Abstract
Tobacco smoking is a common risk factor of cardiovascular diseases, cancers and heart health problems. In Taif, the number of secondary polycythemia patients is increasing dramatically and most of those patients are heavy smokers. Therefore, this study is an attempt to understand the pathophysiological mechanism behind that problem. Whole blood and serum samples were collected from forty healthy people and forty tobacco smokers, voluntary for this study. Complete blood counts revealed a significant increase in the red blood cell count, hemoglobin concentrations, hematocrit and neutrophils with some elevations in total white blood cells, lymphocytes and monocytes. Moreover, serum analysis of both erythropoietin and interleukin-7 showed a significant reduction in their levels among smokers which were about 35% and 65% respectively. Gene expression study showed a significant upregulation of RAG-1, RAG-2 and EPOR-1 genes caused by tobacco smoking. In conclusion, data presented in the current study suggest that tobacco smoking might cause alveolar tissue inflammation and vascular injury causing an immune response that elevates the white blood cells count. Another suggestion is that tobacco smoking defects the pulmonary gaseous exchange mechanism leading to the secondary polycythemia indicated by the increase in red blood cell count, hemoglobin levels, hematocrit and by the low serum erythropoietin levels.
Keywords: Smoking, Secondary, Polycythemia, Leukocytosis, Erythropoietin, IL-7, EPOR-1, RAG-1, RAG-2
1. Introduction
Tobacco smoking is a common risk factor of cardiovascular diseases, cancers and heart health problems. Nowadays, the number of smokers is increasing up among the population over the world. In this habit, smokers are exposed to tens of toxic materials such as carbon monoxide, hydrocarbons and nitrosamine compounds. In Taif, the number of secondary polycythemia patients is increasing dramatically and most of those patients are males and shared the one fact that they are heavy smokers which raises very important questions how and why?
Over more than fifty years researchers have been working hard to answer these two questions properly. In 1978, Smith and Landaw have reported an increase in red blood cell volume in a study conducted on 22 smokers (Smith and Landaw, 1978). Another study was published in 1982 showed a strong relation between cigarette smoking and secondary polycythemia (Calverley et al., 1982). Aitchison and Russell have concluded that smoking underlies the incidence of secondary polycythemia in a study conducted on 14 smokers (Aitchison and Russell, 1988). In the same context, tobacco smoking is associated with high hemoglobin levels as a positive feedback of hypoxia caused by carbon monoxide inhalation and that may underlie the decrease of the erythropoietin serum levels of smokers as negative feedback (Eisenga et al., 2018, Singh et al., 2016, Tanabe et al., 1997).
Furthermore, chronic smoking has been found to be related to persistent polyclonal B cell lymphocytosis (PPBL), for instance, Dasanu CA and Codreanu I have reported that 29% of studied PPBL cases were related to chronic smoking (Casassus et al., 1987, Dasanu and Codreanu, 2012, Delannoy et al., 1993). These studies have been supported by other reports that showed a decrease in Interleukin-7 (IL-7) serum levels in smokers compared with healthy individuals as negative feedback of lymphocytosis (Lehto et al., 2010, Nakamura et al., 2014, Tymkiw et al., 2011). IL-7 is a glycoprotein produced by different types of cells such as hepatocytes, keratinocytes, lymphocytes, dendritic cells and stromal cells (Fry and Mackall, 2002, Lin et al., 2017). IL-7 has an essential role in lymphocyte maturation and pluripotency (Lin et al., 2017). IL-7 binds to a distinct tyrosine kinase receptor on lymphocytes progenitors called IL-7 receptor (IL-7 R) and that binding, in turn, activates either JAK1 or JAK3 leading to the activation of some downstream kinases such as STAT5a/b, PI3K and SRC (Johnson et al., 2012). Ultimately, the signaling pathway triggered by IL-7 protects and activates two crucial targets playing a key role in the maturation and proliferation of lymphocytes which are called the recombination-activating genes 1 and 2 (RAG1 and RAG2) (Fry and Mackall, 2002, Johnson et al., 2012, Lin et al., 2017). Therefore, the current study is an attempt to understand the physiological and genetic factors contribute to the secondary polycythemia caused by chronic smoking.
