Summary
Background
Men who have sex with men (MSM) face a 28-fold higher risk of HIV acquisition than men who have sex with women (MSW). Condoms are the most accessible prevention method, with billions produced annually. Due to potentially high clinical failure, international regulatory agencies do not approve condoms for anal sex. This trial sought to provide data regarding approval of condoms for anal sex.
Methods
We conducted a blinded, crossover randomized trial among MSM and MSW in Atlanta, Georgia, USA. Crossover conditions were standard condoms, thin condoms, and condoms fitted to each user's penile dimensions. The primary outcome was total clinical failure (slippage and/or breakage), assessed using an intention-to-treat analysis. A mixed methods model assessed differences in odds of failure. The study is registered with ClinicalTrials.gov, NCT02753842, and is completed.
Findings
We enrolled 252 MSM and 252 MSW between May 19, 2016 and May 2, 2017. Participants reported a total of 4884 anal or vaginal sex acts using study-provided condoms. For all crossover conditions, clinical failure was lower for anal sex (0•7%, 16/2351) than for vaginal sex (1•9%, 48/2533), (odds ratio 0•40, 95% confidence interval 0•21, 0•75, p < •001)00. There was no difference in odds of failure for anal sex acts between the different types of condoms. Due to study design, nearly all anal sex acts used condom-compatible lubricant (98•3%), yet only a minority of vaginal sex acts (41•6%) used lubricant. Sex acts for which lubricant was used had lower failure for both anal and vaginal sex, with no difference in odds of failure between them.
Interpretation
In the largest trial of effectiveness of condoms for anal sex to date, we found remarkably low levels of failure. Condoms should be approved by regulatory agencies for anal sex. Clinicians may recommend condoms as a highly efficacious HIV and STD prevention tool for anal sex. Differences between failure for anal and vaginal sex were likely due to differential use of lubricant. Condom promotion programs should consider providing additional lubricant for all condoms distributed.
Research in context
Evidence before this study
Despite representing a fraction of most populations, men who have sex with men comprise a majority of new HIV transmissions in many countries. Male condoms are the most accessible and widely used prophylactic to prevent transmission, yet regulatory agencies have not label indicated condoms for anal sex. We searched for English-language publications using the PubMed database, on May 22, 2019, for trials assessing condom failure for anal sex with terms “condom,” “trial,” “anal,” and “failure” in any field and any year. Among 8 articles identified, 1 was a trial with anal sex failure as a primary outcome.
This study found clinical failure to be 6.5% using a paper diary method, a level higher than the 5% threshold regulatory agencies have used to approve condoms for vaginal sex. In the past 40 years, over 300 condoms have been label indicated by the United States Food and Drug administration (FDA) based on vaginal sex data; none have been FDA indicated based on anal sex data or labeled for anal sex use.
Added value of this study
This randomized crossover trial, the largest to date, sought to pursue a label indication for different types of condoms, including standard, thin, and fitted, for anal sex by using mobile-optimized daily diaries to record condom clinical failure outcomes. It showed low clinical failure for anal sex, with each type of condom in the study failing less than 1%. This level was significantly lower than clinical failure levels for vaginal sex. Lubricant use in the study was higher for anal sex than vaginal sex; a secondary analysis only considering sex events in which lubricant was used revealed no difference in condom failure levels for anal sex compared to vaginal sex.
Implications of all the available evidence
We anticipate that our findings will allow for international regulatory agencies to provide a label indication for each of these types of condoms for anal sex. For persons considering condom use, confidence in condoms is essential. In one study, men who have sex with men reported higher willingness to use condoms if the condoms had a label indication for anal sex. We observed very low failure levels when additional lubricant was used for sex events. This raises the question of whether all public health provision of condoms should be accompanied with lubricant. The high efficacy of condoms for anal and vaginal sex indicates their continued relevance for HIV and STD prevention.
1. Introduction
Worldwide, men who have sex with men (MSM) bear a high burden of HIV, with estimated regional prevalence ranging from 3.0% to 25.4% [1]. The use of HIV pre-exposure prophylaxis (PrEP) and condoms to address the co-occurring epidemics of HIV and sexually transmitted diseases (STD) is increasingly recommended. Modeling supports expanded provision of packages of HIV prevention services, indicating that moderate increases in both PrEP and condom use would be complementary rather than duplicative [2]. Condoms are a key HIV prevention tool because many men need protection from HIV and STD but are not indicated for PrEP [3]. Moreover, PrEP is not universally available or accepted. Of the 193 UN member nations, only 38 have approved PrEP. In the US, where emtricitabine/tenofovir for PrEP has been label-indicated since 2012, an estimated 814,000 out of 3,295,000 sexually active MSM in 2015 were indicated for PrEP [3], yet only an estimated 100,000–200,000 individuals had PrEP prescriptions in 2017 [4,5]. Condoms are recommended for use by all sexually active MSM. For MSM on PrEP, concurrent condom use is recommended to prevent the spread of STD and for all others, condoms are the primary prophylactic for HIV and STD.
Estimates of the performance of condoms for anal sex vary widely depending on study design. In two secondary analyses, the estimated range for the reduction in HIV transmission that results from condom use for anal sex was 63–91% [6,7]. Both studies relied on lengthy recall periods to determine condom use or nonuse. Studies assessing per-act total clinical failure (breakage or slippage) found condom failure for anal sex ranging from 1.8% to 8.0%, with median 3•4% [[8], [9], [10], [11], [12], [13], [14]]. The median recall period for these studies was 6 months. Two studies with diary-based assessments of per-act clinical failure, recorded with pen and paper at the event-level, found total clinical failure for anal sex of 6.5% and 6.9% [15,16].
