Abstract
Background
Medicinal plants are important source of drugs with pharmacological activities. Therefore, there is always rising demands to discover more therapeutic agents from various species. Orthosiphon stamineus, Gynura procumbens and Ficus deltoidea are high valued medicinal plants of Malaysia contain rich source of phenolic and flavonoid compounds. The aims of the present study were to evaluate anti-oxidant, antimicrobial and anti-proliferative effects on A549, HeGP2 and MCF7 cell lines of four different extracts of Orthosiphon stamineus, Gynura procumbens and Ficus deltoidea.
Methodology
The leaves of all selected plants were extracted with methanol, chloroform, ethyl acetate and butanol separately with simple cold maceration. Antioxidant activity of all crude extracts were quantitatively measured against DPPH and Ferric Reducing Assay. Antimicrobial evaluation was done by Microdilution and MTT assay and antipoliferative activity of all extracts of selected plant were evaluated against A549, HePG2 and MCF7 cell lines.
Results
Results showed that methanol extract exhibited highest percentage free radical scavenging activity of almost all extracts of selected plants. Antimicrobials results showed chloroform and methanol extracts of O. stamineus extract were the two most active extracts against resistant MRSA but not S. aureus. Only methanol extract of G. procumbens showed antimicrobial activity against the tested pathogens. Chloroform and methanol extracts of F. deltoidea elicited antimicrobial activity against S. aureus but not MRSA. Antiproliferative activity against three tested cell lines results showed that ethyl acetate extract of O. stamineus showed good effect whereas methanol extract of F. deltoidea and G. procumbens exhibited good antiproliferative activity.
Conclusions
The results of the present investigation demonstrated significant variations in the antioxidant, antimicrobial and antiproliferative effects of different solvent extracts. These data could be helpful in isolation of pure potent compounds with good biological activities from the extracts of plants.
Keywords: Orthosiphon stamineus, Gynura procumbens, Ficus deltoidea, Antioxidant, Antiproliferative, Antimicrobials
1. Introduction
The nature provides plenty of herbs and plants which serve as the leading source of traditional medicines that can be used to relief many different types of illnesses. The usages of these plants are continuously increasing day by day because of effectiveness and less side effect as compared to current synthetic drugs. To date, there is still increased demands for discovery of novel agents from various species of medicinal plant (Al-Daihan et al., 2013). Cancer causes about 12.5% deaths of the total population (Balouris et al., 2016). It is increasingly recognized that cancer is associated with oxidative stress which arises from an inequity between the progress and counterbalancing of prooxidants. The high content of free radical species causes oxidative damage, consequently in a cascade of degradative effects that contribute to human diseases which includes cancer. Research is still on going for investigation of potent natural compounds from plants with good antioxidant activity (Shaikh et al., 2014, Bagchi et al., 2000).
In spite of advances in antibiotic therapy, infectious problems remain a vital cause of death and illness amongst hospitalized patients. Currently there is great interest in developing new antimicrobial agents from natural sources to fight against microbial resistance. Antibiotic resistance is a serious problem that affects the healthcare sector in both developing and developed countries. In fact, presence of multidrug-resistant (MDR) bacteria in hospital and community remains an extensively unanswered problem and a substantial load to health services. To address this problem, it is essential to control the use of antibiotics, to elucidate mechanisms of resistance and eventually to develop new therapeutic entities (Balouris et al., 2016, Aslam et al., 2018, Duin and Paterson, 2016).
Testing of compounds and screening of raw plant extracts are necessary. There is always demand of natural products of the highest efficacy and lower side effects (Rafieian-Kopaie and Nasri, 2015, Lachenmayer et al., 2010). The selected plants O. stamineus, G. procumbens and F. deltoidea are found to contain valuable phenolic and flavonoid content, that are responsible for various pharmacological activities. As literature showed, all three selected plants contain different categories of chemical constituents in different solvent extracts due to difference in polarity of solvent (Choo et al., 2012, Algariri et al., 2013, Ashraf et al., 2018, Ashraf, 2019, Yam et al., 2008). Thus, it may exhibit different kind of biological activities. So it is interesting to identify the solvent extract which could show good and effective biological activities. This study aims to evaluate and screen three Malaysian key plants O. stamineus, G. procumbens and F. deltoidea for their antioxidant, antimicrobial and antiproliferative effects.
2. Materials and methods
2.1. Materials
2.1.1. Antibiotics, broth and chemical reagents
Luria Bertani (LB) broth was obtained from Himedia (Mumbai, India). The antibiotics, ampicillin and vancomycin, were supplied by Merck (Darmstadt, Germany) and Naqalai Tesque (Kyota, Japan), respectively. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Merck (Darmstadt, Germany). Dimethyl sulfoxide (DMSO) was acquired from Fisher Scientific (Leicestershire, UK). α,α-diphenyl-β-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). All other chemicals and reagents used were of AR grade and were obtained from commercial sources.
2.1.2. Pathogenic bacteria cultures
The pathogenic bacteria that were used in this study included the gram-positive Staphylococcus aureus (ATCC: 6538) and their resistant strain, methicillin-resistant S. aureus (MRSA, ATCC: 33591). The bacteria cultures were obtained from the culture collections of the CDDR Laboratory, Faculty of Pharmacy, UiTM, Puncak Alam Campus, Selangor Branch. The cultures were sub cultured three times prior to experimental use.
2.1.3. Cell culture
All cell lines used were obtained from American Type Culture Collection (ATCC, Manassas, Virginia). Lung adenocarcinoma cell line (A549) was cultured in Roswell Park Memorial Institute 1640 (RPMI-1640), while hepatocyte carcinoma (HepG2) and breast adenocarcinoma (MCF7) cell lines were cultured in Dulbecco modified Eagle medium (DMEM). All the cultures were supplemented with 10% (v/v) fetal bovine serum. The culture medium was changed twice a week and the cultures were passaged weekly.
2.2. Plant material and extraction
The leaves of Orthosiphon stamineus, Gynura procumbens and Ficus deltoidea were purchased from local market, Malaysia. The leaves were dried in the shade for 5 days and then powdered with a grinder (Panasonic MX-AC400W). About 50 g of the powdered leaves of selected plants were macerated in 500 mL of methanol, butanol, chloroform and ethyl acetate solvent for 48 h separately with occasional shaking. The extracts were collected after filtration using Whatman no: 1 filter paper and extracts were concentrated to dryness under reduced pressure using a rotary evaporator (Heidolph Hei-VAP Expert).
2.3. Determination of total phenolic content (TPC)
The total phenolic content in the extracts was estimated based on Folin–Ciocalteu method but with modifications (Noreen et al., 2017, Ashraf et al., 2018). Calibration curve for the reference standard was plotted with gallic acid. Briefly, 1 mg/mL plant extract (0.5 mL) was mixed with 5 mL of the Folin-Ciocalteu reagent (10 times diluted with de-ionised water) and were neutralised with 5 mL of sodium carbonate solution (7.5%, w/v). The reaction mixture was incubated at room temperature for 30 min and absorbance was read at 760 nm. The amount of total phenolics was calculated as gallic acid equivalent (GAE) in mg per g of dry weight (DW).
2.4. α,α-diphenyl-β-picrylhydrazyl (DPPH) radical scavenging activity
The free radical scavenging activities of all four extracts methanol, chloroform, ethyl acetate and butanol were determined by α,α-diphenyl-β-picrylhydrazyl (DPPH) assay, as described by Noreen et al., 2017, Amir et al., 2013 but with modification. Briefly, 200 μL extracts (25–400 μg/mL) were mixed with 3.8 mL DPPH (0.1 mM) solution and incubated in the dark at room temperature for 30 min. Blank solution was also prepared. The absorbance was read at 517 nm. Ascorbic acid was included as positive control. Free radical scavenging activity (%) was calculated using the formula [(Ao – At)/Ao)] × 100; where by Ao = Absorbance of control and At = Absorbance of samples.
2.5. Ferric reducing power assay
The ferric reducing power activity of methanol, chloroform, ethyl acetate and butanol extracts were evaluated using Ferric Reducing Assay (Amir et al., 2013), but with modifications. Briefly, 1 mL (125–1000 μg/ml), 2.5 mL 0.2 M sodium phosphate buffer (PH 6.6) and 2.5 mL 1% potassium ferricyanide solution were mixed, vortexed and incubated at 50 °C for 20 min after which added, 2.5 mL of trichloroacetic acid (10%) was added and the mixture was centrifuged at 3000 rpm for 10 min and finally (2.5 mL) deionized water and 0.1% ferric chloride (0.5 mL) were added to supernatant. The developed colored solution was read at 700 nm against the blank using spectrophotometer. Ascorbic acid was used as the positive control. The reducing power of the samples were compared with the reference standard.
