Abstract
Root Knot Nematode (RKN, Meloidogyne incognita) is one of the greatest damaging soil pathogens causes severe yield losses in cucumber and many other economic crops. Here, we evaluated the potential antagonistic effect of the root mutualistic fungus Piriformospora indica against RKN and their impact on vegetative growth, yield, photosynthesis, endogenous salicylic acid (SA) and its responsive genes. Our results showed that P. indica dramatically decreased the damage on shoot and root architecture of cucumber plants, which consequently enhanced yield of infested plants. Likewise, P. indica colonization clearly improved the chlorophyll content and delimited the negative impact of RNK on photosynthesis. Moreover, P. indica colonization exhibited a significant reduction of different vital nematological parameters such as soil larva density, amount of eggs/eggmass, eggmasses, females and amount of galls at cucumber roots. Additionally, the results showed that SA level was significantly increased generally in the roots of all treatments especially in plants infested with RKN alone as compared to control. This suggests that P. indica promoting SA levels in host cucumber plant roots to antagonize the RKN and alleviate severity damages occurred in its roots. This higher levels of SA in cucumber roots was consistent with the higher expressional levels of SA pathway genes PR1 and PR3. Furthermore, P. indica colonization reduces PR1, PR3 and increased NPR1 in roots of RKN infested cucumber plants when compared to non-colonized plants. Interestingly, our in vitro results showed that direct application of P. indica suspension against the J2s exhibited a significant increase in mortality ratio. Our results collectively suggest that P. indica promoting morphological, physiological and SA levels that might together play a major important role to alleviate the adverse impact of RKN in cucumber.
Keywords: Piriformospora indica, Root-knot nematode, Cucumber, Salicylic acid
1. Introduction
Cucumber (Cucumis sativus L.) is vital economical vegetal crops (Mukhtar et al., 2013). In addition it consider a typical plant for vascular biology and sex determination studies with a draft genome size 243.5 Mb (∼30% smaller than estimated genome size 367 Mb) (Huang et al., 2009). Root-knot nematodes (RKNs; Meloidogyne spp.) are endoparasites causing a dangerous hazard to agricultural sector. RKN includes more than 80 species, with a diverse devastating ability. Globally, root knot nematode is soil pathogens producing severe yield damages in numerous crops due to their ability to infest many of plant taxa (Abad and Williamson, 2010, Kayani et al., 2017). RKN species cause an estimated yearly damage of $157 billion worldwide (Deau et al., 2008). Moreover, its induce typically root deformations (galls), leaf chlorosis, defoliation, dwarfing, wilting and dropping profit of infested plants (Sasser et al., 1983). Control of phytoparasitic nematodes is a major global challenge for growers all over the world. Various agricultural practises and biological approaches against RKNs have been testified (Collange et al., 2011). One of tactics for managing RKN in vegetable crops is crop rotation (Osei et al., 2010). But due to the wide host range of RKN, efficacy of crop rotation as a phytoparasitic nematode management strategy was decreased (Chen and Tsay, 2006, Salem et al., 2019a). Therefore, selection of resistant varieties would be a standard approach to defend plants against RKN (Guan et al., 2012). To date, no commercial cucumber cultivars are found to resist plant roots against Meloidogyne spp. Moreover, negative impact of synthetic pesticides and chemicals inputs on human and environment restrict their application globally (Nicolopoulou-Stamati et al., 2016). However, some approaches exhibited to cope plants against RKN parasitism such as cover crops (Navarrete et al., 2016), grafting (Liu et al., 2015, Salem et al., 2019b), application of organic amendments (Abdel-Dayem et al., 2012, Abdeldaym et al., 2014) and plant extracts (Javed et al., 2007) as well as bio-control (Daneshkhah et al., 2013; Atia et al., 2016). All these strategies either alone or combined together are considered the most sustainable methods for controlling plant root knot nematode (Escobar et al., 2010, Escobar et al., 2015). However, controlling root knot nematodes is hard because its complicated life cycle and environmental threats of poisonous nematicides. During the last decade, a growing attention has paid to the application of plant growth-promoting fungi (PGPF) by way of a supportable and potential alternative to chemicals pesticides (Dutta and Thakur, 2017). Among antagonists fungi, the symbiotic mycorrhizal fungi has played an important role in the acquisition of nutrients from soils, stimulating plant growth, in addition to their ability to suppress the destructive effect of above and below-ground plant