Abstract
Toxicity of cardamom and clove seed powder and extracted compounds against the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae), was assessed in laboratory exposure experiments. The treatments comprised different amounts of seed powder of cardamom (0.8, 1, 3, and 5 mg) and clove (1, 3, 5, 7 mg), and extract concentrations (0.2, 0.4, 0.5, 0.6, 0.7, and 0.8) for both plants using ether petroleum or chloroform. Data showed that 5 mg of cardamom powdered seed resulted in 93% mortality after one day and 100% mortality after two days. Whereas after two days, lower amounts (0.8, 1, and 3 mg) resulted in 26%, 40%, 46%, respectively. A similar result was obtained for clove seed powder, where 7 mg caused 53% mortality after one day and 100% mortality after three days, other amounts (1, 3, and 5 mg) resulted in 33%, 73%, and 80%, mortality respectively, after three days. We found that all amounts of extract of both plants resulted in 100% mortality after three days. GC-MS analysis of the cardamom and clove extracts revealed the presence of a large number of terpenes of particular note was eugenol and two novel compounds Hydroxy-alpha-Terpenyl Acetate and Labda -8(17),13(E)- Diene- 15. The current work aims at the possibility of benefiting from natural plants pesticides as being safer as well as on the separation of volatile oils, which was known to be important in the control pests.
Keywords: Cardamom, Clove, Rhynchophorus ferrugineus, Terpenes, Hydroxy-alpha-Terpenyl acetate, Labda-8(17), 13(E)-diene-15
1. Introduction
The Red palm weevil (RPW)) Rhynchophorus ferrugineus (Olivier.) (Coleoptera: Dryophthoridae) is a major pest of various palms in the Middle East, South and South East Asia, North Africa and Southern Europe (Nirula, 1956). Females lay eggs in damaged or wounded plants. Upon hatching, the larvae burrow into the fresh tissue and migrate to the bud region and heart of the crown where they feed for two to four months eventually killing the host plant (Abraham, 1971). The RPW has a wide and host range and has been reported to infest 40 species palm worldwide (Vidyasagar and Subaharan, 2000). Infested date palms exhibit several symptoms depending on the stage of attack, such as producing of brown fluid with a fermented odor that is mixed with palm tissue exerted by feeding larvae, tunneling of palm tissue, presence of adults and pupae at the base of fronds, dried of infested offshoots, pupae around the palm base, dried outer leaves, and topping of the trunk in casees of sever and extensive tissue damage (Vidyasagar and Subaharan, 2000).
Management of agricultural pests of field and post-harvest crops over the past half century has largely depended on the use of synthetic pesticides (Ferry et al., 2004). Several reviews of control strategies for RPW including integrated pest management are available. The development of insect resistance to synthetic pesticide and the associated high operational cost and environmental pollution haves created a need alternative approaches to control insect pests. The use of essential oils is a potential alternative to synthetic pesticides in the control of numerous field and household insect pests (Sarwar et al., 2005, Sarwar et al., 2012, Sarwar et al., 2013). Essential oils are natural, volatile and complex compounds, and their characteristic odors are attributed to secondary metabolites in plants. Many plant essential oils and their constituents from terpenes show a broad spectrum of insecticidal, repellents, attractants, inducement and deterrent of oviposition, growth regulating and anti-vector activity against pest insects (Bakkali et al., 2008). Plant essential oils and monoterpenes act as botanical pesticides. One of the most important features of botanical pesticides is the range of effects against specific insects. Most compounds present in essential oils have been shown to be relatively non-toxic to mammals and fish via toxicological tests indicating reduced risk. Monoterpenes, the chemical constituents of essential oils found in plants, are biologically active compounds. Further, essential oils and their constituents demonstrate fumigant and topical toxicity as well as antifeedant and repellent effects (Shayaa et al., 1997, Sharaby and AL-Dosary, 2014, Sharaby and EL-Nujiban, 2015) in insects. Spice essential oils contain a complex mixture of volatile monoterpenes, sesquiterpenes, and phenols, which play important defensive roles against insect herbivory (Isman, 2006). Some of these essential oil constituents were found to exhibit contact or fumigant toxicity, while others had only insect repellent, and antifeedant effects (Isman, 2000, Sedy and Koschier, 2003, Kanat and Alma, 2004, Nerio et al., 2010, Caballero-Gallardo et al., 2011).