2.6. Microdilution and MTT assay
The density of pathogenic bacteria was standardised to 0.5 McFarland standard (1 × 108 CFU/mL) before being seeded (100 µL) onto the 96 flat bottom well plate and treated with extracts (0.01–100 mg/mL). Ampicillin (10−8–10−4 mg/mL) and vancomycin (10−4–1 mg/mL) were included as the positive controls for S. aureus and MRSA, respectively. The treated bacteria were incubated at 37 °C for 24 h after which the MTT assay was performed in accordance to Grela et al. (2018), but with minor modifications. Briefly, 50 µL 0.5 mg/mL MTT solution was added onto each well before incubation at 37 °C for 2 h. The plate was centrifuged at 950g for 5 min. The supernatant was removed and 100 µL DMSO was added. The plate was shaken for 5 min before being subjected to measurement of absorbance at 570 nm using a microplate reader (Tecan Infinite M200, Männedorf, Switzerland). The readings were used to plot the dose-response curves.
2.7. In vitro cytotoxic activity
Dry methanol, ethyl acetate, chloroform and butanol extracts were dissolved in dimethyl sulphoxide (0.5% DMSO) to obtain appropriate solutions of the extracts. 0.5% DMSO was used for all extracts and control solution. O. stamineus, G. procumbens and F. deltoidea (methanol, chloroform, ethyl acetate and butanol) extracts were tested for in vitro cytotoxicity against A549, HePG2 and MCF-7 cells by MTT assay (Coligan et al., 1992, Lee Peter and Houghton, 2005). Briefly, each cell line was seeded at a density of 10,000 cells/well and incubated in a CO2 incubator at 37 °C overnight to allow the cells to adhere to the plate. Cells were treated with extracts (50–1000 µg/mL) for 24 and 48 h. 100 μL of MTT (0.5 mg/mL) was added to each well. The formazan produced was solubilized in DMSO and absorbance was read at 540 nm using an ELISA micro plate reader. The percentage of inhibition (%) was calculated according to the following formula:
Cytotoxicity was expressed as IC50 value the concentration required to inhibit 50% of the cancerous cell.
2.8. Statistical analysis
All the cytotoxicity experiments were repeated at least three times. Statistical analysis was performed using one-way ANOVA. A p-value of less than 0.05 was considered as statistically significant.
3. Results & discussion
3.1. Total phenolic content (TPC)
For O. stamineus, methanol extract yielded the highest amount of total phenolic (173.3 mg GAE/g dry weight). The other extracts yielded TPC in sequence of butanol extract (90.4 mg GAE/g dry weight) > ethyl acetate extract (73.8 mg GAE/g dry weight) > chloroform extract (29.1 mg GAE/g dry weight).
Interestingly, for G. procumbens, ethyl acetate yielded the highest phenolic content (183.764 mg GAE/g dry weight) when compared to other extracts. Chloroform yielded the lowest content (70.94 mg GAE/g dry weight). In case of F. deltoidea, the highest yield of TPC was reported in methanol extract (200.94 mg GAE/g dry weight) whereas butanol yielded the lowest (115.82 mg GAE/g dry weight).
3.2. DPPH radical scavenging activity
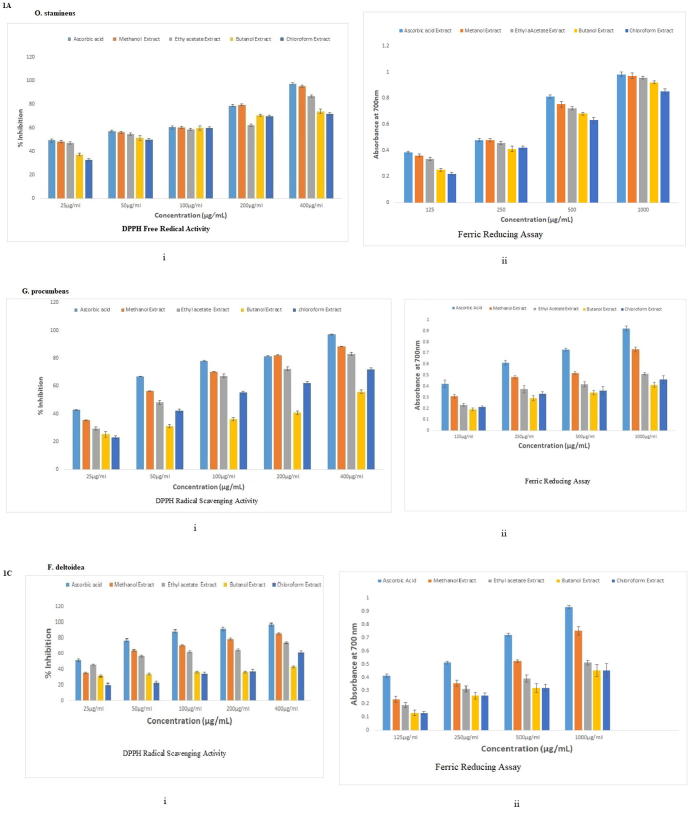
Fig. 1 shows the radical scavenging effects of methanol chloroform, ethyl acetate, butanol at different concentration. The antioxidant activities of the extracts were compared to ascorbic acid. Methanol extract showed the highest scavenging activity. The radical scavenging effect of the methanol extract seemed to be comparable to that of ascorbic acid at concentration 100 µg/mL. The scavenging activities of other extracts were in the order of ethyl acetate > butanol > chloroform (Fig. 1Ai).
Fig. 1.
DPPH Radical Scavenging Activity and Ferric Reducing assays of different extracts of O. stamineus, G. procumbens and F. deltoidea expressed as (n = 3).
For G. procumbens, methanol extracts exhibited highest DPPH radical scavenging activity than other extracts similar to O. stamineus. Ascorbic acid showed the highest scavenging activity when compared to extracts at all concentration (except methanol at 200 μg/mL) (Fig. 1Bi).
For F. deltoidea, methanol extract also showed highest scavenging at 400 µg/mL (85.41%) when compared to other extracts. Butanol extract showed poor scavenging activity (Fig. 1Ci).
3.3. Reducing power assay
Neutralisation of radicals happens once antioxidant donate an electron to free radicals. Reducing power ability of the extract was measured when Fe3+ is converted to Fe2+. (Gomes et al., 2012). The intense Prussian blue colour complex was measured at λ700nm. The strong antioxidant activity reported may be due to presence of phenolic and flavonoid compounds. Strength of antioxidant power is directly related to absorbance of reaction mixtures. All extracts showed concentration-dependent reducing power. Increase in absorbance indicated increased antioxidant activity. Greater absorbance value indicated stronger reducing power of the extract. All four extracts of selected three plants showed similar increasing fashion of reducing power with increase in extract concentrations. Methanol extract showed the highest reducing power when compared to other extracts (Fig. 1Aii).
For G. Procumbens, Methanol extract exhibited the highest reducing power when compared to other extracts. Butanol extract exhibited the least reducing power. This result was also confirmatory to the results of DPPH assay (Fig. 1Bii).
Remarkably, extracts of F. deltoidea also follow the same pattern of reducing power assay as G. procumbens in the order of methanol extract exhibited highest reducing power whereas butanol extract exhibited least reducing power (Fig. 1Cii).
