Abstract
Severe inborn cardiac malformations are typically corrected in cardioplegia, with a cardio-pulmonary bypass (CPB) taking over body circulation. During the operation the arrested hearts are subjected to a global ischemia/reperfusion injury. Although the applied cardioplegic solutions have a certain protective effect, application of additional substances to reduce cardiac damage are of interest.
18 domestic piglets (10–15 kg) were subjected to a 90 min CPB and a 120 min reperfusion phase without or with the application of epigallocatechin-3-gallate (10 mg/kg body weight) or minocycline (4 mg/kg body weight), with both drugs given before and after CPB. 18 additional sham-operated piglets without or with epigallocatechin-3-gallate or minocycline served as controls. In total 36 piglets were analyzed (3 CPB-groups and 3 control groups without or with epigallocatechin-3-gallate or minocycline respectively; 6 piglets per group). Hemodynamic and blood parameters and ATP-measurements were assessed. Moreover, a histological evaluation of the heart muscle was performed.
Results
Piglets of the CPB-group needed more catecholamine support to achieve sufficient blood pressure. Ejection fraction and cardiac output were not different between the 6 groups. However, cardiac ATP-levels and blood lactate were significantly lower and creatine kinase was significantly higher in the three CPB-groups. Markers of apoptosis, hypoxia, nitrosative and oxidative stress were significantly elevated in hearts of the CPB-group. Nevertheless, addition of epigallocatechin-3-gallate or minocycline significantly reduced markers of myocardial damage. Noteworthy, EGCG was more effective in reducing markers of hypoxia, whereas minocycline more efficiently decreased inflammation.
Conclusions
While epigallocatechin-3-gallate or minocycline did not improve cardiac hemodynamics, markers of myocardial damage were significantly lower in the CPB-groups with epigallocatechin-3-gallate or minocycline supplementation.
Keywords: Cardio-pulmonary bypass; Heart; Minocycline; EGCG, ischemia/reperfusion injury
Abbreviations: ACT, activated clotting time; AEC, 3-amino-9-ethylcarbazole; AIF, apoptosis-inducing factor; cC3, cleaved caspase-3; CO, cardiac output; CPB, cardio-pulmonary bypass; DNA, deoxyribonucleic acid; EF, ejection fraction; EGCG, epigallo-3-catechin-gallate; HIF1α, hypoxia-inducible factor α; HPLC, high pressure liquid chromatography; MPTP, mitochondrial permeability transition pore; NT, nitrotyrosine; PAR, poly-ADP-ribose; PARP, poly-ADP-ribose polymerase; ROS, reactive oxygen species; TNFα, tumor necrosis factor α
1. Introduction
Many cardiac operations are performed under cardiac arrest during which a heart-lung machine ensures body circulation and oxygenation. JH Gibbon was the inventor of the heart-lung machine i.e. the cardio-pulmonary bypass (CPB) and also was the first cardiac surgeon who uses this apparatus during closure of an atrial septal defect (Gibbon, 1954). The invention of a heart-lung machine significantly advanced the development of cardiac surgery. Since that time various cardiac operations could be carried out such as coronary bypass surgery or correction of congenital heart diseases. Nowadays, even severe cardiac malformations -like tetralogy of Fallot or transposition of the great arteries- may be corrected with the help of CPB and a lot of those patients, which otherwise would have died, now have a nearly normal life-span or at least may reach adulthood (Bailliard and Anderson, 2009, Martins and Castela, 2008).
When the CPB is connected to the patient, the heart is arrested using cardioplegic solutions with heart and lung being bypassed for a certain period, during which especially the heart is not supplied with blood or oxygen. This means a severe global ischemic injury to the heart muscle and additionally when the patient is de-cannulated from CPB also a severe reperfusion damage (Royster, 1993). To protect the heart against ischemia/reperfusion injury, cold cardioplegic solutions -namely St. Thomas or Bretschneider solution- or warm blood cardioplegia (Calafiore blood cardioplegia) are the most widely implemented procedures in the operation theatre (Braimbridge et al., 1977, Bretschneider, 1980, Calafiore et al., 1995). However, impairment of cardiac function with the need of catecholamine therapy is not uncommon after CPB and worsens patient’s outcome (Salgado et al., 2015).
As cardiomyocytes are not able to store sufficient amounts of ATP but on the other hand have a high demand, ATP-levels drop rapidly during ischemia. Although during CPB the heart is arrested and mostly cooled as described above it comes to ischemic damage which is further aggravated during reperfusion, when the heart is re-warmed and starts contracting. On the cellular level ischemia/reperfusion injury induces several signaling cascades. The transcription factor HIF1α (hypoxia-inducible factor 1α) is activated by hypoxic conditions and translocates into the nucleus thereby promoting gene activation to improve cell survival (Benizri et al., 2008). On the other hand, this factor was also associated with apoptosis and cell death and thus an inhibition of HIF1α might be beneficial for cells (Guo et al., 2001). Furthermore, oxygen and nitrogen radicals, which are elevated mainly during reperfusion, lead to mitochondrial damage and DNA strand breaks. The following activation of apoptosis factors (AIF, apoptosis-inducing factor) by PAR (poly-ADP-ribose) finally lead to cell death (Yu et al., 2006). CPB also induces inflammation via cytokine activation (for example TNFα) thereby triggering the extrinsic apoptosis pathway via caspase cleavage (Cheng et al., 2017). Thus, several signaling cascades involved in ischemia/reperfusion injury could finally lead to a dysfunction of the heart muscle, often seen after CPB. Therefore, cardiac protection during CPB is still a major issue.
The main green tea ingredient EGCG (epigallo-3-catechin-gallate) has anti-oxidant properties and seems to be beneficial in reducing oxygen radicals (Kim et al., 2014a, Kim et al., 2014b). We and others could show that this catechin had a positive influence on kidney injury during CPB (Funamoto et al., 2016, Kasper et al., 2016). Additionally, as significant inflammation is provoked by CPB it might be that inti-inflammatory drugs decrease cytokine production and thus prevent from caspase-induced apoptosis during CPB (Drabek et al., 2014, Hill, 1998).
In a Langendorff-model of isolated perfused rabbit hearts we could demonstrate, that EGCG and minocycline were effective in reducing cardiac ischemia/reperfusion damage (Salameh et al., 2015a, Salameh et al., 2015b, Salameh et al., 2015c, Salameh et al., 2018). Thus, to evaluate both drugs in a more clinical setting, we used piglets subjected to a 90 min CPB with subsequent reperfusion (120 min) without or with either EGCG or minocycline. During the experiment, cardiac hemodynamics were examined. Thereafter, the hearts were removed and histological analysis for apoptosis induction, nitrogen radicals and TNFα was performed. Additionally, cardiac ATP-levels were determined.
2. Materials and methods
The following procedures were approved by the Animal Care Committee of the German Regional Council Leipzig, namely “Landesdirektion Sachsen, Dienststelle Leipzig”, approval number W05/11 and W05/12, which ensured humane treatment of all animals as indicated by the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
For our evaluation of EGCG and minocycline during ischemia/reperfusion injury, 36 domestic piglets (weighting between 10 and 15 kg) were randomly assigned to 6 experimental groups with 6 piglets per group: time control without drugs and without CPB, control group with EGCG, control group with minocycline, CPB group without drugs, CPB group with EGCG and CPB group with minocycline. Hearts of piglets assigned to the CPB groups were arrested for 90 min -while body perfusion was maintained by the heart-lung machine-, followed by a 120 min reperfusion phase according to Salameh et al. (2015a). Control piglets were also anaesthetized and a thoracotomy was carried out without connection to the CPB. In control piglets and piglets undergoing CPB with drug administration EGCG (10 mg/kg body weight) or minocycline (4 mg/kg body weight) were applied intravenously 15 min before baseline measurements. A second dose was applied after disconnection from CPB (EGCG 10 mg/kg body weight or minocycline (2 mg/kg body weight). The control piglets with EGCG or minocycline also received a second drug administration at the appropriate time point.
Anesthesia of the piglets was carried out by veterinarians, connecting and running the CPB was performed by experienced heart surgeons and perfusionists. The piglets were evaluated during the whole experiments by a cardiologist. At baseline (time point 0) and after 120 min of reperfusion hemodynamic measurements and analysis of lactate and cardiac creatine kinase were carried out. Thereafter, at the end of the experiment, the piglets were slaughtered and specimens from the left ventricular free wall were either shock-frozen in liquid nitrogen for ATP measurements or fixed with formalin for histological evaluation.