2. Materials and methods
2.1. Materials
Serum erythropoietin and IL-7 ELISA estimation kits were purchased from RayBiotech 3607 Parkway Lane, Suite 200 Peachtree Corners, GA 30092, Atlanta, USA. RNA extraction and purification kits were purchased from QIAGEN Inc. 19,300 Germantown Road Germantown, MD 20874, USA.
2.2. Sample collection
Forty healthy people and forty tobacco smokers were participated in this study after signing the consent form and ethical approval was obtained from the Professional and Research Ethics Committee (PREC) at King Faisal Hospital in Taif. Three kinds of samples were collected to complete this study. Whole blood samples were collected on EDTA for Complete Blood Count (CBC), fresh whole blood samples were collected from the participants and RNA was extracted immediately using RNA extraction and purification kit and serums were collected and frozen at −20 °C for the ELISA investigations.
2.3. CBC and serum analysis
Complete blood counts were conducted using a fully automated blood counter (Sysmex XN-1000 Hematology analyzer). Serum analysis of erythropoietin and IL-7 were conducted using the ELISA commercial kits purchased from RayBiotech.
2.4. Gene expression study
Total RNA was extracted using an intended commercial kit purchased from QIAGEN Inc. Fresh whole blood samples were collected in the presence of heparin. 1.2 ml of the whole blood sample was used as a starting volume. Then total RNA extraction and purification were conducted via following the protocol provided with the kit. Total RNAs yields were estimated spectrophotometrically using the Bio-Rad spectrophotometer. The integrity of yields was confirmed electrophoretically in 1.5% denaturated agarose gel stained with ethidium bromide. The cDNA was formed by mixing 5 µg of the total RNA with 0.5 ng oligo dT primer (Qiagen Valencia, CA, USA) in a sterilized DEPC water for a total volume of 11 µl. The mixture was incubated in the thermal cycler for 5 min at 70 °C for denaturation. For reverse transcription, samples were mixed 10 mM dNTPs and 100 U Moloney Murine Leukemia Virus (M-MuLV) reverse transcriptase (SibEnzyme. Ak, Novosibirsk, Russia) in a 10X RT-buffer, and incubated in the thermal cycler at 37 °C for 60 min and then at 90 °C for 10 min to inactivate the enzyme. PCR was carried out in a final volume of 25 µl containing 1 µl cDNA, 1 µl of 10 pM of each primer forward and reverse (Table 1), and 12.5 µl PCR master mix provided from Promega Corporation, Madison, WI, USA. The reaction was conducted in a thermal cycler at 94 °C for 5 min for the first cycle and followed by 27 cycles in which one minute at 94 °C for denaturation, one minute at 54 °C for annealing and one minute at 72 °C for extension with an additional 10 min at 72 °C for the final extension. Herein, human beta globulin mRNA was used as a reference gene. The PCR products were run on 2% agarose gel stained with ethidium bromide. PCR products were visualized and documented by a gel documentation system and the intensity of bands was estimated by Image J software version 1.47 (http://imagej.en.softonic.com/).
Table 1.
Forward and reverse primers for EPOR-1,RAG-1,RAG-2and β-globin.
| Gene | Forward primer | Reverse Primer |
|---|---|---|
| EPOR-1, 275 bp | 5′-CTA TGC CTG GAA GAG GAT GGA G-3′ | 5′-TGC GGA AAG TGT CAG CAG TG-3′ |
| RAG-1, 275 bp | 5′-ACA CCT CCA AAT ACC TCC AG-3′ | 5′-ATC TTA CTC CAA CCT ACC ACC-3′ |
| RAG-2, 209 bp | 5′-TCG CTG CAC AGA GAA AGA C-3′ | 5′-CAC ACC CAA ATT CAA AAT CCA C-3′ |
| β-globin,175 bp | 5′-CAA CTT CAT CCA CGT TCA CC-3′ | 5′-GAA GAG CCA AGG ACA GGT AC-3′ |
2.5. Statistical analysis
Data are represented as means ± standard error of means (SEM). Data presented in this study were analyzed via the 2-ways ANOVA (analysis of variance) and the post hoc descriptive tests using SPSS software for Windows. The p values that <0.05 were considered statistically significant.
