Abstract
Background
We conducted a multicentre phase II trial to investigate feasibility and antitumor activity of sequential FOLFIRINOX and Stereotactic Body Radiotherapy (SBRT) in patients with locally advanced pancreatic cancer (LAPC), (LAPC-1 trial).
Methods
Patients with biopsy-proven LAPC treated in four hospitals in the Netherlands between December 2014 and June 2017. Patients received 8 cycles of FOLFIRINOX followed by SBRT (5 fractions/8 Gy) if no tumour progression after the FOLFIRINOX treatment was observed. Primary outcome was 1-year overall survival (OS). Secondary outcomes were median OS, 1-year progression-free survival (PFS), treatment-related toxicity, and resection rate. The study is registered with ClinicalTrials.gov, NCT02292745, and is completed.
Findings
Fifty patients were included. Nineteen (38%) patients did not receive all 8 cycles of FOLFIRINOX, due to toxicity (n = 12), disease progression (n = 6), or patients’ preference (n = 1). Thirty-nine (78%) patients received the SBRT treatment. The 1-year OS and PFS were 64% (95% CI: 50%-76%) and 34% (95% CI: 22%-48%), respectively. Thirty grade 3 or 4 adverse events were observed during FOLFIRINOX. Two (5%) grade 3 or 4 adverse events after SBRT were observed. Two (5%) patients died due to a gastro-intestinal bleeding within three months after SBRT were observed. Six (12%) patients underwent a resection, all resulting in a complete (R0) resection. Two patients had a complete pathological response.
Interpretation
FOLFIRINOX followed by SBRT in patients with LAPC is feasible and shows relevant antitumor activity. In 6 (12%) patients a potentially curative resection could be pursued following this combined treatment, with a complete histological response being observed in two patients.
Keywords: Locally advanced pancreatic cancer, Chemotherapy, Radiotherapy, Surgery, Survival
1. Introduction
Pancreatic cancer is the fourth leading cause of cancer-related death with an estimated 5 year survival rate of approximately 9% [1]. At the time of diagnosis, approximately 15% of patients have (borderline) resectable disease (stage I or II), while 35% and 50% of patients present with irresectable locally advanced pancreatic cancer (LAPC, stage III)or metastatic disease (stage IV), respectively [2]. LAPC is determined by the extent of tumour contact with the superior mesenteric artery (SMA), celiac artery (CA), common hepatic artery (CHA), superior mesenteric vein (SMV), and portal vein (PV) [3]. The risk of a positive resection margin increases with increasing tumour contact with arteries and/or veins. Several definitions for LAPC vary in defining the extent of tumour contact with the surrounding blood vessels [4].
The American Society of Clinical Oncology recommends induction chemotherapy followed by local therapies such as (chemo)radiotherapy or local ablation in the treatment of patients with LAPC [5]. Surgery can be considered as an option following (chemo) radiotherapy in the absence of disease progression, if the resection is technically feasible, with a reasonable likelihood of reaching a radical resection [6].
Based upon the observed activity of FOLFIRINOX in patients with metastatic pancreatic cancer [7], several case series of FOLFIRINOX in patients with LAPC have been published [8]. These case series have shown a potential survival benefit of FOLFIRINOX treatment for patients with LAPC [9].
In patients with LAPC, subsequent consolidation treatment after first-line chemotherapy is often considered in the absence of tumour progression [6]. Conventional (chemo) radiotherapy is most frequently used [8]. However, there is a disadvantage to conventional radiation due to its lack of selective tumour targeting [6, 10]. Stereotactic Body Radiotherapy (SBRT) could possibly improve antitumor activity while limiting dose to surrounding organs [11]. No prospective phase II trials investigating the role of sequential FOLFIRINOX and SBRT in patients with LAPC have been published to date [12].
We conducted a multicentre phase II trial to investigate feasibility and antitumor activity of sequential FOLFIRINOX and Stereotactic Body Radiotherapy (SBRT) in patients with LAPC (LAPC-1 trial).
2. Methods
2.1. Study design
Between December 2014 and June 2017 all consecutive patients with biopsy-proven LAPC from four participating hospitals were enrolled in this study. Ethical approval was given from all local ethical committees of the participating hospitals (Erasmus University Medical Center, Leiden University Medical Center, Maasstad Hospital, and Reinier de Graaf Group). The diagnostic work-up included a tri-phasic CT-scan of abdomen and thorax followed by staging laparoscopy. LAPC was defined according to the Dutch guidelines as tumour contact with the SMA, CA, or CHA exceeding 90° or contact with the SMV or PV exceeding 270° [13]. All patients gave written informed consent before participating in the study (ClinicalTrials.gov Identifier: NCT02292745).
2.2. Patients
The inclusion criteria were biopsy-proven LAPC, age 18–75 years, World Health Organization (WHO) performance status ≤1, ASA classification ≤1, no evidence of metastatic disease, largest diameter of tumour ≤7 cm, normal renal, bone marrow, and liver function. Exclusion criteria were prior abdominal radiotherapy, lymph node metastasis outside the radiation field, tumour ingrowth into stomach, other invasive malignancies diagnosed within 3-years, pregnancy or breastfeeding, serious concomitant disorders that comprise the safety of the patient.