2.1. Experimental protocol
A detailed description of the experimental protocol is given in Salameh et al. (2015b). Briefly, piglets were premedicated with atropine (0.02 mg/kg body weight), midazolam (0.5 mg/kg body weight) and ketamine (25 mg/kg body weight), orally intubated and ventilated with 50% O2 and 50% air (anesthesia apparatus: Cato, Drägerwerk, Lübeck, Germany). General anaesthesia was performed with isoflurane (1.5–2%) and analgesia with sufentanil (initial bolus 3 µg/kg body weight, followed by 1–2 µg/h/10 kg body weight). In the appropriate experimental groups, a single dose of minocycline or EGCG was applied intravenously 15 min before baseline measurements (hemodynamic parameters, blood samples at time point 0). Thereafter, thoracotomy was carried out and heparin was administered to achieve an ACT (activated clotting time) of 400 s. The right auricle and aortic bow were cannulated and the CPB (priming volume: 350 ml whole blood) was connected (Salameh et al., 2017). CPB was started, the aorta was cross-clamped and the heart was arrested with cold cardiolplegic solution (Custodiol, Koehler Chemie, Bensheim, Germany). During CPB the piglets were cooled to 28 °C and warmed up again to 37 °C at the end of CPB. Anaesthesia was switched from isoflurane to propofol (25–35 mg/kg body weight) during the whole bypass time. To achieve the same experimental conditions, control piglets also received propofol for 90 min.
Throughout the entire experiment blood gases (normal range: pO2 75–100 mmHg, pCO2 35–45 mmHg, pH 7.35–7.45), central venous pressure (maximum 12 mmHg), mean arterial pressure (target value > 50 mmHg) and oxygen saturation (>90%) were controlled and therapeutic interventions were carried out if necessary. Monitoring of the piglets during CPB followed standard procedures in cardiac surgery.
At the end of CPB (i.e. after 90 min bypass-time) aortic clamp was opened and reperfusion started. The flow of CPB was consecutively reduced to zero and after 30 min the piglets were disconnected from CPB. In the experimental groups with drugs a second dose was given after disconnection. If necessary, circulation was supported by catecholamines (adrenaline and noradrenaline). All piglets were successfully weaned from CPB. After a reperfusion phase of additional 90 min (i.e. in total 120 min after opening of aortic clamp) blood samples were taken to analyse lactate and creatine kinase levels and hemodynamic parameters were assessed and samples of the left ventricular free wall were taken for histological analysis and ATP measurements.
2.2. Pressure-volume loops
Pressure-volume loops were carried out as described previously (Salameh et al., 2012). In brief, a pressure-volume Millar catheter (Millar Instruments, Houston, USA) was inserted into the left ventricle via the left carotid artery. The catheter was connected to a signal amplifier system (MPVS Ultra, Millar Instruments, Houston, USA) and data acquisition and analysis was performed with the Power Lab and Lab Chart Software from ADInstruments (sales department FMI Föhr Medical Instruments GmbH, Seeheim, Germany). Parallel conductance was corrected by injection of hypertonic saline solution (1 ml/10 kg body weight, 10% NaCl) and calibration of stroke volume by the thermodilution method (Picco-system, Picco Pulsion Medical Systems, Munich, Germany). From the pressure-volume loops ejection fraction (EF) and cardiac output were calculated.
2.3. ATP-measurements
Tissue ATP-content was evaluated using the “High Pressure Liquid Chromatography” (HPLC) method as previously published (Dhein et al., 2015). Briefly, tissue samples were homogenized on ice with 5 ml 0.4 M perchloric acid. Thereafter, the extracts were precipitated with 0.8 ml 0.2 mol/L KOH and centrifuged at 4 °C with 3000 g for 10 min. The supernatant was used for ATP measurements. 20 µl were injected onto a RP18 column (Merck, Darmstadt, Germany). The mobile phase consisted of KH2PO4 (215 mM), tetrabutylammonium hydrogen sulphate (2.3 mM), acetonitrile (4%) and KOH (1 M, 0.4%). The HPLC apparatus and UV-detector were from Knauer (Berlin, Germany). ATP peaks were analyzed at 254 nm. ATP standards at three different concentrations (2, 20 and 60 µg/mL) were used to generate a standard curve. Unknown samples and ATP standards were injected three times and ATP concentrations were determined as the mean of these three chromatograms.
2.4. Histological evaluation
Specimens from the left ventricular free wall, fixed with 4% formalin, were embedded in paraffin and 2 µm slices were cut. The samples, were mounted on microscopic slides, dewaxed, re-hydrated and antigen retrieval was performed according to Dhein et al. (2015). Thereafter, myocardial tissue was incubated with primary antibodies for HIF1α (hypoxia-inducible factor, host rabbit, dilution 1:100, Santa Cruz, Heidelberg, Germany), AIF (apoptosis-inducing factor, host mouse, dilution 1:100, Santa Cruz, Heidelberg, Germany), cC3 (cleaved caspase-3, host rabbit, dilution 1:200, New England Biolabs, Frankfurt, Germany), PAR (poly-ADP-ribose, host mouse, dilution 1:600, BioRad, Munich, Germany), NT (nitrotyrosine, host mouse, dilution 1:50, Merck, Darmstadt, Germany) or TNFα (tumor necrosis factor α, host goat, dilution 1:100, Santa Cruz, Heidelberg, Germany) at 4 °C over night. Afterwards, the slides were washed and the appropriate secondary antibodies (anti-mouse, anti-rabbit or anti-goat) labelled with horseradish peroxidase were applied for 1 h at room temperature. Subsequently, the specimens were washed again and visualization of positive cells was performed with 3-amino-9-ethylcarbazole (AEC, red chromogen, Dako, Hamburg Germany). Cell nuclei were counterstained with hematoxylin.
The specimens were investigated microscopically using the Axioimager M1 microscope from Zeiss (Carl Zeiss, Jena, Germany). Photographs were taken at 400× magnification and at least 10 pictures per slide i.e. 60 pictures per experimental group were evaluated by a blinded observer. Only longitudinal sections of cardiomyocytes were analyzed. As HIF1α, PAR, AIF and cC3 translocate into the nuclei after activation, the ratio of positive red cell nuclei and negative blue cell nuclei was calculated. In contrast, as TNFα and NT are located within the cytosol, we therefore determined the ratio of positive red cells and negative unstained cells.
In this way at least 120 cardiomyocytes per picture were analyzed, which means -as 10 pictures per slide were taken- 1200 cardiomyocytes per animal and 7200 cardiomyocytes per experimental group.
2.5. Statistical analysis
All results are presented as mean value and standard error of mean (SEM) of n = 6 experiments. Normal distribution of the data was tested with Shapiro–Wilḱs test.
Hemodynamic data were evaluated with two-way ANOVA followed by Students t-test with Bonferroni correction for multiple measurements to detect statistical significance at a level of p < 0.05. Blood parameters, histological data and ATP measurements were tested for statistical significance using the non-parametric Kruskal-Wallis test (p < 0.05).
For the statistical analysis Systat for Windows, version 13 (Systat Inc., Evanston, IL, USA) was used.
3. Results
Baseline parameters were not significantly different among the 6 experimental groups. At the beginning of the experiment and after reperfusion (at the end of the experiment) ejection fraction, cardiac output and blood pressure did not differ significantly among the 6 experimental groups (Table 1). However, as vital parameters of the piglets were closely monitored, catecholamines had to be administered especially during and after weaning from CPB in the three CPB-groups to support the cardiovascular system. As a result, total catecholamine requirement was significantly higher in piglets subjected to CPB compared to the corresponding control groups (Table 1). Moreover, lactate an indicator for the metabolic state and creatine kinase an indicator for muscle damage were significantly elevated in the three CPB-groups at the end of the reperfusion period (table1). Only the CPB + EGCG group showed a slight but significant creatine kinase reduction compared to CPB alone.
Table 1.
