Abstract
Reichardia tingitana is an annual plant growing in different habitats of the Egyptian deserts. Little is known about variation among the habitats occupied by this species, its distribution, chemical composition, and allelopathic activity. The present study aimed to (a) assess the vegetation composition of three different habitats (Western Coast, Delta Coast, and Wadi Hagoul) of R. tingitana in Egypt, (b) determine their correlation to soil factors, (c) identify the changes in the bioactive constituents of R. tingitana in the three regions, and (d) evaluate the allelopathic activity regarding the variation in the habitat. Density and cover of all plant species associated with R. tingitana were estimated within 52 plots, representing three regions. Physical and chemical parameters of soil were analyzed in each plot. R. tingitana aboveground biomass was collected from each habitat, and the bioactive composition was analyzed using HPLC. The allelopathic effect against two weeds (Amaranthus lividius and Chenopodium murale) was assessed. The floristic composition showed the presence of 133 species belonging to 27 families. In the Delta Coast habitat of R. tingitana, Zygophyllum aegyptium and Calligonum polygonoides co-dominate, while Lycium shawii dominate the Western Coast habitat and finally the habitat of Wadi Hagoul was dominated by Haloxylon salicornicum. Soil analysis revealed little variations among habitats, especially salinity and organic matter. Fifteen compounds, mainly phenolics (60% of the total identified compounds) were identified from all R. tingitana samples. The major compounds were quercetin, naringenin, ellagic, gallic, chlorogenic, and caffeic acids. These compounds varied in diversity or quantity among different habitats. The Western Coast sample was the richest in species, followed by Delta Coast sample. Our study showed that salinity is the crucial factor that induces the production of bioactive constituents in R. tingitana, especially phenolics and flavonoids. The R. tingitana extracts significantly reduced the germination and growth of Chenopodium and Amaranthus. However, the Western Coast sample showed potent allelopathic activity, where the germination was wholly inhibited at 75 mg L−1 and 50 mg L−1, respectively. Thereby, this extract could be used as eco-friendly bioherbicide and may be integrated into weed control strategies.
Keywords: Reichardia tingitana, Edaphic factors, Phytotoxicity, Seconday compounds, Chemical ecology
1. Introduction
Chemical ecology is the science addressing the role of chemical interactions between organisms and their environment. It includes signaling processes and communication between individuals (Baldwin et al., 2002). For example, after herbivores attack, the plant generates volatiles that attracts carnivorous insects that kill plant enemies (Takabayashi and Dicke, 1996). Also, infected plants can produce a signal that induces the immune system of healthy members (Baldwin et al., 2006). These chemicals that are produced by plants to either inhibit or stimulate others are called allelochemicals, and the phenomenon is known as allelopathy (Rice, 1984). The production of these compounds depends on the activation of specialized genes and is often depends on environmental conditions, such as climatic and edaphic factors (Buchanan et al., 2015).
Allelopathy plays a crucial role in the dynamics of plant species in different environments. Understanding this biological phenomenon could help in the development of applications in both natural and agricultural systems (Wardle et al., 2011). Environmental factors of any habitat can directly or indirectly affect the allelopathic potential of a plant. The most critical ecological factors that influence allelopathy include temperature, water content, salinity, nutrient availability and competition stress (Elshamy et al., 2019, Meiners et al., 2012).
Asteraceae or Compositae is one of the largest families of the flowering plants and has worldwide distribution. Most of the family members are herbaceous although a significant number of shrubs, vines, or trees belong the family. In the flora of Egypt, Asteraceae is represented by 98 genera and 228 species (Boulos, 2009). Plants under this family have been widely utilized in the past and are still used today for their medicinal properties. Members of Asteraceae have been reported to possess many biological activities including antibacterial, antifungal, antidiabetic, antihelminthic, immunostimulatory, and anticancer activities (Cazella et al., 2019, Salazar et al., 2018, Sharma et al., 2018).
Only two species of Reichardia genus were recorded in the flora of Egypt, R. picroides (L.) Roth and R. tingitana (L.) Roth. The latter is a glabrous annual herb growing wildly in the coastal and inland desert of Egypt. The stem branches from the base, the plant reaches up to 40 cm with the taproot, which gives rise to the rosette of large radical leaves first, and its flowering time extends from March to May. Preliminary phytochemical examination on aboveground biomass shows the presence of phenolics, tannins, flavonoids, coumarins, volatile oils, glycosides, flavonoids, lactones, esters, large amounts of sterols and/or triterpenes (Abdel-Mogib et al., 1993, Recio et al., 1992).
Aqueous extract of R. tingitana was reported to inhibit the germination and growth of Bidens pilosa, but the specific bioactive compounds were not determined (Abd El-Gawad et al., 2015). Moreover, little is known about the allelochemicals of R. tingitana, and according to our knowledge, no study had revealed the correlation between the habitats of R. tingitana and their bioacive composition as well as alleopathic activity. Therefore, we test the hypothesis that variations in R. tingitana habitats promote changes in the chemical composition of the plant and in consequence affect its allelopathic activity. The specific objectives of this study were to (i) determine the variations in the vegetational composition and soil factors among three different habitats of R. tingitana in Egypt, (ii) identify and compare the variation in the bioactive compounds of R. tingitana aboveground biomass from these three habitats, and (iii) evaluate the differences in R. tingitana allelopathic activity among three regions.
2. Materials and methods
2.1. Study areas
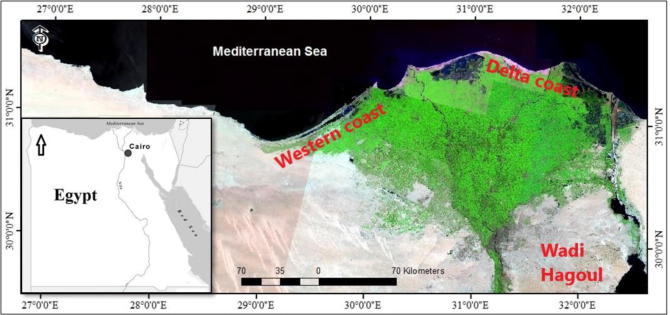
The study areas can be categorized into two sections: the first is the coastal desert which is represented by two regions; Western Coast and Delta Coast, while the second is the inland desert which is represented by Wadi Hagoul (Fig. 1). The Mediterranean coastal desert of Egypt can be classified into three sections: western, delta, and eastern sectors. The Western Coast extends eastwards from Al-Sallum to Abu Qir for about 550 km with a width of about 20 km, constituting the northern coast of the Western Desert of Egypt. The meteorological data (average of >10 years, from 2000 to 2013) of Sidi Abd El-Rahman station showed that mean annual temperature ranges from 14.39 to 25.50 °C, while average annual rainfall was about 192.1 mm per year. The average relative humidity was up to 66.9%. The Delta Coast is characterized by the presence of three shallow lakes, receiving the main bulk of the drainage water from the Nile Delta. This sector extends from Abu Quir to Port Said with a length of about 200 km. The metrological data of Baltim station revealed that the mean annual temperature ranges from 17.3 to 24.0 °C, the average annual rainfall was about 175.2 mm per year, and the mean relative humidity was approximately 57.7%.
Fig. 1.
Map showing the three studied regions namely: Western Coast, Delta Coast, and Wadi Hagoul.
On the other hand, Wadi Hagoul is a drainage system occupying the valley depression between Kahaliya ridge to the south and Gebel Ataqa to the north at the Northwestern part of Gulf of Suez. The area of the wadi is 345 km3, and its main channel extends for 35 km, where it receives water through the running channels from both sides. The wadi extends in a southeast direction toward the Gulf of Suez, crossing limestone beds of the Miocene (Zahran and Willis, 2009). Wadi Hagoul exists in the Eastern Desert, which is characterized by the arid climate. According to the meteorological data of the nearest station (Suez city), the climate is arid and dry, where the mean annual air temperature ranges from 10 to 38 °C. Rainfall is scarce with 34.3 mm as yearly average, while the relative humidity is 54.4%.
2.2. Vegetation analysis
Fifty-two plots were selected to represent the three habitats (Western Coast, Delta Coast, and Wadi Hagoul) of R. tingitana. The area of the plot was 100 m2, estimated according to the minimal area. Within each plot, the density and cover of all species associated with R. tingitana were determined (Canfield, 1941, Shukla and Chandel, 1989). The importance value out of 200 was calculated for each species based on the relative density and cover in each plot. The taxonomic nomenclature, identification, and chorotype of plant species were assessed according to Boulos, 1999–2005., Tackholm, 1974. However, life forms were identified according to the scheme of Raunkiaer (1937).