2.3. Intervention
FOLFIRINOX was started within one month after CT-scan and staging laparoscopy in all patients. Standard FOLFIRINOX (2-h intravenous infusion of oxaliplatin (85 mg/m²) followed by a 2-h intravenous infusion of leucovorin (400 mg/m²) concomitantly with a 90-min intravenous infusion of irinotecan (180 mg/m²), followed by a bolus (400 mg/m²) and a 46-h continuous infusion (2400 mg/m²) of fluorouracil) was given once every two weeks for up to 8 cycles. Dose reductions and delays were according to local practice. In cases of persisting toxicity following two dose reductions, FOLFIRINOX was discontinued.
Routine CT scans were performed after 4 and 8 cycles FOLFIRINOX. Patients in whom no disease progression was observed after the completion of FOLFIRINOX received SBRT. Before the start of SBRT, fiducial markers were inserted in the tumour via endoscopic ultrasonography guidance. Gross tumour volume (GTV) was contoured on a 1.25-mm thick contrast-enhanced CT scan. Clinical target volume (CTV) included the GTV plus geometric expansion of 5 mm to include potential microscopic tumour extension. Planning target volume (PTV) encompassed the CTV plus a 2 mm margin. Prescription dose to the 80% isodose line of the PTV was 40 Gy in 8 Gy daily fractions. At least 95% of the prescribed dose should cover 95% of the PTV, though PTV was allowed to be under dosage to meet the constraints of dose-limiting organs at risk (OAR). Dose constraints are summarized in table 1. Real time tumour tracking was performed using the Synchrony respiratory motion tracking system with the fiducials. Endoscopy was performed to implant three fiducials close to or within the tumour prior to the SBRT. A CT-scan was performed 3 and 6 months after SBRT. If the tumour was deemed resectable and no metastatic lesions were seen, an exploratory laparotomy was performed. Resectability was defined as arterial tumour contact less than 90° and venous tumour contact less than 270°.
Table 1.
Dose constraints for treatment planning of SBRT.
| Organs at risk | Maximum dose/fraction | Total maximum dose |
|---|---|---|
| Spinal Cord | 5.5 Gy | 27.5 Gy |
| Liver | < 17.5 Gy per 700 cc | |
| Bowel | 7 Gy | 35 Gy |
| Kidney | – | ⅓ of kidneys <15 Gray |
| Stomach | 7 Gy | 35 Gy |
2.4. Outcomes
The primary outcome of this study was the 1-year OS rate. The secondary outcomes were 1-year progression free survival (PFS) rate, treatment related toxicity, locoregional PFS, metastatic PFS, and resection rate. OS was calculated from the start of the FOLFIRINOX to the date of death. PFS was calculated from the start of FOLFIRINOX to the date of progression or death. Survival functions were estimated using the Kaplan-Meier method in SPSS (version 21). Adverse events were graded using National Cancer Institute (NCI) Common Toxicity Criteria (CTC 4.0). Radiological responses were assessed using RECIST 1.1 [14]. Histopathological response was graded by the tumour regression grading of the College of American Pathologists. [15]
The 1-year OS rate in a historical cohort of patients within our institution with LAPC treated with the combination of Uracil/Tegafur plus leucovorin and celecoxib in combination with conventional radiotherapy was 40% [16]. We hypothesized that with the current treatment sequence a 1-year survival rate of 60% could be achievable. Calculations were made with a two-sided 5% significance test, a power of 80%, and a 20% dropout rate. Using Hern's design for non-randomized phase II trials, a minimum of 51 patients was needed for this study, to be able to include 42 patients for the final analysis. [17] A log-rank test was used to compare survival data.
This study is written in adherence to the CONSORT guidelines for clinical trials, when applicable.
2.5. The role funding source
There was no funding source for this study.
3. Results
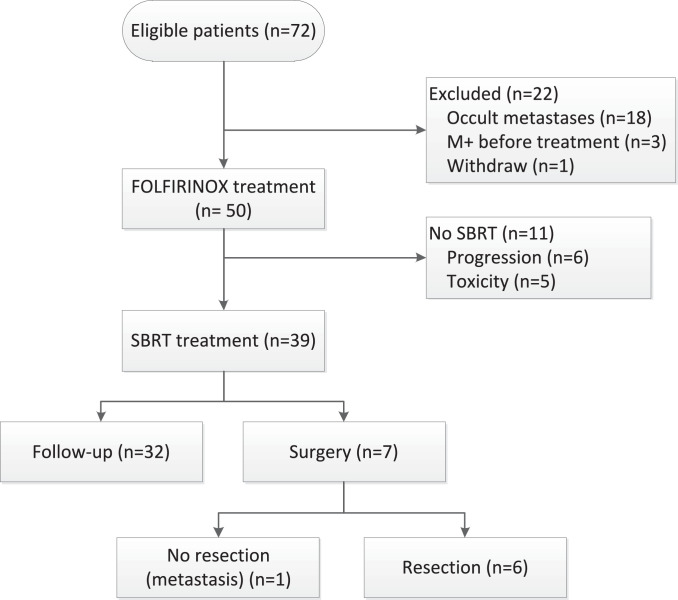
Seventy-two patients were eligible and gave informed consent. Eighteen (25%) patients were found to have metastatic disease at staging laparoscopy, three patients had metastatic disease after restaging imaging before treatment. 51 patients could start the assigned treatments. One patient withdrew consent before treatment (Fig. 1). In the final Intention to treat analysis, 50 patients (50% males, median age 63 years) were included. The tumour was located in the pancreatic head in 29 (58%) patients, pancreatic body in 19 (38%) patients, and pancreatic tail in 2 (4%) patients. Median tumour size was 40 mm [IQR 30–46]. The median pretreatment serum levels of CA19-9 and CEA were 171 kU/l [IQR 56 - 876] and 4.2 ug/l [IQR 3.0 – 10.0], respectively. The median time between staging laparoscopy and start of treatment was 18 days [IQR 12 - 22]. All baseline characteristics are shown in table 2.