3. Results
3.1. Tobacco smoking increases complete blood counts and related measurements
Table 2 shows that tobacco smoking significantly elevates the red blood cells, hemoglobin, hematocrit and neutrophil counts in which p values <0.05 corresponding to nonsmoking participants. Besides, platelets and white blood cell counts are higher in tobacco smokers compared with the nonsmokers as shown in Table 2.
Table 2.
Complete Blood Counts (CBC).
| Parameter | Normal Individuals | Tobacco smokers |
|---|---|---|
| WBCs | 6.1 ± 0.24 × 103/mm3 | 7.1 ± 0.56 × 103/mm3 |
| RBCs | 5.66 ± 0.09 × 106/mm3 | 6.7 ± 0.15 × 106/mm3** |
| Hemoglobin | 15.5 ± 0.18 g/dL | 18.8 ± 0.19 g/dL** |
| HCT | 47.67 ± 0.52% | 57 ± 0.9%** |
| PLT | 269 ± 10.05 × 103/mm3 | 299 ± 22.5 × 103/mm3 |
| Neutrophils | 2.9 ± 0.17 × 103/mm3 | 4 ± 0.5 × 103/mm3** |
| Lymphocytes | 2.37 ± 0.11 × 103/mm3 | 2.2 ± 0.11 × 103/mm3 |
| Monocyte | 0.54 ± 0.02 × 103/mm3 | 0.55 ± 0.03 × 103/mm3 |
| Eosinophils | 0.17 ± 0.01 × 103/mm3 | 0.22 ± 0.03 × 103/mm3 |
| Basophils | 0.04 ± 0.003 × 103/mm3 | 0.07 ± 0.02 × 103/mm3 |
Values are means ± standard error of mean (SEM) for 40 normal individuals and 40 tobacco smokers.
For values that are statistically significant (P < 0.05) corresponding to normal participants.
3.2. Tobacco smoking reduces cytokines related to blood generation
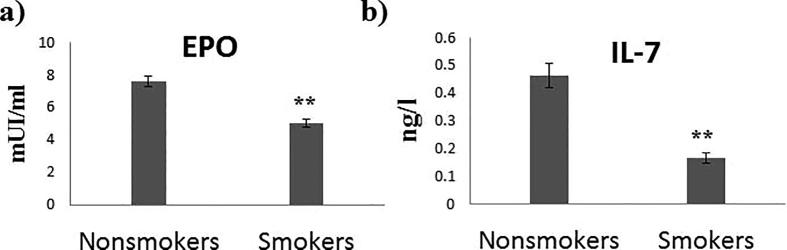
Serum analysis of erythropoietin clarified significant reduction levels in tobacco smokers about 35% compared with the nonsmokers as shown in Fig. 1(a). Similarly, serum levels of interleukine-7 (IL-7) were significantly reduced in tobacco smokers which for about 65% compared to the nonsmokers (Fig. 1(b)).
Fig. 1.
Effect of tobacco smoking on examined serum cytokines levels. (a) Serum erythropoietin levels in mIU/ml. (b) Serum IL-7 levels in ng/L. Data presented in mean ± SEM. ** indicated P value < 0.05.