Blood parameters and hemodynamic data at baseline conditions and after reperfusion. Catecholamine consumption during the entire experiment and tissue ATP-content at the end of reperfusion.
| Parameters | Control | Control + EGCG | Control + Minocycline | CPB | CPB + EGCG | CPB + Minocycline |
|---|---|---|---|---|---|---|
| Creatine kinase (µmol/(L * s) | ||||||
| Baseline | 18 ± 2.9 | 15 ± 2.7 | 16 ± 2.3 | 18 ± 3.2 | 19 ± 3.2 | 20 ± 3.3 |
| Reperfusion | 25 ± 2.4 | 17 ± 2.5 | 24 ± 5.2 | 58 ± 6.3* | 35 ± 6.9& | 45 ± 7.2§ |
| Lactate (mmol/L) | ||||||
| Baseline | 2.1 ± 0.3 | 1.4 ± 0.3 | 2.0 ± 0.4 | 1.7 ± 0.2 | 2.5 ± 0.3 | 1.3 ± 0.2 |
| Reperfusion | 2.3 ± 0.4 | 1.3 ± 0.1 | 2.1 ± 0.3 | 7.3 ± 1.1* | 6.0 ± 1.1+ | 4.9 ± 0.9§ |
| Ejection fraction (%) | ||||||
| Baseline | 64 ± 2.1 | 63 ± 2.8 | 63 ± 1.8 | 62 ± 1.4 | 69 ± 6.2 | 63 ± 2.3 |
| Reperfusion | 64 ± 2.6 | 60 ± 2.5 | 67 ± 4.6 | 61 ± 6.5 | 64 ± 3.3 | 58 ± 2.5 |
| Cardiac output (ml/min) | ||||||
| Baseline | 2359 ± 174 | 2100 ± 108 | 2137 ± 88 | 1949 ± 112 | 2529 ± 238 | 2084 ± 98 |
| Reperfusion | 2199 ± 195 | 2031 ± 124 | 2334 ± 206 | 2245 ± 262 | 2555 ± 382 | 2074 ± 221 |
| Mean arterial blood pressure (mmHg) | ||||||
| Baseline | 66 ± 3.7 | 64 ± 3.8 | 65 ± 2.4 | 70 ± 4.7 | 60 ± 6.4 | 69 ± 3.7 |
| Reperfusion | 70 ± 2.6 | 65 ± 2.5 | 69 ± 4.1 | 71 ± 3.4 | 72 ± 3.7 | 68 ± 4.2 |
| Total catecholamine requirement (µg) | 31 ± 9.1 | 14 ± 8.8 | 32 ± 10.3 | 202 ± 60* | 173 ± 54.6+ | 184 ± 45.8§ |
| Tissue ATP content (µg/mg tissue) | 4.1 ± 0.8 | 5.5 ± 1.3 | 4.8 ± 0.6 | 1.5 ± 0.9* | 1.8 ± 0.2+ | 1.2 ± 0.7§ |
significance (p < 0.05) versus control, & significance (p < 0.05) versus CPB, + significance (p < 0.05) versus control + EGCG, § significance (p < 0.05) versus control + Minocycline.
Interestingly, ATP content of cardiac tissue was significantly lower in piglets undergoing CPB indicating an ATP-deficit after 120 min of reperfusion (Table 1).
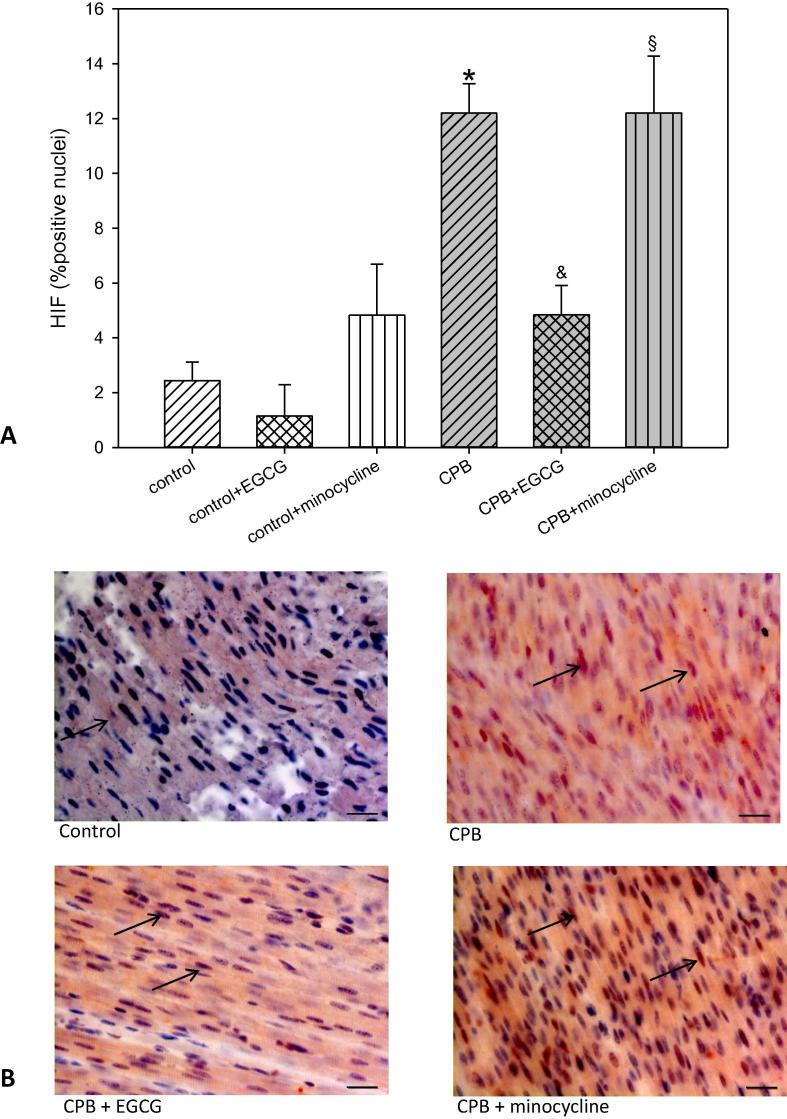
Histological analysis of the cardiac tissue revealed that HIF1α (hypoxia-inducible factor 1α), a transcription factor which is activated by hypoxia, was significantly elevated after CPB. After activation, HIF1α translocates into the nucleus and thus we could detect a nearly six fold increase in HIF1α -positive cell nuclei. This elevation was significantly reduced by EGCG application during CPB but not by minocycline (Fig. 1). Under control conditions both pharmaceuticals did not alter HIF1α expression.
Fig. 1.
Staining and quantification of HIF1α (hypoxia-inducable factor 1α) translocation. (A) upper panel: Bar graphs depict the percentage of nuclei positively stained for HIF1α in specimens from left ventricular free wall. (B) lower panel: Original images showing HIF1α staining of the left ventricle. Arrows indicate positively stained red nuclei. Scale bar = 20 µm. All data are given as means ± SEM. Significant differences (p < 0.05) versus control are indicated by *, significant differences versus CPB by a & and significant differences versus control + minocycline by a §.