2.3. Soil analysis
In each plot, composite soil samples (20 cm depth) were collected, packed in polyethylene bags and returned to the laboratory. The samples were dried in the air at room temperature (25 °C ± 3), sieved through 2 mm sieve to remove any contaminants, and packed in bags till further physical and chemical analyses. Soil particle size, water holding capacity (WHC), soil porosity, organic carbon, and sulphate were determined according to Piper (1947). Chlorides and calcium carbonate contents were determined according to Jackson (1962). Soil pH and electrical conductivity (EC) were measured in water suspension (1:2.5) as described by Jackson (1962). Carbonates and bicarbonates were determined by titration using 0.1 N HCl (Pierce et al., 1958). The extractable cations Na+ and K+ were determined by flame photometry (PHF 80B Biologie Spectrophotometer), while Ca+2 and Mg+2 were estimated using atomic absorption spectrometer (A Perkin-Elmer, Model 2380.USA) according to Allen et al. (1974).
2.4. Plant materials preparation and extraction
The above ground biomass of R. tingitana from five plants was collected from each habitat in a plastic bag and mixed as composite sample. To avoid bias, all the three habitats were sampled at the flowering stage in March and plant individuals were chosen to be of similar size. The samples were dried in room temperature (25 °C ± 3), ground and packed in paper bags. About 200 g of the prepared samples were mixed with one liter of aqueous methanol (70% v/v) and shaken well for 12 h. The suspensions were then centrifuged at 2395 g for 10 min, and evaporated till dryness under rotary evaporator. The residues were collected in a glass vial till further analyses.
2.5. HPLC analysis
The extracted samples of the three Reichardia habitats were analyzed by HPLC. The analysis was accomplished using an Agilent 1260 series. The separation was carried out using a C18 column (4.6 mm × 250 mm i.d., five μm). The mobile phase consisted of water (A) and 0.02% trifluoroacetic acid in acetonitrile (B) at a flow rate of 1 ml min−1. The mobile phase was programmed consecutively in a linear gradient as follows: 0 min (80% A); 0–5 min (80% A); 5–8 min (40% A); 8–12 min (50% A); 12–14 min (80% A) and 14–16 min (80% A). The multi-wavelength detector was scrutinized at 280 nm. The injection volume was ten μl for each of the sample solutions, and the temperature of the column was sustained at 35 °C.
2.6. Allelopathy bioassay
2.6.1. Weed seed source and preparation
To test the allelopathic activity of the three collected Reichardia samples, we targeted two weeds; one infesting the cultivated field in the winter season (Chenopodium murale, hereafter; Chenopodium) and the second one growing in summer season (Amaranthus lividius, hereafter; Amaranthus). The seeds were collected from several healthy plants that grow in cultivated land in Manzalla city, Al-Dakahlia Governorate, Egypt (31°07′08.4″N 31°51′56.5″E). Ripe and uniform seeds with almost of the same size and color were surface-sterilized using 0.3% NaOCl, by immersion for three minutes, then rinsed by sterile distilled water three times, dried on sterilized filter paper, and kept in sterilized vials until further use (Sampietro et al., 2009).
2.6.2. Experimental design of bioassay
For the preparation of plant extracts, different concentrations of 2.5, 5, 10, 25, 50, 75 and 100 mg L−1 of the above-mentioned residues were achieved using dimethyl sulfoxide (DMSO). Also, control treatment was achieved by DMSO. Twenty seeds of each weed were placed in 9 cm Petri dishes lined with sterilized Whatman No. 1 filter paper. The filter paper was moistened with 5 ml of each concentration and DMSO as control, in triplicates. To avoid contamination, the Petri dishes were sealed with parafilm and incubated in the growth chamber at 24 °C and 28 °C for Chenopodium and Amaranthus, respectively (Sampietro et al., 2009). A total of 144 plates [8 treatments (7 concentrations + control) × 3 samples (regions) × 2 species of weed’s seeds × 3 replicates] were obtained. After eight days for Chenopodium and ten days for Amaranthus, the germinated seeds were counted, and the radicle lengths were measured per each plate. The inhibition of germination, and root growth was calculated, concerning control, as follows:
2.7. Data analysis
The plant species diversity indexes were calculated per each habitat of Reichardia based on the vegetation analysis data as follows:
where Pi = ni/ N = proportional abundance of species, i in a habitat made up of s species, ni = the number of plots containing species i and N = S ni.
On the other hand, the data of both soil analysis and allelopathic bioassays were subjected to one-way ANOVA followed by Duncan's post hoc test at probability level 0.05 using CoStat software program (CoHort Software, Monterey, CA, USA). Also, the data derived from HPLC analysis of the three samples were subjected to an agglomerative hierarchical cluster (AHC) and principal component analysis (PCA) to construct a matrix of correlation and to recognize whether a significant difference exists between different treatments. AHC and PCA were performed using XLSTAT statistical computer software package, version 14 (Addinsoft, New York, NY, USA).
3. Results and discussion
3.1. Vegetation composition and soil factors of the three R. tingitana habitats
The floristic analysis of the present study showed that the total number of the recorded plant species is 133 species (64 annuals, three biennials, and 66 perennials) belonging to 105 genera and related to 27 families. The most abundant families were Asteraceae comprising 24 species, followed by Poaceae (22 species), Chenopodiaceae (13 species), Fabaceae (12 species), and Brassicaceae (10 species). However, other families were represented by a few species (Appendix 1). These families were also reported as major families in most of the researches within the same phytogeographical region (Abd El-Gawad and Shehata, 2014, El-Amier and Abd El-Gawad, 2017, Shaltout et al., 1995, Shaltout et al., 2015, Zahran et al., 1990, Zahran and Willis, 2009). According to Raunkiaer (1937), therophytes were the most represented life form (50.38%), followed by chamaephytes (21.80%), hemicryptophytes (12.78%), geophytes (6.77%), and nanophanerophytes (6.02%). However, helophytes, parasites, and phanerophytes were represented by one species only (Appendix 1). The prevalence of therophytes over the other life forms could be attributed to the short life cycle that enables them to resist the instability of the habitat, topography variation and biotic influence. Moreover, therophytes were estimated by 50.3% for the whole Egyptian flora compared with 58.7% for the Mediterranean region and 59.4% for Egyptian Nile region (Ḥasib, 1950).
3.1.1. The habitat of R. tingitana in the Delta Coast
Within the habitat of R. tingitana in the Delta Coast, 66 plant species were recorded (35 annuals, two biennials, and 29 perennials) inhabiting 17 plots that were co-dominated by the halophytic plants; Zygophyllum aegyptium and Calligonum polygonoides. This habitat has average Shannon-evenness value of 0.80 and Simpson diversity value of 0.95 (Table 1). The most important associated annual species (have relatively high importance values) linked to this habitat were Hordeum murinum, Senecio glaucus, Cakile maritima, Mesembryanthemum crystallinum, and M. nodiflorum. The soil analyses of this region showed that it was characterized by significant high levels of sand, salinity, soil pore spaces, pH, SO42−, and HCO3–, while cations except Ca2+ were detected in low concentration (Table 2). No bicarbonates were detected. The northern coast of the Nile Delta of Egypt is mainly characterized with halophytic vegetation as it is affected with the Mediterranean Sea as well as the three shallow lakes of this coastal area that receive the main bulk of the drainage water from the Nile Delta (El-Amier and Abd El-Gawad, 2017, Zahran and Willis, 2009). Therefore, the perennial halophytes are common in this site of study, however, there were also a significant number of annuals which corroborated to the presence of sand dunes that distributed along the shoreline of the delta (El Banna, 2004). The sand dune habitat is characterized by its rich seed bank (Yu et al., 2007). It is worth mentioning here that many studies recorded the C. maritima as a common plant in this area (Abd El-Gawad and Shehata, 2014, El-Amier and Abd El-Gawad, 2017, Zahran et al., 1990). Plants of Fabaceae family tended to persist in soil rather than those of other plant functional groups, where their seeds have strong testa (Yu et al., 2007).
Table 1.