3.3. Effects of tobacco smoking on EPOR-1, RAG-1 and RAG-2 expression
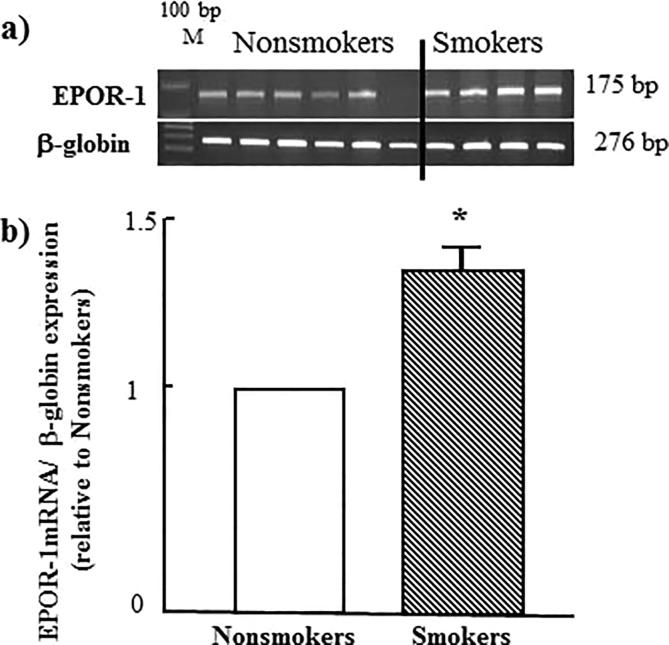
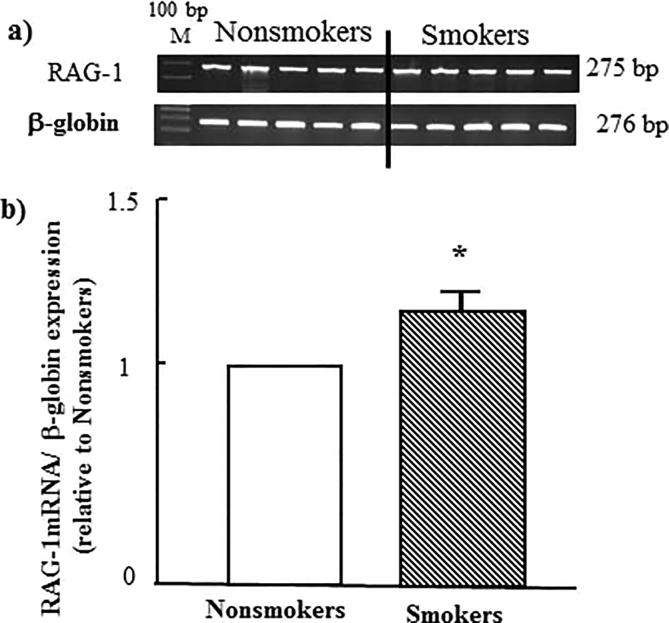
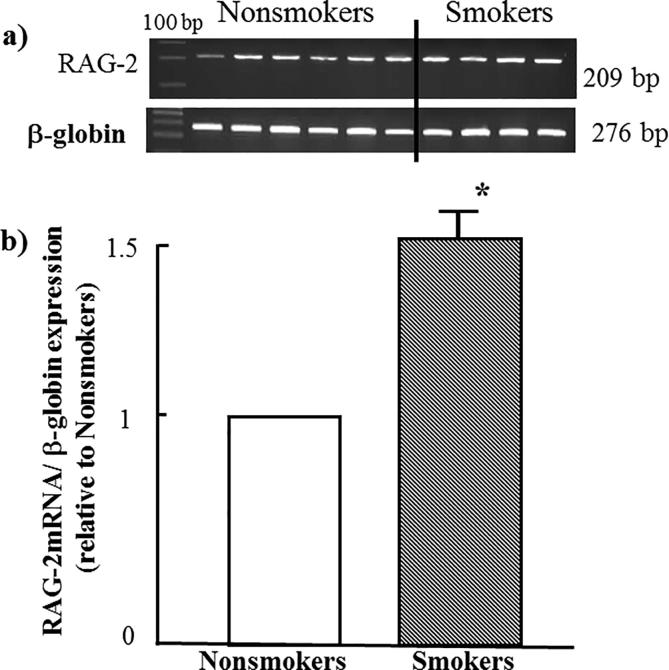
As seen in Fig. 2 tobacco smoking, significantly, induces upregulation of the gene of erythropoietin receptor-1 (EPOR-1) to 50% more. A similar effect was observed when study both RAG-1 and RAG-2 genes as shown in Fig. 3, Fig. 4 respectively.
Fig. 2.
Effect of tobacco smoking on the expression of EPOR-1 gene. (a) electrophoretic emigration picture of the expression of EPOR-1 gene and β-globulin as a control gene. (b) Densitometric analysis of emigrated bands. * indicated P value < 0.05.
Fig. 3.
Effect of tobacco smoking on the expression of RAG-1 gene. (a) electrophoretic emigration picture of the expression of RAG-1 gene and β-globulin as a control gene. (b) Densitometric analysis of emigrated bands. * indicated P value < 0.05.
Fig. 4.