3.4. Antimicrobial activity
3.4.1. Antimicrobial effects of O. stamineus, F. deltoidea and G. procumbens extracts against S. aureus and MRSA
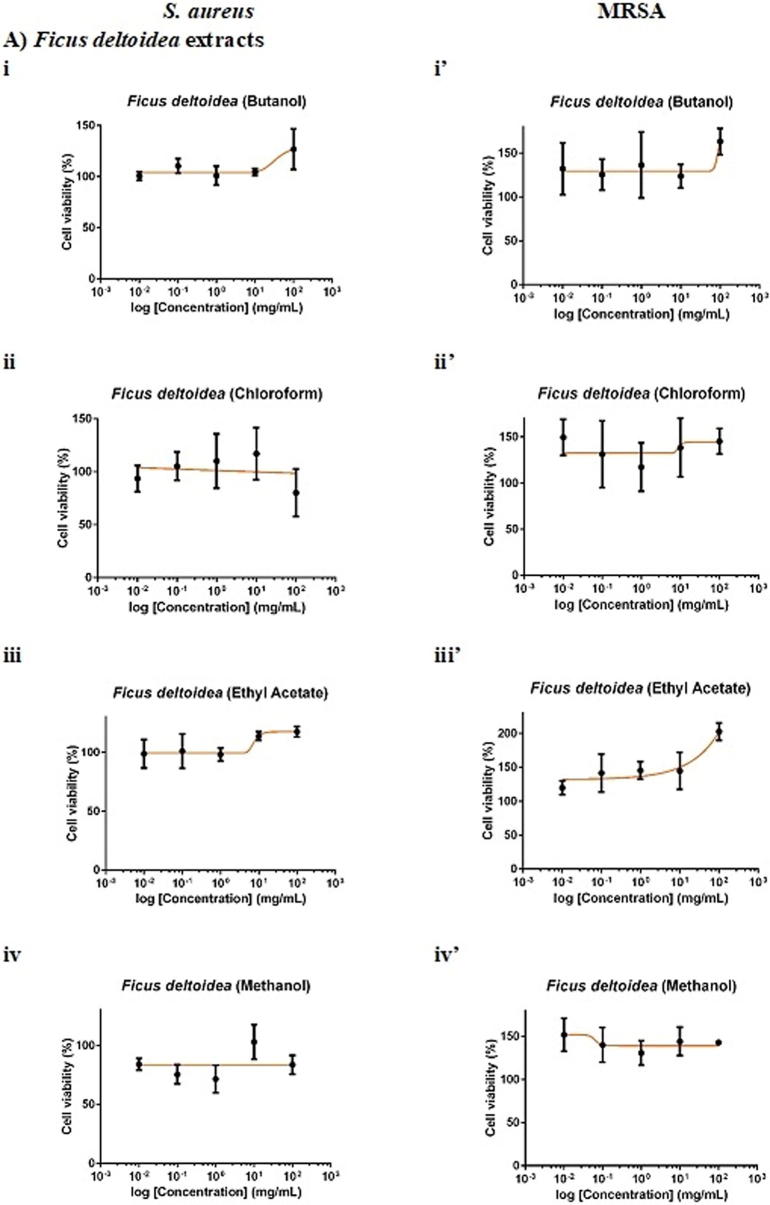
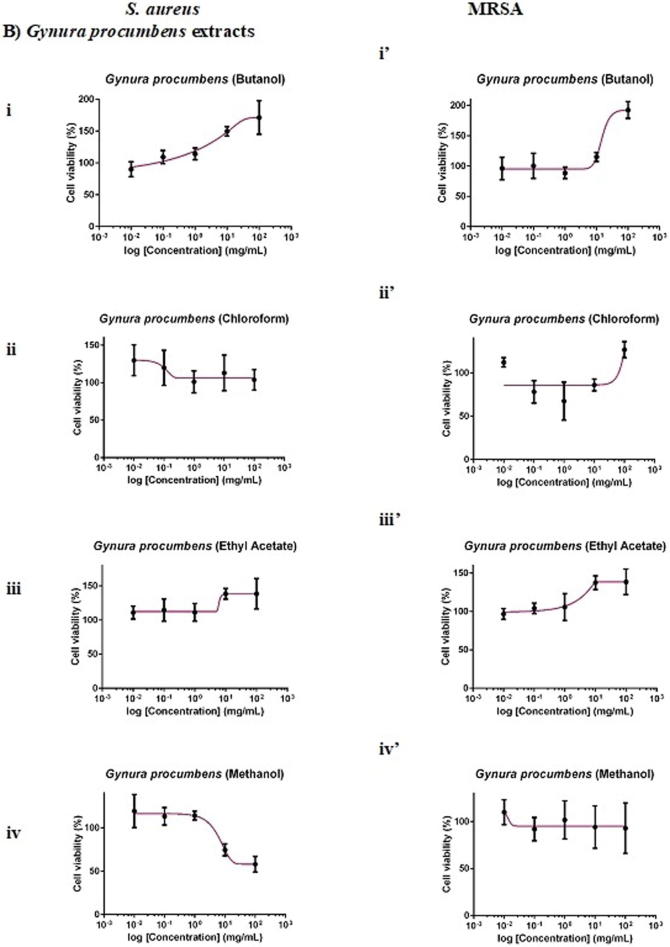
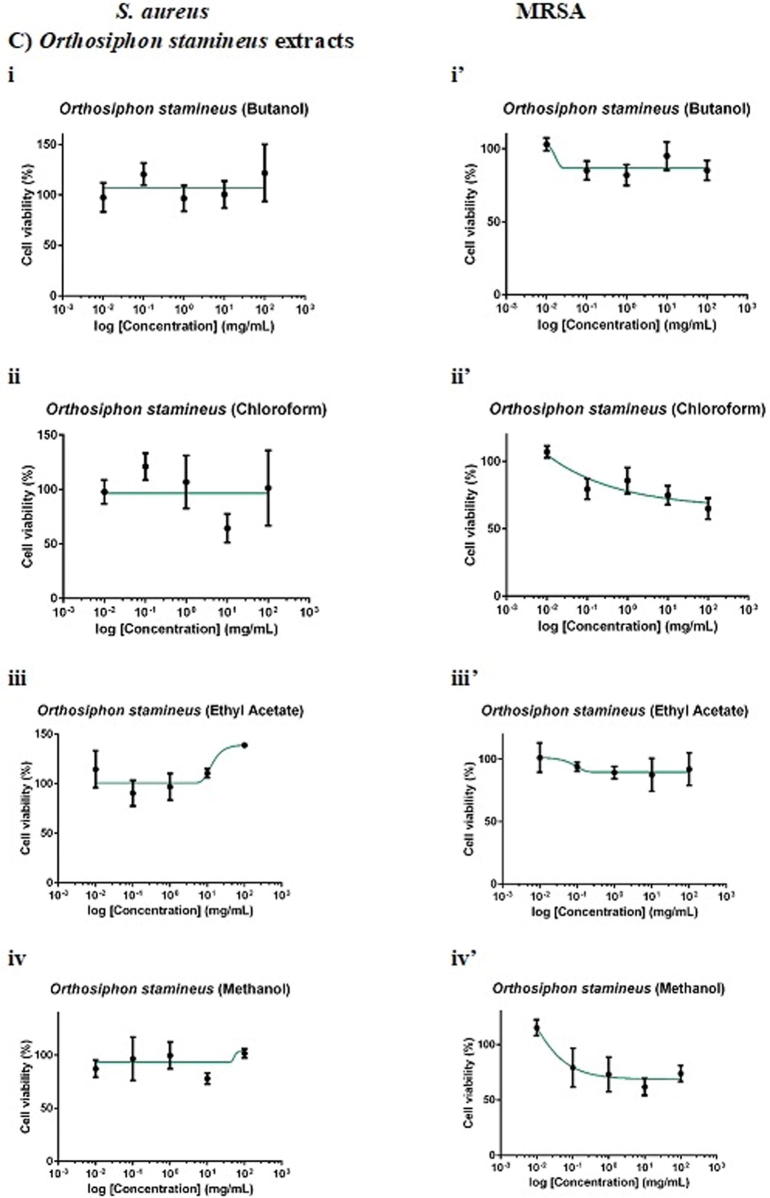
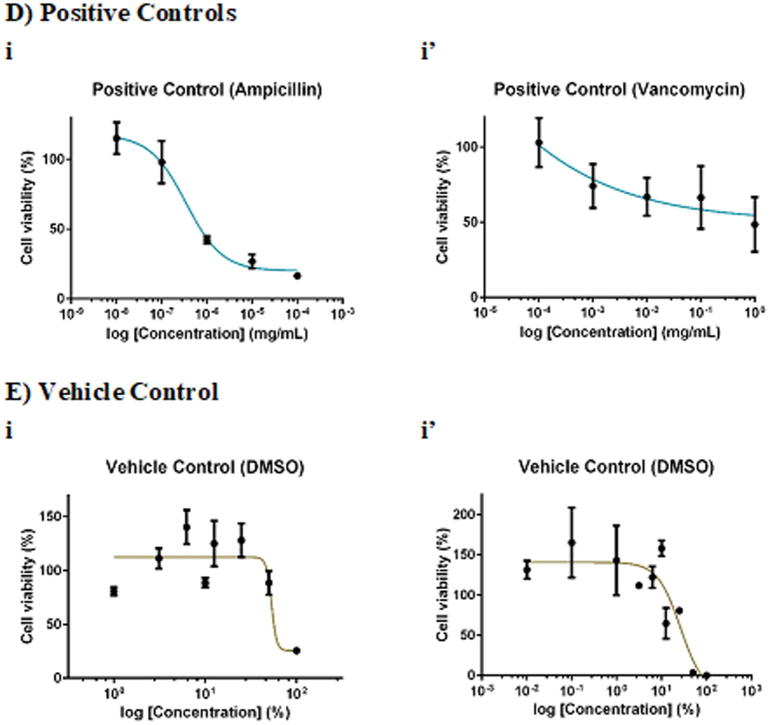
F. deltoidea extract was more selective towards S. aureus than MRSA. Whilst 100 mg/mL chloroform and methanol extracts of F. deltoidea inhibited S. aureus by 20% (Fig. 2Aii) and 16% (Fig. 2Aiv), respectively, the extracts, in general, showed no antimicrobial activity against MRSA. As for G. procumbens, only the methanol extract was effective against the pathogens. It was found that 100 mg/mL methanol extract of G. procumbens inhibited S. aureus by 42% (Fig. 2Biv), 6-times greater than that against MRSA, the resistant strain (by 7%; Fig. 2 Biv′). Interestingly, O. stamineus extract was more selective towards the resistant MRSA than S. aureus. Whilst the extracts, in general, did not inhibit S. aureus growth, 100 mg/mL O. stamineus extract was effective against MRSA, with chloroform extract eliciting the greatest inhibition (35%; Fig. 2Cii′), followed by methanol (26%; Fig. 2Civ′), butanol (15%; Fig. 2Ci′) and ethyl acetate (8%; Fig. 2Ciii′) extracts. Ampicillin yielded an IC50 (concentration required to inhibit 50% pathogens) of 0.0014 mg/mL against S. aureus whereas vancomycin yielded an IC50 of 0.7272 mg/mL against MRSA. Given that 100 mg/mL extract contained only 0.1% DMSO, the vehicle of the extract did not act as confounding factor of the antimicrobial effect against the pathogens.