Plant diversity, dominant and important plant species of the three studied habitats of Reichardia tingitana.
| Regions | No. of plots | Total species | Shannon-evenness | Simpson diversity | Dominant species | Other important species |
|---|---|---|---|---|---|---|
| Delta Coast | 17 | 66 | 0.80 | 0.95 |
Zygophyllum aegyptium (11.51 ± 2.47)* Calligonum polygonoides (10.89 ± 3.14) |
Hordeum murinum (9.99 ± 1.65) Senecio glaucus (9.51 ± 1.00) Cakile maritima (9.17 ± 1.22) Mesembryanthemum crystallinum (8.71 ± 1.2) Mesembryanthemum nodiflorum (8.53 ± 1.00) Reichardia tingitana (7.73 ± 0.58) |
| Western Coast | 19 | 69 | 0.87 | 0.96 | Lycium shawii (20.62 ± 2.58) |
Reichardia tingitana (15.40 ± 0.38) Senecio glaucus (8.95 ± 0.56) Bromus diandrus (8.24 ± 0.73) Suaeda pruinosa (8.05 ± 0.81) Mesembryanthemum nodiflorum (5.37 ± 0.43) Cyondon dactylon (5.23 ± 0.49) Emex spinosa (5.21 ± 0.36) Malva parvifolra (5.12 ± 0.47) |
| Wadi Hagoul | 16 | 63 | 0.86 | 0.96 | Haloxylon salicornicum (14.17 ± 1.11) |
Launaea nudicaulis (12.44 ± 1.08) Cyondon dactylon (9.49 ± 0.81) Bassia muricata (9.34 ± 0.96) Diplotaxis harra (9.23 ± 0.82) Erodium laciniatum (7.45 ± 0.47) Zygophyllum simplex (7.45 ± 0.64) Senecio glaucus (7.28 ± 0.53) Reichardia tingitana (6.77 ± 0.18) |
Values are means ± standard variation.
Table 2.
Soil variables (Mean values ± standard error) of the three studied habitats of Reichardia tingitana. WHC: water holding capacity, and EC: electrical conductivity.
| Soil variables | Delta coast (n = 17) | Western coast (n = 19) | Wadi Hagoul (n = 16) | F-value |
|---|---|---|---|---|
| Sand (%) | 93.91 ± 1.21a | 92.33 ± 0.93a | 93.26 ± 1.08a | 0.54 |
| Silt (%) | 0.90 ± 0.24b | 4.81 ± 0.51a | 1.87 ± 0.31b | 29.71 |
| Clay (%) | 5.19 ± 1.01a | 2.86 ± 0.61a | 4.87 ± 1.18a | 1.71 |
| Porosity (%) | 39.66 ± 0.75a | 25.44 ± 1.01c | 33.88 ± 1.31b | 46.32 |
| WHC (%) | 28.64 ± 1.04a | 29.18 ± 0.78a | 27.69 ± 1.07a | 0.60 |
| CaCO3 (%) | 2.43 ± 0.38b | 2.65 ± 0.19b | 15.25 ± 1.90a | 42.83 |
| Organic carbon (%) | 0.52 ± 0.09b | 1.11 ± 0.13a | 0.35 ± 0.06b | 17.85 |
| pH | 8.22 ± 0.11a | 7.84 ± 0.09b | 8.09 ± 0.11ab | 3.47 |
| EC (mS cm−1) | 1.26 ± 0.01b | 1.85 ± 0.09a | 0.67 ± 0.05c | 199.44 |
| Cl− (%) | 0.06 ± 0.01b | 0.17 ± 0.02a | 0.04 ± 0.01b | 43.15 |
| SO42− (%) | 0.47 ± 0.08a | 0.04 ± 0.01b | 0.40 ± 0.04a | 20.80 |
| HCO3– (%) | 0.14 ± 0.01b | 0.05 ± 0.01c | 0.23 ± 0.02a | 37.15 |
| Na+ (mg g−1) | 5.05 ± 0.14b | 69.17 ± 5.48a | 11.29 ± 0.41b | 124.09 |
| K+ (mg g−1) | 2.25 ± 0.06c | 8.54 ± 0.53a | 5.35 ± 0.32b | 76.30 |
| Ca2+ (mg g−1) | 16.71 ± 0.18b | 14.36 ± 0.92b | 36.44 ± 2.12a | 82.21 |
| Mg2+ (mg g−1) | 4.73 ± 0.09b | 7.02 ± 0.31a | 4.70 ± 0.32b | 25.49 |
The different letter within each row means values with a significant difference after Duncan’s post hoc test (P ≤ 0.05). LSD0.05: least significant difference at a probability level of 0.05.
Moreover, Abd El-Gawad and Shehata (2014) reported that M. crystallinum (important species in the present study) survives well in sandy dune habitats and tolerates the high content of salinity along the Nile Delta Coast. Also, H. murinum, S. glaucus, and M. nodiflorum were reported as predominant species in similar habitats.
3.1.2. The habitat of R. tingitana in the Western Coast
The floristic analysis of R. tingitana habitat at the Western Coast revealed the dominance of Lycium shawii within 19 plots. Also, this habitat attained the highest number of species (69 species; 38 annuals, two biennials, and 29 perennials) as well as the highest diversity, where it has average Shannon-evenness value of 0.87 and Simpson diversity value of 0.96 (Table 1). The other important associated species were S. glaucus, Bromus diandrus, Suaeda pruinosa, M. nodiflorum, Cyondon dactylon, Emex spinosa, and Malva parvifolra. The community structure of this site has some important halophytes such as S. pruinosa and M. nodiflorum as well as some common associates with halophytic vegetation such as S. glaucus, B. diandrus, C. dactylon, E. spinosa, and M. parvifolra (Table 1). This reflects the salt-affected habitat in this site where the soil composition is characterized by high EC (1.84 mS cm−1) as well as high content of WHC, organic carbon, EC, Cl, Na+, K+, Mg2+, and lowest content of SO42−and HCO3– (Table 2). This result is in consistency with other studies related to the vegetation structure of this area (Shaltout et al., 1995, Shaltout et al., 2015).
The Western Coastal belt of the Mediterranean Sea is the wealthiest part of Egypt in its floristic composition due to its relatively high rainfall (Tackholm, 1974). However, most of these species are therophytes (annuals) that flourish during the rainy season.
3.1.3. The habitat of R. tingitana in Wadi Hagoul
The vegetation analysis of the inland desert region (Wadi Hagoul) revealed the dominance of perennials (63 species; 28 annuals, one biennial, and 34 perennials), where the woody bush plant, Haloxylon salicornicum dominate this habitat. In this context, the dominance of perennials is typical in the desert habitat, where these plants have well-adapted strategies against drought, while the other small plants cannot compete with them (Zahran and Willis, 2009). In addition, this habitat has an average Shannon-evenness value of 0.86 and Simpson diversity value of 0.96 (Table 1). The other important associated species were desert plants including; both perennials (Launaea nudicaulis and C. dactylon) and annuals (Bassia muricata, Diplotaxis harra, Erodium laciniatum, Z. simplex, and S. glaucus) that flourish during the rainy season. The soil of the plots representing this region is characterized by high content of CaCO3, HCO3– and Ca2+ and moderate levels of pH, EC, Na+, and K+ (Table 2). These results are in congruence with previous studies of the vegetation of different wadis in the Egyptian deserts (El-Sharkawi et al., 1982, Mashaly, 1996, Zahran and Willis, 2009).
3.2. Bioactive composition of the three R. tingitana samples and their correlations to soil variables
Fifteen compounds were separated and identified from aboveground biomass of R. tingitana collected from the three regions through HPLC analysis (Table 3). These compounds are mainly phenolics (60.0%), while flavonoid compounds come as second rank (26.7%). The identified compounds varied either in the diversity or quantity between the three samples of the R. tingitana. Western Coast sample was the richest sample, followed by Delta Coast sample and finally Wadi Hagoul sample.
Table 3.
Concentration of fifteen identified compounds derived from HPLC analysis of the collected Reichardia tingitana samples from the three habitats.
| Compound name | Chemical formula | Class | Concentration (µg g−1 dry weight) |
||
|---|---|---|---|---|---|
| Delta coast | Western coast | Wadi Hagoul | |||
| Caffeic acid | C9H8O4 | Phenolic | 13.86 | 89.72 | 5.05 |
| Catechin | C15H14O6 | Phenolic | tr* | 4.56 | tr |
| Chlorogenic acid | C16H18O9 | Phenolic | 20.15 | 101.12 | tr |
| Cinnamic Acid | C9H8O2 | Carboxylic acid | 8.01 | 1.88 | 1.23 |
| Coumaric Acid | C9H8O3 | Carboxylic acid | 3.51 | 1.48 | 9.97 |
| Daidzein | C15H10 | Isoflavone | 1.63 | 51.67 | tr |
| Ellagic acid | C14H6O8 | Phenolic | 9.62 | 174.45 | 42.53 |
| Ferulic Acid | C10H10O4 | Phenolic | tr | 0.71 | 1.67 |
| Gallic Acid | C7H6O5 | Phenolic | 6.23 | 116.16 | tr |
| Naringenin | C15H12O5 | Flavanone | 21.14 | 93.72 | tr |
| Propyl Gallate | C10H12O5 | Phenolic | 1.84 | 11.77 | 0.95 |
| Quercetin | C15H10O7 | Flavonol | 291.60 | 349.66 | 40.35 |
| Rutin | C27H30O16 | Flavonol | tr | 14.59 | tr |
| Syringic Acid | C9H10O5 | Phenolic | tr | 29.24 | 3.01 |
| Vanillin | C8H8O3 | Phenolic | tr | 8.33 | tr |
| % respect to all | 66.67 | 100 | 53.33 | ||
tr: detected in trace amount.