The cardamom (Elettaria cardamomum [L.] Maton) from the ginger family (Zingiberaceae) is considered as the queen of spices in India. Its seeds contain a clear to pale yellow essential oil with a pungent odor. The cardamom oil is mainly composed of two major constituents, 1, 8-cineole and α-terpinyl acetate, up to 50% each (Weiss, 2002). It was reported to be toxic to different life stages of the coleopteran and lepidopteran stored-product pests through contact and fumigant actions (Abbasipour et al., 2011). Also, Clove essential oil has been widely studied for its insecticidal and repellent activities against many species of pests (Chaieb et al., 2007, Kafle and Shih, 2013, Cortés-Rojas et al., 2014). However, there is no report on the bioactivity against R. ferrugenius. So this study aims to evaluate the acute toxicity of cardamom and clove extracts against adult red palm weevil in the laboratory.
Here, we evaluated the toxicity of extracted oils and powder of cardamom and clove against adult R. ferrugineus.
2. Materials and methods
2.1. Insects
Adult RPW were obtained from Ministry of Agriculture and Water at- Al Kharj. Transferred to the laboratory entomology Prince Sattam bin Abdel Aziz University, Al-Kharj, KSA. Rearing of adult red palm weevil was according to Al-Rajhy et al. (2005).
2.2. Plants
Cardamom (Elettaria cardamomum) and clove (Eugenia caryophyllus) seed were get from the market, washed in cold water, dried and extracted using petroleum ether or chloroform, and used for bioassays.
2.3. Extractions
Compounds from known weights of seed, washed in cold water, dried and then ground in a high-speed blender. The resulting powder was soaked in different organic solvents for 48 h. For extraction, 500 ml of petroleum-ether (B.P.4-60 °C C) was added to flask containing 250 g of powdered cardamom seeds (Su, 1985). After 48 h, the flask was shaken vigorously for almost with whatman filter paper (No.4) through anhydrous sodium sulphate. The solvent was then evaporated in a water- bath at 40 °C and the filtrate was thoroughly dried and put back into the flask. Extraction was repeated with chloroform. Extracts were then kept in screw-capped glass vials at 4 °C until needed (Islam, 1983, Afifi et al., 1988).
2.4. Chemical analysis
Extractions were analyzed using gas Chromatography-mass spectrometry (GC-MS), where molecules were separated due to differences in molecular weight and ionized. Chemical compounds were identified, based on the relative intensity of the ions using GC-MS ion trap configuration that then allowed mass spec/mass spec (MS/MS) analysis of samples, reduce the rate of false positive. GC-MS parameters included: Shemadzu, 5050 GC-MS 2 supplied samples model 20S-Aoc, and automatic injector 20i-Aoc characteristic fragmentation split/splitless by column separation Rtx-30 m, 0.25 mm ID, detector ETP, helium gas at 1.5 ml/min, temperature 50–310 °C for oven at high 10 m/min and 250 °C for injector, 280 °C for detector injected with 1 µl of each extract separately (Al-Dawsary, 2014).
2.5. Treatments
Three replicates of five adult RPW were exposed to a slice of sugarcane that had been mixed with 0.8, 1, 3 or 5 mg of powdered cardamom seed or 1, 3, 5, or 7 mg of powdered clove seed. Untreated sugarcane was a control. Mortality was assessed after 24, 48, and 72 h of exposure and LC50 and LC95 were calculated.
Three replicates of RPW were treated with the extracted compounds. 1 ml of extract was mixed with 10 ml of distilled water and three drops of Triton × 100 to create an essential oil emulsion that was applied to the insects. Using a small plastic hand sprayer. An aqueous solution of the same amount of water + emulsifier was applied as a control. Following treatment Insects were allowed to dry and then placed in a glass jar for mortality observation after 24, 48, and 72 h. All experiments were conducted in constant temperature at 28 ± 2 °C.
2.6. Statistical analysis
Used SPSS (24), and used Anova two ways for analysis data.
