized controlled trial. Journal of Traumatic Stress, 23(5), 553–563. [DOI] [PubMed] [Google Scholar]
- Cox RC, & Olatunji BO (2016). A systematic review of sleep disturbance in anxiety and related disorders. Journal of Anxiety Disorders, 37, 104–129. [DOI] [PubMed] [Google Scholar]
- Edinger J, Kirby A, Lineberger M, Loiselle M, Wohlgemuth W, & Means M (2004). The duke structured interview for sleep disorders. Durham: University Medical Center. [Google Scholar]
- Ellis TE, Rufino KA, & Nadorff MR (2019). Treatment of nightmares in psychiatric inpatients with imagery rehearsal therapy: an open trial and case series. Behavioral sleep medicine, 17(2), 112–123. [DOI] [PubMed] [Google Scholar]
- Foa E, Hembree E, & Rothbaum B (2007). Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences therapist guide (Treatments that work) Oxford University Press; New York. [Google Scholar]
- Forbes D, Phelps AJ, McHugh AF, Debenham P, Hopwood M, & Creamer M (2003). Imagery Rehearsal in the Treatment of Posttraumatic Nightmares in Australian Veterans with Chronic Combat-Related PTSD: 12-Month Follow-Up Data. Journal of Traumatic Stress, 16(5), 509–513. [DOI] [PubMed] [Google Scholar]
- Galovski TE, Monson C, Bruce SE, & Resick PA (2009). Does cognitive-behavioral therapy for PTSD improve perceived health and sleep impairment? Journal of Traumatic Stress, 22(3), 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A (2013). Sleep disturbances as the hallmark of PTSD: Where are we now? American Journal of Psychiatry, 170(4),372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Hall M, Krakow B, Katherine Shear M, & Buysse DJ (2005). A brief sleep scale for posttraumatic stress disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. Journal of Anxiety Disorders,19(2),233–244. [DOI] [PubMed] [Google Scholar]
- Giosan C, Malta LS, Wyka K, Jayasinghe N, Evans S, Difede J, & Avram E (2015). Sleep Disturbance, Disability, and Posttraumatic Stress Disorder in Utility Workers. Journal of Clinical Psychology, 71(1), 72–84. [DOI] [PubMed] [Google Scholar]
- Gutner CA, Nillni YI, Suvak M, Wiltsey-Stirman S, & Resick PA (2013). Longitudinal course of anxiety sensitivity and PTSD symptoms in cognitive-behavioral therapies for PTSD. Journal of Anxiety Disorders, 27(7), 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, & Koffman RL (2004). Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine, 351(1), 13–22. [DOI] [PubMed] [Google Scholar]
- Hollis S, & Campbell F (1999). What is meant by intention to treat analysis? Survey of published randomised controlled trials. British Medical Journal, 319(7211), 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazis LE, Selim A, Rogers W, Ren XS, Lee A, & Miller DR (2006). Dissemination of methods and results from the Veterans Health Study: Final comments and implications for future monitoring strategies within and outside the Veterans healthcare system. Journal of Ambulatory Care Management, 29(4), 310–319. [DOI] [PubMed] [Google Scholar]
- Kessler RC (2000). Posttraumatic stress disorder: The burden to the individual and to society. Journal of Clinical Psychiatry, 61(SUPPL. 5), 4–12+13–14. [PubMed] [Google Scholar]
- Koffel E, Khawaja IS, & Germain A (2016). Sleep disturbances in posttraumatic stress disorder: Updated review and implications for treatment. Psychiatr Ann, 46(3), 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, & Spitzer RL (2002). The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann, 32(9), 509–515. [Google Scholar]
- Larsen SE, Fleming CJE, & Resick PA (2019). Residual symptoms following empirically supported treatment for PTSD. Psychological Trauma: Theory, Research, Practice, and Policy, 11(2), 207–215. [DOI] [PubMed] [Google Scholar]
- Maher MJ, Rego SA, & Asnis GM (2006). Sleep disturbances in patients with post-traumatic stress disorder: Epidemiology, impact and approaches to management. CNS Drugs, 20(7), 567–590. [DOI] [PubMed] [Google Scholar]
- Marshall AJ, Acheson DT, Risbrough VB, Straus LD, & Drummond SP (2014). Fear conditioning, safety learning, and sleep in humans. J Neurosci, 34(35), 11754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM (1993). Insomnia: Psychological assessment and management. New York: Guildford Press. [Google Scholar]
- Nappi CM, Drummond SP, & Hall JM (2012). Treating nightmares and insomnia in posttraumatic stress disorder: a review of current evidence. Neuropharmacology, 62(2), 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi CM, Drummond SP, Thorp SR, & McQuaid JR (2010). Effectiveness of imagery rehearsal therapy for the treatment of combat-related nightmares in veterans. Behav Ther, 41(2), 237–244. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Benson-Jungquist C, Smith MT, & Posner DA (2005). Cognitive behavioral treatment of insomnia: A session-by-session guide.
- Pruiksma KE, Taylor DJ, Wachen JS, Mintz J, Young-McCaughan S, Peterson AL, … Resick PA (2016). Residual sleep disturbances following PTSD treatment in active duty military personnel. Psychological Trauma: Theory, Research, Practice, and Policy, 8(6), 697–701. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, & Lunney CA (2018). Frontline Science: Residual symptoms following prolonged exposure and present-centered therapy for PTSD in female veterans and soldiers. Depress Anxiety. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, & Montgomery P (2008). Disturbed sleep in post-traumatic stress disorder: Secondary symptom or core feature? Sleep Medicine Reviews, 12(3), 169–184. [DOI] [PubMed] [Google Scholar]
- Straus LD, Acheson DT, Risbrough VB, & Drummond SPA (2017). Sleep deprivation disrupts recall of conditioned fear extinction. Biol Psychiatry Cogn Neurosci Neuroimaging, 2(2),123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus LD, Drummond SPA, Nappi CM, Jenkins MM, & Norman SB (2015). Sleep Variability in Military-Related PTSD: A Comparison to Primary Insomnia and Healthy Controls. Journal of Traumatic Stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot LS, Maguen S, Metzler TJ, Schmitz M, McCaslin SE, Richards A, … Neylan TC (2014). Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: A randomized controlled trial. Sleep, 37(2), 327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, & van Der Helm E (2009). Overnight therapy? The role of sleep in emotional brain processing. Psychological Bulletin, 135(5), 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins KC, Lang AJ, & Norman SB (2011). Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety, 28(7), 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayfert C, & De Viva JC (2004). Residual Insomnia Following Cognitive Behavioral Therapy for PTSD. Journal of Traumatic Stress, 17(1), 69–73. [DOI] [PubMed] [Google Scholar]