Caffeic acid, cinnamic acid, coumaric acid, ellagic acid, gallic acid, propyl gallate, and quercetin were identified in the samples of R. tingitana in the three regions with significant variation in the quantity. However, chlorogenic acid, daidzein, ferulic acid, naringenin, and syringic acid were identified in two regions, while catechin, vanillin, and rutin were only detected in the Western Coast sample (Table 3).
The R. tingitana sample collected from Western Coast attained the highest significant values of constituents compared to the others two samples (Delta Coast and Wadi Hagoul), where quercetin, ellagic acid, gallic acid, chlorogenic acid, naringenin, and caffeic acid are the major compound in this sample. On the other hand, quercetin was detected as major compound in deltaic coast R. tingitana sample, while quercetin and ellagic acid attained the highest concentration in the sample of the Wadi Hagoul. The accumulation of active secondary metabolites could be ascribed to the influences of multiple environmental factors on the wild plant during developmental and growth periods in addition to genetic factors. Certain compounds are synthesized, and their concentration significantly increase under specific environments (Liu et al., 2015).
The environmental stress factors (light, temperature, soil water, soil fertility, and salinity) eventually lead to changes in overall phytochemical composition which thereby play a strategic role in the generation of bioactive compounds (Yang et al., 2018). This is considered as plant behavior that improves the adaptation and survival in response to the harsh environment as well as enhances the immune system of the plant against herbivores and pathogens (Pellissier et al., 2016).
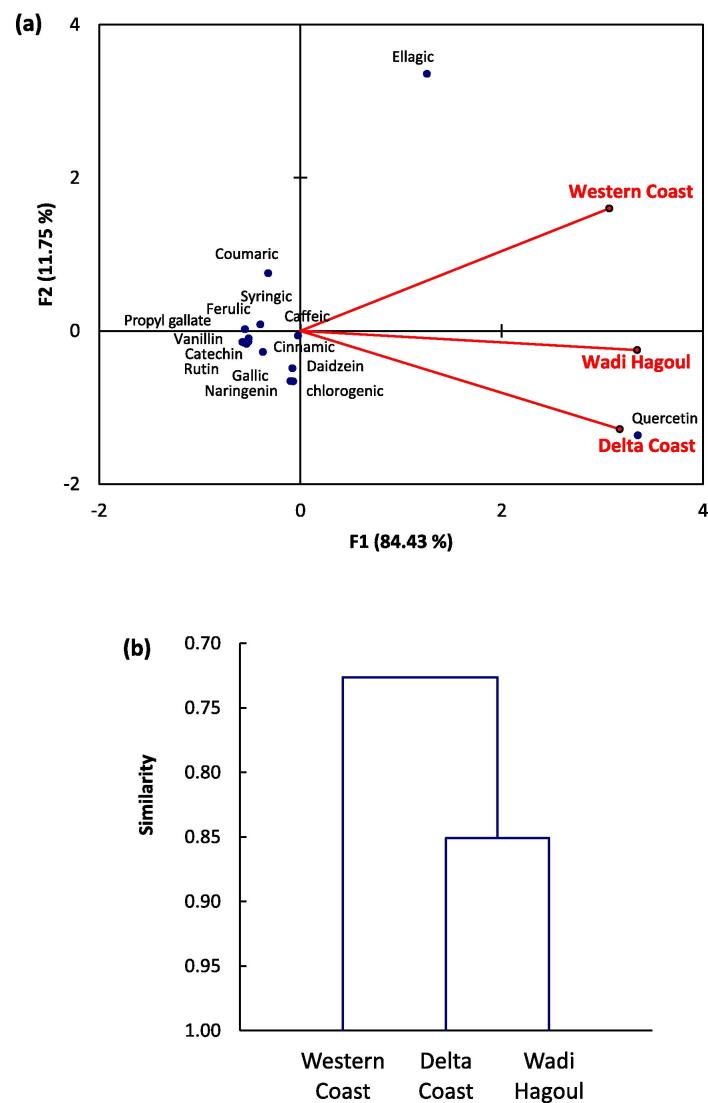
To evaluate the correlation between the different studied habitats and their content of bioactive compounds derived from HPLC analysis, we subjected the data to PCA and AHC. The sample of R. tingitana collected from the Western Coast region was not correlated to the other two samples, which reflect the variation of the chemical composition (Fig. 2a). However, the samples of Delta Coast and Wadi Hagoul were correlated to each other. On the other hand, the application of AHC reflected the same conclusion, where we can classify the samples into two groups; one comprising the Western Coast sample and the other group contained the samples of Delta Coast and Wadi Hagoul (Fig. 2b).
Fig. 2.
(a) Principal component analysis (PCA) and (b) agglomerative hierarchical clustering (AHC) based on the chemical composition derived from HPLC analysis of the three collected samples of Reichardia tingitana.
From the PCA analysis, it was clear that quercetin was the main compounds that showed a close correlation to the Delta Coast sample, while it showed a little bit correlation to Wadi Hagoul sample. This mean that it is considered as an indicator compound in the studied R. tingitana samples. On the other hand, ellagic acid showed a low correlation to the Western Coast sample and did not show any relationship to either Wadi Hagoul or Delta Coast samples. The other compounds showed the same correlation to all samples.
Flavonoids play an essential role in several arrays of plant life such protection against pathogens, insect attraction, pollination, and allelopathy (Winkel-Shirley, 2001). Moreover, under biotic and abiotic stresses, reactive oxygen species (ROS) are generated in the plant cells, and in consequence, flavonoids induced in the plant to scavenge ROS (Baskar et al., 2018, El-Shora and Abd El-Gawad, 2015a, El-Shora and Abd El-Gawad, 2015b). Therefore, the higher concentration of quercetin in the R. tingitana sample from Western Coast region could be ascribed to the high salinity, where quercetin produced as a protecting agent in plants against oxidative stress.
From the other side, caffeic acid, cinnamic acid, coumaric acid, ellagic acid, gallic acid, and propyl gallate were also reported to be induced under salinity stress (Colla et al., 2013), therefore we can say that the higher the content of phenolics in the samples from the Western Coast could be attributed to the salinity level. A compound like ellagic acid has the ability to alleviate the osmotic stress (Abu El-Soud et al., 2013), therefore the higher content of ellagic acid in the samples of R. tingitana from the Western Coast habitat could be considered as a strategy of the plant to cope with salt stress, while for the sample of Wadi Hagoul it could be like a defense against the drought. Our study showed that the salinity, organic matter, and macro-elements (Na+, K+, and Mg2+) may be the crucial factors that induce the production of bioactive constituents in R. tingitana, especially the phenolic acids and flavonoids.
3.3. Allelopathic activity of the three R. tingitana samples
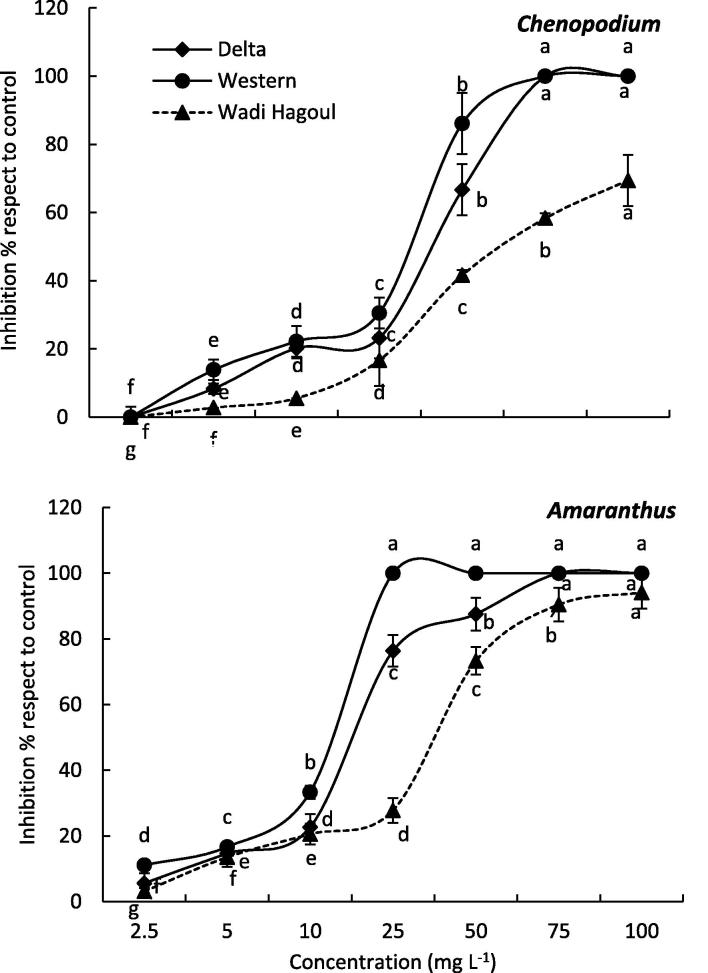
The allelopathic effect of various extracts of R. tingitana aboveground biomass collected from three different regions (Delta Coast, Western Coast, and Wadi Hagoul) on germination of Chenopodium and Amaranthus was illustrated in Fig. 3. In general, R. tingitana extracts from all regions significantly (p ≤ 0.05) reduced the germination of Amaranthus more than Chenopodium (Fig. 3). The seed germination of Chenopodium was completely inhibited at a concentration of 75 mg L−1 under the effect of Delta Coast and Western Coast R. tingitana extracts, while it was inhibited by 58.33% for the Wadi Hagoul R. tingitana extract at the same concentration. The IC50 values (the concentration of a substance that is required for 50% inhibition of a specific biological function) of the germination of Chenopodium were 37.85 mg L−1, 42.33 mg L−1 and 66.06 mg L−1 for Western Coast, Delta Coast, and Wadi Hagoul R. tingitana extracts, respectively. On the other hand, the germination of Amaranthus was completely inhibited at a concentration of 25 mg L−1 under the effect of Western Coast. While it was inhibited by 76.35% and 27.78% under treatments of Delta Coast and Wadi Hagoul R. tingitana extracts, respectively. The IC50 values of the germination of Amaranthus were 20.65 mg L−1, 29.99 mg L−1 and 42.16 mg L−1 for Western Coast, Delta Coast, and Wadi Hagoul R. tingitana extracts, respectively.