Due in part to inconsistent estimates and lack of data with minimal recall bias, condoms are not currently label indicated for anal sex by regulatory agencies, including the US Food and Drug Administration (FDA). ISO guidance, which is used by many countries to inform their clearance of condoms, notes that testing of condoms should include only vaginal sex use, explicitly excluding anal sex [17]. This may be because of a perception, as noted by FDA, that, “condoms may be more likely to break during anal intercourse than during other types of sex.”[18] A search of the FDA approval database reveals that in the past 40 years, over 300 condoms have been label indicated by FDA based on vaginal sex data. No condom has been label indicated based on anal sex data, or labeled for anal sex use, despite anal sex being the predominant mode of HIV transmission among MSM and being increasingly common among heterosexual couples [19].
To clarify the performance of condoms for anal sex and to provide data to inform consideration of indication of condoms for anal sex, we conducted a study that differed in three ways from its predecessors. First, measures were obtained on a daily basis rather than requiring accurate memory of condom use over longer time periods; memory for specific experiences becomes increasingly inaccurate with the passage of time [20]. Another difference was that this was the first study to pursue a label indication for anal sex for condoms. A third difference was that the study was larger than previous clinical trials of condoms for anal and vaginal sex. The study had two a priori hypotheses regarding clinical failure (NCT02753842): (1) the rate of clinical failure for anal sex among MSM would be lower than a threshold to be determined by FDA for fitted, thin, and standard condoms and (2) fitted condoms would improve anal sex clinical failure performance relative to standard condoms.
2. Methods
2.1. Study design
From May 19, 2016 to May 2, 2017, we enrolled 504 participants in the crossover trial: 252 MSM and 252 men who have sex with women (MSW). A crossover design is recommended by FDA for condom clinical failure trials, likely due to the limited potential for carryover effects across different types of condoms. The study was conducted in Atlanta, GA, USA at several sites of the Rollins School of Public Health at Emory University. The study was approved by the Emory University Institutional Review Board, IRB00083754 and adheres to CONSORT reporting guidance. The trial protocol has been published previously [21].
2.2. Participants
The full eligibility assessment with 27 criteria is reported elsewhere [21], and includes male sex at birth, recent insertive sex, age 18–54, being HIV-negative at a baseline test, not using condoms for contraception, and willing to complete all study procedures, including using condoms and lubricant. Recruitment was conducted through a variety of sources including flyers, posters, website banner ads, app-based ads, and social media. To maximize the separation of study arms, MSM that reported recent vaginal sex or MSW that reported recent anal sex were ineligible. Individuals testing HIV positive were provided linkage to care services. The eligibility assessment also included home measurement of fitted condom size, accomplished with a paper template to indicate condom size. All study participants completed an informed consent procedure.
2.3. Randomization and masking
We used permuted block randomization, conducted on a clinical data management system, to determine the order in which the condom sets were distributed. Six crossover orders were used to balance allocation of conditions. More detail on randomization is available in the study protocol [22]. In the double-blind experiment, study condoms in plain foil were provided to participants, with the only identifying information being a two-digit code on the foil. Study staff were blinded based on roles, with the study statistician and Principal Investigators blinded until after completion of primary analyses.
2.4. Procedures
The study design was informed by a pre-submission meeting with FDA. The full study design, as well as power calculations, have been described previously [21]. In brief, over a series of study visits, participants received five fitted condoms, five thin condoms, and five standard condoms. Participants had up to four weeks to use all five condoms of a particular type before being crossed over into the next condition (condom type). Participants using all condoms in a set within two weeks were crossed over to receive the next set of study condoms, so participants were enrolled for a minimum of 6 weeks and a maximum of 12 weeks. Participants were provided up to $50 incentive for each study visit.
Participants completed surveys at biweekly study visits. An electronic daily coital diary was used to track sexual activity between visits. Participants received a daily reminder and incentives to encourage completion. Incentives were provided when participants completed the first question of the diary, specifying their daily sexual activity (yes/no). To avoid bias, we provided incentives for any response, including those indicating no sex. Similarly, participants were incentivized to attend study visits but not incentivized for the use of study condoms. The daily sex diary tracked whether participants had sex on a given day, the type of sex, lubricant use, condom use, condom clinical failure, use of drugs or alcohol immediately preceding or during sex, and errors in condom application. Participants were trained in required components of the study: completion of coital diaries, determination of fitted condom size, and proper condom use. Training, based on WHO guidance [21,22], included instructions for all MSM to use lubricant with all anal sex acts, and for MSW to use lubricant as needed or desired.
All condoms produced for the study were manufactured from latex and had FDA 510(k) clearance. Standard condoms were 185 ± 10 mm length, 53 ± 2 mm width, and 70 ± 10 µm thick. These dimensions represent those for condoms commonly sourced by the United Nations Population Fund (UNFPA) [21]. Thin condom were of identical width and length to “standard”, but 50 ± 5 µm thick. Fitted condoms were produced in a range of 56 sizes, with 70 ± 10 µm thick. Participants’ fitted size was determined by their use of a fitting system consisting of a paper template graduated with non-sequential numbering and lettering. Packets of 10 ml water-based, condom-compatible lubricant were distributed with the condoms.
2.5. Outcomes
Primary outcomes for the study relating to clinical failure were assessed through the daily sex diary. Clinical failure was assessed using standard ISO measures for clinical slippage, clinical breakage, and total clinical failure (slippage and/or breakage) [23], and follows FDA guidance regarding outcome reporting [24]. Event-level correlates of condom failure, such as errors in condom use, were also developed from ISO measures. We used an intention-to-treat analysis that included any anal sex acts among MSM and any vaginal sex acts among MSW in which a study condom was used. We conducted a secondary, per-protocol analysis that used the above criteria but excluded sex acts for which lubricant was used incorrectly: lubricant inside of the condom, use of condom incompatible lubricant, or (for the MSM arm only) not using any lubricant. No substantial differences were found between the intention-to-treat and per-protocol analyses; see Appendix A for per protocol analyses.