pathogens, resulting in an improvement of plant performance and their tolerance (Schmidt et al., 2011, Rai et al., 2014). Piriformospora indica is a plant-root-colonizing fungus belonging to division Basidiomycetes. It could be simply replicates in the absence of any host plant, in addition to its ability to full-grown at different compound and slight substrates, so its asexually stage forms chlamydospores (Varma et al. 1999). Its discovered in 1998 in the Indian Thar desert by Varma and his collaborators, P. indica has attracted great attention due to its growth and yield promoting ability, besides its potential to confer systemic resistance against many of biotic and abiotic stresses (Daneshkhah et al., 2013, Li et al., 2017, Xu et al., 2017, Abdelaziz et al., 2019). In addition to the direct beneficial interaction of P. indica with plant host, application of autoclaved culture filtrates (CF) containing fungal exudates as well as cell-wall extracts (CWE) were proved their powerful to promote plant growth (Vadassery et al., 2009). Furthermore, P. indica had resulted in an intense interest in the implementation of the fungus as biofertilizer and bioprotector (Venneman et al., 2017). Many studied stated the possibility of P. indica to improve plant resistance against powdery mildew (Qiang et al., 2012, Salem et al., 2018), Fusarium oxysporum, Verticillium dahlia and Pepino Mosaic Virus (Sarma et al., 2011, Fakhro et al., 2010, Sun et al., 2014). Therefore, the aim of the current study is to evaluate the potential antagonistic effect of P. indica against root knot nematode (Meloidogyne incognita) and their impact on greenhouse cucumber production. Additionally, to evaluate vegetative growth characteristics, photosynthesis analysis, salicylic hormone levels and some of innate responsive genes.
2. Materials and methods
2.1. Plant, nematode, fungus cultivation and inoculation
Cucumber Seeds (Cucumis melo cv. Hesham) superficial sterilized with 1.5% sodium hypochlorite up to ten min, washed 3 periods by H2O, and then immersed again in SDW for 3 h. After that, placed in hot SDW at 60 °C for 5 min to kill any endophytes inside the seeds, then left for air drying (Huang and Backhouse, 2005). To test the absence of any internal endophytes, the sterilized seeds were sown on potato dextrose agar (PDA) medium. Fifty sterile seeds were spread in sterilized soil mixture of peat moss: sand: vermiculite (1:1:1, v:v:v) at quadrangular elastic pots (7x7x8 cm) till germinate to be used as host for M. incognita. To prepare inoculum of P. indica, the fungus was cultured on PDA plates at 28 ± 5 °C and then incubated in PDA broth medium for half month in 29 °C speed 155 rpm on a shaker. Mycelium of grown fungus was collected, centrifuged and washed 3 times with SDW. Twenty grams of P. indica mycelium was mixed with 1000 ml SDW to produce 2% P. indica suspension. To prepare inoculum of nematode, Meloidogyne Incognita eggs in this test were extracted from infested pumpkin roots (Cucurbita pepo L.) according to Hussey and Barker (1973). The second-stage juveniles (J2s) from eggs were collected and stored at 15 °C till used. The number of J2s in 1 ml SDW was adjusted to 100 J2s/ml.
2.2. Greenhouse experiment
Pot experiment was carried out under greenhouse, controlled conditions at 28 ± 2°C for two successively seasons (2016/2017) at Faculty of Agriculture, Cairo University. The treatments were as follows: Control (CT; without nematode infestation and without P. indica inoculation), P. indica (PI; with P. indica inoculation only), Nematode (RKN; with nematode infestation only) and P. indica with nematode (RKN + PI; with P. indica inoculation and nematode infestation). For P. indica inoculation, 10 ml of 2% P. indica suspension was injected in the root zone for each plant at 3, 7 and 15 days in nursery before transplanting, whereas SD used as a fake test. Afterward, the cucumber seedlings were transplanted in formalin-sterilized polyethylene pots (20 cm) field with sandy soil and each pot was contained one plant. All treatments were distributed randomly and all trials were replicated 40 times. Afterward 14 days of transplantation, pots of both treatments (RKN and RKN + PI) were inoculated with the J2 at the rate of 1000 J2/pot. Plants were irrigated twice weekly and were fertilized once intervals with ½ Hoagland’s nutrition solution to sustain their growth. For nematological analysis, by the end of experiment, cucumber roots were uprooted, washed and number of galls per plant was assessed according to scale of Barker (1985). The number of egg masses isolated from cucumber roots was accounted under microscope (Hussey and Barker, 1973). The J2 population at the soil after each pot was determined by sampling 50 g of soil by means of Cobb’s sieving and transferring protocol, after the adapted Baermann funnel protocol (Southey, 1986). Then, total number of J2 per kilogram of soil was calculated.