3. Results
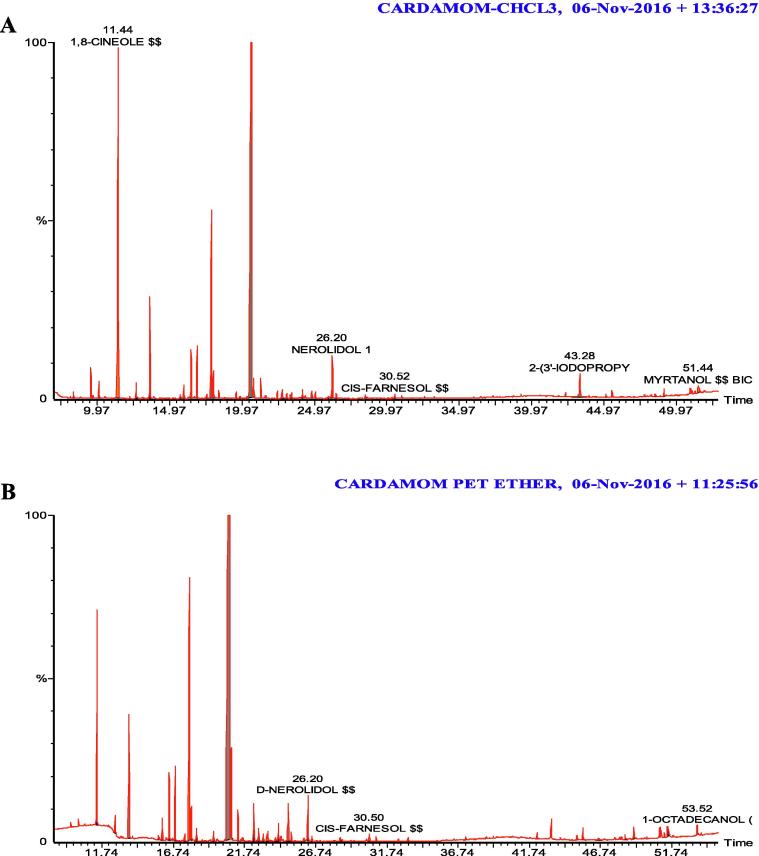
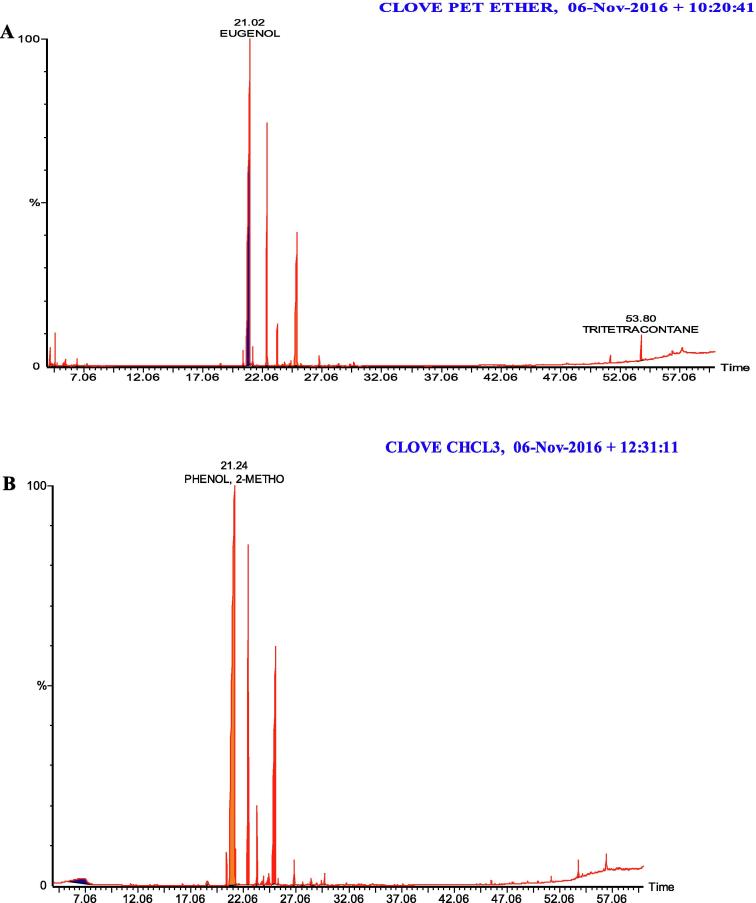
Terpene Content namely eugenol, linalool, 1,8-cineole, chavicol, myrtanol, 1-octadecanol, acetyl eugenol, 3-allyl eugenol, acetyl eugenol, and many compounds were present in all extracts (see Table 1, Table 2, Fig. 1, Fig. 2).
Table 1.
