Abstract
Background
Additive manufacturing (AM) is a rapidly expanding new technology involving challenges to occupational health. Here, metal exposure in an AM facility with large-scale metallic component production was investigated during two consecutive years with preventive actions in between.
Methods
Gravimetric analyzes measured airborne particle concentrations, and filters were analyzed for metal content. In addition, concentrations of airborne particles <300 nm were investigated. Particles from recycled powder were characterized. Biomonitoring of urine and dermal contamination among AM operators, office personnel, and welders was performed.
Results
Total and inhalable dust levels were almost all below occupational exposure limits, but inductively coupled plasma mass spectrometry showed that AM operators had a significant increase in cobalt exposure compared with welders. Airborne particle concentrations (<300 nm) showed transient peaks in the AM facility but were lower than those of the welding facility. Particle characterization of recycled powder showed fragmentation and condensates enriched in volatile metals. Biomonitoring showed a nonsignificant increase in the level of metals in urine in AM operators. Dermal cobalt and a trend for increasing urine metals during Workweek Year 1, but not in Year 2, indicated reduced exposure after preventive actions.
Conclusion
Gravimetric analyses showed low total and inhalable dust exposure in AM operators. However, transient emission of smaller particles constitutes exposure risks. Preventive actions implemented by the company reduced the workers' metal exposure despite unchanged emissions of particles, indicating a need for careful design and regulation of the AM environments. It also emphasizes the need for relevant exposure markers and biomonitoring of health risks.
Keywords: 3D-printing, Additive manufacturing, Metals, Occupational exposure, Particle exposure
1. Introduction
Additive manufacturing (AM), in everyday language often called 3D printing, is a term for processes that involve the creation of objects by different techniques. Those techniques involve sequential addition of layers of materials followed by specific fusing based on digital models. These techniques allow creation of highly complex structures in materials such as metal or ceramics with added functionalities not obtainable via other production means [1]. In addition, there are additional 3D printing technologies, printing polymer-based products mostly used in home and office environments around the world for prototyping and creation of end user products [2].
The use of metal AM technologies in the industry setting has several possible advantages including the ability to construct spare parts on demand, thereby diminishing the need for keeping items in stock, and the ability to replace worn parts by building new components on an existing part to avoid replacing the whole construction. There are many different techniques for metal AM including binder jetting, powder bed fusion, and directed energy deposition that all have different properties and are appropriate for different materials and end products [1]. However, there is still relatively scarce information regarding health and safety issues related to metal AM processes that need to be investigated to minimize the risk for operators. There is, however, a lot of information in the literature regarding health effects of metal exposure in other occupational settings such as an increased risk of lung complications due to cobalt exposure in hard metal workers [3] and risks of health complications among welders [4], [5]. Furthermore, AM may generate airborne nanoscale metal particles that in other occupational settings such as welding are known to be hazardous to human health [6]. Based on the potential risks the metal powder in AM facilities poses, it is important to understand whether the processes cause elevated exposure to metals in the operators and what types of engineering and administrative processes are needed to reduce the risk of eventual health effects.
We have earlier reported a pilot project regarding generation of dust and particles from metal AM processes [7]. In the present study, we have investigated the dust and particle concentrations as well as biomonitored employees' urine and dermal metal levels in an AM facility at two different occasions since the company had moved to a phase of serial production of components. The measurements were performed during two consecutive years, while in between, the company implemented several restrictions and guidelines to the workforce to reduce powder spreading in the AM facility. Furthermore, particle characterization by scanning electron microscopy (SEM) was used to provide more information regarding exposure risks.
2. Materials and methods
2.1. AM process
Similar to what was reported in the pilot study [7], the studied AM process was powder bed fusion using selective laser melting (SLM). The process is based on the addition of 20 μm of metal powder evenly over the building plate, followed by fully melting the metal in a desired shape by a laser beam before a new 20 μm of metal powder is added. These steps are then repeated until the whole structure is complete. The process takes place in an argon-filled chamber that is ventilated after the process is completed, and surplus metal powder is removed by vacuuming. This used metal powder is filtered to remove eventual larger particle complexes created in the processes, and the remaining powder is reused.
The company used different AM machines including Eosint M280, EOS M290, and EOS M400 (EOS GmbH Electro Optical Systems, Krailling, Germany). The M280 instruments were equipped with 200-W lasers, whereas M290 and M400 were equipped with 400-W lasers. Between Year 1 and Year 2, there was a 30% increase in the number of machines and general production. The different types of AM machines were situated in different booths in the same production hall, with one booth with three M290 machines in a row (approximately 1 m apart) and one with four M280 machines (one in each corner of the booth, approximately 2 m apart). In the same production hall, there was also a booth dedicated to dry sawing (e.g., printed products from the building plate) and a separate booth for other types of postprinting modifications. In Year 2, a separate booth with two M400 machines was built in the same production hall. The AM operator operated all AM machines during the day.
In the previously reported pilot study, the nickel alloy powder IN939 was used for the AM process [7]. At the time of the present study, the company had started to use mainly Hastelloy® X (Haynes International, ID, USA), which is an alloy powder containing 47% nickel, 22% chromium, 18% iron, 9% molybdenum, 1.5% cobalt, and other metals (below 1%) (nominal composition according to the producer).
2.2. Preventive actions between Year 1 and Year 2
Between Year 1 and Year 2, the company adjusted its guidelines regarding personal protection equipment (PPE) and work routines. These new guidelines stated that during open handling of the metal powder, PPE with overalls, shoe covers, single-use nitrile gloves, and powered air-purifying respirators with the P3 filter were required. During other operations in the AM facility, similar PPE but without the shoe covers and powered air-purifying respirator was required. The company also enforced restrictions that products created in the AM facility were not allowed to be taken out of the factory before being postprocessed and depowdered.