2.3. Laboratory experiment
In vitro test was conducted twice to investigate the antagonistic activity of P. indica on mortality of M. incognita. Dual culture experiment was performed in Petri dishes inoculated with 10 ml of 2% P. indica solution plus 1000 J2s/ dish. Dishes inoculated with M. incognita or P. indica alone were served as controls. Then, all dishes were incubated at 28 ± 5 °C for 7 days and afterwards survived J2s were counted at 0, 3, 5 and 7 days. Additionally, parallel experiment was conducted twice to evaluate the influence of defense-phytohormone (SA) against larvae of Meloidogyne incognita. Dishes containing 1000 J2s/dish were inoculated with SA alone (500 µM of SA) or combined with 2% P. indica suspension. The number of mortality of J2s was counted at 1, 2, 3 and 7 days.
2.4. Vegetative growth and total yield assessment
Twenty-four plants of each treatment were selected randomly to determine cucumber plant growth and development parameters. Plant height, fresh and dry masses of treated cucumber plants were calculated after 30 days of nematode infestation. Leaf area meter (C1-202 Laser Areameter) was used to measure area of the 5th leaf from top. Fruits of each treatment were daily harvested and weighted till the end of the experiments to calculate the average total yield per meter-square.
2.5. Physiological analysis
2.5.1. Chlorophyll content, photosynthesis, transpiration rate and water use efficiency
After 35 days of RKN infestation, the 5th leaf of 24 plants from each treatment was selected for physiological analysis. Chlorophyll contented of was calculated by Chlorophyll meter (SPAD, 502 Minolta Co, Japan). Photosynthesis rate (μmol CO2 m−2 s−1), transpiration rate (mmol H2O m−2 s−1) and H2O usage efficacy (WUE; μmol CO2 mmol−1 H2O) were measured at mid-day (12:00 am − 14:00 pm) using an infrared gas analyzer LICOR 6400 (Lincoln, Nebraska, USA).
2.6. Endogenous level of salicylic phytohormone
Root of cucumber plants were gathered in liquid nitrogen and keept in freezer. Samples were then ground and 10 mg of powdered tissue was isolated with 9:1 (v/v) methanol–chloroform, then derivatized by BSTFA at 120 °C for 1 h, Forcat et al. (2008). Quantitative study was done by using GC–MS instrument in SIM mode using an inner standard for SA.
2.7. RNA isolation and quantitative PCR analysis
Thirty-five days post RKN infestation, leaves and roots were collected from three replicates in liquid nitrogen for each treatment. RNA was isolated with Trizol reagent and treated with DNase I (Cat Num.: EN0525, Thermo Scientific). The cDNA was synthesized using the SuperScript™ II Reverse Transcriptase as outline by manufacturer’s manual (Cat Num.: 18064014, Thermo Scientific). Gene-specific primers (NPR1, PR1 and PR3: for SA pathway; and Actin as a housekeeping gene were used (Table 1S). The qPCR examination was done using an Mx3000P QPCR System (Agilent Technologies). 15 µL final volume of the reaction. As outlined by (Beaubois et al., 2007). Three biological replicates (3 plants/treatment) were evaluated, and the mean and standard deviation values of statistics were measured.
2.8. Statistical analysis
All experiments and treatments were carried out in three replicates, each replicate consist of 24 plants for greenhouse experiment and 24 dishes for in vitro trial. Data of the two successive seasons (2016 and 2017) were exposed to analysis of variance (ANOVA) and means were linked by Duncan examination (p < 0.05) using the statistic 7 program (version 2004). The significance of differences among gene expressions data sets were assessed using paired student’s t-test based on three replicates.