Fig. 1.
Flowchart of the included patients.
Table 2.
Baseline characteristics.
| Baseline characteristics | N = 50 (%) or [IQR] |
|---|---|
| Age, median | 63 [53–68] |
| Gender, male | 25 (50) |
| BMI | 23.8 [21.6 – 27.6] |
| Tumor origin | |
| Head | 29 (58) |
| Body | 19 (38) |
| Tail | 2 (4) |
| Pretreatment median CA 19.9 (µg/L) | 171 [56–876] |
| Jaundice | 21 (42) |
| Pretreatment median CEA (kU/L) | 4.2 [3.0–10.0] |
| Diabetes | 12 (24) |
| Abdominal pain | |
| Yes | 39 (78) |
| Missing | 1 (2) |
| Weight loss | |
| Yes | 39 (78) |
| Missing | 6 (12) |
| Maximum tumor size (mm), median | 40 [12–22] |
| Vascular involvement | |
| Venous >270 ° | 7 (14) |
| Arterial >90 ° | 10 (20) |
| Both | 33 (66) |
FOLFIRINOX was given to all 50 patients with a median of 8 cycles [IQR 4–8], with 43 (86%) patients completing 4 or more cycles. The reasons for not completing the assigned chemotherapy were toxicity (n = 14), disease progression (n = 6), and patient's preference (n = 1). Seven (14%) patients had partial remission after induction FOLFIRINOX. Dose reductions were applied in 46% of patients. Thirty grade 3 or 4 adverse events during the FOLFIRINOX mainly consisted of diarrhoea (n = 10), infection (n = 8), vomiting (n = 4), hepatic toxicity (n = 2), neuropathy (n = 1), gastro-intestinal perforation (=1), mucositis (n = 1), and fatigue (n = 1). No deaths were attributed to FOLFIRINOX. Two patients received gemcitabine after initial toxicity of FOLFIRINOX. Sequential to induction chemotherapy, 39 (78%) patients received SBRT. The reasons for not starting SBRT were progression under FOLFIRINOX (n = 6), and toxicity from FOLFIRINOX (n = 5). All 39 patients received the assigned dose of 40 Gray. One (3%) patient had a grade 3 vomiting as adverse event, one (3%) patient a grade 4 gastro-intestinal bleeding after SBRT and two (5%) patients had a grade 5 gastro-intestinal bleeding after SBRT. Both events were observed within three months after completing SBRT. In one patient a duodenal-pancreatic fistula with an aneurysm of the SMA was diagnosed, while the other patient refused any further diagnostics. All adverse events of FOLFIRINOX and SBRT are summarized in table 3.
Table 3.
Grade 3 or higher adverse events for FOLFIRINOX and SBRT.
| FOLFIRINOX | SBRT | ||||
|---|---|---|---|---|---|
| Description | Grade 3 | Grade 4 | Grade 3 | Grade 4 | Grade 5 |
| Diarrhea | 9 | 1 | – | – | – |
| Infection | 5 | 3 | – | – | – |
| Vomiting | 1 | 3 | 1 | – | – |
| Liver toxicity | 2 | – | – | – | – |
| Neuropathy | 1 | – | – | – | – |
| GI bleeding | – | 1 | – | 1 | 2 |
| Mucositis | 1 | – | – | – | – |
| Fatigue | – | 1 | – | – | – |
| Other | 2 | – | – | – | – |
| Total | 21 | 9 | 1 | 1 | 2 |
After FOLFIRINOX and SBRT treatment, four (10%) patients showed local progression, 19 (49%) distant progression, and four (10%) patients both distant and local progression. Second-line chemotherapy for progressive disease was given in seven patients, two patients received FOLFIRINOX, three patients gemcitabine, and two patients gemcitabine with nab-paclitaxel. Seven (14%) patients underwent an explorative laparotomy of which six patients underwent a potentially curative resection. One patient did not undergo a resection due to a solitary 3 mm occult liver metastasis found during the operation. The patients who underwent a resection had pancreatic cancer in the head (n = 4), and in the body (n = 2). Histopathological examination showed a complete pathological response in two (33%) patients, moderate response in three (50%) patients, and no pathological response in one (17%) patient. In all patients, resection margins were negative (e.g., closest margin > 1 mm). Postoperative complications were seen in four patients; bile leakage (n = 2), ischemic gastro-intestinal perforation (=2), bleeding (n = 1), and delayed gastric emptying (=1).