Baseline study measures were developed from existing questionnaires, and included sexual history, past sexual dysfunction, and condom use experiences and perceptions. The full baseline instrument has been previously published [21]. Condom use self-efficacy was assessed based on a previously validated instrument, with data dichotomized due to a skewed distribution. Potential covariates for failure, such as circumcision or erectile function, were measured with previously validated measures. To facilitate interpretation of results, we categorized age. Sensitivity analyses found that differences in categorization of age, as well as treating age continuously, provided analogous results. Income, condom width, and condom length were collected categorically; to address data sparsity, some categories of these variables were combined. HIV status was determined with the INSTITM HIV-1/HIV-2 rapid antibody test.
2.6. Statistical analysis
Descriptive data analyses (counts and percentages) were used to construct Table 1 and Fig. 1, Fig. 2. Table 2 displays two logistic mixed effects models to compare condom failure performance, taking into account the crossover study design that led to repeated measures for each participant. The primary analysis adjusted for study design variables: random effects for person nested within randomization block and adjusted for arm (anal versus vaginal sex), condom randomization order (first, second, third), and condom type (fitted, thin, standard). The secondary analysis adjusted for all study design variables and a set of covariates. Covariates were divided into domains (demographics, biological, condom use) and domains with p < 0•1 association with clinical failure were considered for inclusion in the final model. Backwards selection with p < 0•05 reduced covariates in the final model to study design variables and individual covariates significantly associated with clinical failure. Model fit was assessed through generalized chi-square. Model-based estimates and confidence intervals of the odds of failure were used to compare clinical failure performance, according to study aims. A priori power calculations determined a sample size of 504 participants to assess study hypotheses regarding clinical failure, assuming 80% power, 80% retention in the study, and α = 0•05; further details have been published previously [21]. All analyses were performed in SAS v9•4. The study is registered with ClinicalTrials.gov, NCT02753842.
Table 1.
Condom failure by demographics, biological factors, condom use experience, and sexual event variables in a crossover trial of condoms for anal and vaginal sex, United States, 2016–2017.
| Clinical failure | Clinical breakage | Clinical slippage | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Demographics | |||
| Age | |||
| 18–24 | 26 (1.3%) | 19 (1.0%) | 7 (0.4%) |
| 25–39 | 26 (1.2%) | 13 (0.6%) | 13 (0.6%) |
| 40–54 | 12 (1.7%) | 3 (0.4%) | 9 (1.3%) |
| Race and ethnicity | |||
| Hispanic | 4 (0.6%) | 2 (0.3%) | 2 (0.3%) |
| White non-Hispanic | 30 (1.3%) | 17 (0.8%) | 13 (0.6%) |
| African-American non-Hispanic | 20 (1.5%) | 12 (0.9%) | 8 (0.6%) |
| Other non-Hispanic | 10 (1.5%) | 4 (0.6%) | 6 (0.9%) |
| Education | |||
| College, post graduate, or professional school | 34 (1.3%) | 15 (0.6%) | 19 (0.7%) |
| Some college, associate's degree, technical school | 17 (1.2%) | 9 (0.6%) | 8 (0.6%) |
| High school or GED, or less | 13 (1.6%) | 11 (1.4%) | 2 (0.3%) |
| Income | |||
| < $20,000 | 22 (1.6%) | 12 (0.9%) | 10 (0.7%) |
| $20,000–$29,999 | 7 (1.2%) | 0 (0.0%) | 7 (1.2%) |
| $30,000–$39,999 | 6 (1.4) | 3 (0.7%) | 3 (0.7%) |
| $40,000–$49,999 | 8 (1.9%) | 3 (0.7%) | 5 (1.2%) |
| >= $50,000 | 14 (0.8%) | 10 (0.6%) | 4 (0.2%) |
| Marital Status, current | |||
| Legally married or registered partnership/union | 6 (1.3%) | 0 (0.0%) | 6 (1.3%) |
| Divorced/Separated | 2 (1.1%) | 2 (1.1%) | 0 (0.0%) |
| Never married | 56 (1.3%) | 33 (0.8%) | 23 (0.5%) |
| Biological Factors | |||
| Circumcised | |||
| Circumcised (cut) | 52 (1.3%) | 28 (0.7%) | 24 (0.6%) |
| Uncircumcised (uncut) | 12 (1.4%) | 7 (0.8%) | 5 (0.6%) |
| Erectile function scale, with condom, past 6 months | |||
| No erectile dysfunction | 43 (1.2%) | 28 (0.8%) | 15 (0.4%) |
| Mild, moderate or severe erectile dysfunction | 16 (1.8%) | 5 (0.6%) | 11 (1.2%) |
| Missing | 5 (1.1%) | 2 (0.4%) | 3 (0.7%) |
| Penile dimensions | |||
| Fitted condom width | |||
| < 11.7 cm | 5 (0.6%) | 3 (0.4%) | 2 (0.3%) |
| 11.7–13.49 cm | 27 (1.0%) | 13 (0.5%) | 14 (0.5%) |
| >= 13.5 cm | 32 (2.3%) | 19 (1.3%) | 13 (0.9%) |
| Fitted condom length | |||
| < 12.2 cm | 1 (0.7%) | 1 (0.7%) | 0 (0.0%) |
| 12.2–14.19 cm | 14 (1.3%) | 6 (0.5%) | 8 (0.7%) |
| 14.2–16.69 cm | 29 (1.4%) | 17 (0.8%) | 12 (0.6%) |
| >= 16.7 cm | 20 (1.3%) | 11 (0.7%) | 9 (0.6%) |
| Condom use experiences | |||
| Used a condom for insertive sex, past 30 days | |||
| Yes | 47 (1.2%) | 26 (0.7%) | 21 (0.5%) |
| No | 17 (2.2%) | 9 (1.2%) | 8 (1.1%) |
| Missing | |||
| Removed condom before finishing sex, past 6 months | |||
| Yes | 25 (1.8%) | 12 (0.9%) | 13 (0.9%) |
| No | 34 (1.1%) | 21 (0.7%) | 13 (0.4%) |
| Missing | 5 (1.1%) | 2 (0.4%) | 3 (0.7%) |
| Condom broke, slipped, or both during sex, past 6 months | |||
| Yes | 35 (2.1%) | 21 (1.3%) | 14 (0.9%) |
| No | 24 (0.9%) | 12 (0.4%) | 12 (0.4%) |
| Missing | 5 (1.1%) | 2 (0.4%) | 3 (0.7%) |
| Condom self efficacy score | |||
| Scored below 16 | 36 (1.6%) | 18 (0.8%) | 18 (0.8%) |
| Scored 16 | 28 (1.1%) | 17 (0.7%) | 11 (0.4%) |
| Sexual-event variables from daily coital logs | |||
| Lubricant type | |||
| Condom compatible lubricant | 28 (0.8%) | 13 (0.4%) | 15 (0.4%) |
| No lubricant* | 34 (2.3%) | 20 (1.3%) | 14 (0.9%) |
| Non-condom compatible lubricants | 2 (12.5%) | 2 (12.5%) | 0 (0.0%) |
| Incorrect condom use⁎⁎ | |||
| Yes | 9 (3.7%) | 6 (2.5%) | 3 (1.2%) |
| No | 55 (1.2%) | 29 (0.6%) | 26 (0.6%) |
| Alcohol or drug use before or during sex act | |||
| Yes | 11 (2.3%) | 6 (1.2%) | 5 (1.0%) |
| No | 53 (1.2%) | 29 (0.7%) | 24 (0.5%) |
Use of saliva only was classified as 'no lubricant'.