3. Results
3.1. Effect of P. Indica colonization on vegetative performance
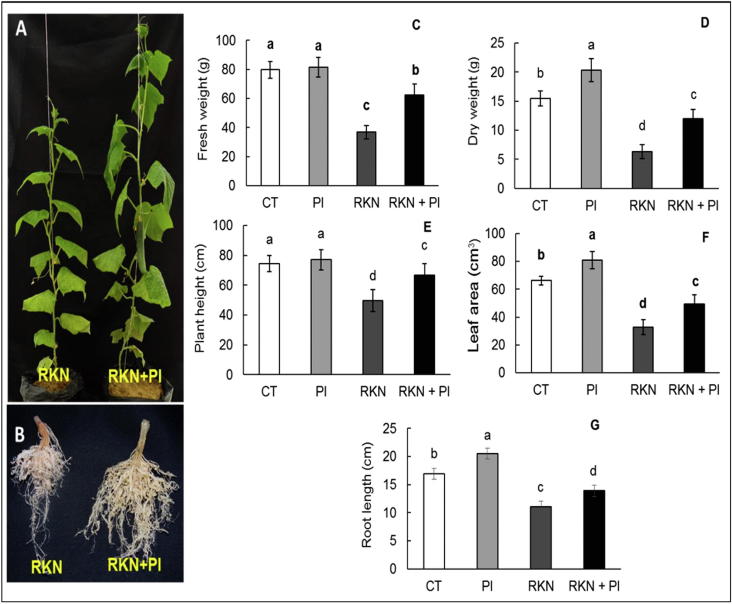
Generally, P. indica has a significant positive influence on shoot and root architecture of cucumber plants; which consequently enhance the vegetative growth through motivation to produce more roots and branches (Fig. 2a, b). Particularly, the RKN + PI plants (simultaneously inoculated with M. incognita and P. indica) were less affected by nematode infection and exhibit significant higher levels of leaf area, plant high, fresh and dry biomasses comparing to plants inoculated with M. incognita alone (RKN plants) (Fig. 1a,b). Obviously, we showed an important rise, i.e. 1.5-fold in plant height; 1.7-fold in both fresh weight and leaf area; and 1.9-fold in dry weight in cucumber plants simultaneously colonized with M. incognita and P. indica related to the plants injected with M. incognita alone (Fig. 2c, d, e). Furthermore, we observed non-significant increase in plant height (1.2-fold); fresh weight (1.0-fold) in cucumber plants colonized with P. indica alone (PI) related to the control plants (CT), (Fig. 2 c,e,f.). Meanwhile, we found significant increase in both dry weight (1.4-fold), leaf area (1.2-fold) in cucumber plants colonized with P. indica alone related to the control.
Fig. 2.
Effect of P. indica colonization and/or RKN infestation on cucumber architecture. Treatments were arranged as follow: control (CT), P. indica inoculation (PI), nematode infestation (RKN), and dual inoculation of P. indica and M. incognita (RKN + PI). (A) and (B) photos represent the difference in the vegetative growth and root architecture of cucumber plants under RKN infestation alone or in combination with P. indica colonization, respectively. Fresh weight (C), dry weight (D), plant height (E) leaf area of the 5th leaf (E), root length (G) were measured at 35 post RKN infestation. Columns with the similar letter are not significantly dissimilar rendering to Tukey test (p < 0.05).
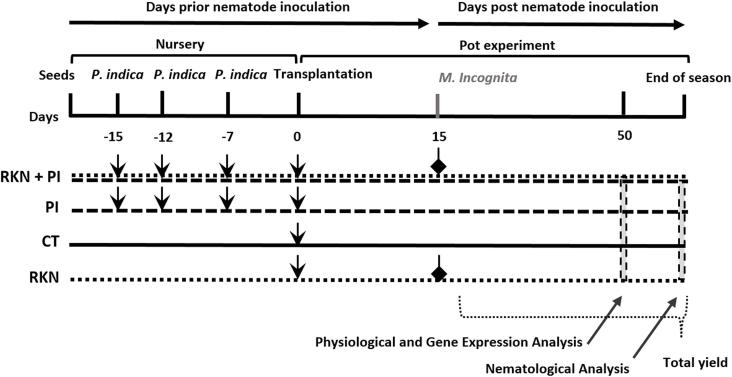
Fig. 1.
Scheme of the experimental set up. Horizontal lines characterise different plant treatments as follow: Control (CT), P. indica inoculation (PI), nematode infestation (RKN), and dual inoculation of P. indica and M. incognita (RKN + PI). Samples for physiological and gene expression analysis were collected at 35 days after inoculation with M. incognita, while nematological analysis were done at the end of experiments. Cucumber fruits were harvested daily and were weighted till the end of experiments to calculate the total yield per plant.