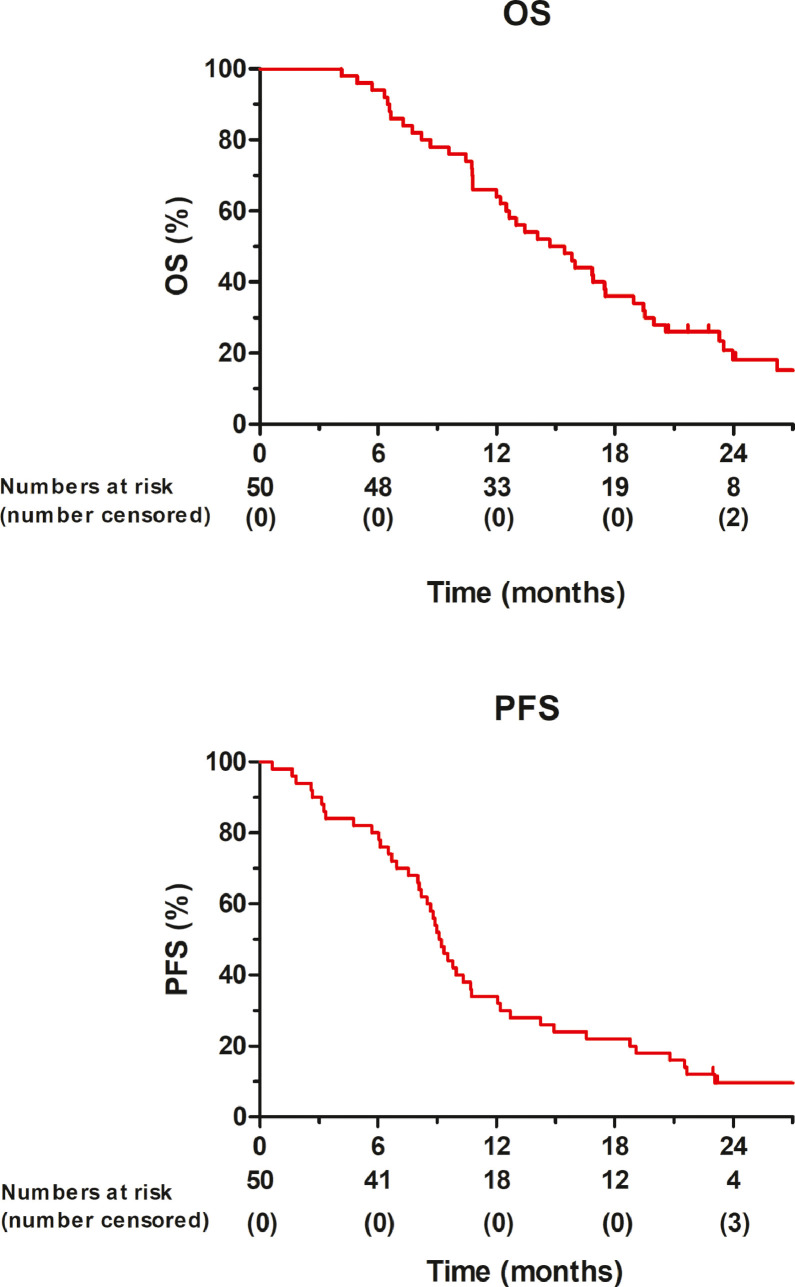
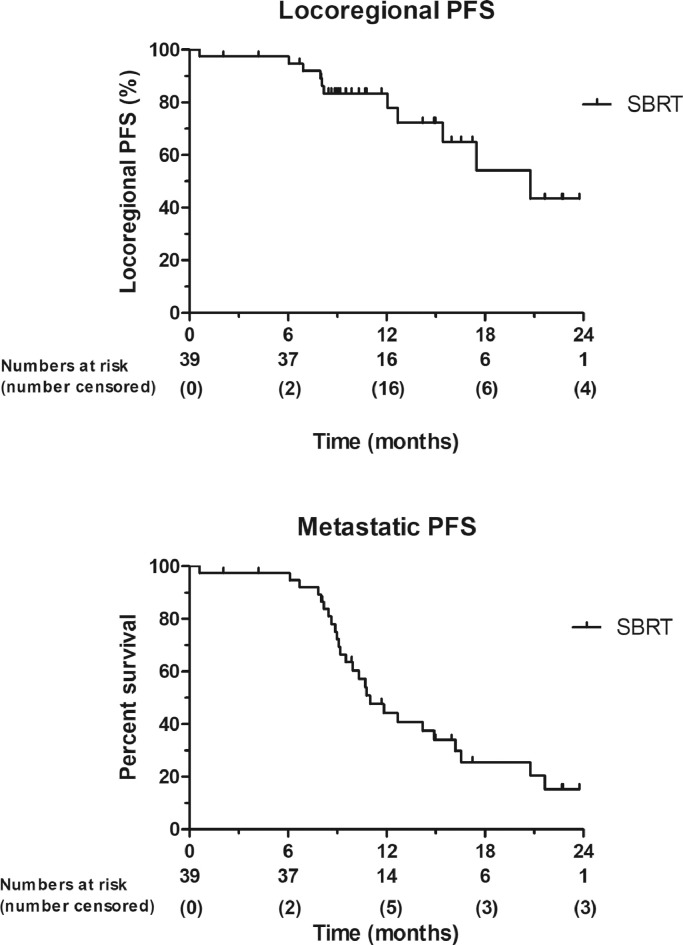
All patients had a minimum follow-up of 1 year, with a median follow-up of 29 months (95% CI 23–36) of patients alive at last follow-up. The 1-year OS rate in the intention to treat population was 64% (95% CI 50–76). The 1-year PFS rate was 34% (95% CI 22–48). OS and PFS rates are shown in Fig. 2. The median OS and PFS were 15 (95% CI 11–18) and 9 months (95% CI: 8–10), respectively. The 1-year OS rates for patients who had completed SBRT was 79% (95% CI 65–89), while the 1-year OS rate for patients who had also undergone curative resection was 83% (95% CI 44–97). The median OS for patients who had completed SBRT was 17 months (95% CI 14–21) and was 7 months (95% CI 6–8) in patients who had not received SBRT (p<0.001). The median OS for the six patients that underwent resection was 23 months (95% CI 13–34). The median OS after starting SBRT was 10 months (95% CI 7–12). Median locoregional PFS in all patients was 17 months (95% CI 11–24), 20 months (95% CI 14–28) for the SBRT group and 3 months (95% CI 2–4) for the non-SBRT group (p<0.001). The median distant PFS in all patients was 11 months (95% CI 10–12), for the SBRT group 11 months (95% CI 9–13), and 3 months (95% CI 2–4) in the non SBRT group (p<0.001). The locoregional and metastasis PFS are shown in Fig. 3.
Fig. 2.
Kaplan-Meier plot of overall survival (OS), and progression free survival (PFS) of all patients (N = 50).
Fig. 3.
Kaplan-Meier of locoregional progression free survival (PFS), and metastatic PFS in patients after stereotactic body radiotherapy (SBRT) treatment (N = 39).
4. Discussion
In this multicentre open-label phase II trial, patients with LAPC were sequentially treated with FOLFIRINOX and SBRT consisting 5 fractions of 8 Gray. To our knowledge, this is the first trial that has prospectively investigated feasibility and antitumor activity of this combined approach in patients with LAPC. The 1-year OS rate of 64% is significantly higher than the 1-year OS rate of 40% achieved in our own historic cohort of patients treated with