Fig. 2.
Dose-response curves of 24-hour of F. deltoidea, G. procumbens and O. stamineus extracts against S. aureus and MRSA. S. aureus and MRSA were seeded at density standardised to 0.5 McFarland standard (1 × 108 CFU/mL). The pathogenic bacteria were being treated with (A) F. deltoidea, (B) G. procumbens, (C) O. stamineus extracts, (D) positive and (E) vehicle controls. The plates were incubated at 37 °C for 24 h after which the MTT assay was performed. Each point represents mean SD of quadruplicates.
3.5. Cytotoxic activities of extracts
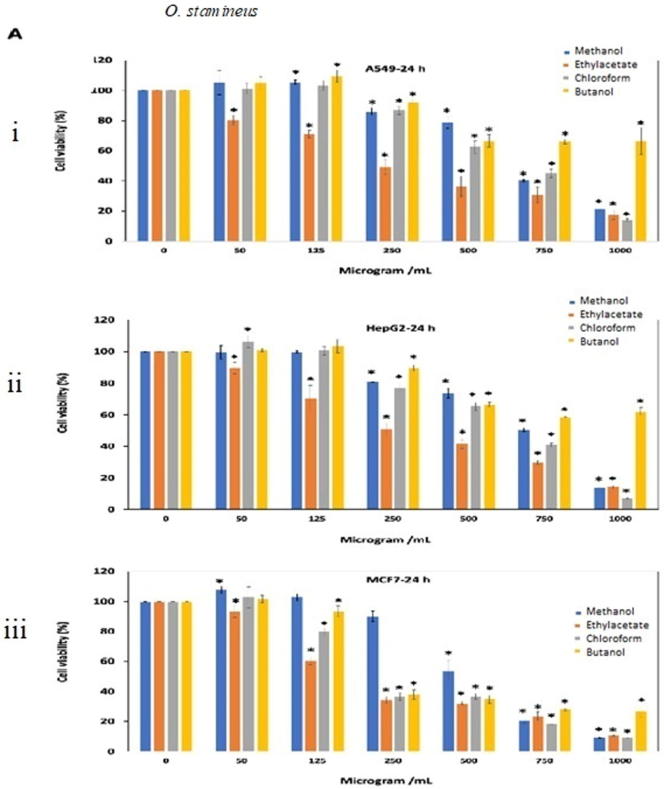
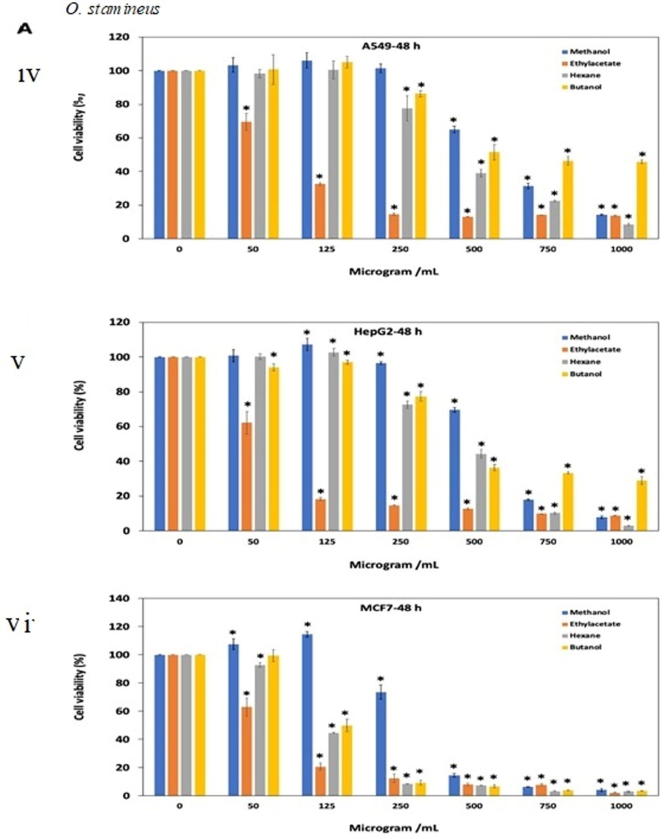
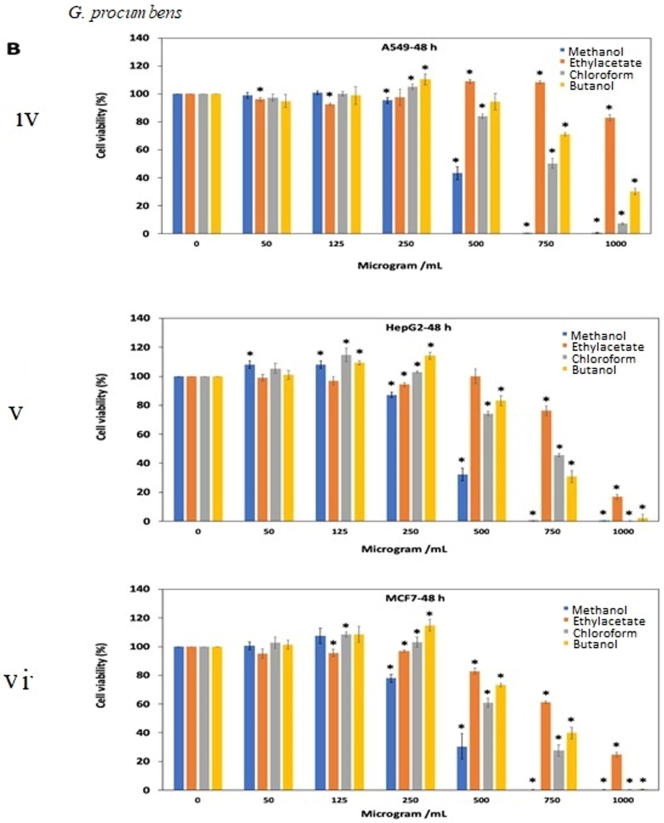
The extracts resulted in decrease cell viability and cell growth inhibition in a dose dependent manner. For O. stamineus, methanol extract had more than 90% cell viability (125 μg/mL) after 24 and 48 h exposure against A549, HePG2 and MCF-7 cell lines (Fig. 3A. i, ii, iii, iv, v, vi). Interestingly, ethyl acetate extract showed comparatively good antiproliferative effect (IC50=81.92, 61.47 and 65.28 μg/mL) against A549, HePG2 and MCF-7 cancer cell lines, respectively after 48 h of treatment (Fig. 3A. iv, v, vi). Chloroform showed antipoliferative activity (IC50= 256.5 and 116.4 μg/mL) against MCF7 cell line after treatment for 24 and 48 h. Table 1A, Table 1B, Table 1C shows the antiproliferative activity (IC50 value) of all extracts tested on A549, HeGP2 and MCF-7 cancer cell lines.