3.2. Effect of P. indica colonization on yield
The conducted experiment results revealed that RKN + PI plants produced relatively higher yield comparing to RKN plants suggesting that even the PI enhancing the plant resistance against the RKN infestation or that PI directly affecting the Root-knot nematodes viability and productivity; and both two suggestions will be examined in our study (Fig. 3). The fruits yield was found to increase by 38% (in PI vs CT treatment) and increase by 167% (in RKN + PI vs RKN treatment). This increase in total yield found to be positively correlated with the increase in all tested vegetative growth parameters (leaf area, plant high, root length, fresh and dry).
Fig. 3.
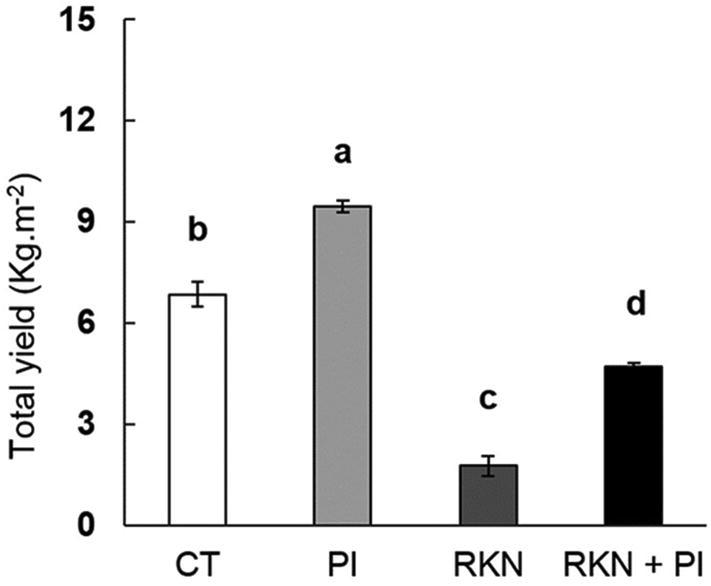
Effect of P. indica colonization and/or RKN infestation on cucumber fruit yield. Treatments were arranged as follow: control (CT), P. indica inoculation (PI), nematode infestation (RKN), and dual inoculation of P. indica and M. incognita (RKN + PI). Columns with the similar letter are not significantly dissimilar rendering to Tukey test (p < 0.05).
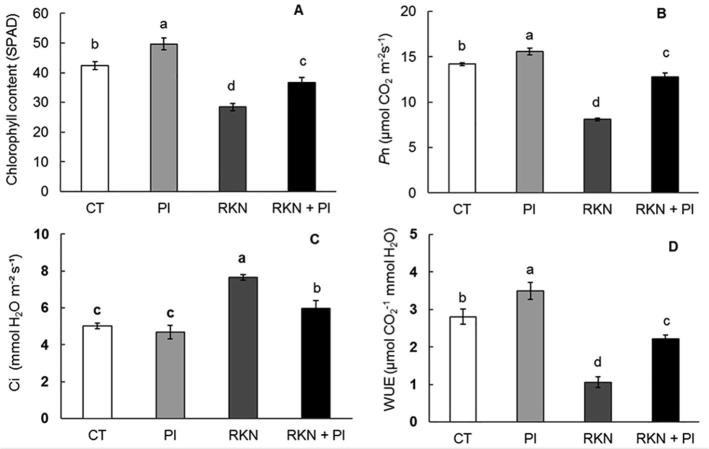
3.3. Effect of P. indica colonization on chlorophyll content and photosynthetic parameters
The effect of P. indica inoculation on chlorophyll content (SPAD), photosynthesis rate (Pn) and water use efficiency (WUE) as given in Fig. (4a, b, d) clearly indicated that, plants inoculated with P. indica alone (PI) significantly showed higher records comparing to control (CT). Meanwhile, plants infested with RKN alone (RKN) showed significant reduction in these parameters as compared to all other treatments. Fascinatingly the interaction effect on the simultaneously inoculated plants with both RKN and PI (RKN + PI) resulted in significant rising of chlorophyll content, photosynthesis rate and water use efficiency over plants infected with RKN alone (RKN).
On the other hand, there was no significant change among the inoculated plants with PI alone (PI) and un-inoculated (CT) with respect to the transpiration rate (Ci). While the plants infested with RKN alone (RKN) showed significant rising in their transpiration rate parameters as compared to other treatments. Interestingly, the plants simultaneously inoculated with both RKN and PI (RKN + PI) revealed a clear reduction in their transpiration rate compared to plants infested with RKN alone (RKN) (Fig. 4c).