2.3. Exposure measurements
2.3.1. Gravimetric analyses
Gravimetric analyses of airborne particles were performed by pumping air over filters in accordance with Swedish standards [8], [9]. Both personal and stationary measurements were performed in the AM facility and in the welding facility. Sampling of total dust was performed according to a modified version of the National Institute of Occupational Safety and Health Manual of Analytical Methods 0500 using an open-faced cassette. Inhalable dust was collected using an IOM sampler (SKC Ltd, Dorset, UK). Both samplers were used with a 25-mm mixed cellulose ester filter and an airflow rate of 2 L/min. The samplers were placed in the breathing zone for the personal exposure measurements; for the stationary measurement, the pumps were placed on the worktable integrated in the machines (at approximately 1.5-m height). Calibrated flow meters were used for measuring the airflow rate for all samplers before and after sampling.
Filters were analyzed gravimetrically for particulate mass. Metal analysis was performed by inductively coupled plasma mass spectrometry (iCAP™ Q; Thermo Fisher Scientific, Waltham, MA, USA) [10], [11], [12].
2.3.2. Particle counting
Two different particle-counting instruments were used: Lighthouse 3016-IAQ for the measurement of particles between 0.3–10 μm and Nanotracer for the detection of ultrafine particles (nanoparticles) between 10–300 nm. The Lighthouse 3016-IAQ (Lighthouse Worldwide Solutions, CA, USA) is a handheld optical particle-counting instrument with six different size channels for particles (0.3, 0.5, 1.0, 2.5, 5.0, and 10.0 μm) that allows simultaneous detection across the different sizes. The Nanotracer (Phillips, Best, the Netherlands) is a diffusion charge device that measures particles in the range of 10–300 nm [13]. This instrument allows measurements of both particle concentration and average particle diameters. The particle counters where placed at the worktable for the machines close to the stationary gravimetric sampling.
2.3.3. Particle characterization using SEM
Both new and recycled powders were investigated by SEM. Samples were prepared by dispersing particles in water, sonicating in an ultrasonic bath for 3 min, and finally filtering onto 25 mm-diameter polycarbonate filters with a pore size of 0.6 μm. The filters were precoated with a thin layer of platinum in a Cressington 208HR sputter coater (Cressington Scientific Instruments Ltd., Watford, UK). A piece of approximately 1 cm2 was cut out from the filter and mounted on an aluminum stub with a carbon tab. The samples were imaged and analyzed using a Hitachi SU6600 field emission scanning electron microscope (Hitachi, Tokyo, Japan) equipped with a Bruker energy-dispersive X-ray spectrometry (EDX) detector (Bruker Nano GmbH, Berlin, Germany).
2.4. Biomonitoring
2.4.1. Study population
All participants were recruited from the available workforce at the company. AM operators and welders were invited to participate. The office staff in the AM facility was invited to participate as controls.
2.4.2. Ethical considerations
All participants signed a written consent, and the study was approved by the local ethics committee in Linköping (Dnr 2016/112-31).
2.4.3. Dermal exposure
Dermal exposure was investigated by taping the index fingers of the participants with three pieces of tape (Scotch Magic; 3M, MN, USA) in sequence. Additional tapes were taken on different areas around the AM facility. Metal concentrations on the third tape on fingers and the first tape on surfaces were measured using a NitonTM XL3t X-ray fluorescence Analyzer (Thermo Fisher Scientific) with a portable test stand. For each tape, 3 random chosen points were measured for 90 seconds using the “thin-film sample”–analyzing application.
2.4.4. Metals in urine
Urine was collected in acid-cleaned sampling tubes at the start and at the end of the workweek according to a protocol used at the clinic to reduce risk of contamination of the samples. The urine samples were kept cold until arrival at the laboratory where they were frozen (-20°C) until analysis. Before analysis, specific gravity of the urine samples was measured. Metal levels were assessed by ICP-MS (iCAP™ Q; Thermo Fisher Scientific).
2.5. Statistical analyses
Comparisons between groups of workers were performed using the Student t test in Statistica 13 (Dell, TX, USA). The Bonferroni correction was used for p-values to account for multiple analyses. Continuous variables were log-transformed before analysis.
3. Results
3.1. Exposure measurements
3.1.1. Gravimetric analyses
The filter-based particle measurements were performed for comparison with current Swedish occupational exposure limits (OELs) for dust and metals. During Year 1, personal exposure and stationary measurements were performed in both the AM and welding facility. During Year 2, measurements were only performed in the AM facility for comparison with Year 1.
Almost all gravimetric analyses were within the OEL, with the exception of one personal exposure measurement of cobalt in inhalable dust in the AM facility during Year 2 (Table 1). The AM operators showed a significantly higher level of cobalt and nickel in inhalable dust and lower level of manganese in total and inhalable dust compared with welders (Table 1). The AM operators showed a significant increase in the level of cadmium and decrease in the level of iron in inhalable dust when comparing Year 1 and Year 2 (Table 1).
Table 1.