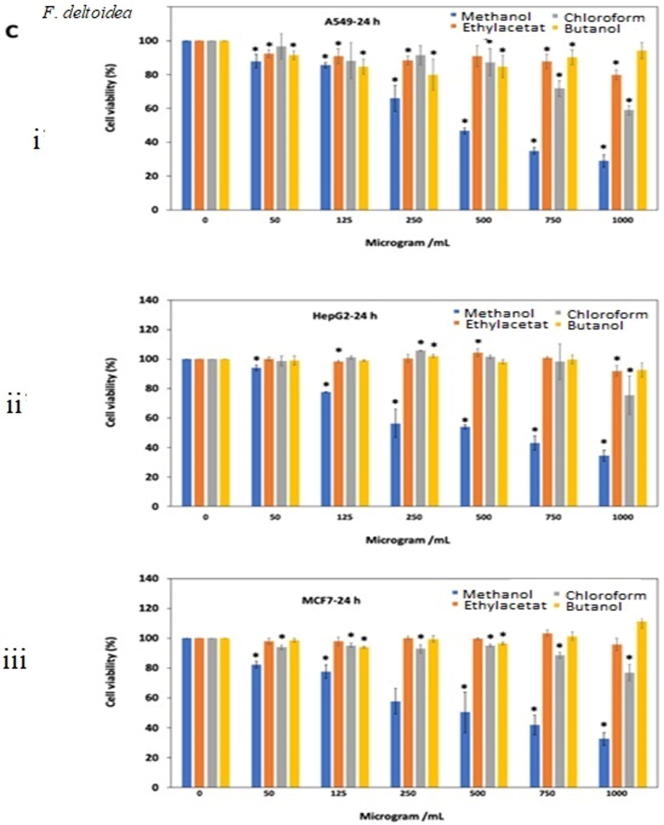
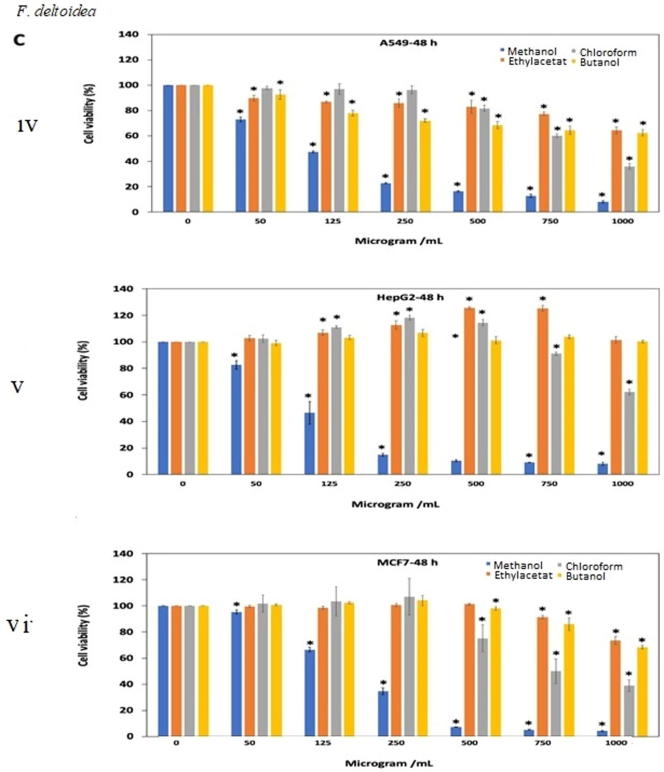
Fig. 3.
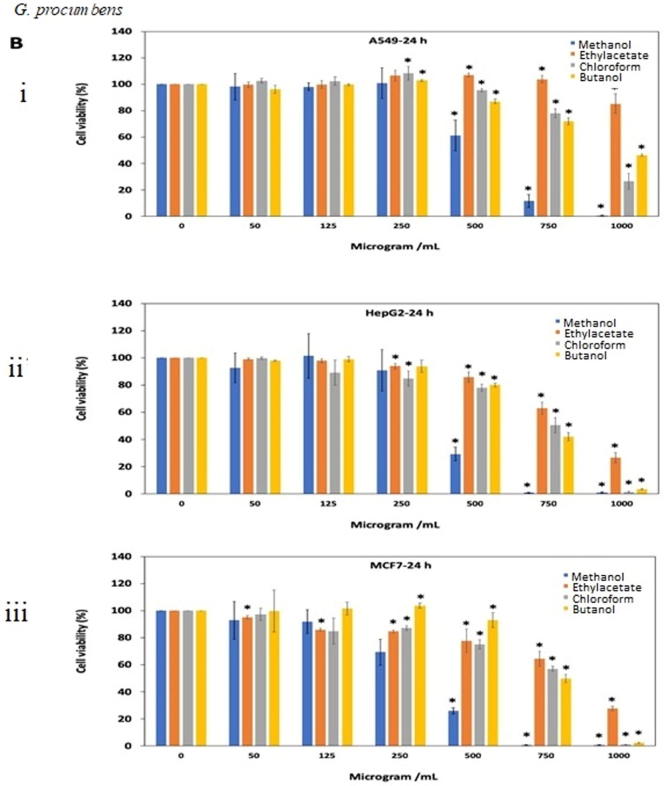
Antiproliferative effects of the extracts of (A) O. stamineus, (B) G. procumbens and (C) F. deltoidea on three human cancer cell lines at 24 h and 48 h. Cells were treated with various concentrations (50, 125, 250, 500, 750, 1000 µg/mL) of methanol, ethyl acetate, chloroform and butanol extracts. Cell proliferation was measured at 24 h and 48 h by MTT assay. The data were expressed as mean ± SD deviation values obtained from three independent determinations. *, P < 0.05 versus controls.
Table 1A.
IC50 (µg/mL) of Orthosiphon stamineus extracts on human cancer cell lines at 24 and 48 h.
| Cells | Time (h) | IC50 of Orthosiphon stamineus extracts (µg/mL) |
|||
|---|---|---|---|---|---|
| Methanol extract | Ethyl acetate extract | Chloroform extract | Butanol extract | ||
| A549 | 24 | 683.8 ± 21.2 | 266.1 ± 15.5 | 616.4 ± 20.2 | >1000 |
| 48 | 604.6 ± 12.5 | 81.9 ± 7.4 | 425.8 ± 11.9 | 709.9 ± 51.8 | |
| HepG2 | 24 | 676.9 ± 28.0 | 293.2 ± 17.1 | 577.0 ± 30.6 | >1000 |
| 48 | 578.8 ± 8.6 | 61.5 ± 6.1 | 407.1 ± 15.9 | 469.5 ± 24.3 | |
| MCF7 | 24 | 514.8 ± 12.8 | 202.0 ± 15.5 | 256.5 ± 20.8 | 322.4 ± 36.1 |
| 48 | 327.6 ± 15.6 | 65.3 ± 3.6 | 116.4 ± 2.6 | 126.4 ± 3.7 | |
Table 1B.