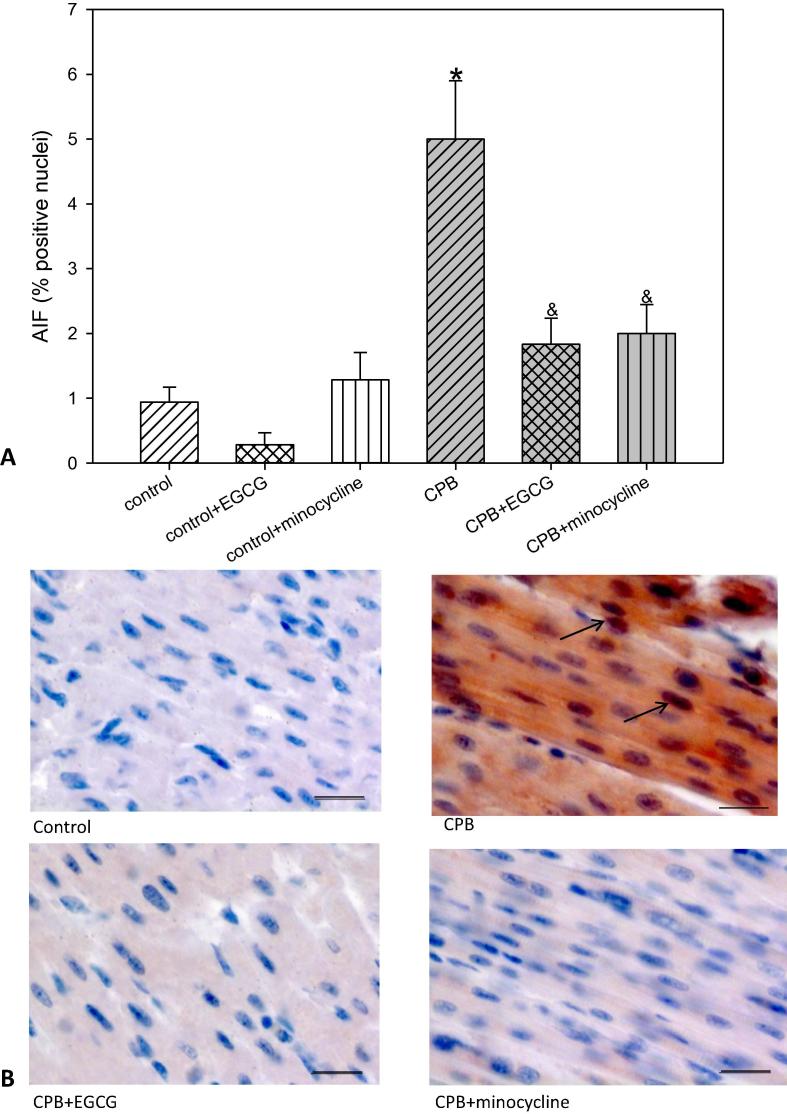
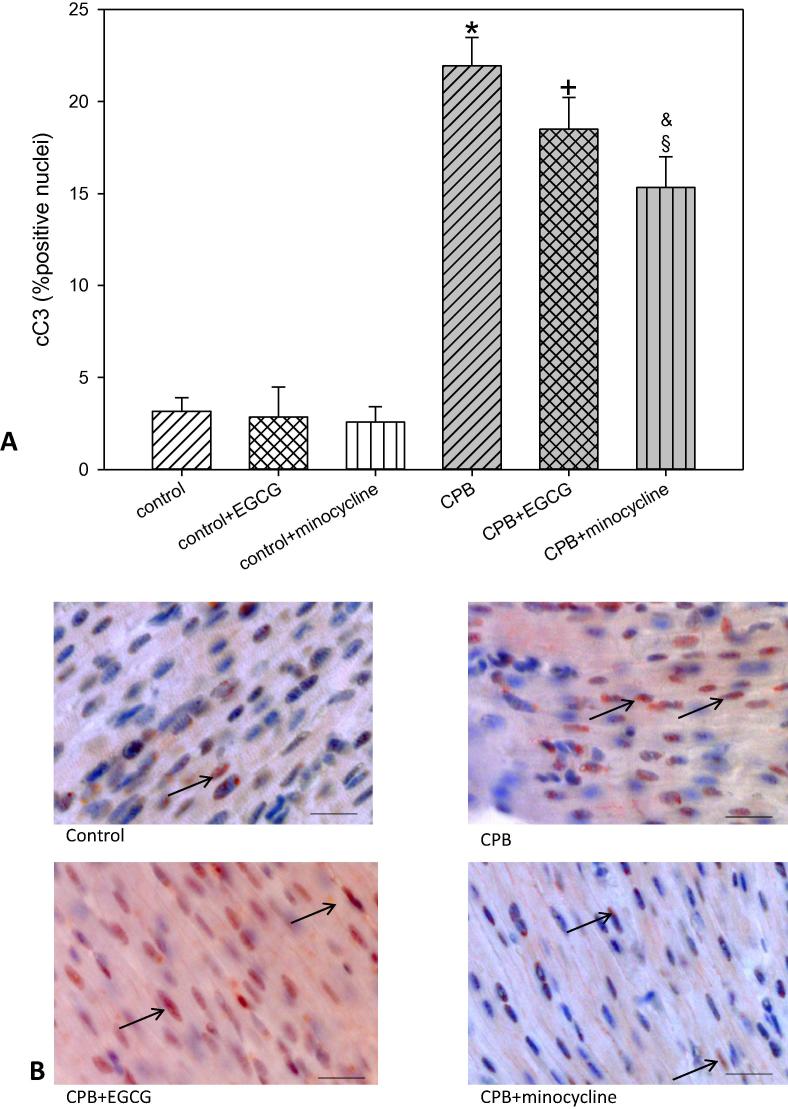
To evaluate a possible induction of apoptosis we analyzed AIF (apoptosis-inducing factor) and cC3 (cleaved caspase 3). Both factors induce apoptosis: AIF representing the intrinsic and cC3 representing the extrinsic pathway. Our data show that after CPB the number of cells possibly undergoing apoptosis -as indicated by an increase in AIF and/or cC3- was significantly elevated. This elevation could be blocked by both drugs EGCG and minocycline in the case of AIF but not in the case of cC3. The latter one was only slightly reduced by minocycline (Fig. 2, Fig. 3). In piglets without CPB EGCG and minocycline did not influence the number of AIF or cC3 positive cells.
Fig. 2.
Staining and quantification of AIF (apoptosis-inducing factor) translocation. (A) upper panel: Bar graphs depict the percentage of nuclei positively stained for AIF in specimens from left ventricular free wall. (B) lower panel: Original images showing AIF staining of the left ventricle. Arrows indicate positively stained red nuclei. Scale bar = 20 µm. All data are given as means ± SEM. Significant differences (p < 0.05) versus control are indicated by *, significant differences versus CPB by a &.
Fig. 3.
Staining and quantification of cC3 (cleaved-caspase 3) translocation. (A) upper panel: Bar graphs depict the percentage of nuclei positively stained for cC3 in specimens from left ventricular free wall. (B) lower panel: Original images showing cC3 staining of the left ventricle. Arrows indicate positively stained red nuclei. Scale bar = 20 µm. All data are given as means ± SEM. Significant differences (p < 0.05) versus control are indicated by *, significant differences versus control + EGCG by a +, significant differences versus control + minocycline by a §, significant differences versus CPB by a &.
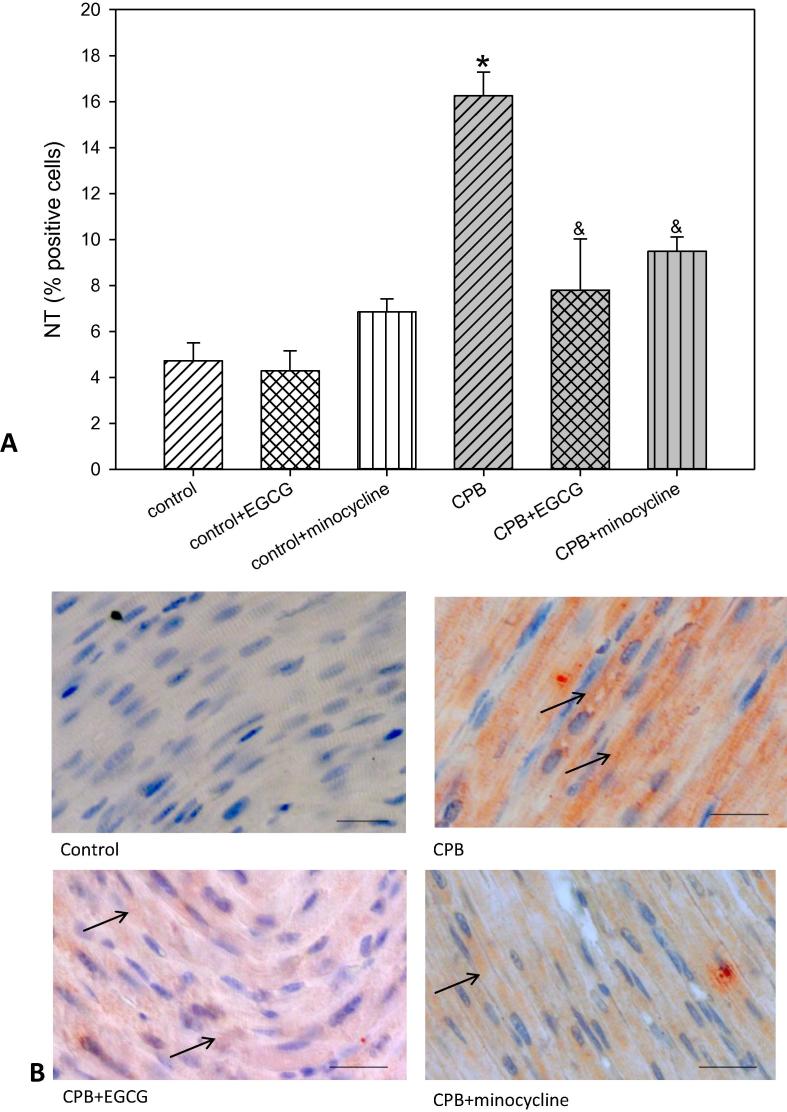
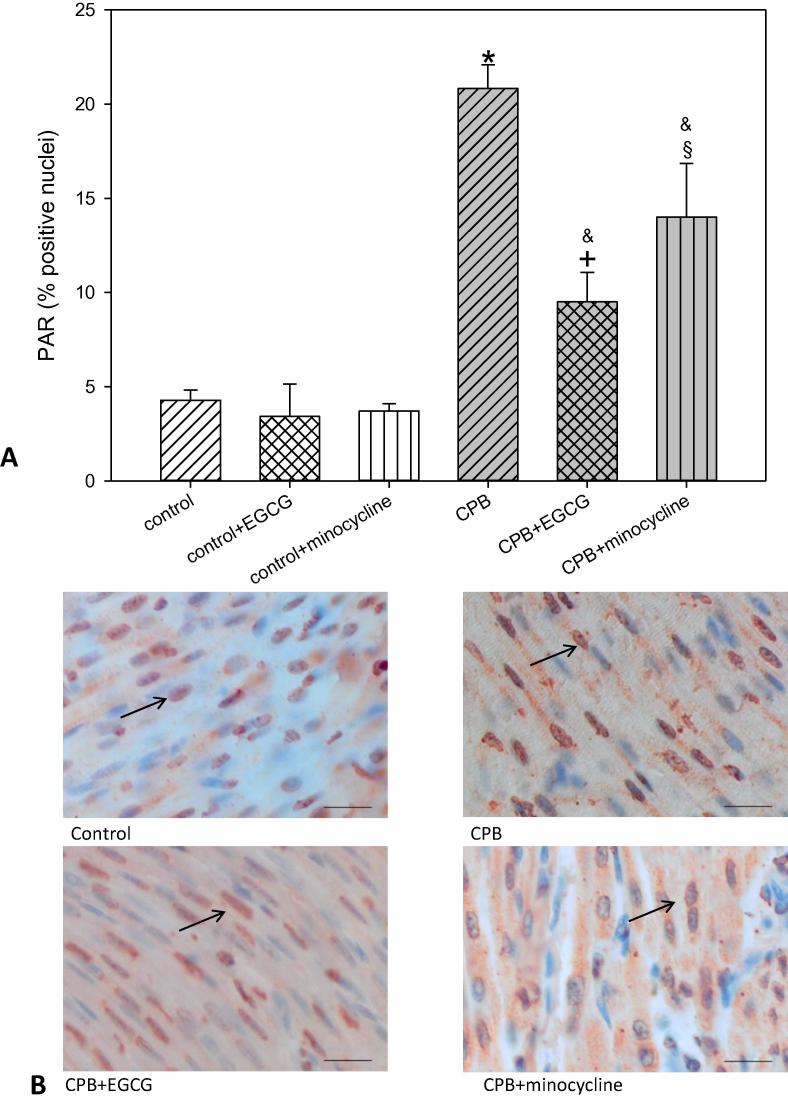
Reactive nitrogen species lead to protein nitrosylation i.e. nitrotyrosine (NT) formation and DNA strand breaks. In turn the enzyme poly-ADP-ribose polymerase (PARP), which is responsible for the repair of DNA strand breaks, is activated. This PARP activation leads to the formation of PAR (poly-ADP-ribose). In our experimental set-up we could determine a significant NT and PAR formation as indicators for reactive nitrogen species possibly inducing DNA strand breaks. EGCG and minocycline significantly reduced NT and PAR (Fig. 4, Fig. 5). Again, under control conditions EGCG and minocycline did not alter NT or PAR.
Fig. 4.