Air concentrations of dust and metals collected using personal sampling for 8 hours using inhalable dust samplers (IOM, for inhalable fraction) or open-faced cassettes (for total dust fraction).
| Year | Location | n | Dust (mg/m3) | Cd (μg/m3) | Co (μg/m3) | Cr (μg/m3) | Fe (μg/m3) | Mn (μg/m3) | Mo (μg/m3) | Ni (μg/m3) | Pb (μg/m3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhalable fraction | |||||||||||
| Swedish occupational exposure limits | 5 | 20 | 200 | 100 | |||||||
| 1 | AM | 6 | 0.7 (0.6–1.3) | 0.1 (0.1-0.3) | 3.6 (0.6-15.9) | 21.3 (6.8-86.8) | 134.6 (114.7-253.8) | 0.4 (0.1-17.1) | 6.9 (2.6-19.6) | 38.4 (6.6-268.9) | 0.6 (0.3-12.2) |
| Welding | 8 | 0.8 (0.5-1.8) | 0.1 (0.1-0.3) | 0.2 (0.1-1.6)### | 6.8 (2.0-134.6) | 173.0 (99.8-579.7) | 3.1 (1.4-12.4)# | 3.4 (2.0-9.5) | 5.7 (2.0-99.4)# | 0.4 (0.3-0.8) | |
| 2 | AM | 8 | 0.9 (0.6-2.2) | 1.5 (1.2–2.0)*** | 1.5 (0.1-28.3) | 21.1 (3.0-331.0) | 48.7 (23.9-283.3)* | 1.5 (1.2-2.0) | 5.2 (1.2-49.1) | 38.3 (5.1-715.7) | 0.5 (0.4-0.6) |
| Total dust fraction | |||||||||||
| Swedish occupational exposure limits | 20 | 500 | 10 000 | 500 | |||||||
| 1 | AM | 6 | 0.2 (0.10-0.4) | 0.1 (0.1-0.3) | 0.7 (0.1-17.5) | 6.7 (2.0-59.4) | 128.7 (100.8-253.8) | 0.2 (0.1-0.9) | 2.8 (2.0-5.1) | 12.1 (2.0-256.1) | 0.4 (0.3-0.8) |
| Welding | 8 | 0.2 (0.2-0.6) | 0.1 (0.1-0.3) | 0.2 (0.1-0.6) | 4.7 (2.1-44.7) | 143.0 (105.1-280.9) | 1.3 (0.1-5.0)## | 2.7 (2.1-5.6) | 4.6 (2.1-30.0) | 0.4 (0.2-0.8) | |
*p < 0.05, Year 1 vs Year 2 in the AM facility at the same location and dust type.
**p < 0.01, Year 1 vs Year 2 in the AM facility at the same location and dust type.
***p < 0.001, Year 1 vs Year 2 in the AM facility at the same location and dust type.
#p < 0.05, AM vs welding in the same year at the same location and dust type.
##p < 0.01, AM vs welding in the same year at the same location and dust type.
###p < 0.001, AM vs welding in the same year at the same location and dust type.
AM, additive manufacturing.
Analyses were performed using the Student t test. Metals were analyzed by ICP-MS. Values are the geometric mean (minimum–maximum).
The stationary measurements showed generally lower levels compared with personal exposure (Supplemental table 1). Some of the statistically significant differences found in personal exposure assessments were also present in the stationary measurements, including decreased levels of manganese in inhalable dust in the AM facility compared with the welding facility and increased levels of cadmium and decreased levels of iron in inhalable dust when comparing Year 1 and Year 2 (Supplemental table 1).
3.1.2. Particle counting
Particle-counting instruments were used in both the AM and welding facility during the whole week of the first year. At the follow-up, particle counting was only performed in the AM facility.
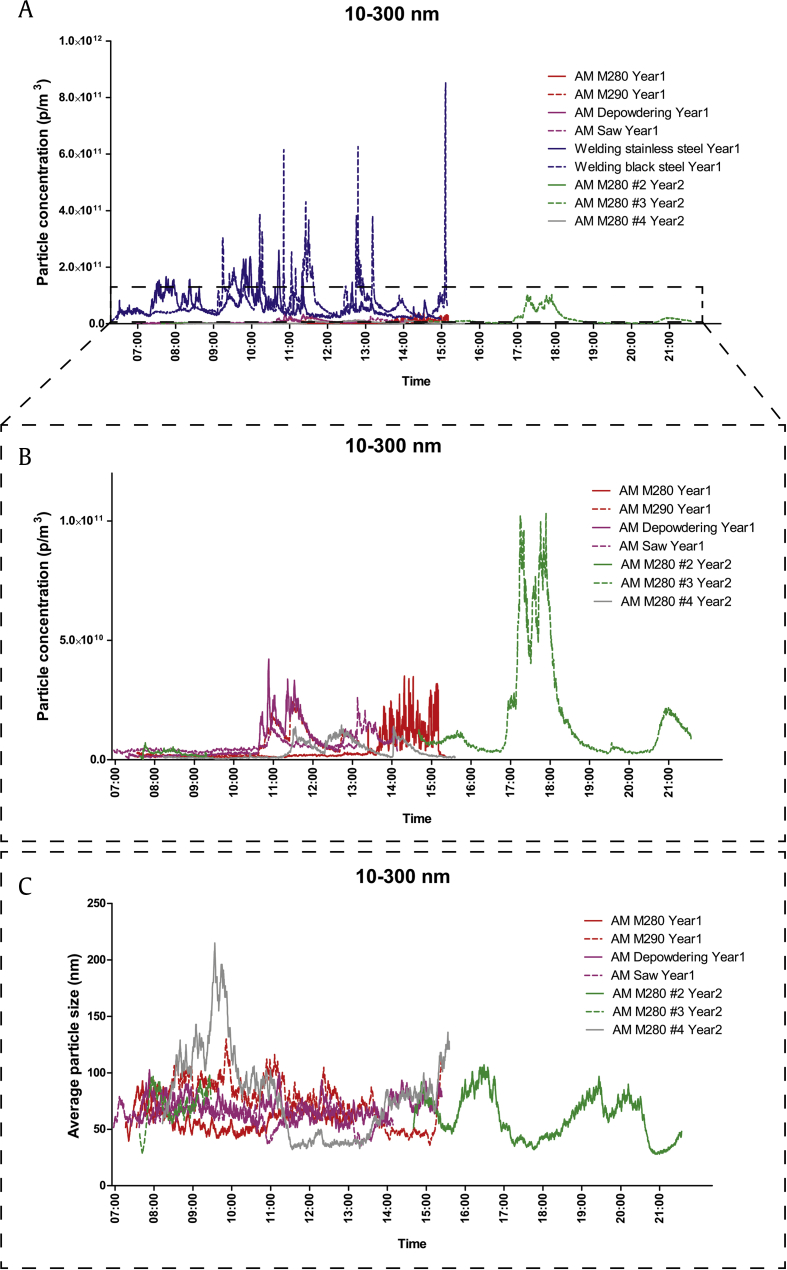
For the smallest particles (10–300 nm, measured using the Nanotracer), the welding facility showed much higher levels of particles compared with the AM facility (Fig. 1A) during normal production. In the AM facility, the highest levels were found during the second year (Fig. 1B). Elevated levels of particles could be detected at different work tasks, for example, sawing of printed products from the construction plate and removing of excess powder from the finished product. The highest levels of particle levels coincided with the smallest particle sizes (Fig. 1C).