Uracil/Tegafur plus leucovorin and celecoxib in combination with conventional radiotherapy. Most patients (78%) completed the assigned treatment of FOLFIRINOX and SBRT. No deaths were attributed to the FOLFIRINOX treatment, while two deaths (5%) were possibly attributable to SBRT. The resection rate was 12%, and in all patients the resection turned out to be radical.
In the last decade, FOLFIRINOX has emerged as a possible new standard therapy for LAPC [6]. Although no RCTs have been published to confirm this finding, many case series have demonstrated promising survival rates of FOLFIRINOX in patients with LAPC [8]. In this study, the progression rate after FOLFIRINOX was only 12%, which is very high compared to gemcitabine [10]., which again points to the enhanced efficacy of FOLFIRINOX compared to gemcitabine. Moreover, staging laparoscopy prior to systemic treatment results in more accurate patient selection, which could have influenced the progression rate. Recent patient-level meta-analyses of 315 patients showed even a 1-year OS rate of 80%. This somewhat unexpected finding most likewise is the result of patient selection due to the retrospective design of most of the included studies, as only one prospective study comprising 11 patients with LAPC was included in this analysis [18]. In the patient-level meta-analysis about 60% of the patients received subsequent (chemo) radiotherapy after FOLFIRINOX. However, in the studies that applied RT more frequently no improvement of OS was reported [8].
Radiotherapy can be considered as a rational local treatment approach in patients with LAPC in whom no metastatic disease is seen after systemic therapy [6]. Staging laparoscopy could be included in the diagnostic work-up in patients staged as LAPC on imaging, as it gives better patient selection [19]. Our study confirms that staging laparoscopy frequently (25% in this study) discovers metastatic disease that was not seen on initial radiologic analyses. It is unlikely that localized treatment options alone after systemic therapy in patients with metastatic disease will improve survival.