Terpenoids found in cardamom extracts.
| Chloroform |
Petrolium ether |
||
|---|---|---|---|
| Name compound | RT | Name compound | RT |
| .ALPHA.-PINENE | 8.34 | 1,8-CINEOLE | 11.40 |
| SABINENE | 9.56 | TRANS-SABINENE HYDRATE | 12.68 |
| .BETA.-MYRCENE | 10.10 | LINALOOL | 13.64 |
| 1,8-CINEOLE | 11.44 | .DELTA.-TERPINEOL | 15.70 |
| TRANS-SABINENE HYDRATE | 12.68 | 3-CYCLOHEXEN-1-OL | 15.96 |
| LINALOOL | 13.64 | LINALYL PROPIONATE | |
| .DELTA.-TERPINEOL | 15.72 | 4-TERPINENYL ACETATE | 16.88 |
| 3-CYCLOHEXEN-1-OL | 15.98 | Z-CITRAL | 17.54 |
| LINALYL PROPIONATE | LINALYL ACETATE | 17.88 | |
| CIS-SABINENE HYDRATE ACETATE | 16.88 | TRANS-GERANIOL | 18.02 |
| Z-CITRAL | 17.56 | Z-CITRAL | 18.38 |
| LINALYL ACETATE | 17.86 | .DELTA.-TERPINYL ACETATE | 19.58 |
| TRANS-GERANIOL | 18.00 | ALPHA-TERPINYL ACETATE | 20.70 |
| CITRAL | 18.38 | EUGENOL | 20.82 |
| .DELTA.-TERPINYL ACETATE | 19.58 | NERYL ACETATE | 21.28 |
| 2,6-OCTADIENOIC ACID | TRANS-CARYOPHYLLENE | 22.40 | |
| 1-P-MENTHEN-8-YL ACETATE | 1-METHYL-4-(1-ACETOXY-1-METHYL | 22.74 | |
| EUGENOL | 20.78 | GERMACRENE-D | 32.92 |
| NERYL ACETATE | 21.28 | .BETA.-SELINENE | 24.14 |
| 2-OCTENYL ACETATE | 21.60 | (-)-.ALPHA.-SELINENE | 24.32 |
| TRANS-CARYOPHYLLENE | 22.40 | 3-ALLYL-6-METHOXYPHENOL | 24.82 |
| 1-METHYL-4-(1-ACETOXY-1-METHYL | 22.76 | HYDROXY-.ALPHA.-TERPENYL ACETATE | 25.04 |
| .BETA.-SELINENE | 24.14 | D-NEROLIDOL | 26.20 |
| .ALPHA.-SELINENE | 24.32 | (E,E)-4,8,12-TRIMETHYL-1,3,7,1 | |
| ACETIISOEUGENOL | 24.80 | (. + -.) 2-EXO-HYDROXYCINEOLE | |
| HYDROXY-.ALPHA.-TERPENYL ACETATE | 25.04 | CIS-FARNESOL | 30.50 |
| NEROLIDOL 1 | 26.20 | TRANS, TRANS-FARNESAL | 31.00 |
| (E,E)-4,8,12-TRIMETHYL- | FARNESYL ACETATE 3 | ||
| 1,3,7,1,1-TRIDECATETRAENE | 26.48 | GERANYL LINALOOL ISOMER B | 37.26 |
| (. + -.) 2-EXO-HYDROXYCINEOLE | NONADECANE | 42.28 | |
| CIS-FARNESOL | 30.52 | 1-OCTADECANOL | 45.08 |
| TRANS-CARYOPHYLLENE | 31.00 | DOCOSANE, 11-DECYL- | 45.50 |
| (+)-LABDA-8(17),13(E)-DIENE-15 | 32.56 | HEXADECANOIC ACID | 46.60 |
| FARNESYL ACETATE 3 | TRITETRACONTANE | 48.46 | |
Table 2.
Terpenoids found in clove extracts:
| Chloroform |
Petrolium ether |
||
|---|---|---|---|
| Name compound | RT | Name compound | RT |
| CHAVICOL | 18.62 | CYCLOHEXANE | 4.26 |
| .ALPHA.-COPAENE | 20.48 | CYCLOHEPTANE | 4.44 |
| 3-ALLYL GUAIACOL | 21.24 | HEPTANE | 5.38 |
| TRANS-CARYOPHYLLENE | 22.54 | BENZENE | 6.52 |
| .ALPHA.-HUMULENE | 23.36 | NONANE | 7.36 |
| GERMACRENE-D | 23.96 | CHAVICOL | 18.54 |
| 1-NAPHTHALENOL | 24.38 | ALPHA.-COPAENE | 20.46 |
| E,E-.ALPHA.-FARNESENE | 24.48 | EUGENOL | 21.02 |
| ACETYL EUGENOL | 25.12 | ALPHA.-COPAENE | 21.26 |
| CADINA-1,4-DIENE | 25.36 | BICYCLO[7.2.0]UNDEC-4-ENE | 22.46 |
| 1-METHYLENE-2B-HYDROXYMETHYL-3 | ALPHA.-HUMULENE | 23.30 | |
| −4B-(3-METHYLBUT- 2-ENYL)CYCLOHEXANE | 26.88 | GAMMA.-CADINENE | 23.76 |
| HUMULENE OXIDE | 27.68 | GERMACRENE-D | 23.92 |
| 2′,3′,4′ TRIMETHOXYACETOPHENON | 29.76 | CYCLOHEXANOL | 24.32 |
| 53.82 | (E,E)-.ALPHA.-FARNESENE | 24.46 | |
| PHENOL, 2-METHOXY-4-(2-PROPENYL | |||
| CADINA-1,4-DIENE | 25.30 | ||
| CARYOPHYLLENE OXIDE | 26.80 | ||
| 9-METHYL-10,12-HEXADECADIEN-1- | 27.62 | ||
| CARYOPHYLLA-4(12),8(13)-DIEN-5 | 28.46 | ||
| (-)-CARYOPHYLLENE OXIDE | 29.42 | ||
| 2′,3′,4′ TRIMETHOXYACETOPHENONE | |||
| TRITETRACONTANE | 53.80 | ||
Fig. 1.