Fig. 1.
Time-resolved measures of 10- to 300-nm particle concentrations in the AM facility in Year 1 (red and purple lines), welding facility in Year 1 (blue lines), and AM facility in Year 2 (green and gray lines). (A) Particle concentrations in the AM and welding facilities. (B) Particle concentrations in the AM facility. (C) Average particle sizes in the AM facility. AM, additive manufacturing.
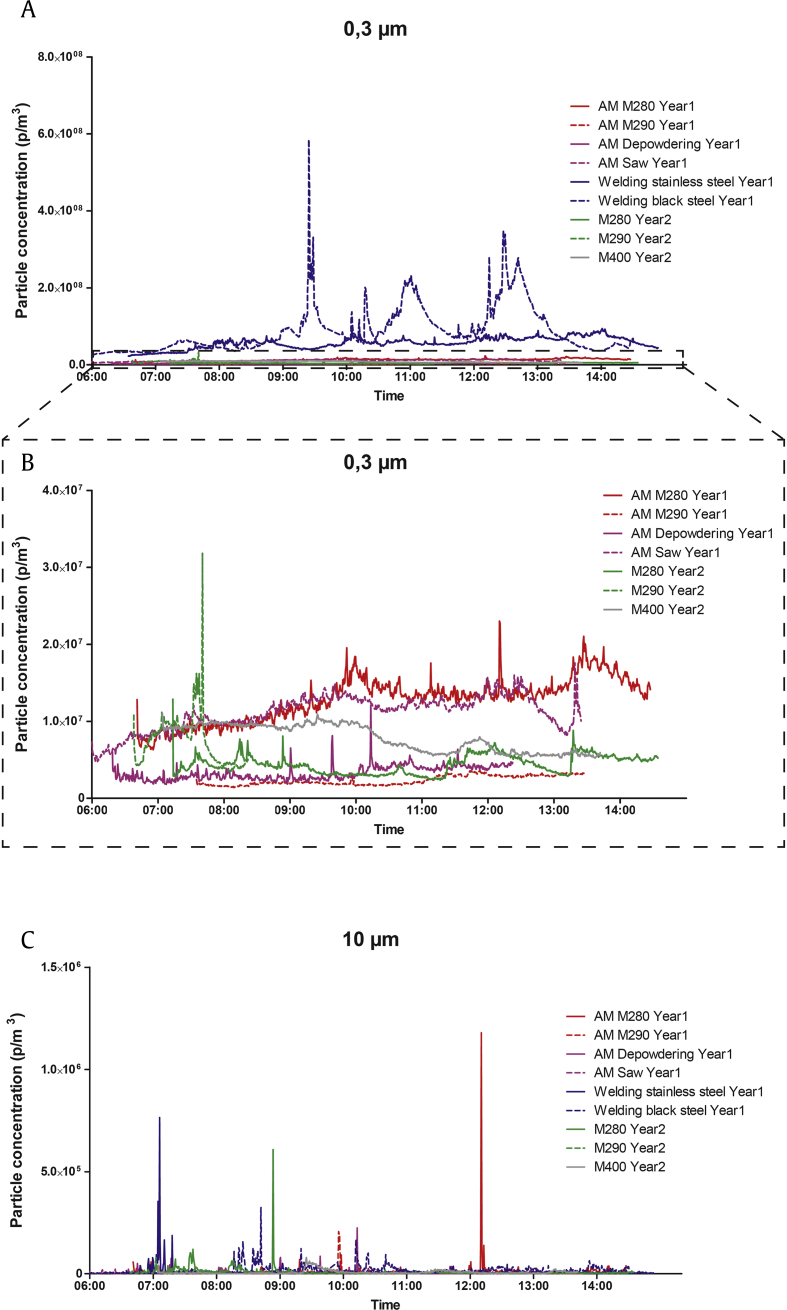
For slightly larger particles (0.3 μm, measured using the Lighthouse), a similar pattern as for the 10- to 300-nm particles was found with much higher levels in the welding facility compared with the AM facility (Fig. 2A). In the AM facility, the single highest peak was in the second year. However, there were sustained elevated levels throughout the day at some locations during Year 1 (Fig. 2B). For the largest measured particles (10 μm, measured using the Lighthouse), the highest peak was found in the AM facility in Year 2, followed by the welding facility and AM facility in Year 1 (Fig. 2C).
Fig. 2.
Time-resolved measures of 0.3- and 10-μm particle concentrations in the AM facility in Year 1 (red and purple lines), welding facility in Year 1 (blue lines), and AM facility in Year 2 (green and gray lines). (A) 0.3-μm particle concentrations in the AM and welding facilities. (B) 0.3-μm particle concentrations in the AM facility. (C) 10-μm particle concentrations in the AM and welding facilities. AM, additive manufacturing.