The first cases of stereotactic radiotherapy for pancreatic cancer were reported in 2000 from Stanford University and showed basic feasibility of single fraction with stereotactic radiotherapy in the treatment of pancreatic cancer [20]. This study was followed by an initial phase I dose escalation study with stereotactic radiotherapy for pancreatic cancer also from Stanford University [21]. A total of fifteen patients were enrolled and received doses of 15, 20, or 25 Gy to the primary tumour. Five patients had acute toxicity, which consisted of grade 2 nausea, pain, or diarrhoea. The 6 (38%) patients who received 25 Gy had local control of their primary pancreatic tumour, but all died due distant metastases. The median survival for the cohort was 11 months. Following this, a phase II trial from the Stanford group aimed to integrate standard gemcitabine chemotherapy with stereotactic radiotherapy to address the high propensity of distant metastasis in pancreatic carcinoma [22]. Two weeks or more after the stereotactic radiotherapy (25 Gy), gemcitabine was restarted and continued until disease progression. Sixteen patients were enrolled and all patients completed treatment with a median survival of 11.4 months, and 1-year OS of 50%. Thirteen (81%) of 16 patients had local control, but these patients developed distant progression. None of the patients had sufficient response to undergo resection. Acute toxicity was low, but late toxicity was severe including five grade 2 duodenal ulcers, one grade 3 duodenal stenosis requiring stenting, and one grade 4 duodenal perforation requiring surgery. Mahadevan et al. retrospectively analysed a planned strategy of initial chemotherapy with restaging and followed by SBRT for those patients with no evidence of distant progression [23]. Patients without metastases after two cycles were treated with SBRT (tolerance-based dose of 24–36 Gy in 3 fractions) between the third and fourth cycle without interrupting the chemotherapy cycles. Eight (17%) of the 47 patients were found to have metastatic disease after two cycles of gemcitabine; the remaining 39 patients received SBRT. The median OS for all patients who received SBRT was 20 months. The local control rate was 85%, while 54% of patients developed metastases. Late grade 3 toxicities such as gastro-intestinal bleeding and obstruction were observed in three (9%) of the 39 patients. Recently, FOLFIRINOX followed by SBRT have been reported in some retrospective cohort studies. Mellon et al. showed a promising response rate with 21 patients receiving FOLFIRINOX followed by SBRT (5 fraction/6 Gy) [24]. Six (24%) of the 21 patients had partial response. The median OS in this study was 15 months for all patients. Our study, being the first phase II trial that explored this combination regimen, shows a similar antitumor activity in patients with LAPC.
In our study 6 (12%) patients underwent resection, all resulting in radical (R0) resections. This rate is lower than the 28% resection rate in a recent meta-analysis [25]. In our study, surgical exploration after FOLFIRINOX and SBRT was only considered if imaging showed “disencasement” with arterial tumour contact not exceeding 90° and venous tumour contact not exceeding 270°, as defined by our national guidelines [13]. The decision to perform the exploration was made during our multidisciplinary tumour board meetings, where the surgeon makes the final call on the technical feasibility of the surgery. Imaging after induction therapy is unreliable to determine local progression or response, and therefore some centres consider surgical exploration more liberally, provided that distant metastatic disease is absent [6, 26]. However, it remains uncertain whether patients will benefit from surgical exploration [8]. The most recent ASCO guideline suggests that patients should undergo a resection only after radiological response to induction therapy [6]. There is a need for prospective trials to investigate the role of surgical exploration and resection in patients with LAPC without distant disease progression after induction therapy.
Grade 3 or 4 adverse event rate during FOLFIRINOX our study was 30 events in 50 patients, which is comparable with the previously reported series [25]. Four (10%) grade 3 or higher adverse events after SBRT were observed, with two patients suffering from a fatal GI bleeding within 3 months after completing SBRT. These mortality rates are comparable to reported results from the literature [27].
Several ablative therapies such as radiofrequency ablation (RFA) and irreversible electroporation (IRE) are currently being assessed in clinical studies in patients with LAPC. [12] The median OS in patients with LAPC treated in one single centre with RFA varies from 19 to 26 months [12]. In this series no specified treatment protocol was used as patients could have been treated with RFA after chemotherapy or could have received RFA as first-line treatment [28]. Therefore, a comparison between our study and the published studies on RFA is difficult. Morbidity after RFA is reported between 0 and 28%, while 30-day mortality ranges from 0 to 3% [28], [29], [30].
Several studies on IRE treatment in LAPC patients are published, with largest cohort that of Martin et al. consisting of 200 patients [31]. The study reports on patients receiving IRE after chemo (radiotherapy) treatment. After initial systemic treatment, patients underwent either IRE treatment or IRE combined with resection for margin accentuation with a median OS of 23 months and 28 months, respectively. Other studies reported median OS between 15 and 27 months after IRE treatment in LAPC [12]. Morbidity after IRE was reported between 10 and 57%, while mortality was found between 1 and 3% [12]. However, these studies are prone to selection bias, because patients with rapid progression on systemic chemotherapy were not included.
The main limitation of the current study is that it was designed as a single-arm non-randomized phase II study making any comparison to other treatment options virtually impossible. Another relevant issue is that our current definition of LAPC differs significantly; the definition for LAPC in this trial is based on the Dutch Pancreatic Cancer Group definition, which is more conservative than the National Comprehensive Cancer Network and AHPBA/SSO/SSAT definitions for LAPC. Finally, all patients in this study received FOLFIRINOX according to the schedule that was described in the PRODIGE 4 trial [18]. However, several studies have meanwhile reported on a so-called modified FOLFIRINOX schedule which uses a 25% reduction of 5-FU gives comparable survival outcomes, but with a decreased toxicity profile [32].