Chromatograph analysis (GC-MS) of chardamom extracts. (A) Chloroform; (B) Petroleum ether.
Fig. 2.
Chromatograph analysis (GC-MS) of clove extracts. (A) Petroleum ether; (B) Chloroform.
3.1. Effect of seeds powder
Effect of cardamom seed powder on RPW mortality rate varied with concentration. After one day of treatment mortality rate at the highest concentration 5 mg was 93%, but zero at the other concentration; after two days mortality at the highest concentration had reached 100%. A three days mortality rates for the 0.8- 1- and 3- mg treatments were 40%, 26%, and 46% respectively (Table 3) the effect of cardamom seed powder was significantly different from that at 0.05%.
Table 3.
Toxicity of cardamom seeds and clove against adult red palm weevil.
| Conc. | %Mortality after Exposure to cardamom seeds |
LC50 | LC95 | F* | Conc. | %Mortality after Exposure to clove seeds |
LC50 | LC95 | F* | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||||||||
| 0.8 | 0 | 20% | 40% | 1 | 13% | 26% | 33% | ||||||
| 1 | 0 | 7% | 26% | 3 | 7% | 33% | 80% | ||||||
| 3 | 26% | 26% | 46% | 2.2 | 5.2 | 132.827 | 5 | 0 | 20% | 73% | |||
| 5 | 93% | 100% | 100% | 7 | 53% | 73.3% | 100% | 1.78 | 6.42 | 8.283 | |||
| control | 0 | 0 | 0 | control | 0 | 0 | 0 | ||||||
Significantly different between treatments at 0.05%.
3.2. Effects of clove
Seeds powder on RPW mortality also varied with concentration mortality rate at the highest concentration (7 mg) after one day was, and 100% after three days. Mortality rates in the 1- 3- and 5 mg treatments were 33%, 80%, and 73% respectively (Table 3) the effect of cardamom seed powder was significantly different from that at 0.05%.
3.3. Effect of extracted compounds
Cardamom compounds extracted using petroleum ether mortality after one day of exposure; after three days, mortality rates had risen to 100% response to the 0.4-0.6, and 0.7 ml treatments (Table 4). In contrast, compounds extracted using chloroform achieved 100% mortality rates for the 0.7, and 0.8 ml treatments after one day of exposure. After three days of exposure, 100% mortality was observed in response to all treatments for both types of extract (Table 4) the effect of petroleum ether and chloroform was not significantly different from that 0.05%.
Table 4.
Toxicity of cardamom extracts against adult red palm weevil.
| Conc. | %Mortality after treatment with petroleum ether extract |
LC50 | LC95 | F | %Mortality after treatment with chloroform extract |
LC50 | LC95 | F | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |||||||
| 0.2 | 13% | 13% | 13% | 13% | 13% | 86.6% | ||||||
| 0.4 | 26.6% | 53.3% | 100% | 0.33 | 0.62 | 2.282 | 33.3% | 73.3% | 100% | |||
| 0.5 | 33.3% | 40% | 53.3% | 40% | 53.3% | 100% | 1.96 | 0.83 | 0.301 | |||
| 0.6 | 46.6% | 73.3% | 100% | 60% | 100% | – | ||||||
| 0.7 | 86.6% | 100% | – | 100% | – | – | ||||||
| 0.8 | 80% | 100% | – | 100% | – | – | ||||||
| control | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
Clove compounds extracted using petroleum ether extract resulted in low levels of adult mortality, after one day, for the 0.1, 0.2, and 0.3 ml treatments; the mortality rate the lower levels of concentration after one day of exposure, ranging between 40 and 100% across the treatments (Table 5). After three days, mortality was observed in response to all treatments for both types extract all concentrations with the exception of the 0.2 and 0.5 ml chloroform extracts. The effect of petroleum ether extract was not significantly at 0.05%, while chloroform extract were significant different from that of 0.05%.