Using a single study condom for multiple sex acts (e.g. for oral and anal sex) or placing lubricant inside the study condom were considered to be incorrect use.
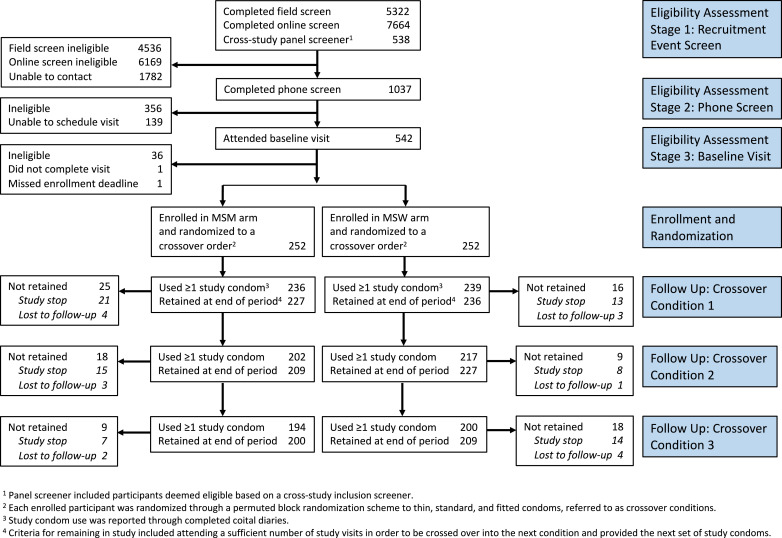
Fig. 1.
Study Flow-Chart.
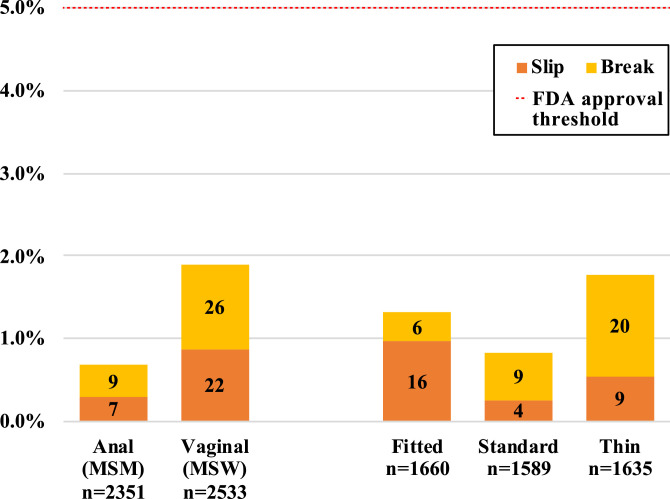
Fig. 2.
Condom Failure by Type of Sex and Study Arm, intention-to-treat analysis.
Table 2.
Logistic mixed effects models of study design and potential confounders predicting condom failure in a crossover trial of condoms for anal and vaginal sex, United States, 2016–2017.
| Primary analysis modela | Secondary analysis (covariate adjusted) modela | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Type of sex | ||||
| Anal (MSM)b | 0.40 | 0.21, 0.75 | 0.37 | 0.19, 0.72 |
| Vaginal (MSW)c | ref | ref | ||
| Condom Type | ||||
| Fitted | 1.67 | 0.83, 3.38 | 1.57 | 0.75, 3.29 |
| Thin | 2.17 | 1.11, 4.25 | 2.25 | 1.12, 4.51 |
| Standard | ref | ref | ||
| Baseline characteristics | ||||
| Condom failure, past 6 months | na | na | 2.27 | 1.07, 5.22 |
| No condom use, past 30 days | na | na | 2.36 | 1.07, 5.22 |
| Fitted condom width | ||||
| Large (>13.5 cm) | na | na | 3.97 | 1.28, 12.29 |
| Medium (11.7–13.5 cm) | na | na | 1.74 | 0.565, 5.39 |
| Small (<11.7 cm) | na | na | ref | |
| Sexual event characteristics | ||||
| Incorrect lubricant use during sex | na | na | 7.00 | 1.21, 40.65 |
Note: This table is based on an intent-to-treat analysis.
The primary (a priori) analysis controlled only for study design variables: type of sex, condom type, the randomized order in which condoms were received, and repeated measures on individuals. The secondary analysis also adjusted for significant covariates (condom failure, condom use, fitted condom width, incorrect lubricant use).