In conclusion, FOLFIRINOX followed by SBRT existing of 5 fractions of 8 Gray was safe and feasible. This resulted in a 1-year OS rate of 64%, and a 1-year PFS rate of 34%. Furthermore, ultimately 6 (12%) patients underwent potentially curative R0 resection. In our view, this warrants further investigation of a more aggressive surgical approach in randomized trials. Nonetheless, distant progression remains the biggest concern in LAPC patients. Therefore, studies are needed to further explore the potential role of this protocolled regimen combined with other systemic therapies. For instance, immunotherapy is emerging as a synergetic treatment to radiotherapy with promising results [33, 34]. Sequential treatment of chemotherapy and SBRT combined with immunotherapy, could potentially improve outcomes in this group of patients.
5. Research in context
5.1. Evidence before this study
Pancreatic cancer is projected to be the second most common cancer related cause of death by 2030. One-third of all pancreatic cancer patients have locally advanced pancreatic cancer at presentation. FOLFIRINOX has been the first-choice systemic therapy for locally advanced pancreatic cancer, although level I evidence is still needed to confirm superiority of FOLFIRINOX. Local ablative therapies in the locally advanced setting could be considered in addition to systemic therapy, however, prospective clinical trials are scarce.
5.2. Added value of this study
This is the first phase II trial that evaluated the FOLFIRINOX followed by stereotactic body radiotherapy in patients with locally advanced pancreatic cancer. The 1-year OS rate in the intention to treat population was 64% (95% CI 50–76).
5.3. Implications of all the available evidence
FOLFIRINOX followed by stereotactic body radiotherapy for patients with locally advanced pancreatic cancer is feasible. This protocolled regimen is promising and should be further explored in randomized controlled trials.
Contributors
M. Suker: literature search, patient inclusion, data collection, data analysis, tables, figures, data, writing, revisions
J.J. Nuyttens: study design, literature search, patient inclusion, data collection, data analysis, revisions
F.A.L.M. Eskens: literature search, patient inclusion, writing, revisions
B.C.M. Haberkorn: patient inclusion, writing
P.P.L.O. Coene: patient inclusion, writing
E. van der Harst: patient inclusion, writing
B.A. Bonsing: patient inclusion, writing
A. Vahrmeijer: patient inclusion, writing
J.S.D. Mieog: patient inclusion, writing
RJ. Swijnenburg: patient inclusion, writing
D. Roos: patient inclusion, writing
B. Groot Koerkamp: patient inclusion, supervision, writing, revisions
C.H.J. van Eijck: literature search, patient inclusion, data, writing, revisions, supervision
All authors have read and approved the final version of this manuscript.
Funding
No
Declaration of interests
We declare no competing interests.
Acknowledgements
We would like to thank J.H.E. Verhagen-Oldenampsen for her administrative aid for this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2019.10.013.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Vincent A., Herman J., Schulick R., Hruban R.H., Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilimoria K.Y., Bentrem D.J., Ko C.Y., Ritchey J., Stewart A.K., Winchester D.P. Validation of the 6th edition ajcc pancreatic cancer staging system: report from the national cancer database. Cancer. 2007;110(4):738–744. doi: 10.1002/cncr.22852. [DOI] [PubMed] [Google Scholar]
- 4.Shaib W.L., Ip A., Cardona K., Alese O.B., Maithel S.K., Kooby D. Contemporary management of borderline resectable and locally advanced unresectable pancreatic cancer. Oncologist. 2016;21(2):178–187. doi: 10.1634/theoncologist.2015-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rombouts S.J., Vogel J.A., van Santvoort H.C., van Lienden K.P., van Hillegersberg R., Busch O.R. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br J Surg. 2015;102(3):182–193. doi: 10.1002/bjs.9716. [DOI] [PubMed] [Google Scholar]
- 6.Balaban E.P., Mangu P.B., Khorana A.A., Shah M.A., Mukherjee S., Crane C.H. Locally advanced, unresectable pancreatic cancer: american society of clinical oncology clinical practice guideline. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(22):2654–2668. doi: 10.1200/JCO.2016.67.5561. [DOI] [PubMed] [Google Scholar]
- 7.Conroy T., Desseigne F., Ychou M., Bouche O., Guimbaud R., Becouarn Y. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Suker M., Beumer B.R., Sadot E., Marthey L., Faris J.E., Mellon E.A. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. The Lancet Oncology. 2016;17(6):801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadot E., Doussot A., O'Reilly E.M., Lowery M.A., Goodman K.A., Do R.K. FOLFIRINOX induction therapy for stage 3 pancreatic adenocarcinoma. Ann. Surg. Oncol. 2015;22(11):3512–3521. doi: 10.1245/s10434-015-4647-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammel P., Huguet F., van Laethem J.L., Goldstein D., Glimelius B., Artru P. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 11.Liu F., Erickson B., Peng C., Li X.A. Characterization and management of interfractional anatomic changes for pancreatic cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83(3):e423–e429. doi: 10.1016/j.ijrobp.2011.12.073. [DOI] [PubMed] [Google Scholar]