Effect of tobacco smoking on the expression of RAG-2 gene. (a) electrophoretic emigration picture of the expression of RAG-2 gene and β-globulin as a control gene. (b) Densitometric analysis of emigrated bands. * indicated P value < 0.05.
4. Discussion
Nowadays, tobacco smoking is implicated in many chronic and acute diseases such as atherosclerosis, heart attack and cancers. Recently, Michael Fricker and his colleagues have reported that tobacco smoking causes systemic hypoxia (Fricker et al., 2018). A similar conclusion has been published by E. Ferrer, et al in seven years back (Ferrer et al., 2011). In 1973, Arthur L. Sagone Jr. and his group have reported that tobacco smoking reduces tissue oxygen supply and they found significant elevations in hemoglobin, red blood cell counts, hematocrit and red cell mass among smokers compared to non-smokers (Sagone et al., 1973). The current study is consistent with those reports and several others whereas Table 2 presented a significant increase in red blood cell counts, hematocrit, and hemoglobin levels in which p values were <0.05 compared to the non-smokers. These data suggest an increase in bone marrow activity to avoid the hypoxia induced by smoking. Besides, erythropoietin is another cytokine released from the kidney to stimulate red blood cell production. Several studies have demonstrated that smoking decreases the serum levels of erythropoietin as a result of the negative feedback of hemoglobin and red blood cells increase (Eisenga et al., 2018, Singh et al., 2016, Tanabe et al., 1997). The current study has shown a significant reduction in erythropoietin levels in smokers serums compared to the non-smokers which are consistent with those studies. However, Fig. 2 shows an increase in the expression of mRNA of erythropoietin receptor in smokers which clarifies the molecular principle of the increase of red blood cell count and hemoglobin levels presented in Table 2 and that might be positive feedback of the low serum erythropoietin levels shown in Fig. 2. These data are nicely consistent with some previously published work (de La Chapelle et al., 1993, Hämäläinen et al., 2012, Izzotti et al., 2003).
Moreover, the whole blood analysis of both participated groups revealed an elevation in white blood cell counts and differential in smokers and that elevation was statistically significant in neutrophil counts as shown in Table 2. These findings are highly consistent with several published studies (Higuchi et al., 2016, Schwartz and Weiss, 1991, Schwartz and Weiss, 1994, Stemmelin et al., 2004). In fact, several reports emphasized that tobacco smoking causes vascular injury which in turn elevates inflammatory markers and leukocyte counts and that may interpret the neutrophil count increase in smokers as found in this study (Lee et al., 2012, Smoking and inflammation, 2005, Tibuakuu et al., 2017).
In a related context, the recombination-activating genes 1 and 2 (RAG1 and RAG2) are key proteins for maturation and proliferation of the lymphocytes. Delannoy and his colleagues have reported a link between the chronic polyclonal b- cell lymphocytosis and cigarette smoking (Delannoy et al., 1993). That report is supported by other studies concluded that tobacco smoking is associated with either monoclonal or polyclonal lymphocytosis (Casassus et al., 1987, Dasanu, 2013, Dasanu and Codreanu, 2012). In the current study, the genetic expression investigation on both RAG1 and RAG2 genes was striking when showed a significant increase in the mRNA expression of both genes in tobacco smokers compared to the non-smokers as shown in Fig. 3, Fig. 4. These results are nicely fit the leukocyte counts shown in Table 2 and adding further support to the idea of the association between tobacco smoking and lymphocytosis. IL-7 is an important cytokine released from the stromal cells in the bone marrow to regulate b-cell development. However, IL-7 downregulates the expression of both RAG1 and RAG2 genes (Amin and Schlissel, 2008, Johnson et al., 2008, Ye and Graf, 2007). Fig. 2(b) clearly shows that the serum levels of IL-7 in smokers are significantly lower than in non-smokers which clarifies how the expression of these genes among smokers is increased.
5. Conclusion
In conclusion, data presented in this study suggest that tobacco smoking can cause inflammatory conditions in either alveolar tissues or vascular injury leading to an immune response indicated by leukocytosis and lymphopoiesis. Furthermore, smoking is leading to secondary polycythemia that diagnosed via the increase of the red blood cell count, hemoglobin levels, the expression of the EPOR gene and the low serum erythropoietin levels depending on several published references.