Staining and quantification of NT (nitrotyrosine) positive cells. (A) upper panel: Bar graphs depict the percentage of cells positively stained for NT in specimens from left ventricular free wall. (B) lower panel: Original images showing NT staining of the left ventricle. Arrows indicate positively stained red cells. Scale bar = 20 µm. All data are given as means ± SEM. Significant differences (p < 0.05) versus control are indicated by *, significant differences versus CPB by a &.
Fig. 5.
Staining and quantification of PAR (pol-ADP-ribose) positive cells. (A) upper panel: Bar graphs depict the percentage of cells positively stained for PAR in specimens from left ventricular free wall. (B) lower panel: Original images showing PAR staining of the left ventricle. Arrows indicate positively stained red cells. Scale bar = 20 µm. All data are given as means ± SEM. Significant differences (p < 0.05) versus control are indicated by *, significant differences versus control + EGCG by a +, significant differences versus control-minocycline by a §, significant differences versus CPB by a &.
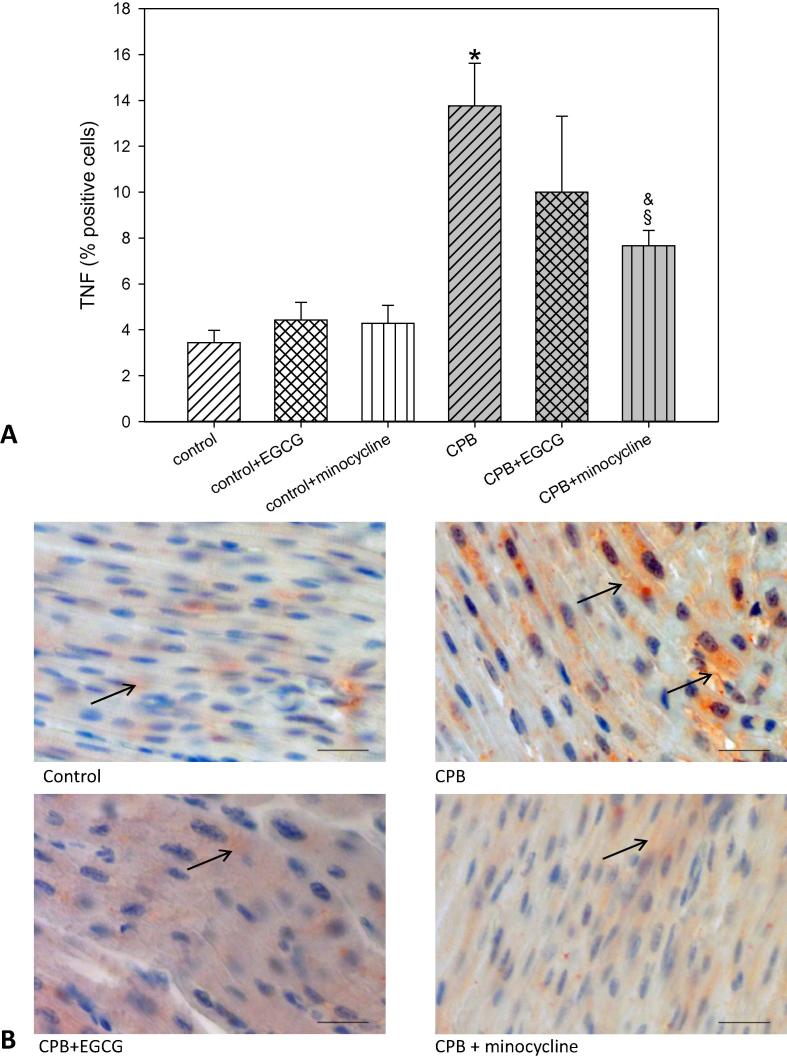
TNFα (tumor necrosis factor 1α), a cytokine involved in inflammatory processes, was also significantly elevated after CPB. Application of EGCG only slightly reduced TNFα. In contrast, minocycline significantly decreased this cytokine, although control levels were not reached (Fig. 6). In control piglets, EGCG and minocycline had no influence of TNFα expression.
Fig. 6.
Staining and quantification of TNFα (tumor necrosis factorα) positive cells. (A) upper panel: Bar graphs depict the percentage of cells positively stained for TNFα in specimens from left ventricular free wall. (B) lower panel: Original images showing TNFα staining of the left ventricle. Arrows indicate positively stained red cells. Scale bar = 20 µm. All data are given as means ± SEM. Significant differences (p < 0.05) versus control are indicated by *, significant differences versus control-minocycline by a §, significant differences versus CPB by a &.
4. Discussion
In our present animal study we could demonstrate that application of CPB led to a severe ischemia/reperfusion injury of the heart with ATP depletion, and the necessity of catecholamine therapy to stabilize contractile function. Moreover, histological analysis of the hearts revealed a significant increase in nitrogen radicals, enhanced PAR formation and finally to enhanced apoptosis. Additionally, CPB also resulted in elevated TNFα levels, as a sign of inflammation.
Both drugs EGCG and minocycline, although no direct effect on contractile function or ATP-levels was seen, seemed to reduce nitrosative stress, apoptosis and inflammation. Nevertheless, it worth mentioning that EGCG and minocycline have different action profiles: with EGCG being more effective in reducing HIF1α-translocation whereas minocycline was more potent in decreasing TNFα levels.