Fig. 4.
Effect of P. indica colonization and/or RKN infestation on (A) estimated chlorophyll content (SPAD), (B) photosynthesis rate (Pn), (C) transpiration rate (Ci) and (D) water use effectiveness (WUE) at 35 days post RKN infestation. Treatments were arranged as follow: control (CT), P. indica inoculation (PI), nematode infestation (RKN), and dual inoculation of P. indica and M. incognita (RKN + PI). Columns with the similar letter are not pointedly dissimilar according to Tukey test (p < 0.05).
3.4. Effect of P. Indica colonization on defense-phytohormone (SA)
Plant-microbe interactions are commonly correlated with alterations in levels of phytohormones. In order to investigate whether the P. indica affects the level of defense-phytohormones, the amounts of SA was measured across all treatments including control. Interestingly, our results demonstrated that the defence-phytohormone SA is significantly up-regulated in plant roots inoculated with P. indica alone (PI) comparing to control (CT) plants. As a response of cucumber plants infested with RKN alone, we showed a significant rise, i.e. 24-fold in SA hormone level as compared to control. On the other side, as a result of colonization with PI of the cucumber plants infested with RKN, we clearly observed that PI colonization successfully decreased level of SA hormone (−7)-fold in roots as compared to plants infested only with RKN (Fig. 5).
Fig. 5.
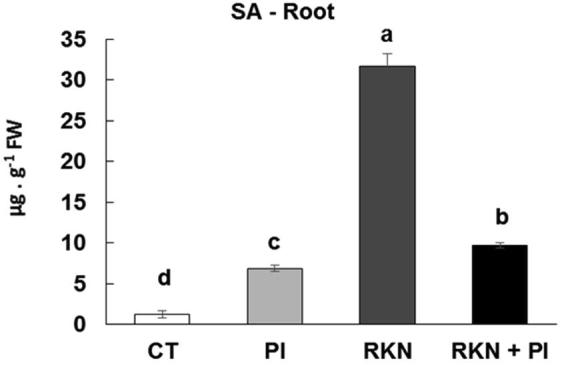
Effect of P. indica colonization and/or RKN infestation on salicylic acid content (SA) in roots of cucumber; control (CT), P. indica inoculation (PI), nematode infestation (RKN), and dual inoculation of P. indica and M. incognita (RKN + PI). Columns with the similar letter are not significantly different according to Tukey test (p < 0.05).
3.5. Effect of P. indica colonization and SA on M. incognita eggs and J2
In vitro experiments, the obtained results showed that the direct application of P. indica, and SA separately or combined together significantly increased the mortality ratio of J2. After 7 days of exposure period, the highest mortality ratio in J2 population density was achieved by mixing P. indica suspension with salicylic acid (75.8%), followed by P. indica alone (69.3%), and then by salicylic acid alone (48.3%), as compared with the control (1.6%) at 7 days (Fig. 6). On the other hand, the obtained results from in vivo experiment indicated that soil larva density, developmental stages, no. of eggs/eggmass, no. of eggmasses, no. of females in root and no. of galls were significantly decreased in RKN + PI vs. RKN treatment (Fig. 7).
Fig. 6.
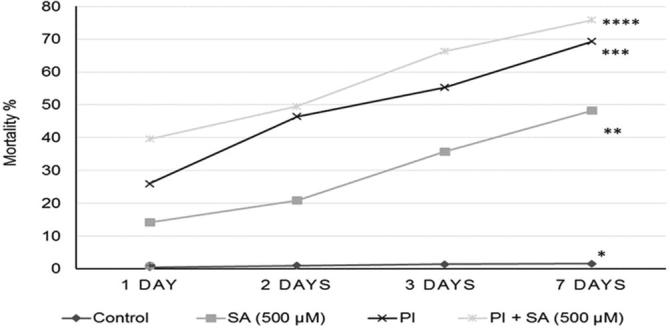
| An in vitro experiment shows the effect of direct applications of P. indica suspension (PI) and salicylic acid (SA) on mortality percentage of J2. * No. of stars indicate the degree of significance as related to control at p < 0.001(***), p < 0.01(**) and p < 0.05 (*) according to Tukey test.
Fig. 7.