Declaration of Competing Interest
The author declare that he has no competing interests and this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aitchison R., Russell N. Smoking–a major cause of polycythaemia. J. R. Soc. Med. 1988;81(2):89–91. doi: 10.1177/014107688808100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin R.H., Schlissel M.S. Foxo1 directly regulates the transcription of recombination-activating genes during b cell development. Nat. Immunol. 2008;9(6):613–622. doi: 10.1038/ni.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calverley P.M.A., Leggett R.J., McElderry L., Flenley D.C. Cigarette smoking and secondary polycythemia in hypoxic cor pulmonale. American Rev. Respirat. Dis. 1982;125(5):507–510. doi: 10.1164/arrd.1982.125.5.507. [DOI] [PubMed] [Google Scholar]
- Casassus P., Lortholary P., Komarover H., Lejeune F., Hors J. Cigarette smoking-related persistent polyclonal b lymphocytosis. A premalignant state. Arch. Pathol. Lab. Med. 1987;111(11):1081. [PubMed] [Google Scholar]
- Dasanu C.A. Smoking-induced monoclonal b-lymphocytosis in two female smokers: what are the odds? Conn Med. 2013;77(6):353–355. [PubMed] [Google Scholar]
- Dasanu C.A., Codreanu I. Persistent polyclonal b-cell lymphocytosis in chronic smokers: More than meets the eye. Conn. Med. 2012;76(2):69–72. [PubMed] [Google Scholar]
- de La Chapelle A., Träskelin A., Juvonen E. Truncated erythropoietin receptor causes dominantly inherited benign human erythrocytosis. Proc. Natl. Acad. Sci. 1993;90(10):4495–4499. doi: 10.1073/pnas.90.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delannoy A., Djian D., Wallef G., Deneys V., Fally P., Martiat P., Michaux J.L. Cigarette smoking and chronic polyclonal b-cell lymphocytosis. Nouv. Rev. Fr. Hematol. 1993;35(2):141–144. [PubMed] [Google Scholar]
- Eisenga M.F., Kieneker L.M., Touw D.J., Nolte I.M., van der Meer P., Huls G. Active smoking and hematocrit and fasting circulating erythropoietin concentrations in the general population. Mayo Clin. Proc. 2018;93(3):337–343. doi: 10.1016/j.mayocp.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Ferrer E., Peinado V.I., Castañeda J., Prieto-Lloret J., Olea E., González-Martín M.C. Effects of cigarette smoke and hypoxia on pulmonary circulation in the guinea pig. Eur. Respir. J. 2011;38(3):617–627. doi: 10.1183/09031936.00105110. [DOI] [PubMed] [Google Scholar]
- Fricker M., Goggins B.J., Mateer S., Jones B., Kim R.Y., Gellatly S.L. Chronic cigarette smoke exposure induces systemic hypoxia that drives intestinal dysfunction. JCI insight. 2018;3(3) doi: 10.1172/jci.insight.94040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry T.J., Mackall C.L. Interleukin-7: From bench to clinic. Blood. 2002;99(11):3892–3904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- Hämäläinen P., Saltevo J., Kautiainen H., Mäntyselkä P., Vanhala M. Erythropoietin, ferritin, haptoglobin, hemoglobin and transferrin receptor in metabolic syndrome: A case control study. Cardiovascular Diabetol. 2012;11(1):116. doi: 10.1186/1475-2840-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi T., Omata F., Tsuchihashi K., Higashioka K., Koyamada R., Okada S. Current cigarette smoking is a reversible cause of elevated white blood cell count: Cross-sectional and longitudinal studies. Prevent. Med. Rep. 2016;4:417–422. doi: 10.1016/j.pmedr.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzotti A., Balansky R.M., Cartiglia C., Camoirano A., Longobardi M., De Flora S. Genomic and transcriptional alterations in mouse fetus liver after transplacental exposure to cigarette smoke. FASEB J. 2003;17(9):1127–1129. doi: 10.1096/fj.02-0967fje. [DOI] [PubMed] [Google Scholar]