3.1.3. SEM analysis
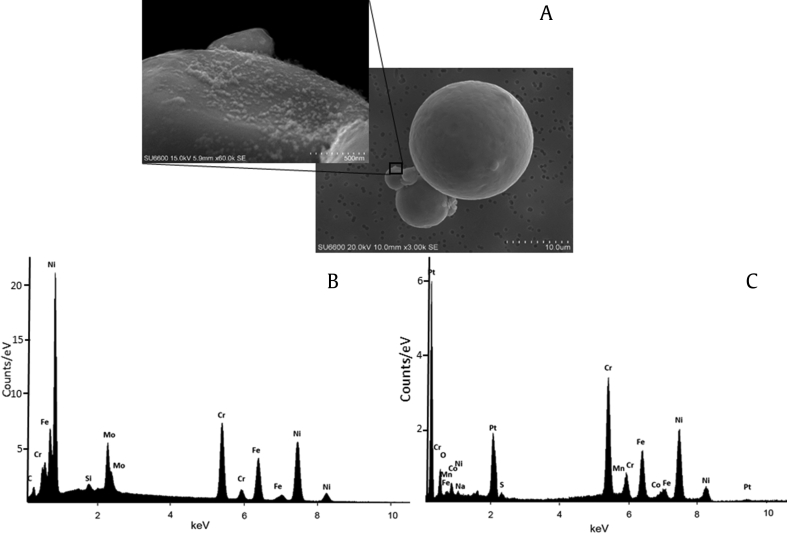
One fraction of particles in the recycled powder showed the presence of agglomerates composed of ultrafine particles attached to larger particles (Fig. 3A). The elemental analysis by EDX revealed that the ultrafine particles contained similar elements as larger particles in new powder with detectable manganese (Mn) but not molybdenum (Mo) (Fig. 3B and C). Furthermore, sodium (Na) and sulfur (S) were found in the ultrafine particle agglomerates but not in the larger particles of new powder.
Fig. 3.
SEM analysis. (A) Recycled powder with the inlay depicting the presence of ultrafine particle agglomerates on the larger particles. (B) Elemental analysis of unused powder using EDX. (C) Elemental analysis of ultrafine particle agglomerates from the inlay of (A). The detected Pt probably derives from the precoating. EDX, energy-dispersive X-ray spectrometry; SEM, scanning electron microscopy.
3.2. Biomonitoring
3.2.1. Participants
The participants in the study were voluntarily recruited from the available workforce. The number of participants and their gender distribution as well as age are displayed in Table 2.
Table 2.
Gender and age distribution of participating individuals, at Year 1 and Year 2.
| Participants | AM operators | Controls | Welders |
|---|---|---|---|
| Year 1 | |||
| Gender (F/M) | 1/6 | 5/6 | 0/11 |
| Age (mean ± SD) | 40 ± 12 | 40 ± 8 | 40 ± 13 |
| Year 2 | |||
| Gender (F/M) | 3/8 | 5/10 | |
| Age (mean ± SD) | 37 ± 9 | 44 ± 10 | |
AM, additive manufacturing; SD, standard deviation.
3.2.2. Urine metals
Urine metal levels were measured at the beginning and the end of the workweek in controls and AM operators at both Year 1 and Year 2 as well as in the welders at Year 1. The AM operators had a trend for a higher geometric mean of chromium, cobalt, and nickel compared with the controls (Table 3). The AM operators showed a trend toward a decreased geometric mean of cobalt and nickel in Year 2 than that in Year 1.
Table 3.
Density-adjusted urine metal values.
| Study group | Year | Beginning/end of the week | n | Cr (nmol/L) | Mn (nmol/L) | Co (nmol/L) | Ni (nmol/L) |
|---|---|---|---|---|---|---|---|
| Controls | 1 | Beginning | 11 | 1.3 (0.3–7.8) | 2.6 (0.56-219,3) | 3.9 (1.4-15.0) | 8.9 (1.0-49.8) |
| 2 | Beginning | 5 | 0.7 (0.3-2.4) | 8.1 (0.7-118.7) | 2.2 (0.6-5.7) | 9.2 (0.8-31.1) | |
| End | 6 | 0.5 (0.2-2.3) | 4.4 (0.1-129.5) | 2.8 (1.5-5.8) | 8.6 (1.5-30.3) | ||
| AM operators | 1 | Beginning | 7 | 1.5 (0.2-18.7) | 1.6 (0.4-10.7) | 5.6 (1.3-30.0) | 25.4 (3.6-107.1) |
| End | 6 | 1.8 (0.2-13.6) | 1.4 (0.7-2.2) | 7.3 (1.9-42.8) | 33 (6.9-94.9) | ||
| 2 | Beginning | 10 | 2.6 (0.3-20.1) | 8.6 (0.4-221.5) | 5.5 (1.4-11.5) | 22.5 (10.9-65.4) | |
| End | 9 | 1.3 (0.3-15.1) | 4.6 (0.4-258.6) | 4.7 (0.7-22.4) | 23.2 (5.0-115.7) | ||
| Welders | 1 | Beginning | 10 | 2.8 (1.2-5.3) | 1.2 (0.3-2.3) | 2.9 (1.9-5.0) | 16.7 (7.9-33.9) |
| End | 8 | 3.3 (1.1-12.6) | 1.6 (0.6-3.6) | 3.7 (2.2-6.4) | 24.9 (8.9-2118.4) |
AM, additive manufacturing.
Values are the geometric mean (minimum–maximum).