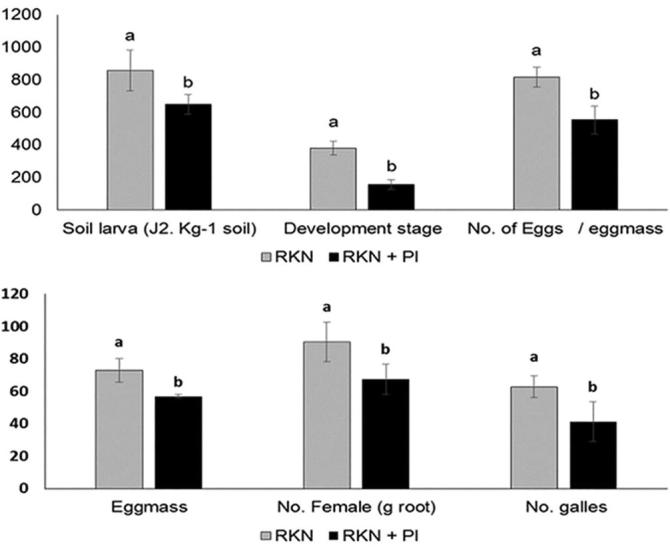
Effect of P. indica colonization on soil larva density, developmental stage, no. of eggs/egg mass, no. of egg masses, no. of females and no. of galls. RKN = plants infested by nematode alone; RKN + PI = plants inoculated by both P. indica and RKN. Columns followed by the same letter are not pointedly dissimilar as to Tukey test (p < 0.05).
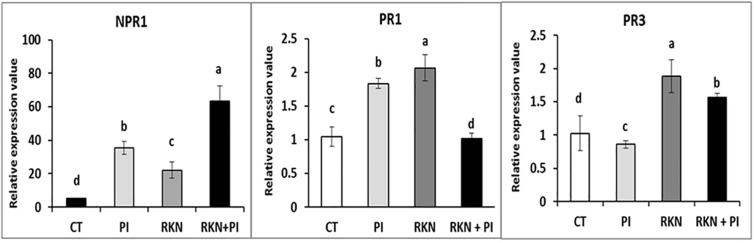
3.6. Effect of P. indica colonization on expression levels of SA related genes
The effect of P. indica inoculation on expression levels of NPR1, PR1, PR3 genes in roots clearly indicated that PI showed significant higher records (i.e. 7-fold for NPR1 and 0.7-fold for PR1) comparing to control (CT). While, a slight reduction in the expression level was observed in PR3 gene. Meanwhile, plants infested with RKN alone (RKN) showed a importantsignificant rise in the expression levels of both PR1 and PR3 as compared to other treatments. On the contrary, the NPR1 expression level was significantly down-regulated as compared to all other treatments except the CT. Interestingly, the interaction effect on the simultaneously inoculated plants with both RKN and PI (RKN + PI) resulted in significant rising of NPR1 expression over plants infected with RKN alone (i.e. 2.5-fold). Conversely, the effect of simultaneous inoculation with both RKN and PI resulted in significant mitigation of PR1 and PR3 expression levels when compared to plants infected with RKN alone (i.e. −1-fold and −0.2-fold, respectively) (Fig. 8).
Fig. 8.
Relative gene expression of NPR1, PR1 and PR3 genes in roots of the P. indica colonized and non-colonized cucumber plants at 35 days post RKN infestation. Significance of differences, P < 0.001 (**), between means was evaluated by Student’s t-test, ± SE (n = 4), based on three independent experiments.