Fig. 3.
Allelopathic effect of Reichardia tingitana aboveground biomass collected from three habitats on the germination of Chenopodium murale (above) and Amaranthus lividius (below). The different letters within each line means significant difference at p ≤ 0.05.
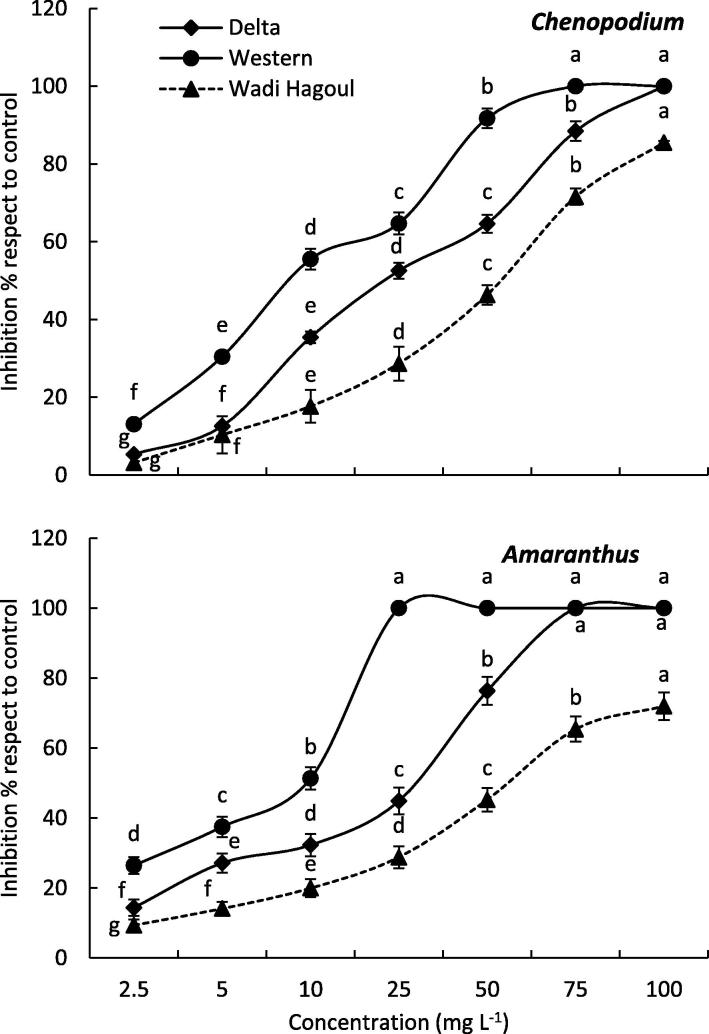
Various extracts from R. tingitana showed significant (p ≤ 0.05) inhibition of root growth of Chenopodium and Amaranthus after treatment, and the degree of inhibition of plant extracts increased with increasing concentration (Fig. 4). The R. tingitana extracts from the Western Coast and Delta Coast samples expressed total inhibition of Chenopodium root growth at concentration of 75 mg L−1, while it was inhibited by 88.45% and 71.57% for the Delta Coast and Wadi Hagoul R. tingitana extracts, respectively. The IC50 values of the root growth of Chenopodium were 19.66 mg L−1, 36.80 mg ml−1 and 53.27 mg L−1 for Western Coast, Delta Coast, and Wadi Hagoul R. tingitana extracts, respectively.
Fig. 4.
Allelopathic effect of Reichardia tingitana aboveground biomass collected from the three habitats on the root growth of Chenopodium murale (above) and Amaranthus lividius (below). The different letters within each line means significant difference at p ≤ 0.05.
On the other hand, the root growth of Amaranthus was entirely inhibited at a concentration of 25 mg L−1 under the effect of Western Coast. However, the extracts of Delta Coast and Wadi Hagoul R. tingitana samples inhibited it by 76.34% and 45.23%, respectively at the same concentration. The IC50 values of the germination of Amaranthus were 4.60 mg L−1, 31.12 mg L−1 and 59.11 mg L−1 for Western Coast, Delta Coast, and Wadi Hagoul R. tingitana extracts, respectively.
Generally, the extracts from the R. tingitana collected from the Western Coast revealed more allelopathic effect against the tested plants than the samples from Delta Coast or Wadi Hagoul regions. This activity might be attributed to the higher concentrations of phenolics and flavonoids reported in this sample. Phenolic compounds, especially quercetin, ellagic acid, gallic acid, chlorogenic acid, caffeic acid, and naringenin have been reported to possess various biological activities. Moreover, the bioactive ingredients enable the plant to face or survive in the variable stressful conditions, and they are considered as an important part of the plant defense system against pathogenic attacks and environmental stresses (Yang et al., 2018). In addition, most of the identified compounds in this sample were reported as allelochemicals (Inderjit, , 1996, Li et al., 2010). Phenolics and flavonoids were reported as defense compounds against herbivores and pathogens such as bacteria and fungi (Agrawal, 2007). Quercetin and naringenin were reported as the most often cited allelopathic flavonoids (Berhow and Vaughn (1999)). Naringenin was reported to inhibit the growth of soybean (Bido et al 2010). These bioactive compounds might play a role in the allelopathic activity of R. tingitana either singular or in a synergetic manner.
4. Conclusion
The vegetation analysis of the three habitats of R. tingitana within three different regions (Western Coast, Delta Coast, Wadi Hagoul) revealed a significant variation in the vegetation composition as well as soil variables, while salinity, organic matter, and macro-elements (Na+, K+, and Mg2+) were the most controlling factor. Fifteen identified secondary compounds were determined using HPLC, where they are varied in the diversity and quantity between the three samples of the R. tingitana. Western Coast sample had the highest number of the bioactive compounds, followed by Delta Coast sample and finally Wadi Hagoul sample. Therefore, we can say that R. tingitana extract from the same species but from different conditions caused different allelopathic (inhibitory) effect, and this supposed to be related to the variation in the bioactive constituents. Our study showed that the salinity is the key factor that induce the production of bioactive constituents in R. tingitana, especially the phenolic acids and flavonoids as well. The R. tingitana methanol extract, especially Western Delta sample, showed potent allelopathic activity against Chenopodium and Amaranthus, where the germination was utterly inhibited at a concentration of 75 mg L−1 and 50 mg L−1, respectively. Therefore, these extracts could be used as a green source, eco-friendly bioherbicide and to be integrated into the weed control program of weeds. However, further study is needed for characterization of the specific allelochemical(s), evaluation the modes of action, and the biosafety. Also, further study is needed to confirm the mechanism(s) of salinity to induce the bioactive compounds.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1440-113). Further, the authors like to sincerely thanks the Department of Botany, Faculty of Science, Mansoura University, Egypt.
Footnotes
Peer review under responsibility of King Saud University.
Appendix 1.