Table 5.
Toxicity of clove extracts against adult red palm weevil.
| Conc. | %Mortality after treatment with petroleum ether extract |
LC50 | LC95 | F | Conc. | %Mortality after treatment with chloroform extract |
LC50 | LC95 | F* | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||||||||
| 0.1 | 6.6% | 13% | 33.3% | 0.2 | 40% | 66.6% | 86.6% | ||||||
| 0.2 | 6.6% | 6.6% | 20% | 0.4 | 73.3% | 73.3% | 100% | ||||||
| 0.3 | 6.6% | 6.6% | 26.6% | 0.44 | 0.74 | 0.383 | 0.5 | 53.3% | 66.6% | 93.3% | |||
| 0.4 | 86.6% | 100% | – | 0.6 | 100% | – | – | 1.7 | 0.45 | 16.810 | |||
| 0.6 | 100% | – | – | 0.7 | 100% | – | – | ||||||
| 0.8 | 100% | – | – | 0.8 | 80% | 100% | – | ||||||
| control | 0 | 0 | 0 | control | 0 | 0 | 0 | ||||||
Significantly different between treatments at 0.05%.
4. Discussion
Our study supports previous works on the use of aromatic oils and herbicides in pest control, that have demonstrated the efficacy of natural products insect pests. Consistent with previous studies, our results demonstrated mortality of up to 100% using extracts of cardamom and cloves against adult RPW. Further, we report the presence of a large number of terpenes, including eugenol in the extracts (Table 1, Table 2) that are known to exert insecticidal effects against a large number of insects.
(Shayaa et al., 1991, Phillips et al., 1995, Huange et al., 2002, Waliwitya et al., 2005).
Similar to our results, Olivero-Verbel et al. (2010) stated that the main components found in the volatile oil from E. cardamomum were 1,8-cineol and α-terpineol acetate. Korikontimath et al., 1999, Lawrence, 1979.
In our study cardamom seed extract using chloroform solvent was initially more effective than extracted using petroleum ether solvent at the highest concentrations (0.7 and 0.8 ml). All insects died after 24 h of treatment; however, after 3days of treatment mortality was 100% at all concentrations of the method of extraction.
The effects of clove seed extract were similar to those of cardamom seeds: 100% mortality was recorded after 24 h using highest concentrations (Table 4, Table 5) and mortality at the lower concentrations increased after 72 h, occasionally reaching 100%. Powdered seed of both plants was effective against adult RPW mortality for both treatments were 100% high concentrations after 72 h. However; cardamom was initially most effective 5 g of powdered cardamom seeds (93% mortality) after 24 h (Table 3).
Chromatographic analysis of the plant extracts (Table 1, Table 2) showed the presence of terpenes that were likely to have caused the recorded mortalities in this experiment. The most important terpene detected was eugenol, which has been to be an effective insecticide. Huang et al. (2002) reported contact toxic effects of eugenol, iso eugenol, and methyl eugenol adult Sitophilus zeamais. Waliwitiya et al. (2005) noted toxic effects of a number of mono terpines and volatiles including: thymol, cetronellal, and eugenol on final larval instars of Agriotes obscurus. Here, we observed high mortality rates in adult RPW exposed to a number of terpenes: our results support those Sharaby and Al Dosary (2014) who showed that camphene is attractive to both sexes of RPW adults, eliciting 60 and 40% mortality on males and females respectively. Camphene has a range of effects on insects; the most important is the blocking of the air holes (spiracles) through which the insect breathes, causing asphyxiation. AL-Sharook et al. (1991) recorded effects of essential oils on insect respiratory and nervous systems and hormone regulation that lead to death. In some cases essential oils may also act as poisons, interacting with the fatty acids of the insect and interfering with normal metabolism. Previous research has demonstrated that essential oils have neurotoxic, cytotoxic and mutagenic effects in different organism, since they act at multiple levels the possibility of developing resistance is (Bakkali et al., 2008). Here, we identified novel terpenes in clove and cardamom extracts: hydroxy-alpha-Terpenyl acetate, and labda -8(17), 13(E)-diene-15, (Table 1) that may exert synergistic toxic effects insects.