There was an elevated concentration of manganese in some controls and AM operators at Year 2 compared with Year 1. This was later found to depend on high concentrations of manganese in a coffee blend used in these locations in Year 2, where coffee drinkers showed elevated levels, whereas noncoffee drinkers showed no such trend (data not shown).
3.2.3. Dermal exposure
In Year 1, three of the AM operators had detectable levels of cobalt, nickel, and chromium at the end of the workweek on the index finger of their dominant hand (mean concentration of cobalt: 110 ng/cm2, chromium: 370 ng/cm2, and nickel: 630 ng/cm2) as analyzed by XRF. Elevated levels of cobalt were also found on various surfaces in the production area (machine display and floor). In the second year, none of the study participants had detectable levels of cobalt on the skin of their index fingers, as analyzed with XRF.
4. Discussion
Along with increased use of AM technologies, in many different industrial sectors, there is a need for characterization of potential exposure risks and relevant preventive actions that reduce eventual health risks. Here, we present data on occupational exposure of AM operators working with metal powder in a serial production unit.
4.1. Gravimetric analyses
The gravimetric analyses showed that the levels of total and inhalable dust in the AM facility were all below the Swedish OEL. Furthermore, the filter-based metal analyses were all but one within the OEL. There was also a significant increase in the level of cobalt in inhalable dust when comparing AM and welding environments, but the level was relatively low, reaching about 20% of the Swedish OEL. However, because cobalt is a possible carcinogen in the presence of other metals [14], even low levels may be detrimental. The major components of the metal powder (including nickel, chromium, and molybdenum, but not iron) were higher in the AM environment than in the welding environment, indicating exposure risks to metal powder in the AM facility. A positive finding was that no difference was found in the AM facility between Year 1 and Year 2 regarding particle dust, despite a 30% increase in the number of units produced during the two years. The unchanged dust levels likely result from improved work routines and investment in safer machines but may also mirror the fact that gravimetric analyses are not the optimal method for measurement of small particles [7]. The levels of metals on the filters were higher from the personal samplers compared with stationary sampling at the AM machines for inhalable dust during both years, indicating an increased exposure for operators not only in direct contact with AM machines.
4.2. Particle counting analyses
Besides the gravimetric analyses, particle-counting instruments were used to investigate smaller particles and to get a time-resolved image of particles generated in the AM and welding facilities. The levels of both nanoparticles (average size in general between 50 and 100 nm, as measured using the Nanotracer) and slightly larger particles (0.3 μm measured using the Lighthouse) were constantly much higher in the welding facility compared with the AM facility. High levels of nanoparticles in welding facilities are in line with what we found in the pilot study [7] and what is known from recent studies focusing on welding [15]. This may depend on the fact that in the welding facility, there are a wide array of different processes including welding, metal cutting, and combustion engines that may contribute to the bulk of produced small particles. The AM facility on the other hand is specialized for AM, and here, the powder sizes range from 10 nm up to 60 μm or larger, depending on powder characteristics. The main bulk of the smallest particles seems to be generated during the AM process (which takes place in an enclosed chamber that is ventilated after the process is completed) and is then accumulated owing to sieving and recycling of the powder. Owing to the lack of OELs for nanoparticles and the expanding use of AM, the Nanosafety Research Centre of the Finnish Institute of Occupational Health (FIOH) has defined a target value of 20000 nanoparticles/cm3 (at a density of >6000 kg/m3) for an 8-h exposure time [16]. In the present study, the levels of nanoparticles (10–300 nm measured using the Nanotracer) in the AM facility reached an average of 6300 particles/cm3 in all measurements that lasted for more than 5 h (range, 3500–17000 particles/cm3), that is, below the target limit proposed by the FIOH. This is good news because nanoscaled particles are believed to possess different toxicological properties compared with larger particles and may be more toxic at the cellular level [17]. However, the levels of 10-μm particles in the AM facility must not be neglected because these particles could still be harmful if inhaled or ingested. Inhalation of metal particles alters nasal lavage fluid acute-phase proteins [15] and may increase cancer risk in the lungs, kidneys, and bladder [18].
4.3. SEM analysis
The SEM analysis showed particles in the size range of 4–10 μm, where recycling of the powder caused an apparent fragmentation of particles to smaller sizes, which is similar to what we found in the pilot study. (Note that another metal alloy powder was used.) [7] Furthermore, we identified ultrafine particles (condensates) agglomerated onto larger particles in recycled powder, similar to what has been shown in other studies using SLM [19], [20]. The sampling location in these studies was on the powder bed or on the chamber wall, where the concentration of condensates probably will be much higher than that on the powder. Sutton et al. [19] investigated the condensate by SLM of 304-L stainless steel powder by EDX and reported significant amounts of all elements found in the base powder, with the exception of Ni. They concluded that vaporization of all elements occurred. In our study, the agglomerated ultrafine particles appeared to be enriched in more volatile elements such as manganese. This may be expected because the particles are formed by evaporation and condensation of alloying elements from the starting powder. In addition, sodium and sulfur, which only exist as trace elements in the starting powder, were detected in the agglomerates. It is important to keep in mind that the background signal from larger particles can affect the EDX results. Further investigations are necessary to draw conclusions about the condensate particles found in this study.
4.4. Urine metal analysis
Measurements of urine metals indicated that AM operators had a higher body burden of the metals present in the metal powder (although nonsignificant) than the office controls. In the AM operators, there was a trend for increased levels of nickel, chromium, and cobalt during the Workweek Year 1 (20–30% increase on the group level). At follow-up, the metal levels were in general lower and did not increase during the workweek. This indicates that the preventive actions taken by the company with work routine restrictions and use of PPE at the most critical work tasks successfully decreased the exposure of the operators. However, the AM operators still had a trend for elevated levels compared with the controls, indicating that there could be some persistent exposure not prevented by the interventions. It should be noted that the production rate was increased in the second year compared with the first, possibly masking differences between Year 1 and Year 2.