Vegetation composition of the three habitatis of Reicharadia tingitana representing both coastal and inland desert of Egypt, and the life forms of different associated plants. Values are mean of the importance values (based on density and cover of the plant species) ± standard error. Th: therophytes, Ch: chamaephytes, H: hemicryptophytes, G: geophytes, Nph: nanophanerophytes, He: helophytes, P: parasites, and Ph: phanerophytes.
| Plant species | Life form | Coastal desert |
Inland desert |
||
|---|---|---|---|---|---|
| Delta Coast | Western Coast | Wadi Hagoul | |||
| Perennials | |||||
| 1 | Achillea santolina L. | Ch | 0.83 ± 0.19 | ||
| 2 | Alhagi graecorum Boiss. | H | 0.32 ± 0.19 | ||
| 3 | Anabasis articulata (Forssk.) Moq. | Ch | 2.18 ± 0.54 | ||
| 4 | Artemisia judiaca L. | Ch | 3.27 ± 0.40 | 1.05 ± 0.26 | |
| 5 | Astragalus spinosus (Forssk.) Muschl. | Ch | 1.53 ± 0.27 | ||
| 6 | Atractylis carduus (Forssk.) C.Chr. | H | 0.30 ± 0.12 | 2.13 ± 0.23 | |
| 7 | Atriplex halimus L. | Nph | 2.28 ± 1.34 | 4.87 ± 0.47 | |
| 8 | Atriplex semibaccata R.Br. | H | 6.76 ± 1.96 | 1.10 ± 0.20 | |
| 9 | Calotropi sprocera (Willd.) R.Br. | Ph | 3.38 ± 0.58 | ||
| 10 | Calligonum polygonoides L. subsp. comosum (L’ Her.) Soskov | Nph | 10.89 ± 3.14 | 0.16 ± 0.04 | |
| 11 | Lepidium draba L. | H | 0.25 ± 0.06 | ||
| 12 | Cenchrus cillaris L. | He | 3.91 ± 0.66 | ||
| 13 | Cistanche phelypaea (L.) Cout. | P, G | 0.76 ± 0.23 | ||
| 14 | Cleome droserifolia (Forssk.) Delile | Ch | 1.24 ± 0.31 | ||
| 15 | Convolvulus arvensis L. | H | 3.54 ± 0.41 | ||
| 16 | Convolvulus lanatus Vahl | Ch | 1.26 ± 0.31 | ||
| 17 | Cynanchum acutum L. | H | 0.79 ± 0.64 | 5.16 ± 0.58 | |
| 18 | Cyondon dactylon (L.) Pers. | G | 4.63 ± 1.25 | 5.23 ± 0.49 | 9.49 ± 0.81 |
| 19 | Deverra tortuosa (Desf.) DC. | Ch | 1.25 ± 0.23 | ||
| 20 | Diplotaxis harra (Forssk.) Boiss. | Ch | 9.23 ± 0.82 | ||
| 21 | Echinops spinosus L. | H | 5.20 ± 1.49 | 0.64 ± 0.14 | 1.33 ± 0.33 |
| 22 | Elymus farctus (Viv.) Runem. ex Melderis | G | 0.20 ± 0.12 | ||
| 23 | Euphorbia terracina L. | H | 1.31 ± 0.22 | ||
| 24 | Euphorbia retusa Forssk. | H | 1.48 ± 0.16 | ||
| 25 | Fagonia arabica L. | Ch | 4.40 ± 0.75 | 1.33 ± 0.33 | |
| 26 | Fagonia cretica L. | Ch | 4.59 ± 0.47 | ||
| 27 | Farsetia aegyptia Turra | Ch | 2.20 ± 0.30 | ||
| 28 | Gypsopila capillaris (Forssk.) C.Chr | H | 0.58 ± 0.14 | ||
| 29 | Halocnemum strobilaceum (Pall.) M. Bieb. | Ch | 4.74 ± 2.08 | 1.15 ± 0.14 | |
| 30 | Haloxylon salicornicum (Moq.) Bunge ex Boiss. | Ch | 14.17 ± 1.11 | ||
| 31 | Heliotropium curassavicum L. | Ch | 1.47 ± 0.60 | ||
| 32 | Hyoscyamus muticus L. | Ch | 1.28 ± 0.22 | ||
| 33 | Imperata cylindrical (L.) Raeusch. | H | 1.30 ± 0.22 | ||
| 34 | Iphiona mucronata (Forssk.) Asch. & Schweinf. | Ch | 0.23 ± 0.13 | ||
| 35 | Kickxia aegyptiaca (L.) Nάbelek. | Ch | 0.19 ± 0.05 | ||
| 36 | Lasiurus scindicus Henrard | G | 3.01 ± 0.44 | ||
| 37 | Launaea mucronata (Forssk.) Muschl. | H | 5.04 ± 0.89 | 1.74 ± 0.21 | |
| 38 | Launaea nudicaulis (L.) Hook.f. | H | 4.36 ± 0.92 | 2.24 ± 0.24 | 12.44 ± 1.08 |
| 39 | Launaea spinosa (Forssk.) Sch. Bip. ex Kuntze | Ch | 1.85 ± 0.37 | ||
| 40 | Limbarda crithmoides (L.) Dumort. | Ch | 2.26 ± 1.08 | ||
| 41 | Limonium pruinosum (L.) Chaz. | G, He | 1.23 ± 0.19 | ||
| 42 | Lotus creticus L. | H | 1.65 ± 0.97 | ||
| 43 | Lycium shawii Roem & Schult. | Nph | 9.92 ± 0.24 | 20.62 ± 2.58 | |
| 44 | Moltkiopsis ciliata (Forssk.) I. M. Johnst. | Ch | 3.51 ± 2.07 | 2.82 ± 0.35 | |
| 45 | Ochradenus baccatus Delile | Nph | 4.54 ± 0.78 | ||
| 46 | Pancratium maritimum L. | G | 0.25 ± 0.15 | ||
| 47 | Panicum coloratum L. | G | 2.59 ± 0.30 | ||
| 48 | Panicum turgidum Forssk. | H | 4.73 ± 1.93 | 3.59 ± 0.53 | |
| 49 | Phragmites australis (Cav.) Trin. ex Steud. | G, He | 2.23 ± 0.74 | 3.69 ± 0.47 | 2.92 ± 0.39 |
| 50 | Pluchea dioscoridis (L.) DC. | Nph | 1.09 ± 0.24 | 1.56 ± 0.39 | |
| 51 | Polycarpaea repens (Forssk.) Asch. & Schweinf. | Ch | 0.49 ± 0.12 | ||
| 52 | Polygonum equsetiforme Sm. | G | 4.23 ± 2.49 | 1.01 ± 0.16 | |
| 53 | Retama raetam (Forssk.) Webb & Berthel. | Nph | 0.47 ± 0.28 | 1.14 ± 0.25 | 2.27 ± 0.42 |
| 54 | Silene succulenta Forssk. | H | 0.91 ± 0.53 | ||
| 55 | Silybum marianum (L.) Gaertn. | H | 0.92 ± 0.14 | ||
| 56 | Stipagrostis lanata (Forssk.) De Winter | G | 0.33 ± 0.19 | ||
| 57 | Suaeda monoica Forssk. | Ch | 0.15 ± 0.04 | ||
| 58 | Suaeda pruinosa Lange | Ch | 8.05 ± 0.81 | ||
| 59 | Symphyotrichum squamatum (Spreng.) Nesom | Ch | 0.43 ± 0.10 | ||
| 60 | Tamarix nilotica (Ehrenb.) Bunge | Nph | 1.30 ± 0.22 | 2.22 ± 0.38 | |
| 61 | Thymelaea hirsuta (L.) Endl. | Nph | 3.25 ± 1.92 | ||
| 62 | Zilla spinosa (L.) Prantl | Ch | 5.25 ± 0.56 | ||
| 63 | Zygophyllum aegyptium Hosny | Ch | 11.51 ± 2.47 | 0.76 ± 0.14 | |
| 64 | Zygophyllum album L. | Ch | 7.45 ± 2.15 | ||
| 65 | Zygophyllum coccineum L. | Ch | 4.97 ± 0.47 | ||
| 66 | Zygophyllum decumbens Delile | Ch | 1.19 ± 0.24 | ||
| Biennials | |||||
| 67 | Beta vulgaris L. | Th | 1.79 ± 1.05 | ||
| 68 | Centaurea aegyptiaca L. | Th | 3.93 ± 0.69 | 2.17 ± 0.23 | |
| 69 | Spergularia marina (L.) Griseb. | Th | 0.37 ± 0.22 | 1.40 ± 0.19 | |
| Annuals | |||||
| 70 | Aegilops bicornis (Forssk.) Jaub. & Spach | Th | 2.71 ± 1.13 | 2.30 ± 0.32 | |
| 71 | Aegilops crassa Boiss | Th | 4.01 ± 0.50 | ||
| 72 | Aegilops kotschyi Boiss. | Th | 2.30 ± 1.03 | ||
| 73 | Anchusa humilis (Desf.) I.M. Johnst. | Th | 1.43 ± 0.32 | ||
| 74 | Anthemis cotula L. | Th | 0.83 ± 0.14 | ||
| 75 | Astragalus bombycinus Boiss. | Th | 1.32 ± 0.20 | 2.22 ± 0.22 | |
| 76 | Astragalus peregrinus Vahl | Th | 1.47 ± 0.26 | ||
| 77 | Atriplex lindleyi Moq. subsp. inflata (Muell.) Willson. | Th | 0.28 ± 0.17 | 1.29 ± 0.17 | |
| 78 | Avena fatua L. | Th | 0.13 ± 0.08 | 5.79 ± 0.34 | |
| 79 | Bassia indica (Wight) Scott. | Th | 6.63 ± 0.99 | 1.44 ± 0.24 | 1.17 ± 0.16 |
| 80 | Bassia muricata (L.) Asch. | Th | 0.58 ± 0.34 | 9.34 ± 0.96 | |
| 81 | Brassica tournefortii Gouan. | Th | 0.54 ± 0.32 | 2.77 ± 0.40 | 4.45 ± 0.76 |
| 82 | Bromus diandrus Roth | Th | 3.37 ± 1.12 | 8.24 ± 0.73 | |