IC50 (µg/mL) of Gynura procumbens extracts on human cancer cell lines at 24 and 48 h.
| Cells | Time (h) | IC50 of Gynura procumbens extracts (µg/mL) |
|||
|---|---|---|---|---|---|
| Methanol extract | Ethyl acetate extract | Chloroform extract | Butanol extract | ||
| A549 | 24 | 538.2 ± 12.8 | >1000 | 878.9 ± 12.3 | 966.4 ± 17.4 |
| 48 | 486.5 ± 5.12 | >1000 | 727 ± 12.3 | 869.9 ± 16.8 | |
| HepG2 | 24 | 414 ± 22.9 | 817.3 ± 13.7 | 695.8 ± 28.9 | 676.6 ± 13.1 |
| 48 | 411.5 ± 14.6 | 846.6 ± 8.0 | 667.4 ± 25.0 | 652.3 ± 17.9 | |
| MCF7 | 24 | 326.9 ± 16.7 | 806.9 ± 51.0 | 710.1 ± 34.6 | 746.9 ± 10.8 |
| 48 | 375.3 ± 15.1 | 797.3 ± 14.4 | 567.1 ± 15.2 | 648.4 ± 24.0 | |
Table 1C.
IC50 (µg/mL) of Ficus deltoidea extracts on human cancer cell lines at 24 and 48 h.
| Cells | Time (h) | IC50 of Ficus deltoidea extracts (µg/mL) |
|||
|---|---|---|---|---|---|
| Methanol extract | Ethyl acetate extract | Chloroform extract | Butanol extract | ||
| A549 | 24 | 452.4 ± 18.9 | >1000 | >1000 | >1000 |
| 48 | 111.3 ± 3.8 | >1000 | 839.7 ± 12.7 | >1000 | |
| HepG2 | 24 | 501.3 ± 37.9 | >1000 | >1000 | >1000 |
| 48 | 114.6 ± 5.9 | >1000 | >1000 | >1000 | |
| MCF7 | 24 | 455.4 ± 43.1 | >1000 | >1000 | >1000 |
| 48 | 178.1 ± 3.1 | >1000 | 791.6 ± 42.2 | >1000 | |
For G. procumbens methanol extract was found to show good antiproliferative effects as compared to other extracts. IC50 value of methanol extract against all three A549, HeGP2 and MCF-7 cell line at 24 and 48 h treatments were 538.2–486.5 μg/mL, 414–411.5 μg/mL and 326.9–375.3 μg/mL, respectively (Fig. 3B. i, ii, iii, iv, v, vi). F. deltoidea extracts also showed antiproliferative activity in dose dependent manner. Butanol and ethyl acetate extracts were almost ineffective against all three cell lines tested (IC50 value > 1000 μg/mL). Methanol extract yielded quite good result after 48 h of treatment against all three cell lines (Fig. 3C. i, ii, iii, iv, v, vi).
3.6. Morphological changes
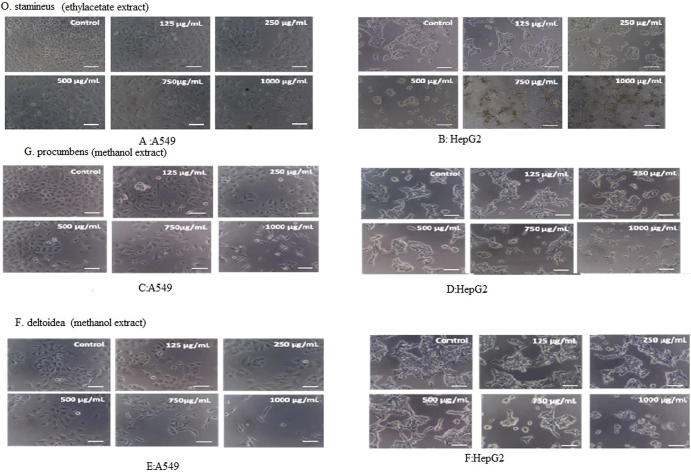
Confocal microscopic images of treated cells showed that the treatment with the extracts resulted in dramatic morphological changes in A549 and HepG2 cell lines after 24 h. Morphological changes of cells from epithelial-like shape to spindle and rounded shape were observed. Cells treated with high concentration of extracts caused cytoplasmic condensation, shrinkage, reduction in size and tendency to float in the medium after extracts treatment for 24 h compared to control. Taken together, the extracts induced morphological changes in a dose dependent manner (Fig. 4 A-E).
Fig. 4.
Morphological changes in A549 and HepG2 cells by ethyl acetate of O. stamineus (A & B), methanol extract of G. procumbens (C & D) and F. deltoidea (E & F), respectively. The cells were incubated with indicated concentration of the extract for 24 h, and then visualized by light microscopy.
4. Discussion
In the current study, results showed that solvent extracts play very important role in exhibiting the biological activities. In the case of antioxidant activity, it was found that antioxidant and total phenolic content (TPC) depends on the polarity of solvent. The results indicated that antioxidant and TPC were highest in methanol extract of almost all selected plants as compared to other extracts. The reason could be due to phenolics are polar in nature. Polar solvents such as methanol could solvate polar solutes and ions more efficiently and orient themselves towards the solute through electrostatic attraction. In other words, phenolics and flavonoids could dissolve better in the polar solvents (Zhang et al., 2016). Our finding is also in accordance with Ong et al. (2011) whereby methanol extract of F. deltoidea exhibited the highest phenolic content followed by chloroform and hexane extract.
Our present finding of G. procumbens are also in line with previous finding. Kaewseejan and Siriamornpun (2015) have reported that G. procumbens extracts could show antioxidant capacity and reductive ability. It was also found that reducing power assay and DPPH, follows the same pattern of scavenging for different extracts. In both the cases, methanol solvent extract showed greater activity. Previous study by Rosidah Yam et al. (2009) also stated the direct correlation of free radical scavenging and reducing capacity of G. procumbens plant extract. Another results also revealed the reducing power assay of G. procumbens (Prior et al., 2005). A study by Teoh and his fellows also showed that methanol and its fractionated hexane, ethyl acetate and water extract also exhibited good total phenolic content, antioxidant power, cytotoxicity. Result showed that out of all extracts, ethyl acetate exhibited good antioxidant and antiproliferative effect (Teoh et al., 2013). In another experiment, three different extracts hexane dichloromethane and methanol of G. divaricata leaves were chosen for the evaluation of the antioxidant, cytotoxicity of the essential oil. The extract showed good antiproliferative effect of essential oil against KB, MCF-7 and NCI-H187 cancer cell lines (Jiangseubchatveera et al., 2015). It also revealed inhibition of tongue carcinogenesis and prevent osteosarcoma cancerous tumour in a bone (Li et al., 2017). Algariri et al. (2013) also found the reduction of cytochrome P-450 enzymes such as CYP3A4, CYP1A2, and CYP1A1 cytochrome P-450 by G. procumbens. It can also hinder the start phase of carcinogenesis by rouse the expression of glutathione-transferase as it helps in detoxification of carcinogenic compounds.
Antimicrobial assay of theses extracts, produced very interesting results. In the case of F. deltoidea, the present findings indicated chloroform and methanol extracts to be effective against S. aureus but not MRSA. It was reported that chloroform extract of F. deltoidea leaves did not inhibit S. aureus but methanol extract of the leaves inhibited the pathogens with an inhibition zone diameter of 7.0 ± 0.1 mm (Uyub et al., 2010). Elsewhere, methanol extract of whole F. deltoidea significantly inhibited the growth of S. aureus, forming a wide inhibition zone of 15.67 ± 0.58 mm and lowest minimum inhibitory concentration (MIC) value of 3.125 mg/mL (Abdsamah et al., 2012). As for G. procumbens, the present study found methanol extracts to be more effective against S. aureus than MRSA. It was reported that 400 μg/disc crude methanol extracts of G. procumbens leaves showed no activity against S. aureus (Mustafizur Rahman and Al Asad, 2013). Recent findings, however, reported otherwise whereby 100 mg/mL methanol extracts of G. procumbens leaves yielded inhibition zone of 8.0 ± 0.03 mm against S. aureus (Nawi et al., 2019). Interestingly, the current study found chloroform and methanol extracts of O. stamineus to be the two most active extracts against resistant MRSA but not S. aureus. Previous studies (spot test), however, found no activity of 50 mg/mL methanol O. stamineus whole plant extract against S. aureus ATCC 25,923 and MRSA ATCC 43300. Nevertheless, subsequent microdilution method indicated MIC of 12.5 mg/mL against S. aureus and MIC of 25 mg/mL against MRSA (BM3, WM2, K32 and K52) (Md Nor and Mohd Yasin, 2018). The positive controls, ampicillin and vancomycin exhibited more potent antimicrobial activities when compared to the plant extracts. Previous studies reported MIC ≤ 0.25 µg/mL for ampicillin against S. aureus (Pengov and Ceru, 2003) and MIC < 2 μg/mL for vancomycin against MRSA (Aminzadeh et al., 2014).