At the beginning of the experiments all piglets were in a comparable clinical condition. However, after CPB and within the first minutes after reperfusion it became obvious that the CPB-groups needed significantly more catecholamines to maintain arterial blood pressure and cardiac output than the control piglets without CPB. Deterioration of left ventricular function after CPB -even if the patient had a normal cardiac function preoperative- is well known in human medicine and is usually treated with catecholamines (Salgo et al., 2015). However, a prolonged catecholamine therapy is in the long term not advantageous for the heart muscle. The need of catecholamine support to maintain ventricular function corresponds well with the reduced cardiac ATP concentrations, which we found in our piglet model of CPB. Surprisingly, even 120 min reperfusion was not enough to completely restore intracellular energy rich phosphates, which suggests an ongoing metabolic dysregulation. Moreover, we saw a nearly threefold increase in blood lactate levels, which point to disturbed (i.e. anaerobic) metabolic processes of the bodýs periphery. The elevated creatine kinase concentrations also fit into the clinical picture of reduced peripheral perfusion.
Both pharmaceutical which we applied had no significant effects on clinical parameters; however, we saw considerable positive results in our histological analysis.
EGCG is the main ingredient of green tea, which is one of the most popular beverages in the Asian countries. Green tea is said to have many positive characteristics: lowering of blood lipids, arteriosclerosis reduction, anti-inflammatory and anti-oxidative properties as well as cancer prevention. Moreover, we and others found that it also positively affects cardiac outcome after ischemia/reperfusion injury: after global or regional ischemia, the EGCG treated hearts showed a better contraction behavior and in case of regional ischemia a reduced infarct size (Kim et al., 2014a, Kim et al., 2014b, Lee et al., 2012, Piao et al., 2011, Salameh et al., 2018). However, these studies were carried out with isolated saline solution-perfused hearts and not with whole animals undergoing CPB in a clinical setting. Interestingly, in a recently published study Funamoto et al. conducted animal experiments using CPB in diabetic rats, and they could demonstrate that CPB induced acute kidney injury which was significantly diminished by pre-treatment of the rats with EGCG (Funamoto et al., 2016). Funamoto et al. ascribed their positive EGCG effects on renal function to the anti-oxidative properties of EGCG. Additionally, they also found reduced oxidative stress against DNA (deoxyribonucleic acid) (Funamoto et al., 2016). These data fit well with our CPB-study: nitrotyrosine, a marker for oxidative and nitrosative stress- was significantly reduced in the CPB-groups treated with EGCG. Similarly, PAR, which is an indicator for DNA strand breaks and also induces apoptosis via mitochondrial AIF release, was significantly lowered by EGCG. Tyrosine nitration is typically found in conditions where oxygen radicals and NO form peroxinitrite, which itself nitrosylates several cellular proteins and which is also responsible for DNA damage (Pacher et al., 2007). PARP enzymes attempt to repair DNA nicks, which is an ATP-consuming process and at the end might lead to cell necrosis. PAR is produced as a result of DNA repair by PARP enzymes and triggers the programmed cell death via AIF release (Yu et al., 2006). Thus, EGCG with its anti-oxidative properties might be of advantage for cardiac cells as it lowered AIF, PAR and NT thereby inhibiting deleterious pathways for cell death. However, in our study tissue ATP-levels were low and not different among the three CPB groups. This phenomenon might point to a still disturbed ATP metabolism even after 120 min of reperfusion and is consistent with the clinical observation of myocardial stunning in patients after CPB (Eberhardt et al., 2000).
Another apoptosis inducing factor is cC3, which can be activated by various stimuli including CPB (Malmberg et al., 2011). In our study we also found enhanced cC3 levels after CPB. However EGCG only slightly lowered cC3, which might indicate that the extrinsic apoptosis pathway is still active. On the other hand, as cC3 is able to cleave PARP, thereby hindering an ATP-consuming process, the metabolic state of the cell might be better (Garnier et al., 2003). However, as we did not see differences in ATP-levels among our CPB-groups it is questionable if this process plays a role in our study.
Typically, during ischemia significant HIF1α translocation into cell nuclei occurs. This transcription factor is activated by low oxygen levels and initiates cell rescuing processes (Ong et al., 2014). Thus, the six-fold enhanced HIF1α nuclear staining in our CPB-group compared to the control group indicates an ischemic injury of cardiomyocytes. Addition of EGCG lowered HIF1α to control levels, although the ischemic burden of hearts undergoing CPB with or without EGCG is obviously not different. However, it is to say that also reactive oxygen species (ROS) activate HIF1α nuclear translocation (Zepeda et al., 2013). As EGCG has excellent anti-oxidative properties, it might be that the radical scavenging features of this catechin reduce the impact of free radicals, which develop during ischemia and re-oxygenation. At least, less ROS could mean a reduced incidence of DNA strand breaks and a reduced mitochondrial AIF release (An et al., 2014). Decreased NT and PAR formation as well as decreased AIF translocation, which we also found, would therefore be consistent with the hypothesis of less oxidative stress.
It is well known in cardiac surgery that CPB induces a considerable inflammatory response (Durandy, 2014). Consistent with this clinical observation, we also found an enhanced number of TNFα -positive cells in our cardiac samples. Addition of EGCG during CPB did not reduce TNFα to a significant extent in cardiac tissue. In contrast, in several animal models (diabetes and aging), it was shown that EGCG significantly decreased TNFα serum levels (Niu et al., 2013, Othman et al., 2017). However, cardiac samples were not evaluated in these studies. In a former study of our working group lung samples of piglets undergoing CPB were analyzed and we demonstrated that TNFα was elevated especially in the bronchial epithelium after CPB (Kasper et al., 2016). Although, EGCG treated piglets also had no benefit regarding bronchial TNFα levels, they had a lower rate of alveolar infiltration of neutrophil granulocytes (Kasper et al., 2016). Thus, EGCG had a certain anti-inflammatory effect, which was not evident in our cardiomyocyte samples.
Compared with this observation, minocycline addition showed a significant reduction in TNFα positivity of cardiac samples. The antibiotic minocycline has strong anti-inflammatory properties and we and others could demonstrate that tissue TNFα was significantly reduced by minocycline (Drabek et al., 2014, Salameh et al., 2017). This might suggest that the inflammatory response following CPB is suppressed or at least reduced by minocycline, which has a high relevance for the patients.
In addition, minocycline has a considerable anti-oxidative potential and also inhibits the mitochondrial permeability transition pore (MPTP) (Gieseler et al., 2009). This could mean that mitochondrial metabolic processes are less disturbed by ROS or nitric radicals, which might improve the metabolic state of the cells. Consistent with this result, nitrotyrosine formation was diminished, which indicates less peroxynitrite formation and presumably less DNA strand breaks. Moreover, as minocycline is a direct inhibitor of PARP, PAR formation should also be reduced, which we also demonstrated (Alano et al., 2006). Both, inhibitory roles of minocycline (anti-oxidation and PARP-inhibition) should then lead to enhanced ATP-levels, which we did not see after reperfusion. However, in a former manuscript of our working group we could demonstrate, that minocycline improved ATP-levels in lung tissue (Salameh et al., 2017). The reason for this discrepancy might be that the contracting heart -in contrast to the lung- consumes high amounts of ATP, which perhaps cannot be provided within the time span of 120 min of reperfusion. However, reduced PAR should result in reduced AIF release which we found in our study in the minocycline treated CPB-groups. Additionally, we also saw a slight but significant reduction in cC3. Thus, both apoptotic pathways seemed to be reduced by minocycline. The capability of minocycline in reducing the intrinsic and extrinsic pathway of apoptosis was also described by Heo et al. (2006). Furthermore, it also fits to the reduced TNFα -levels which we saw, as TNFα might initiate the extrinsic pathway of apoptosis.
Interestingly, in contrast to EGCG minocycline did not alter HIF1α translocation. This is in line with at study of Dhein et al., who also found that minocycline did not inhibit CPB-induced HIF1α nuclear translocation in renal tubules (Dhein et al., 2015). However, these authors also described that in liver cells minocycline significantly inhibited HIF1α. Thus, the effect of minocycline on HIF1α might be tissue dependent and also dependent on the experimental conditions, since in the CPB-situation organs like liver, kidney and brain are still perfused, while in the hearts perfusion is completely stopped resulting in global (cold) ischemia.
Nevertheless, the divergent action of both administered drugs regarding HIF1α and TNFα is interesting. EGCG is a flavan-3-ol derivative composed as a carbonic acid ester of the epigallocatechin (with 3 ring structures) bound to gallic acid via a hydrophilic bridge. EGCG belongs to the group of catechins. These molecules include (as a general characteristic) a substituted benzopyrane. In a recently published manuscript Ferguson et al. (2017) described the necessity of a benzopyrane group for HIF1α inhibition. These authors could show that certain substituted benzopyrane derivates can dock to HIF1α thereby inhibiting the binding of HIF1α to the DNA. The effective structures in their investigation show similarities to the structure of the catechin EGCG with regard to the position of hydrophobic and hydrophilic groups. Thus, such an inhibition of the interaction of activated HIF1α with the DNA might be an explanation for the anti-HIF1α effects of EGCG. As not only EGCG has protective effects against ischemia/reperfusion injury but also catechin and epicatechin. Thus, it might be that the benzopyran structure is necessary to reduce nuclear HIF1α. However, it must be stated that a direct HIF1α inhibitory action of catechin and epicatechin has not been demonstrated until now.