| 83 | Cakile maritima Scop. subsp.aegyptiaca (Willd.) Nyman | Th | 9.17 ± 1.22 | 3.13 ± 0.37 | |
| 84 | Carduus getulus Pomel | Th | 0.15 ± 0.09 | 0.33 ± 0.07 | |
| 85 | Carthamus tenuis (Boiss & Blanche) Bornm. | Th | 2.73 ± 0.70 | 4.26 ± 0.61 | |
| 86 | Chenopodium murale L. | Th | 4.11 ± 0.70 | 4.68 ± 0.35 | 1.25 ± 0.15 |
| 87 | Cutandia memphitica (Spreng.) Benth. | Th | 0.54± | ||
| 88 | Emex spinosa (L.) Campd. | Th | 1.22 ± 0.49 | 5.21 ± 0.36 | 0.52 ± 0.13 |
| 89 | Enarthrocarpus lyratus (Forssk.) DC. | Th | 0.48 ± 0.11 | ||
| 90 | Erodium laciniatum (Cav.) Wild. | Th | 0.27 ± 0.16 | 1.47 ± 0.25 | 7.45 ± 0.47 |
| 91 | Euphorbia heliscopia L. | Th | 2.37 ± 0.39 | ||
| 92 | Euphorbia peplus L. | Th | 3.45 ± 0.47 | ||
| 93 | Herniaria hemistemon J. Gay | Th | 0.36 ± 0.09 | ||
| 94 | Hordeum murinum L. | Th | 10.02 ± 1.65 | 2.42 ± 0.36 | |
| 95 | Hordeum spontaneum K. Koch | Th | 1.18 ± 0.22 | ||
| 96 | Ifloga spicata (Forssk.) Sch. Bip. | Th | 2.57 ± 1.09 | 4.89 ± 0.68 | |
| 97 | Lactuca serriola L. | Th | 0.25 ± 0.15 | 4.70 ± 0.59 | |
| 98 | Lavatera cretica L. | Th | 2.90 ± 0.31 | ||
| 99 | Legousia speculum-veneris (L.) Chaix | Th | 1.43 ± 0.32 | ||
| 100 | Lobularia arabica (Boiss.) Muschl. | Th | 1.31 ± 0.29 | ||
| 101 | Lolium multiflorum Lam. | Th | 4.30 ± 1.25 | 0.96 ± 0.17 | |
| 102 | Lolium perenne L. | Th | 3.66 ± 0.55 | ||
| 103 | Lotus glinoides Delile | Th | 1.29 ± 0.15 | ||
| 104 | Lotus halophilus Boiss. | Th | 0.70 ± 0.29 | ||
| 105 | Lotus polyphyllos E. D. Clarke | Th | 2.57 ± 0.35 | ||
| 106 | Malva parvifolra L. | Th | 4.27 ± 0.74 | 5.12 ± 0.47 | 6.51 ± 0.61 |
| 107 | Matthiola longipetala (Vent.) DC. subsp. livida (Delile) Maire | Th | 6.99 ± 0.61 | ||
| 108 | Medicago polymorpha L. | Th | 0.46 ± 0.27 | ||
| 109 | Melilotus indicus (L.) All. | Th | 2.17 ± 0.29 | ||
| 110 | Mesembryanthemum crystallinum L. | Th | 8.71 ± 1.22 | 3.89 ± 0.41 | |
| 111 | Mesembryanthemum forsskaolii Hochst. ex Boiss. | Th | 4.57 ± 0.49 | ||
| 112 | Mesembryanthemum nodiflorum L. | Th | 8.53 ± 1.00 | 5.37 ± 0.43 | |
| 113 | Orobanche crenata Forssk. | Th | 0.24 ± 0.06 | ||
| 114 | Parapholis incurva (L.) C.E. Hubb | Th | 1.20 ± 0.71 | 0.12 ± 0.03 | |
| 115 | Paronychia arabica (L.) DC. | Th | 0.09 ± 0.05 | ||
| 116 | Phalaris minor Retz. | Th | 1.05 ± 0.47 | ||
| 117 | Plantago lagopus L. | Th | 4.24 ± 0.58 | 1.45 ± 0.36 | |
| 118 | Poa annua L. | Th | 1.43 ± 0.84 | 0.22 ± 0.05 | 1.14 ± 0.21 |
| 119 | Reichardia tingitana (L.) Roth | Th | 7.73 ± 0.58 | 15.40 ± 0.38 | 6.77 ± 0.18 |
| 120 | Reseda decursiva Forssk. | Th | 0.65 ± 0.10 | ||
| 121 | Rumex pictus Forssk. | Th | 6.71 ± 0.73 | ||
| 122 | Rumex vesicarius L. | Th | 4.86 ± 0.52 | ||
| 123 | Salsola kali L. | Th | 2.81 ± 0.58 | 0.49 ± 0.12 | |
| 124 | Senecio glaucus L. | Th | 9.51 ± 1.00 | 8.95 ± 0.56 | 7.28 ± 0.53 |
| 125 | Silene vivianii Steud. | Th | 0.94 ± 0.42 | ||
| 126 | Sisymbrium irio L. | Th | 4.50 ± 0.32 | ||
| 127 | Sonchus oleraceus L. | Th | 0.07 ± 0.04 | ||
| 128 | Spergularia rubra (L.) J. & C.Presl | Th | 0.11 ± 0.03 | ||
| 129 | Trigonella stellata Forssk. | Th | 1.93 ± 0.25 | ||
| 130 | Urospermum picroides (L.) F.W. Schmidt | Th | 1.04 ± 0.41 | 4.64 ± 0.44 | |
| 131 | Urtica urens L. | Th | 0.19 ± 0.04 | ||
| 132 | Volutaria lippii (L.) Cass. Ex Maire | Th | 1.29 ± 0.25 | ||
| 133 | Zygophyllum simplex L. | Th | 7.45 ± 0.64 | ||
References
- Abd El-Gawad A., Mashaly I.A., Al-Nafie R.I. Antioxidant activity and allelopathic potential of five wild plants on germination and growth Bidens pilosa L. Int. J. Curr. Res. 2015;7:21019–21024. [Google Scholar]
- Abd El-Gawad A.M., Shehata H.S. Ecology and development of Mesembryanthemum crystallinum L. in the Deltaic Mediterranean coast of Egypt. Egyptian J. Basic Appl. Sci. 2014;1:29–37. [Google Scholar]
- Abdel-Mogib M., Abou-Elzahab M., Dawidar A., Ayyad S. A sesquiterpene glucoside from Reichardia tingitana. Phytochemistry. 1993;34:1434–1435. [Google Scholar]
- Abu El-Soud W., Hegab M.M., AbdElgawad H., Zinta G., Asard H.J.P.P. Ability of ellagic acid to alleviate osmotic stress on chickpea seedlings. Plant Physiol. Biochem. 2013;71:173–183. doi: 10.1016/j.plaphy.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Agrawal A.A. Macroevolution of plant defense strategies. Trends Ecol. Evol. 2007;22:103–109. doi: 10.1016/j.tree.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Allen S.E., Grimshaw H., Parkinson J.A., Quarmby C. Blackwell Scientific Publications; 1974. Chemical Analysis of Ecological Materials. [Google Scholar]
- Baldwin I.T., Halitschke R., Paschold A., Von Dahl C.C., Preston C.A. Volatile signaling in plant-plant interactions:“ talking trees” in the genomics era. Science. 2006;311:812–815. doi: 10.1126/science.1118446. [DOI] [PubMed] [Google Scholar]
- Baldwin I.T., Kessler A., Halitschke R. Volatile signaling in plant–plant–herbivore interactions: what is real? Curr. Opin. Plant Biol. 2002;5:351–354. doi: 10.1016/s1369-5266(02)00263-7. [DOI] [PubMed] [Google Scholar]
- Baskar V., Venkatesh R., Ramalingam S. Flavonoids (antioxidants systems) in higher plants and their response to stresses. In: Gupta D.K., Palma J.M., Corpas F.J., editors. Antioxidants and Antioxidant Enzymes in Higher Plants. Springer International Publishing; Cham: 2018. pp. 253–268. [Google Scholar]
- Bido G.D.S., Ferrarese M.D.L.L., Marchiosi R., Ferrarese-Filho O. Naringenin inhibits the growth and stimulates the lignification of soybean root. Brazilian Arch. Biol. Technol. 2010;53:533–542. [Google Scholar]
- Berhow M., Vaughn S. Higher plant flavonoids: biosynthesis and chemical ecology. In: Inderjit K.M.M.D., Foy C.L., editors. Principles Practices in Plant Ecology: Allelochemical Interactions. CRC Press; Boca Raton: 1999. [Google Scholar]
- Boulos L. Al Hadara Publishing; Cairo, Egypt: 1999–2005.. Flora of Egypt; pp. 1–4. [Google Scholar]
- Boulos L. Al Hadara Publishing; Cairo, Egypt: 2009. Flora of Egypt Checklist. [Google Scholar]
- Buchanan B.B., Gruissem W., Jones R.L. John Wiley & Sons; UK: 2015. Biochemistry and Molecular Biology of Plants. [Google Scholar]
- Canfield R. Application of the line interception method in sampling range vegetation. J. Forest. 1941;39:288–394. [Google Scholar]