The absolute levels of metals in the investigated workers were low, for example, the geometric mean of Co in the AM operators was lower than what has been found in different nonoccupationally exposed populations (geometric mean: 7.3–13.2 nmol/L) [21], [22], [23] and much lower than that in different occupationally exposed individuals (median/geometric mean: 62.7–228 nmol/L) [24], [25]. In contrast, one of the welders had very high nickel values in urine at the end of the Workweek Year 1 (>2000 nmol/L), by far exceeding the biomonitoring action limit of 100 nmol/L used by the FIOH [26]. Further investigation indicated that this individual had worked without any PPE throughout the week. This illustrates how important it is to wear gas and particle full-face protection while welding. It also points out the importance of how a welding facility is designed or equipped to avoid release of nanoparticles into the surrounding air.
4.5. Dermal exposure
Because some earlier studies have shown a possible contribution to the uptake of cobalt from skin exposure [27], [28], dermal exposure was investigated for the AM operators. The investigation of dermal exposure indicated that AM operators during Year 1 had a detectable dermal exposure. The participants with the highest levels of cobalt on their hands in Year 1 were the same individuals with the highest level of cobalt in urine at the same time. This indicates a link between dermal exposure and urine metal levels, similar to what has been found by others [27], [28]. The reason for the reduced levels of dermal exposure between Year 1 and Year 2 probably depends on stricter safety instructions and increased use of gloves by the operators.
4.6. Limitations and future perspectives
The study has limitations such as the relative low number of participants for biomonitoring. The AM site in which the measurements have been performed is one of the world-leading sites using AM not just for prototyping but also for actual serial production of metal components. The production is expanding, which can be seen in the larger number of individuals available for measurements during Year 2 compared with Year 1. We managed to recruit all available AM operators at the site, thereby reducing the risk for skewing of data owing to selective participation.
The present study deals with the need for information regarding health and safety related to metal AM. As AM is a relatively new technique that is rapidly expanding and most likely will involve a large number of workers in the near future, it is of great importance to provide the industry with information regarding exposure and best practice to reduce eventual health risks. This includes finding out what type of exposure and biomonitoring measurements may be used to test facilities and workers to reduce the risk. The present study has revealed a need for particle-counting instruments, as well as limit values, as a complement to gravimetric analyses for identification of particles that do not contribute to a mass or for identification of work tasks with increased exposure. Optimal design and ventilation for welding facilities and obligatory PPE should be considered. Regarding AM, machines with closed powder-handling systems should be of high priority, and owing to the complex powder distribution, optimal ventilation systems should be further investigated. The study also shows that biomonitoring using urine and dermal metals may be a good way to ensure that workers are minimally exposed.
In summary, the present study shows that the AM operators and welders have relatively low dust exposure based on gravimetric analyses. However, particle-counting instruments showed high numbers of nanoparticles in welding environments and peaks of particles ranging from 10 nm to 10 μm or larger at specific work tasks in the AM environment. Biomonitoring revealed elevated exposure during the first year, which was reduced by control measures the second year. This emphasizes the need for careful design and regulation, as well as the need for relevant exposure and biomonitoring markers of health risks, in the AM environments.
Conflicts of interest
The authors declare no conflict of interest. All authors, except E.N., work in regional or state financed organizations and do not have any ties with companies that produce or sell 3D printing/additive manufacturing equipment. E.N. works in a company that uses 3D printing in the production, but do not produce or sell 3D printing/additive manufacturing equipment.
Acknowledgments
The present study was supported by AFA Insurance, Sweden (grant number, 150246), and the employers Occupational and Environmental Medicine in Linköping, Region Östergötland, Sweden, Occupational and Environmental Medicine in Örebro, Sweden, and the National Institute of Occupational Health, Oslo, Norway.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.shaw.2019.07.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary information is available at Journal of Exposure Science and Environmental Epidemiology's website.
References
- 1.Gokuldoss P.K., Kolla S., Eckert J. Additive manufacturing processes: selective laser melting, electron beam melting and binder jetting-selection guidelines. Materials (Basel) 2017;10:E672. doi: 10.3390/ma10060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipson H., Kurman M. Wiley; Indianapolis, IN, USA: 2013. Fabricated: the new world of 3D printing. [Google Scholar]
- 3.Rehfisch P., Anderson M., Berg P., Lampa E., Nordling Y., Svartengren M. Lung function and respiratory symptoms in hard metalworkers exposed to cobalt. J Occupat Environ Med. 2012;54:409–413. doi: 10.1097/JOM.0b013e31824d2d7e. [DOI] [PubMed] [Google Scholar]
- 4.Christensen S.W., Bonde J.P., Omland O.A. Prospective study of decline in lung function in relation to welding emissions. J Occup Med Toxicol. 2008;3:69–78. doi: 10.1186/1745-6673-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Zein M., Gautrin D., Infante-Rivard C., Malo J. Prevalence and association of welding related systemic and respiratory symptoms in welders. Occup Environ Med. 2003;60:655–661. doi: 10.1136/oem.60.9.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andujar P., Simon-Deckers A., Galateau-Salle F., Fayard B., Beaune G., Clin B. Role of metal oxide nanoparticles in histopathological changes observed in the lung of welders. Part Fibre Toxicol. 2014;11:23. doi: 10.1186/1743-8977-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graff P., Ståhlbom B., Nordenberg E., Graichen A., Johansson P., Karlsson H. Evaluating measuring techniques for occupational exposure during additive manufacturing of metals: a pilot study. J Indust Ecol. 2017;21:S120–S129. [Google Scholar]
- 8.SS-EN ISO 10882-1 . 2011. Health and safety in welding and allied processes – sampling of airborne particles and gases in the operator's breathing zone – Part 1: sampling of airborne particles. [Google Scholar]
- 9.SS-ISO 15767 . 2010. Workplace atmospheres – controlling and characterizing uncertainty in weighing collected aerosols. [Google Scholar]
- 10.NIOSH 2003, Method 7300: elements by ICP. In NIOSH Manual of analytical methods. 4 ed. Cincinatti: National Institute of Occupational Safety and Health (NIOSH).