Anal sex for MSM only, per study design. MSW reported 7 anal-only sex acts with 1 clinical failure. These acts were excluded from this intent-to-treat analysis. Sensitivity analyses indicate this exclusion did not impact study conclusions.
Vaginal sex for MSW only, per study design. No MSM reported vaginal sex. MSW reported 14 instances of vaginal and anal sex in the same act with 1 clinical failure. These acts were included in the vaginal sex arm for these intent-to-treat analyses. Per protocol analyses excluding these acts can be found in Appendix A.
2.7. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
A total of 1037 individuals completed a phone screen to determine eligibility, 504 were enrolled in the study and received a randomized crossover condition order, and 409 (81.1%) of these were retained until the end of the study period. A full flowchart detailing participant recruitment and retention can be seen in Fig. 1. Among all enrolled participants in each arm, 200 MSM (79.4%) and 209 MSW (82.9%) were retained throughout the study period. Of the total enrolled per arm, 43 MSM (17.1%) and 35 MSW (13.9%) were study stopped, and 9 MSM (3.6%) and 8 MSW (3.2%) were lost to follow-up. Among MSM, the most common reasons for study stopping were voluntary withdrawal (n = 27), new HIV-positive partner [6], STI symptoms or recent diagnosis [5], and moving out of the study area [5]. Among MSW, the most common reasons for study stopping were voluntary withdrawal [21] and moving out of the study area [10].
Demographic and baseline data, broken down by MSM and MSW arms, are reported elsewhere [21]. In brief, the study sample was predominantly single (87.3% never married), identified as homosexual for MSM (90.5%) or heterosexual for MSW (97.2%,), and diverse (47.8% White non-Hispanic, 12.3% Hispanic, 26.0% African American non-Hispanic, and 13.7% other, non-Hispanic). Annual earnings varied considerably: ≥$50,000 (37.2%), $20,000 to $49,999 (31.6%), and <$20,000 (31.2%). Nearly three-quarters (74.0%) of participants in both arms rated themselves as “very experienced” using condoms. Despite this experience, 36.3% of MSM and 38.1% of MSW reported condom slippage, condom breakage or both in the past 6 months. MSM and MSW were similar in terms of race/ethnicity, income, marital status, homelessness, and variables relating to condom use including recent condom use, condom self-efficacy, recent condom errors, and recent condom failure. Likely an artifact of higher levels of MSW recruitment on college campuses relative to MSM, there were demographic differences between the study arms. More MSW were students (53% versus 11%), aged 18–24 (60% versus 25%), and had only achieved high school or less education (22% versus 8%).
Table 1 displays the proportion of sex acts in which study condoms were used and clinical failure occurred, by demographics, biological factors, past condom use experiences, and experiences during the sexual event. Overall, study condom total clinical failure (slippage, breakage, or slippage and breakage) occurred in 1.3% (64/4884) of sex acts. Total clinical failure proportions were low across a broad range of baseline variables, ranging from 0.6% to 2.3%. More variability was observed in sexual-event level measures. For sex in which non-condom compatible lubricant (e.g. oil-based lubricant) was used, failure occurred 2/16 times (12.5%). For sexual events in which condoms were used incorrectly, condoms failed 9/242 times (3.7%). There were no cases of clinical slippage and clinical breakage in the same sex act. MSM reported no vaginal sex acts with a study condom. MSW reported 7 anal-only sex acts with 1 clinical failure, which were excluded from intent-to-treat analyses presented in Table 1 based on our intent to maximize separation of study arms. Moreover, MSW reported 14 instances of anal and vaginal sex in the same act with 1 failure, which were included in the vaginal sex arm for intention-to-treat analyses. Per protocol analyses that excluded all anal sex acts reported by MSW (Appendix A) were not substantially different than intention-to-treat analyses.
Fig. 2 shows failure levels by study design variables of type of sex and type of condom. For anal sex, there were 16/2351 (0.7%) condom failures, with failure levels ranging across the three types of condoms from 0.62% to 0.76%. For vaginal sex, there were 48/2533 (1.9%) condom failures, with failure levels ranging across the three types of condoms from 0.95% to 2.72%. Based on these low failure levels, the study supports the a priori hypothesis that condoms fail less than an acceptable threshold for anal sex. The exact level of acceptable failure remains to be determined by FDA, but for vaginal sex, condoms have been previously cleared based on a clinical failure threshold of <5.0%.
Table 2 reports the results from the logistic mixed effects models for total clinical condom failure. The primary analysis model (PA) adjusts for study design variables and a secondary analysis (SA) model adjusts for these and other variables expected to be associated with condom failure. In both models, anal sex was associated with lower failure relative to vaginal sex (PA OR: 0.40, 95%CI: 0•21, 0.75; SA OR: 0.37, 95%CI 0.19, 0.72). Fitted condoms did not have different levels of failure than standard condoms for vaginal and anal acts (PA 1.67, 95% CI 0.83–3.83) or for anal sex acts only (PA OR: 0.93, 95% CI: 0.27, 3.26), suggesting rejection of the a priori hypothesis that fitted condoms would fail at lower rates than standard condoms for anal sex. Thin condoms were associated with higher failure rates in both models relative to standard condoms (PA OR: 2.17, 95%CI: 1.11, 4.25; SA OR: 2.25, 95%CI 1.12, 4.51). In the SA model, higher levels of clinical failure were associated with baseline characteristics of having experienced condom failure in the previous 6 months, not having used condoms in the 30 days prior to study initiation, and having larger self-measured condom width. Among variables assessed after each sex event, using condom lubricant incorrectly was associated with higher odds of failure.
Due to study design, nearly all anal sex acts included condom-compatible lubricant (2307/2347, 98.3%) and a minority of vaginal sex acts included condom-compatible lubricant (1053/253, 41.6%). A post-hoc analysis among vaginal sex acts for which study or other condom-compatible lubricant was used indicated clinical failure of 1.1% (12/1053) for these acts (data not displayed in tables). Conversely, vaginal sex acts with either nonuse of lubricant or use of condom-incompatible lubricant had failure rates of 2.5% (36/1467). When controlling for use of condom-compatible lubricant, there was no difference in the odds of failure for anal versus vaginal sex (OR 0.83 95% CI −0•3, 2.2, p = 0.70).