Regarding the tetracyclin minocycline and its action of TNFα there is only little information. The synthetic drug minocyclin belongs to the octa-hydro-naphthacenes which have been first identified in streptomyces species. Anti-TNFα activity of such drugs and of minocycline in particular has been found also by others (Naderi et al., 2017, Xiao et al., 2016, Pabreja et al., 2011). This action is also found with other molecules chemically not related to minocycline (Shamim and Laskowski, 2017), so that the exact mechanism behind this inhibitory effect against TNFα presently remains unclear. There is however one interesting report on an inhibitory effect of tetracycline, minocycline and doxycycline, but not of makrolide antibiotic like clarithromycin or roxithromycin, on the PAR2-IL8-axis (protease activated receptor-2 - interleukin 8 axis) resulting in decreased TNFα (Ishikawa et al., 2009). Whether this mechanism might be involved in the cardioprotective actions must be investigated in future studies.
Taken together, anti-oxidative and anti-apoptotic drugs as EGCG and minocycline can help to reduce the catecholamine demand after CPB by reduction of the CPB-induced ischemia/reperfusion damage. EGCG and minocycline, thus, may be of interest in particular in cases which require a long operation period/cardiac arrest period or in critically ill patients.
5. Conclusions
CPB -despite cold cardiac arrest- results in a global ischemia/reperfusion damage of the heart inducing HIF1α translocation, PAR generation, activation of apoptosis pathways and the need of catecholamine support. These negative effects of CPB can be avoided by pre-treatment with EGCG or minocycline. Our findings might open new perspectives for complex and long cardiac operations and for critically ill patients undergoing cardiac surgery.
Declarations of interest
None.
Author contributions
Aida Salameh conceived and designed the experiments, analyzed the data and wrote the manuscript; Stefan Dhein designed the experiments, analyzed the data and edited the manuscript. Marie Mewes and Sophie Sigusch analyzed the data; Marcel Vollroth and Philipp Kiefer carried out the experiments, Ingo Dähnert and Johannes Seeger conceived the experiments.
Acknowledgments
Authors have nothing to disclose with regard to commercial support. This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Aida Salameh, Email: aida.salameh@medizin.uni-leipzig.de.
Stefan Dhein, Email: stefan.dhein@medizin.uni-leipzig.de.
Marie Mewes, Email: mewes_marie@yahoo.no.
Sophie Sigusch, Email: sophie.sigusch@uni-wh.de.
Philipp Kiefer, Email: philipp.kiefer@medizin.uni-leipzig.de.
Marcel Vollroth, Email: marcel.vollroth@medizin.uni-leipzig.de.
Johannes Seeger, Email: seeger@vetmed.uni-leipzig.de.
Ingo Dähnert, Email: ingo.daehnert@medizin.uni-leipzig.de.
References
- Alano C.C., Kauppinen T.M., Valls A.V., Swanson R.A. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc. Natl. Acad. Sci. USA. 2006;103(25):9685–9690. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Z., Qi Y., Huang D., Gu X., Tian Y., Li P., Li H., Zhang Y. EGCG inhibits Cd(2+)-induced apoptosis through scavenging ROS rather than chelating Cd(2+) in HL-7702 cells. Toxicol. Mech. Methods. 2014;24:259–267. doi: 10.3109/15376516.2013.879975. [DOI] [PubMed] [Google Scholar]
- Bailliard F., Anderson R.H. Tetralogy of fallot. Orphanet. J. Rare. Dis. 2009;4:2. doi: 10.1186/1750-1172-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benizri E., Ginouvès A., Berra E. The magic of the hypoxia-signaling cascade. Cell. Mol. Life. Sci. 2008;65(7–8):1133–1149. doi: 10.1007/s00018-008-7472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braimbridge M.V., Chayen J., Bitensky L., Hearse D.J., Jynge P., Canković-Darracott S. Cold cardioplegia or continuous coronary perfusion? report on preliminary clinical experience as assessed cytochemically. J. Thorac. Cardiovasc. Surg. 1977;74:900–906. [PubMed] [Google Scholar]
- Bretschneider H.J. Myocardial protection. Thorac. Cardiovasc. Surg. 1980;28:295–302. doi: 10.1055/s-2007-1022099. [DOI] [PubMed] [Google Scholar]
- Calafiore A.M., Teodori G., Mezzetti A., Bosco G., Verna A.M., Di Giammarco G., Lapenna D. Intermittent antegrade warm blood cardioplegia. Ann. Thorac. Surg. 1995;59:398–402. doi: 10.1016/0003-4975(94)00843-v. [DOI] [PubMed] [Google Scholar]
- Cheng C., Xu J.M., Yu T. Neutralizing IL-6 reduces heart injury by decreasing nerve growth factor precursor in the heart and hypothalamus during rat cardiopulmonary bypass. Life Sci. 2017;178:61–69. doi: 10.1016/j.lfs.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Dhein S., Grassl M., Gerdom M., Vollroth M., Bakhtiary F., von Salisch S., Krämer K., Sobiraj A., Kostelka M., Mohr F.W., Salameh A. Organ-protective effects on the liver and kidney by minocycline in small piglets undergoing cardiopulonary bypass. Naunyn. Schmiedebergs. Arch. Pharmacol. 2015;388(6):663–676. doi: 10.1007/s00210-015-1115-4. [DOI] [PubMed] [Google Scholar]
- Drabek T., Janata A., Wilson C.D., Stezoski J., Janesko-Feldman K., Tisherman S.A., Foley L.M., Verrier J.D., Kochanek P.M. Minocycline attenuates brain tissue levels of TNF-α produced by neurons after prolonged hypothermic cardiac arrest in rats. Resuscitation. 2014;85(2):284–291. doi: 10.1016/j.resuscitation.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durandy Y. Minimizing systemic inflammation during cardiopulmonary bypass in the pediatric population. Artif. Organs. 2014;38(1):11–18. doi: 10.1111/aor.12195. [DOI] [PubMed] [Google Scholar]
- Eberhardt F., Mehlhorn U., Larosé K., De Vivie E.R., Dhein S. Structural myocardial changes after coronary artery surgery. Eur. J. Clin. Invest. 2000;30(11):938–946. [PubMed] [Google Scholar]
- Ferguson J., De Los Santos Z., Devi N., Van Meir E., Zingales S., Wang B. Examining the structure-activity relationship of benzopyran-based inhibitors of the hypoxia inducible factor-1 pathway. Bioorg. Med. Chem. Lett. 2017;27(8):1731–1736. doi: 10.1016/j.bmcl.2017.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto M., Masumoto H., Takaori K., Taki T., Setozaki S., Yamazaki K., Minakata K., Ikeda T., Hyon S.H., Sakata R. Green tea polyphenol prevents diabetic rats from acute kidney injury after cardiopulmonary bypass. Ann. Thorac. Surg. 2016;101(4):1507–1513. doi: 10.1016/j.athoracsur.2015.09.080. [DOI] [PubMed] [Google Scholar]
- Garnier P., Ying W., Swanson R.A. Ischemic preconditioning by caspase cleavage of poly(ADP-ribose) polymerase-1. J. Neurosci. 2003;23:7967–7973. doi: 10.1523/JNEUROSCI.23-22-07967.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J.H., Jr. The application of a mechanical heart and lung apparatus to cardiac surgery. Minn. Med. 1954;37:171–185. [PubMed] [Google Scholar]
- Gieseler A., Schultze A.T., Kupsch K., Haroon M.F., Wolf G., Siemen D., Kreutzmann P. Inhibitory modulation of the mitochondrial permeability transition by minocycline. Biochem. Pharmacol. 2009;77(5):888–896. doi: 10.1016/j.bcp.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Guo K., Searfoss G., Krolikowski D., Pagnoni M., Franks C., Clark K., Yu K.T., Jaye M., Ivashchenko Y. Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell. Death. Differ. 2001;8(4):367–376. doi: 10.1038/sj.cdd.4400810. [DOI] [PubMed] [Google Scholar]
- Heo K., Cho Y.J., Cho K.J., Kim H.W., Kim H.J., Shin H.Y., Lee B.I., Kim G.W. Minocycline inhibits caspase-dependent and -independent cell death pathways and is neuroprotective against hippocampal damage after treatment with kainic acid in mice. Neurosci. Lett. 2006;398(3):195–200. doi: 10.1016/j.neulet.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Hill G.E. Cardiopulmonary bypass-induced inflammation: is it important? J. Cardiothorac. Vasc. Anesth. 1998;12(2 Suppl 1):21–25. [PubMed] [Google Scholar]