- 12.Ruarus A., Vroomen L., Puijk R., Scheffer H., Meijerink M. Locally advanced pancreatic cancer: a review of local ablative therapies. Cancers (Basel) 2018;10(1) doi: 10.3390/cancers10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Versteijne E., van Eijck C.H., Punt C.J., Suker M., Zwinderman A.H., Dohmen M.A. Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials. 2016;17(1):127. doi: 10.1186/s13063-016-1262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R. New response evaluation criteria in solid tumours: revised recist guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Kalimuthu S., Serra S., Dhani N., Hafezi-Bakhtiari S., Szentgyorgyi E., Vajpeyi R. Regression grading in neoadjuvant treated pancreatic cancer: an interobserver study. J Clin Pathol. 2017;70(3):237–243. doi: 10.1136/jclinpath-2016-203947. [DOI] [PubMed] [Google Scholar]
- 16.Morak M.J., Richel D.J., van Eijck C.H., Nuyttens J.J., van der Gaast A., Vervenne W.L. Phase ii trial of uracil/tegafur plus leucovorin and celecoxib combined with radiotherapy in locally advanced pancreatic cancer. Radiother Oncol. 2011;98(2):261–264. doi: 10.1016/j.radonc.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 17.A'Hern R.P. Sample size tables for exact single-stage phase ii designs. Stat Med. 2001;20(6):859–866. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
- 18.Conroy T., Paillot B., Francois E., Bugat R., Jacob J.H., Stein U. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer–a groupe tumeurs digestives of the federation nationale des centres de lutte contre le cancer study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(6):1228–1236. doi: 10.1200/JCO.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 19.Callery M.P., Chang K.J., Fishman E.K., Talamonti M.S., William Traverso L., Linehan D.C. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann. Surg. Oncol. 2009;16(7):1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 20.Koong A.C., Christofferson E., Le Q.T., Goodman K.A., Ho A., Kuo T. Phase ii study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63(2):320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Koong A.C., Le Q.T., Ho A., Fong B., Fisher G., Cho C. Phase i study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58(4):1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Schellenberg D., Goodman K.A., Lee F., Chang S., Kuo T., Ford J.M. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72(3):678–686. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 23.Mahadevan A., Miksad R., Goldstein M., Sullivan R., Bullock A., Buchbinder E. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):e615–e622. doi: 10.1016/j.ijrobp.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 24.Mellon E.A., Hoffe S.E., Springett G.M., Frakes J.M., Strom T.J., Hodul P.J. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015;54(7):979–985. doi: 10.3109/0284186X.2015.1004367. [DOI] [PubMed] [Google Scholar]
- 25.Suker M., Beumer B.R., Sadot E., Marthey L., Faris J.E., Mellon E.A. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. The Lancet Oncology. 2016;17(6):801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rangelova E., Wefer A., Persson S., Valente R., Tanaka K., Orsini N. Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single institution experience. Ann Surg. 2019 doi: 10.1097/SLA.0000000000003301. [DOI] [PubMed] [Google Scholar]
- 27.Herman J.M., Chang D.T., Goodman K.A., Dholakia A.S., Raman S.P., Hacker-Prietz A. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121(7):1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantore M., Girelli R., Mambrini A., Frigerio I., Boz G., Salvia R. Combined modality treatment for patients with locally advanced pancreatic adenocarcinoma. Br J Surg. 2012;99(8):1083–1088. doi: 10.1002/bjs.8789. [DOI] [PubMed] [Google Scholar]
- 29.Girelli R., Frigerio I., Giardino A., Regi P., Gobbo S., Malleo G. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage iii ductal adenocarcinoma. Langenbecks Arch Surg. 2013;398(1):63–69. doi: 10.1007/s00423-012-1011-z. [DOI] [PubMed] [Google Scholar]
- 30.Girelli R., Frigerio I., Salvia R., Barbi E., Tinazzi Martini P., Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97(2):220–225. doi: 10.1002/bjs.6800. [DOI] [PubMed] [Google Scholar]
- 31.Martin R.C., Kwon D., Chalikonda S., Sellers M., Kotz E., Scoggins C. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg. 2015;262(3):486–494. doi: 10.1097/SLA.0000000000001441. discussion 92-4. [DOI] [PubMed] [Google Scholar]
- 32.Stein S.M., James E.S., Deng Y., Cong X., Kortmansky J.S., Li J. Final analysis of a phase ii study of modified folfirinox in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114(7):737–743. doi: 10.1038/bjc.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gandhi S.J., Minn A.J., Vonderheide R.H., Wherry E.J., Hahn S.M., Maity A. Awakening the immune system with radiation: optimal dose and fractionation. Cancer Lett. 2015;368(2):185–190. doi: 10.1016/j.canlet.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Formenti S.C., Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys. 2012;84(4):879–880. doi: 10.1016/j.ijrobp.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.