4. Discussion
In the largest clinical trial of condoms for anal sex to date, total clinical failure for anal sex was less than 1% for fitted, thin, and standard condoms. Previously, regulatory agencies have approved non-inferiority applications for vaginal sex based on a less than 5% failure rate [21,25]. We therefore anticipate that our findings will allow for international regulatory agencies to provide a label indication for each of these types of condoms for anal sex. Our previous survey found that 69% of MSM reported they would be more likely to use condoms more frequently if the condoms were FDA label-indicated for anal sex, so a label indication may provide public health utility [26]. A label indication could have utility for women as well, and we see no reason to anticipate biological differences in failure levels for anal sex due to the sex (male or female) of the receptive partner. A label indication will clarify the high efficacy of condoms for anal sex when used properly, potentially promoting their use in combination prevention strategies that encourage choice of efficacious interventions.
In our trial, clinical failure was significantly lower for anal sex than for vaginal sex. This confirmed our hypothesis that condoms would fail at an acceptable level for anal sex, but was contrary to our expectation that condoms might fail more often for anal sex. In multivariate analyses that controlled for potential confounders such as past failure experiences, the effect of lower failure for anal sex remained significant. This finding is contrary to previous studies that found higher failure for anal sex than for vaginal sex.
One likely reason for the difference between the present and previous results involves provision of lubricant. The FDA website notes that condoms may fail more for anal sex than for vaginal sex due to higher levels of friction [18]. We provided all study participants with water-based, condom-compatible study lubricant, which should reduce friction and stress on the condom. MSM were instructed to always use study lubricant, and MSW were instructed to use study lubricant as needed or preferred, per WHO guidance [21, 22]. Differential lubricant use could account for differences in clinical failure rates: post hoc analyses indicated no difference in failure between anal and vaginal sex acts for which condom-compatible lubricant was used. Similarly, previous studies have found additional lubricant use to be associated with lower condom failure for vaginal sex [27,28]. Taken together, these findings raise the question of whether the billions of condoms distributed as part of HIV and STD prevention efforts should be accompanied by lubricant to minimize potential failure. Additional research should be conducted to determine whether such an approach is merited, potentially including studies to explore how additional lubricant influences clinical failure for vaginal sex and how distribution of additional lubricant with condoms for vaginal sex would be perceived by stakeholders and condom users.
Another likely reason for differences between the present and previous studies is improved measurement of clinical failure. The majority of previous studies used recall periods of 1–6 months [[8], [9], [10], [11], [12], [13], [14]], with a minority using paper forms collected at two-week intervals [15,16]. By providing participants with a convenient mobile phone-based daily diary, outcomes were reported shortly following sex acts. More immediate reporting of sexual event data improves the quality of reporting by minimizing recall bias [20]. Our incentive system for attending study visits and completing diary entries also supported better measurement by encouraging timely reporting and removing incentivizes to fabricate data. Equal incentives were provided regardless of whether study condom use was reported; in fact, some participants remained in the study for multiple periods without any reports of condom use (while still receiving study incentives). In contrast, some previous studies provided additional incentives for each condom outcome reported, or required outcome reporting prior to distribution of the next study incentive [15,29]. Such systems could introduce bias if participants fabricated sex events to maximize their incentive, a particularly problematic issue given that MSM over-estimate the frequency of condom failure for anal sex [26)]
The present study has a number of strengths, including large sample size, use of standard measures, regulation-compliant data systems, and use of incentives, item design, and crossover study design that sought to minimize bias. This study also has a number of limitations. Study outcomes are limited to self-report. This concern is partially mitigated because the outcome measure of condom failure demonstrated validity. Concurrent validity is observed in the association of sex-event level variables such as use of condom-incompatible lubricant being associated with higher condom failure. Moreover, predictive validity is observed in associations of baseline variables such as recent use of condoms with lower condom failure in the study period.
By design and based on WHO guidance, MSM received instructions to use lubricant for every sex act and MSW received instructions to use lubricant as needed or preferred. This resulted in highly differential lubricant use. Our secondary analysis indicates that if levels of lubricant use were equal between the two arms, there may have been no finding of different levels of failure between anal and vaginal sex. FDA guidance for clinical trials of condoms led to participant training and strict inclusion criteria (e.g. no genital piercings) that make this trial an assessment of condom efficacy, rather than real-world condom effectiveness.
Overall, condoms performed well across all types of sex, bringing to mind the truism that the predominant reason for condom failure is nonuse [30]. For those considering condom use, confidence in condoms is essential. MSM at-risk for HIV transmission have reported higher willingness to use condoms for anal sex if they carried an FDA label indication for that purpose [26]. The high protection against disease transmission indicated by the extremely low condom failure rates for anal sex in this optimal use study may be similar to the extremely low PrEP failure rates seen in prior optimal use studies, with both having <1% failure. The high efficacy of condoms indicates their continued relevance for HIV and STD prevention.
Data sharing
Deidentified data and explanatory documentation will be shared based on written request, and after review and approval of the lead investigators (AJS, PSS, MPC, and EMR). Access will be granted after approval by the lead investigators of an analysis proposal and execution of a signed data sharing agreement. The study protocol, and relevant documentation such as study measurement instruments and detailed procedures, have been previously published [21].
Funding
NICHD, R44HD078154.
CRediT authorship contribution statement
Aaron J. Siegler: Conceptualization, Writing - original draft, Writing - review & editing. Elizabeth M. Rosenthal: Project administration, Data curation, Writing - review & editing. Patrick S. Sullivan: Conceptualization, Writing - review & editing. C. Christina Mehta: Data curation, Formal analysis, Writing - review & editing. Reneé H. Moore: Data curation, Formal analysis, Writing - review & editing. Lauren Ahlschlager: Writing - review & editing. Colleen F. Kelley: Writing - review & editing. Eli S. Rosenberg: Writing - review & editing. Michael P. Cecil: Conceptualization, Writing - review & editing.