As O. stamineus extract contains valuable compound flavones and vitamin E, this may be helpful in showing secondary chemical evidence for the cytotoxic efficacy and antioxidant potential. Because of presence of phenolic and flavonoid constituents, O. stamineus extract could help in arresting the growth of cell proliferations. In vitro MTT assay result exhibited that ethyl acetate extract of the of O. stamineus has produced good antiproliferative effect as compare to other extracts tested. Additionally, similar earlier studies have also support our present finding (Akowuah et al., 2005a, Akowuah et al., 2005b). Extraction method and solvent extract plays very significant role in determining the strength of free radical scavenging ability of extract (Hossain et al., 2015). Salleh et al., (2011) have studied the ethyl acetate fraction of O. stamineus and found good anti proliferative growth of human hepatocellular carcinoma cell line (HepG2). Stampoulis et al. (1996) also reported the cytotoxic activity of methanolic extract of O. stamineus leaves against liver metastatic colon 26-L5 carcinoma cells. In an another experiment, Awale et al. (2002) studied an isolated compound of Japanese O. stamineus and reported the good antiproliferative effect on liver metastatic murine colon 26-L5 carcinoma and human HT-1080 fibrosarcoma cell lines. Their outcomes are also in agreement with our current finding. Our results are also in accordance with results of Sahib et al. (2009) which indicated the antipoliferative activity of methanolic extract of O. stamineus. Our results are also supported by Abdelwahab et al. (2011) who correlated the antiapoptotic property with antioxidant activities of O. stamineus. A study in Maynmar reported the weak to mild antiproliferative activity of compound of O. stamineus toward highly malignant liver metastatic colon 26-L5 carcinoma and human HT-1080 fibrosarcoma cell lines (Thirugnanasampandan and Selvi, 2012). According to Awale et al. (2003) finding, different percentage of methanol of extract of O. stamineus produces different DPPH free radical scavenging activities. Our results are also in accordance with finding of Akowuah et al., 2005a, Akowuah et al., 2005b which revealed that different antioxidative potency due to different solvent extracts tested. Recently Soib et al. (2019) have checked cytotoxicity study against prostate cancer cell lines DU145 of fractionated aqueous extract of F. deltoidea. Results suggest that flavonoids content of F. deltoidea var. kunstleri could potentially be developed as an anti-cancer agent for prostate cancer. Abrahim et al. (2018) have also carried out antioxidant and cytotoxic study of different extracts of three varieties F. deltoidea and reported highest phenolic and flavonoid content in ethyl acetate fraction. Their finding also explicated that F. deltoidea varieties may have good antioxidant activity but no cytotoxic effects on normal liver cells. Cytotoxicity of O. stamineus extract was also evaluated on normal fibroblast cells and found negligible effect (Al-Suede et al., 2014). Teoh et al. (2016) evaluated cytotoxicity study of G. procumbens extracts on human normal colon cells (CCD-18Co) and reported very low toxicity of ethylacetate against CCD-18Co normal colon cells.
5. Conclusions
Altogether, methanol extract provides excellent free radical scavenging activity in all three selected plants. This could be significant source of natural antioxidant.
MTT assay used in this study, is a simple, standard colorimetric assay (an assay which measures changes in color) for measuring cellular proliferation (cell growth). Ethyl acetate of O. stamineus have good property of antiproliferative effect where as methanolic extract of F. deltoidea and G. procumbens showed comparatively good results against selected cell lines. Chloroform and methanol extracts of F. deltoidea elicited antimicrobial activity against S. aureus but not MRSA. Only methanol extract G. procumbens showed antimicrobial activity against the tested pathogens. Chloroform and methanol extracts of O. stamineus extracts were the two most active extracts against resistant MRSA but not S. aureus. This collected information could help in isolation of pure compounds of more potent pharmacological activity.
Acknowledgement
The Universiti Teknologi MARA (UiTM) is thanked for the financial support under the reference number 600-IRMI/MyRA 5/3/LESTARI (079/2017).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelwahab S.I., Mohan S., Elhassan M.M., Al-Mekhlafi N., Mariod A.A., Abdul A.B. Antiapoptotic and antioxidant properties of Orthosiphon stamineus Benth (Cat's Whiskers): intervention in the Bcl-2-mediated apoptotic Pathway. Evid. Based. Compl. Alternat. Med. 2011;2011 doi: 10.1155/2011/156765. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Abdsamah O., Zaidi N.T., Sule A.B. Antimicrobial activity of Ficus deltoidea jack (mas cotek) Pak. J. Pharma. Sci. 2012;25:675–678. [PubMed] [Google Scholar]
- Abrahim N.N., Abdul-Rahman P.S., Aminudin N. The antioxidant activities, cytotoxic properties, and identification of water-soluble compounds of Ficus deltoidea leaves. Peer J. 2018;11 doi: 10.7717/peerj.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akowuah G.A., Ismail Z., Norhayati I., Sadikun A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical scavenging activity. Food Chem. 2005;93:311–317. [Google Scholar]
- Akowuah G.A., Zhari I., Norhayati I., Sadikun A. Radical scavenging activity of methanol leaf extracts of Orthosiphon stamineus. Pharm. Biol. 2005;42:629–635. [Google Scholar]
- Al-Daihan S., Al-Faham M., Al-shawi N., Almayman R., Brnawi A., Zargar S., Shafi Bhat R. Antibacterial activity and phytochemical screening of some medicinal plants commonly used in Saudi Arabia against selected pathogenic microorganisms. J. King Saud Univ. Sci. 2013;25:115–120. [Google Scholar]
- Algariri K., Meng K.Y., Atangwho I.J., Asmawi M.Z., Sadikun A., Murugaiyah V., Ismail N. Hypoglycemic and anti-hyperglycemic study of Gynura procumbens leaf extracts Asian Pac. J. Trop. Biomed. 2013;3:358–366. doi: 10.1016/S2221-1691(13)60077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminzadeh Z., Yadegarynia D., Fatemi A., Tahmasebian Dehkordi E., Azad Armaki S. Vancomycin minimum inhibitory concentration for methicillin-resistant Staphylococcus aureus infections; is there difference in mortality between patients. Jundis. J. Microb. 2014;7 doi: 10.5812/jjm.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Suede F.S.R., Mohamed B., Ahamed K., Aman S., Majid A., Baharetha H.M., Hassan L.E.A., Kadir M.Q.A., Nassar Z.D., Abdul A.M.S. Majid optimization of Cat's Whiskers Tea (Orthosiphon stamineus) Using supercritical carbon dioxide and selective chemotherapeutic potential against prostate cancer cells. Evid. Based Compl. Alter. Med. 2014 doi: 10.1155/2014/396016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir M., Mujeeb M., Khan A., Ashraf K., Sharma D., Aqil M. Phytochemical analysis and in vitro antioxidant activity of Uncaria gambir. Int. J. Green Pharm. 2013;6:1–6. [Google Scholar]
- Ashraf K., Jameel M., Mujeeb M. Evaluation of pharmacognostical variations in eight accession of Curcuma longa L. Int. J. Green Pharm. 2018;12:1. [Google Scholar]
- Ashraf K. An updated phytochemical and pharmacological review on Gynura procumbens. Asian J. Pharm. Clin. Res. 2019;12:9–14. [Google Scholar]
- Aslam B., Wang W., Arshad M.I., Khurshid M.K., Muzammil S., Rasool M.H., Nisar M.A., Alvi R.F., Aslam M.A., Qamar M.U., Salamat M.K.F., Baloch Z. Antibiotic resistance: a rundown of a global crisis. Infect. Drug Resis. 2018;11:1645–1658. doi: 10.2147/IDR.S173867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awale S., Tezuka Y., Banskota A.H., Shimoji S., Taira K., Kadota S. Norstaminane- and isopimarane-type diterpenes of Orthosiphon stamineus from Okinawa. Tetrahedron. 2002;58:5503–5512. [Google Scholar]
- Awale S.A., Tezuka Y., Banskota A.H., Kadota S. Inhibition of NO production by highly-oxygenated diterpenes of Orthosiphon stamineus and their structure-activity relationship. Biol. Pharma Bull. 2003;26:468–473. doi: 10.1248/bpb.26.468. [DOI] [PubMed] [Google Scholar]
- Bagchi D., Bagchi M., Stohs S.J., Das D.K., Ray S.D., Kuszynski C.A., Joshi S.S., Pruess H.G. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicol. 2000;148:187–197. doi: 10.1016/s0300-483x(00)00210-9. [DOI] [PubMed] [Google Scholar]
- Balouris M., Sadiki M., Ibnsouda S.K. Methods for in vitro evaluating antimicrobial activity: a review. J. Pharma Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J.E. Coligan, A.M. Kruisbeek, D.H. Margulis, E.M. Shevach, W. Strober, Trypan blue exclusion test of cell viability In: Current Protocols in Immun, vol. 3. John Wiley and Son, New York, 1992, pp. 3–4.