- 11.SS-ISO 15202-1, Workplace air – determination of metals and metalloids in airborne particulate matter by inductively coupled plasma atomic emission spectrometry – Part 1: Sampling.
- 12.SS-ISO 15202-2 . 2017. Workplace air – determination of metals and metalloids in airborne particulate matter by inductively coupled plasma atomic emission spectrometry – Part 2: sample preparation. [Google Scholar]
- 13.Marra J., Voetz M., Kiesling H.-J. Monitor for detecting and assessing exposure to airborne nanoparticles. J Nanopart Res. 2010;12:21–37. [Google Scholar]
- 14.Wild P., Bourgkard E., Paris C. Lung cancer and exposure to metals: the epidemiological evidence. Methods Mol Biol. 2009;472:139–167. doi: 10.1007/978-1-60327-492-0_6. [DOI] [PubMed] [Google Scholar]
- 15.Ali N., Ljunggren S.A., Karlsson H., Wierzbicka A., Pagels J., Isaxon C. Comprehensive proteome analysis of nasal lavage samples after controlled exposure to welding nanoparticles shows an induced acute phase and a nuclear receptor, LXR/RXR, activation that influence the status of the extracellular matrix. Clin Proteomics. 2018;15:20. doi: 10.1186/s12014-018-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FIOH Control Approach – chemical safety of 3D printing at workplaces. https://www.ttl.fi/wp-content/uploads/2016/11/Chemical-safety-in-3D-printing-at-workplaces.pdf, accessed 10th October 2018.
- 17.Peixe T.S., de Souza Nascimento E., Schofield K.L., Arcuri A.S.A., Bulcão R.P. Nanotoxicology and exposure in the occupational setting. Occup Dis Environ Med. 2015;3:35–48. [Google Scholar]
- 18.MacLeod J., Harris A.M., Tjepkema M., Peters P.A., Demers P.A. Cancer risks among welders and occasional welders in a national population-based cohort study: Canadian census health and environmental cohort. Saf Health Work. 2017;8:258–266. doi: 10.1016/j.shaw.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton A., Kriewall C., Leu M.-C., Newkirk J.W. Proceedings of the 28th solid freeform fabrication symposium (2017. University of Texas at Austin -- Laboratory for Freeform Fabrication (LFF); Austin, TX): Aug 2017. Characterization of heat-affected powder generated during selective laser melting of 304L stainless steel powder; pp. 261–276. [Google Scholar]
- 20.Gunenthiram V., Peyre P., Schneider M., Dal M., Coste F., Fabbro R. Analysis of laser-melt pool-powder bed interaction during the selective laser melting of a stainless steel. J Laser Appl. 2017;29 022303. [Google Scholar]
- 21.Aprea M.C., Apostoli P., Bettinelli M., Lovreglio P., Negri S., Perbellini L. Urinary levels of metal elements in the non-smoking general population in Italy: SIVR study 2012-2015. Toxicol Lett. 2018;298:177–185. doi: 10.1016/j.toxlet.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Nisse C., Tagne-Fotso R., Howsam M. Members of Health Examination Centres of the Nord − Pas-de-Calais region network, Richeval C, Labat L, Leroyer A. Blood and urinary levels of metals and metalloids in the general adult population of Northern France: the IMEPOGE study, 2008-2010. Int J Hyg Environ Health. 2017;220:341–363. doi: 10.1016/j.ijheh.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Paschal D.C., Ting B.G., Morrow J.C., Pirkle J.L., Jackson R.J., Sampson E.J. Trace metals in urine of United States residents: reference range concentrations. Environ Res. 1998;76:53–59. doi: 10.1006/enrs.1997.3793. [DOI] [PubMed] [Google Scholar]
- 24.Princivalle A., Iavicoli I., Cerpelloni M., Franceschi A., Manno M., Perbellini L. Biological monitoring of cobalt in hard metal factory workers. Int Arch Occup Environ Health. 2017;90:243–254. doi: 10.1007/s00420-016-1190-y. [DOI] [PubMed] [Google Scholar]
- 25.Hutter H.P., Wallner P., Moshammer H., Marsh G. Dust and cobalt levels in the Austrian tungsten industry: workplace and human biomonitoring data. Int J Environ Res Public Health. 2016;13:E931. doi: 10.3390/ijerph13090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.FIOH Biomonitoring of exposure to chemicals. https://www.ttl.fi/wp-content/uploads/2017/11/Biomonitoring-of-exposure-to-chemicals-Guideline-for-specimen-collection.pdf , accessed 12th December 2018.
- 27.Kettelarij J., Midander K., Liden C., Bottai M., Julander A. Neglected exposure route: cobalt on skin and its associations with urinary cobalt levels. Occup Environ Med. 2018;75:837–842. doi: 10.1136/oemed-2018-105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klasson M., Lindberg M., Bryngelsson I.L., Arvidsson H., Pettersson C., Husby B. Biological monitoring of dermal and air exposure to cobalt at a Swedish hard metal production plant: does dermal exposure contribute to uptake? Contact Dermat. 2017;77:201–207. doi: 10.1111/cod.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information is available at Journal of Exposure Science and Environmental Epidemiology's website.