Declaration of Competing Interests
AJS, EMR, PSS, LA, CFK, CCM, RHM, and ESR have no conflicts of interest to declare. MPC is the owner of TheyFit LLC. On January 26, 2016, TheyFit LLC sold all assets pertaining to the study aims including trademarks, intellectual property, inventory, website, and regulatory approvals to Karex Berhad. MPC has no financial interest in Karex Berhad. Karex Berhad manufactured condoms for the study, but had no input regarding trial design, had no access to trial data, and had no access to or input regarding this manuscript.
Acknowledgements
We appreciate and acknowledge the contributions of our study participants. We want to acknowledge the excellent and dedicated work of many research staff on the project, especially Elana Wilder Spaulding and Jessica Swiniarski. Our sincere thanks to Karex Berhad for manufacturing condoms for this study. Thanks also to our clinical laboratory partner, the Emory Center for AIDS Research Clinical Virology Laboratory, led by Dr Colleen Kraft. This study was funded by a Small Business Innovative Research grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R44HD078154). The funder had no involvement with the study design, data collection, data analysis, data interpretation or writing of the manuscript. The study was jointly conceptualized by Emory and TheyFit, informed by a pre-submission discussion with the FDA. Emory investigators held the final decision-making authority in study design and conduct, and were solely responsible for data analysis. All authors had full access to study data, and AJS and MPC had final responsibility for the decision to submit for publication. The study was facilitated by the Center for AIDS Research at Emory University (P30AI050409). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Aaron J. Siegler, Email: asiegle@emory.edu.
Patrick S. Sullivan, Email: pssulli@emory.edu.
C. Christina Mehta, Email: christina.mehta@emory.edu.
Reneé H. Moore, Email: renee.moore@emory.edu.
Lauren Ahlschlager, Email: lauren_ahlschlager@med.unc.edu.
Colleen F. Kelley, Email: cfkelle@emory.edu.
Eli S. Rosenberg, Email: erosenberg2@albany.edu.
Michael P. Cecil, Email: mpcecil@earthlink.net.
Appendix A. Per protocol analysis of a logistic mixed effects models predicting condom failure in a crossover trial of condoms for anal and vaginal sex, United States, 2016–2017
| Primary analysis modela |
Secondary analysis (covariate adjusted) modela |
|||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Type of sex | ||||
| Anal (MSM)b | 0.34 | 0.17, 0.68 | 0.32 | 0.15, 0.67 |
| Vaginal (MSW)c | ref | ref | ||
| Condom type | ||||
| Fitted | 1.99 | 0.91, 4.35 | 1.76 | 0.78, 3.96 |
| Thin | 2.61 | 1.23, 5.55 | 2.42 | 1.12, 5.22 |
| Standard | ref | ref | ||
| Baseline characteristics | ||||
| Condom failure, past 6 months | 2.45 | 1.26, 4.78 | ||
| No condom use, past 30 days | 2.81 | 1.18, 6.66 | ||
| Fitted condom width | ||||
| Large (>13.5 cm) | 3.56 | 1.10, 11.56 | ||
| Medium (11.7 mm to 13.5 cm) | 1.49 | 0.46, 4.82 | ||
| Small (<11.7 cm) | ref | |||
| Sexual event characteristics | ||||
| Drug or alcohol use before/during sex | 2.23 | 1.02, 4.89 | ||
| Incorrect lubricant use during sex | nad | nad | ||
The primary (a priori) analysis controlled only for study design variables: type of sex, condom type, the randomized order in which condoms were received, and repeated measures on individuals. The secondary analysis also adjusted for significant covariates (condom failure, condom use, fitted condom width, drug or alcohol use before sex).
Anal sex for MSM only, per study design. MSW reported 7 anal-only sex acts with 1 clinical failure. These acts were excluded from this per protocol analysis. Sensitivity analyses indicate this exclusion did not impact study conclusions.
Vaginal sex for MSW only, per study design. No MSM reported vaginal sex. MSW reported 14 instances of vaginal and anal sex in the same act with 1 clinical failure. These acts were excluded from these per protocol analyses.
Not applicable because instances of incorrect lubricant use were not eligible for the per-protocol analysis.