In conclusion, as a natural insecticide, the cardamom and clove extracted could be exploited in developing more effective strategies to prevent and control on insects. Furthermore, as clove and cardamom extracted is widely used as an herbal medicine and spice, it is generally recognized as safe to human health (Zheng et al., 1992, Naveena et al., 2006).
Acknowledgement
I would like to thank Prince Sattam bin Abdelaziz University for its support of this research and the facilities to reach the current results.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbasipour H., Mahmoud M., Rastegar F., Hosseinpour M.H. Fumigant toxicity and oviposition deterrency of the essential oil from cardamom, Elettaria cardamomum, against three stored-product insects. J. Insect Sci. 2011;11:165. doi: 10.1673/031.011.16501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham V.A. Note on an effect method of preventing entry by red weevil Rhynchophorus ferrugineus into the stem of Coconut palm through cut petioles. Indian J. Agric. Sci. 1971;41:1130–1131. [Google Scholar]
- Afifi F.A., Hekal A.M., Salem M. Fenuricgreek seed extracts as protectants of wheat grains against stored product insects. Ann. Agric. Sci., Fac. Agric. Ain Shams Univ.; Cairo, Egypt. 1988;33(2):1331–1344. [Google Scholar]
- Al Dawsary M.M. Functional compounds from the integument of adult red palm weevil Rhynchophorus ferrugineus. Saudi J. Biol. Sci. 2014;21:275–279. doi: 10.1016/j.sjbs.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-SHarook Z., Balan K., Jiang Y., Rembold H. Insect growth inhibitors from two tropical Meliaceae: effects of crude seed extracts on mosquito larvae. J. Appl. Ent. 1991;111:425–530. [Google Scholar]
- Bakkali F., Averbeck S., Idaomar M. Biological effects of essential oils A review. J. Food. Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Caballero-Gallardo K., Olivero-Verbel J., Stashenko E.E. Repellent activity of essential oils and some of their individual constituents against Tribolium castaneum Herbst. J. Agric. Food. Chem. 2011;59:1690–1696. doi: 10.1021/jf103937p. [DOI] [PubMed] [Google Scholar]
- Chaieb K., Hajlaoui H., Zmantar T., Kahla-Nakbi A.B., Rouabhia M., Mahdouani K., Bakhrouf A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytother. Res. 2007;21:501–506. doi: 10.1002/ptr.2124. [DOI] [PubMed] [Google Scholar]
- Cortés-Rojas D.F., Souza C.R.F.de, Oliveira W.P. Clove (Syzygium aromaticum): a precious spice. Asian Pac. J. Trop. Biomed. 2014;4:90–96. doi: 10.1016/S2221-1691(14)60215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry N., Edwards M.G., Gatehouse J.A., Gatehouse A.M. Plant insect interaction: molecular approaches to insect resistance. Biotechnol. 2004;15:155–161. doi: 10.1016/j.copbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Huange Y., Ho S.H., Lee H.C., Yap Y.L. Insecticidal properties of eugenol, isoeugenol and methyleugenol and their effects on nutrition of Sitophilus zeamais Motsch. (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) J. Stored Prod. Res. 2002;38:403–412. [Google Scholar]
- Islam, B.N., 1983. Pesticidal action of neem and certain indigenous plants. In: Proc. 2nd Int. Neem Conf., Rauischhlzhausen, p. 263–290.
- Isman M.B. Essential oils for pest and disease management. Crop Prot. 2000;19:603–608. [Google Scholar]
- Isman M.B. Botanical insecticides, deterrents and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Kafle L., Shih C.J. Toxicity and repellency of compounds from clove (Syzygium aromaticum) to red imported fire ants Solenopsis invicta (Hymenoptera: Formicidae) J. Econ. Entomol. 2013;106:131–135. doi: 10.1603/ec12230. [DOI] [PubMed] [Google Scholar]
- Kanat M., Alma M.H. Insecticidal effects of essential oils from various plants against larvae of pine processionary moth, Thaumetopoea pityocampa (Schiff.) (Lepidoptera: Thaumetopoeidae) Pest Manage. Sci. 2004;60:173–177. doi: 10.1002/ps.802. [DOI] [PubMed] [Google Scholar]
- Korikontimath V.S., Mulge R., Zachariah J.T. Variations in essential oil constituents in high yielding selections of cardamom. J. Plant. Crops. 1999;27:230–232. [Google Scholar]
- Lawrence BM. Essential Oils. Allured Publishing Corporation; 1979. Major tropical spices cardamom (Eletteria cardamomum). p. 104.