- Cazella L.N., Glamočlija J., Sokovic M., Gonçalves J.E., Linde G.A., Colauto N.B., Gazim Z.C. Antimicrobial activity of essential oil of Baccharis dracunculifolia DC (Asteraceae) aerial parts at flowering period. Front. Plant Sci. 2019;10:27. doi: 10.3389/fpls.2019.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colla G., Rouphael Y., Cardarelli M., Svecova E., Rea E., Lucini L. Effects of saline stress on mineral composition, phenolic acids and flavonoids in leaves of artichoke and cardoon genotypes grown in floating system. J. Sci. Food Agric. 2013;93:1119–1127. doi: 10.1002/jsfa.5861. [DOI] [PubMed] [Google Scholar]
- El-Amier Y.A., Abd El-Gawad A.M. Plant communities along the international coastal highway of Nile delta, Egypt. J. Sci. Agric. 2017;1:117–131. [Google Scholar]
- Elshamy A.I., Abd-ElGawad A.M., El-Amier Y.A., El Gendy A.E., Al-Rowaily S.L. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragrance J. 2019;34:1–13. [Google Scholar]
- El-Shora H.M., Abd El-Gawad A.M. Physiological and biochemical responses of Cucurbita pepo L. mediated by Portulaca oleracea L. allelopathy. Fresenius Environ. Bull. 2015;24:386–393. [Google Scholar]
- El-Shora H.M., Abd El-Gawad A.M. Response of Cicer arietinum L. to allelopathic effect of Portulaca oleracea L. root extract. Phyton-Annales Rei Botanicae. 2015;55:215–232. [Google Scholar]
- El-Sharkawi H., Salama F., Fayed A. Vegetation of inland desert wadies in Egypt III. Wadi Gimal and Wadi El-Miyah. Feddes Repertorium. 1982;93:135–145. [Google Scholar]
- El Banna M.M. Nature and human impact on Nile Delta coastal sand dunes, Egypt. J. Environ. Geol. 2004;45:690–695. [Google Scholar]
- Ḥasib M. Fouad I University Press; Egypt: 1950. Distribution of Plant Communities in Egypt. [Google Scholar]
- Inderjit Plant phenolics in allelopathy. Bot. Rev. 1996;62:186–202. [Google Scholar]
- Jackson M.L. Constable and Co; LTD, London: 1962. Soil Chemical Analysis. [Google Scholar]
- Li Z.-H., Wang Q., Ruan X., Pan C.-D., Jiang D.-A. Phenolics and plant allelopathy. Molecules. 2010;15:8933–8952. doi: 10.3390/molecules15128933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Liu J., Yin D., Zhao X. Influence of ecological factors on the production of active substances in the anti-cancer plant Sinopodophyllum hexandrum (Royle) TS Ying. PLoS ONE. 2015;10:e0122981. doi: 10.1371/journal.pone.0122981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashaly I. On the phytosociology of Wadi Hagul, Red Sea Coast. Egyptian J. Environ. Sci. 1996;12:31–54. [Google Scholar]
- Meiners S.J., Kong C.-H., Ladwig L.M., Pisula N.L., Lang K.A. Developing an ecological context for allelopathy. Plant Ecol. 2012;213:1221–1227. [Google Scholar]
- Pellissier L., Moreira X., Danner H., Serrano M., Salamin N., Dam N.M., Rasmann S. The simultaneous inducibility of phytochemicals related to plant direct and indirect defences against herbivores is stronger at low elevation. J. Ecol. 2016;104:1116–1125. [Google Scholar]
- Pierce W.C., Haenisch E.L., Sawyer D.T. Wiley Toppen; Tokyo: 1958. Quantitative Analysis. [Google Scholar]
- Piper C.S. Interscience Publishers Inc; New York: 1947. Soil and Plant Analysis. [Google Scholar]
- Raunkiaer C. Clarendon Press; Oxford: 1937. The Life Forms of Plant and Statistical Plant Geography. [Google Scholar]
- Recio M.C., Giner R.M., Hermenegildo M., Peris J.B., Mañez S., Rios J.-L. Phenolics of Reichardia and their taxonomic implications. Biochem. Syst. Ecol. 1992;20:449–452. [Google Scholar]
- Rice E. 2nd ed. Academic Press; New York, USA: 1984. Allelopathy. [Google Scholar]
- Salazar G.J.T., de Sousa J.P., Lima C.N.F., Lemos I.C.S., da Silva A.R.P., de Freitas T.S., Coutinho H.D.M., da Silva L.E., do Amaral W., Deschamps C.J.I.C. Phytochemical characterization of the Baccharis dracunculifolia DC (Asteraceae) essential oil and antibacterial activity evaluation. Ind. Crops Products. 2018;122:591–595. [Google Scholar]
- Sampietro D.A., Catalan C.A., Vattuone M.A. Science Publishers; Enfield, NH, USA: 2009. Isolation Identification and Characterization of Allelochemicals/Natural Products. [Google Scholar]
- Shaltout K., El-Kady H., Al-Sodany Y. Vegetation analysis of the Mediterranean region of Nile Delta. Vegetatio. 1995;116:73–83. [Google Scholar]
- Shaltout K.H., Hosni H.A., El-Fahar R., Ahmed D.A. Flora and vegetation of the different habitats of the western Mediterranean region of Egypt. Taeckholmia. 2015;35:45–76. [Google Scholar]
- Sharma P., Prasad G., Archana S. A comprehensive review: Medicinal plants with potential antidiabetic activity. J. Herbal Sci. 2018;4:29–57. [Google Scholar]
- Shukla R.S., Chandel P.S. S. Chand & Company LTD; Ram Nagar, New Delhi, India: 1989. Plant Ecology and Soil Science. [Google Scholar]
- Tackholm V. 2nd ed. Cairo University Press; Cairo, Egypt: 1974. Students’ Flora of Egypt. [Google Scholar]
- Takabayashi J., Dicke M. Plant-carnivore mutualism through herbivore-induced carnivore attractants. Trends Plant Sci. 1996;1:109–113. [Google Scholar]
- Wardle D.A., Karban R., Callaway R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evolut. 2011;26:655–662. doi: 10.1016/j.tree.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B. It takes a garden. How work on diverse plant species has contributed to an understanding of flavonoid metabolism. J. Plant Physiol. 2001;127:1399–1404. [PMC free article] [PubMed] [Google Scholar]
- Yang L., Wen K.-S., Ruan X., Zhao Y.-X., Wei F., Wang Q. Response of plant secondary metabolites to environmental factors. Molecules. 2018;23:762. doi: 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Sternberg M., Kutiel P., Chen H. Seed mass, shape, and persistence in the soil seed bank of Israeli coastal sand dune flora. Evol. Ecol. Res. 2007;9:325–340. [Google Scholar]
- Zahran M., El-Demerdash M., Mashaly I. Vegetation types of the deltaic Mediterranean coast of Egypt and their environment. J. Veg. Sci. 1990;1:305–310. [Google Scholar]
- Zahran M.A., Willis A.J. 2nd ed. Springer Science & Business Media; Netherlands: 2009. The Vegetation of Egypt. [Google Scholar]