- Ishikawa C., Tsuda T., Konishi H., Nakagawa N., Yamanishi K. Tetracyclines modulate protease-activated receptor 2-mediated proinflammatory reactions in epidermal keratinocytes. Antimicrob. Agents Chemother. 2009;53(5):1760–1765. doi: 10.1128/AAC.01540-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper B., Salameh A., Krausch M., Kiefer P., Kostelka M., Mohr F.W., Dhein S. Epigallocatechin gallate attenuates cardiopulmonary bypass-associated lung injury. J. Surg. Res. 2016;201(2):313–325. doi: 10.1016/j.jss.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Li M., Jeong C.W., Bae H.B., Kwak S.H., Lee S.H., Lee H.J., Heo B.H., Yook K.B., Yoo K.Y. Epigallocatechin-3-gallate, a green tea catechin, protects the heart against regional ischemia-reperfusion injuries through activation of RISK survival pathways in rats. Arch. Pharm. Res. 2014;37(8):1079–1085. doi: 10.1007/s12272-013-0309-x. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Quon M.J., Kim J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox. Biol. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.K., Kim J.H., Kim J.S., Jang Y., Kim J., Park Y.H., Chun K.J., Lee M.Y. Polyphenol (-)-epigallocatechin gallate-induced cardioprotection may attenuate ischemia-reperfusion injury through adenosine receptor activation: a preliminary study. Kor. J. Anesthesiol. 2012;63(4):340–345. doi: 10.4097/kjae.2012.63.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg M., Parkka J., Vahasilta T., Saraste A., Laitio T., Kiss J., Latva-Hirvela J., Saukko P., Savunen T. Cardiomyocyte apoptosis after cardioplegic ischemia: comparison to unprotected regional ischemia-reperfusion. Eur. Surg. Res. 2011;46(1):19–25. doi: 10.1159/000321875. [DOI] [PubMed] [Google Scholar]
- Martins P., Castela E. Transposition of the great arteries. Orphanet. J. Rare. Dis. 2008;3:27. doi: 10.1186/1750-1172-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi Y., Sabetkasaei M., Parvardeh S., Zanjani T.M. Neuroprotective effect of minocycline on cognitive impairments induced by transient cerebral ischemia/reperfusion through its anti-inflammatory and anti-oxidant properties in male rat. Brain Res. Bull. 2017;131:207–213. doi: 10.1016/j.brainresbull.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Niu Y., Na L., Feng R., Gong L., Zhao Y., Li Q., Li Y., Sun C. The phytochemical, EGCG, extends lifespan by reducing liver and kidney function damage and improving age-associated inflammation and oxidative stress in healthy rats. Aging Cell. 2013;12(6):1041–1049. doi: 10.1111/acel.12133. [DOI] [PubMed] [Google Scholar]
- Ong S.G., Lee W.H., Theodorou L., Kodo K., Lim S.Y., Shukla D.H., Briston T., Kiriakidis S., Ashcroft M., Davidson S.M., Maxwell P.H., Yellon D.M., Hausenloy D.J. HIF-1 reduces ischaemia-reperfusion injury in the heart by targeting the mitochondrial permeability transition pore. Cardiovasc. Res. 2014;104(1):24–36. doi: 10.1093/cvr/cvu172. [DOI] [PubMed] [Google Scholar]
- Othman A.I., El-Sawi M.R., El-Missiry M.A., Abukhalil M.H. Epigallocatechin-3-gallate protects against diabetic cardiomyopathy through modulating the cardiometabolic risk factors, oxidative stress, inflammation, cell death and fibrosis in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2017;94:362–373. doi: 10.1016/j.biopha.2017.07.129. [DOI] [PubMed] [Google Scholar]
- Pabreja K., Dua K., Sharma S., Padi S.S., Kulkarni S.K. Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and anti-oxidant mechanisms. Eur. J. Pharmacol. 2011;661(1–3):15–21. doi: 10.1016/j.ejphar.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao C.S., Kim D.S., Ha K.C., Kim H.R., Chae H.J., Chae S.W. The protective effect of epigallocatechin-3 gallate on ischemia/reperfusion injury in isolated rat hearts: an ex vivo approach. Kor. J. Physiol. Pharmacol. 2011;15(5):259–266. doi: 10.4196/kjpp.2011.15.5.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royster R.L. Myocardial dysfunction following cardiopulmonary bypass: recovery patterns, predictors of inotropic need, theoretical concepts of inotropic administration. J. Cardiothorac. Vasc. Anesth. 1993;7:19–25. doi: 10.1016/1053-0770(93)90093-z. [DOI] [PubMed] [Google Scholar]
- Salameh A., Dhein S., Blanke K., Rastan A., Hiyasat B., Dietze A., Sobiraij A., Dähnert I., Janousek J. Right or left ventricular pacing in young minipigs with chronic atrioventricular block: long-term in vivo cardiac performance, morphology, electrophysiology, and cellular biology. Circulation. 2012;125(21):2578–2587. doi: 10.1161/CIRCULATIONAHA.111.079087. [DOI] [PubMed] [Google Scholar]
- Salameh A., Halling M., Seidel T., Dhein S. Effects of minocycline on parameters of cardiovascular recovery after cardioplegic arrest in a rabbit Langendorff heart model. Clin. Exp. Pharmacol. Physiol. 2015;42(12):1258–1265. doi: 10.1111/1440-1681.12485. [DOI] [PubMed] [Google Scholar]
- Salameh A., Kühne L., Grassl M., Gerdom M., von Salisch S., Vollroth M., Bakhtiary F., Mohr F.W., Dähnert I., Dhein S. Protective effects of pulsatile flow during cardiopulmonary bypass. Ann. Thorac. Surg. 2015;99(1):192–199. doi: 10.1016/j.athoracsur.2014.07.070. [DOI] [PubMed] [Google Scholar]
- Salameh A., Einenkel A., Kühne L., Grassl M., von Salisch S., Kiefer P., Vollroth M., Dähnert I., Dhein S. Hippocampal neuroprotection by minocycline and epigallo-catechin-3-gallate against cardiopulmonary bypass-associated injury. Brain Pathol. 2015;25(6):733–742. doi: 10.1111/bpa.12242. Epub 2015 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh A., Greimann W., Vollroth M., Dhein S., Bahramsoltani M., Dahnert I. Lung protection in cardio-pulmonary bypass. J. Physiol. Pharmacol. 2017;68(1):99–116. [PubMed] [Google Scholar]
- Salameh A., Schuster R., Dähnert I., Seeger J., Dhein S. Epigallocatechin gallate reduces ischemia/reperfusion injury in isolated perfused rabbit hearts. Int. J. Mol. Sci. 2018;19(2) doi: 10.3390/ijms19020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado Filho M.F., Barral M., Barrucand L., Cavalcanti I.L., Verçosa N. A randomized blinded study of the left ventricular myocardial performance index comparing epinephrine to levosimendan following cardiopulmonary bypass. PLoS One. 2015;10(12):e0143315. doi: 10.1371/journal.pone.0143315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamim D., Laskowski M. Inhibition of inflammation mediated through the tumor necrosis factor α biochemical pathway can lead to favorable outcomes in Alzheimer. Dis. J. Cent. Nerv. Syst. Dis. 2017;9:1–10. doi: 10.1177/1179573517722512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q., Tan Y., Lin Z., Zhou J., Zhou F., Liu Z., Tang L. Minocycline Inhibits inflammation and squamous metaplasia of conjunctival tissue in airlift conditions. Cornea. 2016;35(2):249–256. doi: 10.1097/ICO.0000000000000687. [DOI] [PubMed] [Google Scholar]
- Yu S.W., Andrabi S.A., Wang H., Kim N.S., Poirier G.G., Dawson T.M., Dawson V.L. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc. Natl. Acad. Sci. USA. 2006;103:18314–21839. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepeda A.B., Pessoa A., Jr., Castillo R.L., Figueroa C.A., Pulgar V.M., Farías J.G. Cellular and molecular mechanisms in the hypoxic tissue: role of HIF-1 and ROS. Cell. Biochem. Funct. 2013;31:451–459. doi: 10.1002/cbf.2985. [DOI] [PubMed] [Google Scholar]