References
- 1.Beyrer C., Baral S.D., van Griensven F., Goodreau S.M., Chariyalertsak S., Wirtz A.L. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–377. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan P.S., Carballo-Dieguez A., Coates T., Goodreau S.M., McGowan I., Sanders E.J. Successes and challenges of HIV prevention in men who have sex with men. Lancet. 2012;380(9839):388–399. doi: 10.1016/S0140-6736(12)60955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith D.K., Van Handel M., Grey J. Estimates of adults with indications for HIV pre-exposure prophylaxis by jurisdiction, transmission risk group, and race/ethnicity, United States, 2015. Ann Epidemiol. 2018 doi: 10.1016/j.annepidem.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Siegler A.J., Mouhanna F., Giler R.M., Weiss K., Pembleton E., Guest J. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28(12):841–849. doi: 10.1016/j.annepidem.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan P.S., Mera Giler R., Mouhanna F., Pembleton E., Guest J., Jones J. Trends in active prescriptions of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis against hiv infections, United States, 2012–2016. Ann Epidemiol. 2018;28(12):833–840. doi: 10.1016/j.annepidem.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson W.D., O'Leary A., Flores S.A. Per-partner condom effectiveness against HIV for men who have sex with men. Aids. 2018;32(11):1499–1505. doi: 10.1097/QAD.0000000000001832. [DOI] [PubMed] [Google Scholar]
- 7.Smith D.K., Herbst J.H., Zhang X., Rose C.E. Condom effectiveness for HIV prevention by consistency of use among men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2015;68(3):337–344. doi: 10.1097/QAI.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 8.van Griensven G.J., de Vroome E.M., Tielman R.A., Coutinho R.A. Failure rate of condoms during anogenital intercourse in homosexual men. Genitourin Med. 1988;64(5):344–346. doi: 10.1136/sti.64.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golombok S., Sketchley J., Rust J. Condom failure among homosexual men. J Acquir Immune Defic Syndr. 1989;2(4):404–409. [PubMed] [Google Scholar]
- 10.Thompson J.L., Yager T.J., Martin J.L. Estimated condom failure and frequency of condom use among gay men. Am J Public Health. 1993;83(10):1409–1413. doi: 10.2105/ajph.83.10.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchbinder S.P., Douglas J.M., Jr., McKirnan D.J., Judson F.N., Katz M.H., MacQueen K.M. Feasibility of human immunodeficiency virus vaccine trials in homosexual men in the United States: risk behavior, seroincidence, and willingness to participate. J Infect Dis. 1996;174(5):954–961. doi: 10.1093/infdis/174.5.954. [DOI] [PubMed] [Google Scholar]
- 12.Stone E., Heagerty P., Vittinghoff E., Douglas J.M., Jr., Koblin B.A., Mayer K.H. Correlates of condom failure in a sexually active cohort of men who have sex with men. J Acqui Immune Defic Syndr Hum Retrovirol. 1999;20(5):495–501. doi: 10.1097/00042560-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 13.Calzavara L., Burchell A.N., Remis R.S., Major C., Corey P., Myers T. Delayed application of condoms is a risk factor for human immunodeficiency virus infection among homosexual and bisexual men. Am J Epidemiol. 2003;157(3):210–217. doi: 10.1093/aje/kwf195. [DOI] [PubMed] [Google Scholar]
- 14.D'Anna L.H., Margolis A.D., Warner L., Korosteleva O.A., O'Donnell L., Rietmeijer C.A. Condom use problems during anal sex among men who have sex with men (MSM): findings from the safe in the city study. AIDS Care. 2012;24(8):1028–1038. doi: 10.1080/09540121.2012.668285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golombok S., Harding R., Sheldon J. An evaluation of a thicker versus a standard condom with gay men. AIDS. 2001;15(2):245–250. doi: 10.1097/00002030-200101260-00015. [DOI] [PubMed] [Google Scholar]
- 16.Reece M., Herbenick D., Sanders S.A., Monahan P., Temkit M., Yarber W.L. Breakage, slippage and acceptability outcomes of a condom fitted to penile dimensions. Sex Transm Infect. 2008;84(2):143–149. doi: 10.1136/sti.2007.028316. [DOI] [PubMed] [Google Scholar]
- 17.ISO. Condoms — Guidance on clinical studies — Part 1: Male condoms, clinical function studies based on self-reports. TC 157N 770. 2012. Contract No.: ISO/WD 29943-1.
- 18.U.S. Food and Drug Administration. Condoms and sexually transmitted diseases 2018[Available from: https://www.fda.gov/forpatients/illness/hivaids/ucm126372.htm.
- 19.Herbenick D., Bowling J., Fu T.J., Dodge B., Guerra-Reyes L., Sanders S. Sexual diversity in the United States: results from a nationally representative probability sample of adult women and men. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groves R.M., Fowler F.J., Couper M.P., Lepkowski J.M., Singer E., Tourangeau R. Survey methodology. In: Groves R.M., Kalton G., Rao J.N.K., Schwarz N., Skinner C., editors. John Wiley & Sons, Inc; Hoboken: 2004. p. 415. [Google Scholar]
- 21.Siegler A.J., Rosenthal E.M., Sullivan P.S., Ahlschlager L., Kelley C.F., Mehta C. Vol. 8. 2019. A double-blind, single center, randomized 3-way crossover trial of fitted, thin and standard condoms for vaginal and anal sex: c-pleasure study protocol and baseline data; p. e12205. (JMIR Res Protoc). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Organization World Health. Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA/FHI360: advisory note. 2012 [Google Scholar]
- 23.International Organization for Standardization. Condoms — guidance on clinical studies — part 1: male condoms, clinical function studies based on self-reports. 2012.
- 24.U.S. Food and Drug Administration (FDA). Testing guidance for male condoms made from new material (Non-Latex)1995[Available from:http://www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm080618.htm.
- 25.U.S. Food and Drug Administration. 510(k) Premarket Notification Database 2018 [Available from:https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm.
- 26.Siegler A.J., Ahlschlager L., Rosenthal E.M., Cecil M.P., Kelley C.F., Rosenberg E.S. Utility of a US Food and Drug Administration (FDA) label indication for condoms for anal sex. Sex Health. 2019 doi: 10.1071/SH18152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabbay M.B., Thomas J., Gibbs A., Hold P. A randomized crossover trial of the impact of additional spermicide on condom failure rates. Sex Transm Dis. 2008;35(10):862–868. doi: 10.1097/OLQ.0b013e31817fb802. [DOI] [PubMed] [Google Scholar]
- 28.Crosby R., Shrier L.A., Charnigo R.J., Weathers C., Sanders S.A., Graham C.A. A prospective event-level analysis of condom use experiences following STI testing among patients in three US cities. Sex Transm Dis. 2012;39(10):756–760. doi: 10.1097/OLQ.0b013e318265a951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macaluso M., Blackwell R., Jamieson D.J., Kulczycki A., Chen M.P., Akers R. Efficacy of the male latex condom and of the female polyurethane condom as barriers to semen during intercourse: a randomized clinical trial. Am J Epidemiol. 2007;166(1):88–96. doi: 10.1093/aje/kwm046. [DOI] [PubMed] [Google Scholar]
- 30.Steiner M.J., Cates W., Jr., Warner L. The real problem with male condoms is nonuse. Sex Transm Dis. 1999;26(8):459–462. doi: 10.1097/00007435-199909000-00007. [DOI] [PubMed] [Google Scholar]