- Choo C.Y., Sulong N.Y., Man F., Wong T.W. Wong Vitexin and isovitexin from the Leaves of Ficus deltoidea with in-vivo α-glucosidase inhibition. J Ethnopharmacol. 2012;142:776–781. doi: 10.1016/j.jep.2012.05.062. [DOI] [PubMed] [Google Scholar]
- Duin D.V., Paterson D. Multidrug resistant bacteria in the community: trends and lessons learned. Infect. Dis. Clin. North Am. 2016;30:377–390. doi: 10.1016/j.idc.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes E.C., Silva A.N., de Oliveira M.R. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid. Med. Cell Longev. 2012;75:6132. doi: 10.1155/2012/756132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grela E., Kozłowska J., Grabowiecka A. Current methodology of mtt assay in bacteria – a review. Acta Histochem. 2018;120:303–311. doi: 10.1016/j.acthis.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Hossain M.A., Mizanur Rahman S.M. Isolation and characterisation of flavonoids from the leaves of medicinal plant Orthosiphon stamineus. Arab. J. Chem. 2015;8:218–221. [Google Scholar]
- Jiangseubchatveera N., Liawruangrath B., Liawruangrath S., Korth J., Pyne S.J. The chemical constituents and biological activities of the essential oil and the extracts from leaves of Gynura divaricata (L.) DC. Growing in Thailand. J. Essen. Oil-Bearing Plants. 2015;18:3543–3555. [Google Scholar]
- Kaewseejan N., Siriamornpun S. Bioactive components and properties of ethanolic extract and its fractions from Gynura procumbens leaves. Indust. Crops Prodt. 2015;74:271–278. [Google Scholar]
- Lachenmayer A., Alsinet C., Chang C.Y., Liovit J.M. Molecular approaches to treatment of hepatocellular carcinoma. Dig. Liver Dis. 2010;42:264–272. doi: 10.1016/S1590-8658(10)60515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Peter C.C., Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J. Ethnopharmacol. 2005;100:237–243. doi: 10.1016/j.jep.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Li J.E., Wang W.J., Zheng G.D., Li L.Y. Physicochemical properties and antioxidant activities of polysaccharides from Gynura procumbens leaves by fractional precipitation. Int. J. Bio. Macromol. 2017;95:719–724. doi: 10.1016/j.ijbiomac.2016.11.113. [DOI] [PubMed] [Google Scholar]
- Md Nor N.S., Mohd Yasin R.A. Antibacterial activity of orthosiphon stamineus towards Staphylococcus aureus and methicilin resistant Stahpylococcus aureus (mrsa) J. Chem. Pharma. Sci. 2018:11. [Google Scholar]
- Mustafizur Rahman A.F.M., Al Asad M.S. Chemical and biological investigations of the leaves of Gynura procumbens. Int. J. Bio. 2013;3:36–43. [Google Scholar]
- Nawi L., Md Isa N.N., Musa N.L.W., Mohd Jan S.L., Shaikh Nasir N.T. Antimicrobial activities of Gynura procumbens leaves extract against selected bacteria. Gad. J. Sci. Tech. 2019;2:17–22. [Google Scholar]
- Noreen H., Semmar N., Farman M., James McCullagh J.S.O. Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant. Coronopus didymus. Asian Pac. J. Trop. Med. 2017;10:792–801. doi: 10.1016/j.apjtm.2017.07.024. [DOI] [PubMed] [Google Scholar]
- Ong S., Ling A., Poospooragi R., Moosa S. Production of Flavonoid compounds in cell cultures of Ficus deltoidea as influenced by medium composition. Int. J. Med. Arom. Plants. 2011;1:2249–4340. [Google Scholar]
- Pengov A., Ceru S. Antimicrobial drug susceptibility of Staphylococcus aureus strains isolated from bovine and ovine mammary glands. J. Dairy Sci. 2003;86:3157–3163. doi: 10.3168/jds.S0022-0302(03)73917-4. [DOI] [PubMed] [Google Scholar]
- Prior Ronald L., Schaich K., Wu Xianli. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Rafieian-Kopaie M., Nasri H. On the occasion of World Cancer Day 2015: the possibility of cancer prevention or treatment with antioxidants: the ongoing cancer prevention researches. Int. J. Prev. Med. 2015;6:108. doi: 10.4103/2008-7802.169077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam M., Sadikun A., Asmawi M. Antioxidant Potential of Gynura procumbens. Pharm. Bio. 2008;46:616–625. [Google Scholar]
- Rosidah Yam F., Sadikun A., Ahmad M., Akowuah G.A., Asmawi M.Z. Toxicology evaluation of standardized methanol extract of Gynura procumbens. J. Ethnopharmacol. 2009;123:244–249. doi: 10.1016/j.jep.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Sahib H.B., Ismail Z., Othman N.H., Abdul Majid A.M.S. Orthosiphon stamineus Benth. methanolic extract enhances the anti-proliferative effects of tamoxifen on human hormone dependent breast cancer. Int. J. Pharmacol. 2009;5:273–276. [Google Scholar]
- Salleh S.A., Rajab N.F., Abdullah N.R., Ismail Z.P., Mouatt P., Dowell A., Muhamad S. In vitro chemopreventive activity of an ethyl acetate fraction derived from hot water extract of Orthosiphon stamineus in HepG2 cells. J. Med. Plants Res. 2011;5:1892–1899. [Google Scholar]
- Shaikh R., Pund M., Dawane A., Iliyas S. Evaluation of anticancer, antioxidant, and possible antiinflammatory properties of selected medicinal plants used in indian traditional medication. J. Trad. Compl. Med. 2014;253 doi: 10.4103/2225-4110.128904. pp. 257.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soib H.H., Yaakob H., Sarmidi M.R., Rosdi M.N.M. Fractionation of aqueous extract of Ficus deltoidea var. Kunstleri’s leaves using solid phase extraction method for anticancer activity on du145 cell line. Malay J Analy Sci. 2019;23:534–547. [Google Scholar]
- Stampoulis P., Tezuk Y., Banskota A.H., Tran K.Q., Saiki S., Kadoka S. Staminolactones A and B and nor staminol A: three highly oxygenated staminane-type diterpenes from Orthosiphon stamineus. Org. Lett. 1996;1:1367–1370. doi: 10.1021/ol990216+. [DOI] [PubMed] [Google Scholar]
- Teoh W.Y., Sim K.S., Richardson J.S.M., Wahab N.A., Hoe S.Z. Antioxidant capacity, cytotoxicity, and acute oral toxicity of Gynura bicolor. Evid.-Based Comp. Alter. Med. 2013;95:8407. doi: 10.1155/2013/958407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh W.Y., Wahab N.A., Richardson J.S.M., Sim K.S. Evaluation of antioxidant properties, cytotoxicity and acute oral toxicity of Gynura procumbens (Compositae) Sains Malaysiana. 2016;45:229–235. [Google Scholar]
- Thirugnanasampandan R., Selvi M.T. Chemical composition analysis and antioxidant activity evaluation of essential oil from Orthosiphon thymiflorus (Roth.) Sleesen. Asian Pac. J. Trop. Biomed. 2012:112–115. [Google Scholar]
- Uyub A.M., Nwachukwu I.N.A.A., Fariza S.S. In-vitro antibacterial activity and cytotoxicity of selected medicinal plant extracts from Penang island Malaysia on metronidazole-resistant-helicobacter pylori and some pathogenic bacteria. Ethnobot Res. Appl. 2010;8:95–106. [Google Scholar]
- Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. [Google Scholar]