- Naveena B.M., Muthukumar M., Sen A.R., Babji Y., Murthy T.R.K. Improvement of shelf-life of buffalo meat using lactic acid, clove oil and vitamin C during retail display. Meat Sci. 2006;74:409–415. doi: 10.1016/j.meatsci.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Nerio L.S., Olivero-Verbel J., Stashenko E. Repellent activity of essential oils. Bioresour. Technol. 2010;101:372–378. doi: 10.1016/j.biortech.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Nirula K.K. Investigation on the pests of coconut palm, Part-IV. Rhynchophorus ferrugineus. Indian Coconut J. 1956;9:229–247. [Google Scholar]
- Olivero-Verbel J., González-Cervera T., Güette-Fernandez J., Jaramillo-Colorado B., Stashenko E. Chemical composition and antioxidant activity of essential oils isolated from Colombian plants. Brazilian J. Pharmacognosy. 2010;20:568–574. [Google Scholar]
- Phillips T.W., Parajulee M.N., Weaver D.K. Toxicity of terpenes secreted by the pr edator Xylocoris flavipes (Reuter) to Tribolium castaneum (Herbst) and Oryzaphilus surinamensis (L.) J. Stored Prod. Res. 1995;31:131–138. [Google Scholar]
- Sarwar, M., Ali, A. Ahmed, N., Tofique, M. 2005. Expediency of different botanical products intended for managing the population of rice stem borers.
- Sarwar M., Ashfaq M., Ahmed A., Randhawa M.A.M. Assessing the potential of assorted plant powders on survival of Caloglyphus grain mite (Acari: Acaridae) in wheat grain Intern. Agric. Sci. Bioresource Eng. Res. 2013;2:106. [Google Scholar]
- Sarwar M., Ahmed N., Bux M., Tofique M. Potential of plant materials for management of Cowpea bruchid Callosobruchus analis (Coleoptera: Bruchidae) in gram Cicer arietinum during storage. The Nucleus. 2012;49:61–64. [Google Scholar]
- Sedy K.A., Koschier E.K. Bioactivity of carvacrol and thymol against Frankliniella occidentalis and Thrips tabaci. J. Appl. Entomol. 2003;127:313–316. [Google Scholar]
- Su H.C.F. Laboratory evaluation of biological activity of Cinnamomum cassia to four species of stored- product insects. J. Entomol. Sci. 1985;20(2):247–253. [Google Scholar]
- Sharaby A., EL-Nujiban A. Biological activity of essential oil of sage plan leaves Salvia offecinalis L. against the black cutworm Agrotis ipsilon (Hubn.) Int. J. Sci & Res. 2015;4:737–741. [Google Scholar]
- Sharaby A., AL-Dosary An electric air flow olfactometer and the olfactory Response of Rhynchophorous ferrugineus weevil to some volatile compounds. J. Agric. Ecol, Res, Int. 2014;1:40–50. [Google Scholar]
- Shayaa E., Kostjukovski M., Eilberg J., Surkprakarn C. Plant oils as fumigant and contact insecticides for the control of stored product insects. Stored Prod. Res. 1997;33:7–15. [Google Scholar]
- Shayaa E., Ravid U., Paster N., Juven B., Zisman U., Pissarev V. Fumigant toxicity of essential oils against four major stored- Product insect. J. Chem. Ecol. 1991;17(3):499–504. doi: 10.1007/BF00982120. [DOI] [PubMed] [Google Scholar]
- Vidyasagar P.S.P.V., Subaharan K. Role of behavior modifying chemicals in management of key pests of palm. In: Narsimban S., Suresh G., Wesley S.D., editors. Innovative pest and disease management in horticultural and plantation crop-SPIC Science Foundation, Chennai, The British Council, The British Deputy High Commission, Chennai, India. 2000. pp. 121–126. [Google Scholar]
- Waliwitya R., Isman M.B.I., Vernon R.S., Riseman A. Insecticidal activity of selected monoterpenoides and rosmary oil to Agriotes obscurus (Coleoptera: Elateridae) J. Econ. Entomol. 2005;98:1560–1565. doi: 10.1093/jee/98.5.1560. [DOI] [PubMed] [Google Scholar]
- Weiss E.A. CABI Publishing; Wallingford, UK: 2002. Spice crops. [Google Scholar]
- Zheng G.Q., Kenney P.M., Lam L.K.T. Sesquiterpenes from clove (Eugenia caryophyllata) as potential anticarcinogenic agents. J. Nat. Prod. 1992;55:999–1003. doi: 10.1021/np50085a029. [DOI] [PubMed] [Google Scholar]