- Johnson K., Chaumeil J., Micsinai M., Wang J.M.H., Ramsey L.B., Baracho G.V. Il-7 functionally segregates the pro-b cell stage by regulating transcription of recombination mediators across cell cycle. J. Immunol. (Baltimore, Md : 1950) 2012;188(12):6084–6092. doi: 10.4049/jimmunol.1200368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K., Hashimshony T., Sawai C.M., Pongubala J.M., Skok J.A., Aifantis I., Singh H. Regulation of immunoglobulin light-chain recombination by the transcription factor irf-4 and the attenuation of interleukin-7 signaling. Immunity. 2008;28(3):335–345. doi: 10.1016/j.immuni.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Lee J., Taneja V., Vassallo R. Cigarette smoking and inflammation: Cellular and molecular mechanisms. J. Dent. Res. 2012;91(2):142–149. doi: 10.1177/0022034511421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto S.M., Huotari A., Niskanen L., Herzig K.-H., Tolmunen T., Viinamäki H. Serum il-7 and g-csf in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2010;34(6):846–851. doi: 10.1016/j.pnpbp.2010.03.033. [DOI] [PubMed] [Google Scholar]
- Lin J., Zhu Z., Xiao H., Wakefield M.R., Ding V.A., Bai Q., Fang Y. The role of il-7 in immunity and cancer. Anticancer Res. 2017;37(3):963–967. doi: 10.21873/anticanres.11405. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Nakamura H., Minematsu N., Chubachi S., Miyazaki M., Yoshida S. Plasma cytokine profiles related to smoking-sensitivity and phenotypes of chronic obstructive pulmonary disease. Biomarkers. 2014;19(5):368–377. doi: 10.3109/1354750X.2014.915342. [DOI] [PubMed] [Google Scholar]
- Sagone A.L., Lawrence T., Balcerzak S.P. Effect of smoking on tissue oxygen supply. Blood. 1973;41(6):845–851. [PubMed] [Google Scholar]
- Schwartz J., Weiss S.T. Host and environmental factors influencing the peripheral blood leukocyte count. Am. J. Epidemiol. 1991;134(12):1402–1409. doi: 10.1093/oxfordjournals.aje.a116045. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Weiss S.T. Cigarette smoking and peripheral blood leukocyte differentials. Ann. Epidemiol. 1994;4(3):236–242. doi: 10.1016/1047-2797(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Singh V., Tanwar A., Hungund A., Hungund S., Nagaraja C. Comparison of serum erythropoietin levels in smokers and nonsmokers with periodontitis: a biochemical study. J. Indian Soc. Periodontol. 2016;20(3):249–253. doi: 10.4103/0972-124X.181242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.R., Landaw S.A. Smokers' polycythemia. N. Engl. J. Med. 1978;298(1):6–10. doi: 10.1056/NEJM197801052980102. [DOI] [PubMed] [Google Scholar]
- Smoking and inflammation. (2005). PLoS Medicine, 2(6), e198. doi:10.1371/journal.pmed.0020198.
- Stemmelin G.R., Doti C.A., Shanley C.M., Ceresetto J.M., Rabinovich O.M., Vicente Reparaz M.A. Smoking as a cause for mild chronic neutrophilia. Blood. 2004;104(11) 3796-3796. [Google Scholar]
- Tanabe N., Ohnishi K., Fukui H., Ohno R. Effect of smoking on the serum concentration of erythropoietin and granulocyte-colony stimulating factor. Intern. Med. 1997;36(10):680–684. doi: 10.2169/internalmedicine.36.680. [DOI] [PubMed] [Google Scholar]
- Tibuakuu M., Kamimura D., Kianoush S., DeFilippis A.P., Al Rifai M., Reynolds L.M. The association between cigarette smoking and inflammation: The genetic epidemiology network of arteriopathy (genoa) study. PLoS ONE. 2017;12(9) doi: 10.1371/journal.pone.0184914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tymkiw K.D., Thunell D.H., Johnson G.K., Joly S., Burnell K.K., Cavanaugh J.E. Influence of smoking on gingival crevicular fluid cytokines in severe chronic periodontitis. J. Clin. Periodontol. 2011;38(3):219–228. doi: 10.1111/j.1600-051X.2010.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Graf T. Early decisions in lymphoid development. Curr. Opin. Immunol. 2007;19(2):123–128. doi: 10.1016/j.coi.2007.02.007. [DOI] [PubMed] [Google Scholar]