4. Discussion
Global concerns about the overusing of synthetic pesticides have given a great attention to looking for new environmentally-friendly nematicides. The utilization of useful antagonistic micro-organisms as mycorrhizal fungi, rhizobacteria and endophytic bacteria against phytoparasitic nematodes has been considered as a promising alternative to the globally available toxic nematicides (Le et al., 2009, Vos et al., 2012). In this regard, a powerful biocontrol fungus; nonpathogenic P. indica was described to positively promote and improve the response of different biotic and abiotic stresses in many crops. Therefore, this study was conducted to investigate the antagonistic power of P. indica against Meloidogyne incognita and their powerful impact on cucumber agro-physiological parameters as well as some molecular innate responsive genes. Overall results clearly showed a suppressive effect of P. indica on RKN infestation as well as enhancing the host plant agro-physiological characteristics through modulation of different host responsive genes. Regarding the vegetative growth characteristics, in a completely agree with previous reports, our results confirmed that the beneficial root endophyte P. indica significantly enhancing plant height (i.e. 1.2-fold), leaf area (i.e. 1.6-fold), root length (i.e. 1.3-fold), fresh and dry weight (i.e. 1.9-fold and 2.6-fold, respectively) in dual infested plants (RKN + PI) as compared to RKN plants. This growth promotion not only might be attributed to enhancement in water absorption, nutrients uptakes, and physiological processes but also to the modulation of phytohormones as well as alleviation of RKN infestation (Fig. 2 and Fig. 4). The similar findings were observed by (Achatz et al., 2010, Ansari et al., 2013, Bajaj et al., 2015, Xu et al., 2018). Furthermore, infested plants with RKN alone appeared in lowest fruit yield and plant growth (Fig. 3). This possibly is due to high population density of RKN attack root of cucumber plants and impaired of nutrient uptake and water absorption (Melakeberhan et al., 1987, Bartlem et al., 2014, Strajnar et al., 2012). Contrary, it has been found that P. indica colonization enhanced cucumber yield by increasing both number and weight of fruits generally as similar findings by Fakhro et al. (2010). On the other hand, RKN infestation clearly reduced chlorophyllous pigment (total; SPAD) and impaired the different photosynthetic parameters (photosynthesis rate, and water use efficiency). Conversely, the P. indica colonization improved the chlorophyll content (in case of control) and delimited the negative impact of RNK on photosynthetic parameters (in case of RKN + PI). Alike remarks have been documented previously on injection with P. indica (Arora et al., 2016, Arora et al., 2018). Interestingly, the P. indica also alleviated the sever effect of RKN on transpiration rate and chlorophyll contented might caused in rise biomass accumulation in the tested black pepper plants (Anith et al., 2018). In this respect also, it had reported that phytoparasitic nematodes induced creation of reactive oxygen species (ROS) that could be decompose the photosynthetic pigment (i.e. chlorophyll) as well as impairment of photosynthetic equipment, decrease of electron transport, carbon fixation capacity and photophosphorylation (Gillet et al., 2017). Interestingly, the reduction in value of photosynthesis rate of RKN plants was also strongly correlated leaf chlorophyll concentration, which consequently limits the gas exchange as many previous reports described (Loveys and Bird, 1973, Bowden and Rouse, 1991, Abouseadaa et al., 2015). On the other side, a significant reduction in nematological parameters includes, soil larva density, developmental stage, no. of eggs/eggmass, no. of eggmasses, no. of females in root and no. of galls in duel colonized plants (RNK + PI) as compared to plants colonized with RNK alone. The mechanism of control RKN using P. indica is still unclear enough. Few studies reported that phytoparasitic nematode reduction induced by P. indica colonization could be attributed to physical barriers formed by its hyphae reduced J2 mobility or syncytium expansion. Likewise, it might be also attributed to the release of fungus nematicidal compounds mixture that significantly reduced egg hatching and J2 mortality (Daneshkhah et al., 2013). Numerous reports have elucidated that fungal endophytes might change chemical possessions of root exudates or may inspire plants to yield chemicals or hormones which repel or disturb nematode attraction (Le et al., 2009). Likewise, repellent impact of root exudates of tomato plants tested with beneficial endophytic Fusarium oxysporum against M. incognita was described (Dababat and Sikora, 2007). Interestingly, the in vitro experiment showed that the direct application of salicylic acid against the J2s of M. incognita exhibited a significant increase in mortality ratio, which might be explain 70% of the P. indica effect to antagonize the root knot nematode. However, biotic stress defense hormones are considered a part of complex network of synergistic and antagonistic interactions (Fraire-Velázquez et al., 2011). Salicylic is a key player in the stimulation of dissimilar signing paths in resistance feed back in plants, when they encounter nematode infections (Ali et al., 2018). It improves the host immune response, compromises the plant defense system whereas and reported to enhance a set of pathogenesis-related defense genes against the phytoparasitic nematode in tomato (Branch et al., 2004) and the suppression of host resistance SA was connected with the development of nematode in tomato (De Medeiros et al., 2017). Under greenhouse conditions, our obtained results showed that the trend of SA significantly increased in the roots of all treatments as compared to control. These results suggest that P. indica promoting SA production inside the host cucumber plant post inoculation. However, the maximum level of SA was observed in plants infested with RKN alone, due to sever damages occurred in its roots. These high levels of damage in roots may stimulate plants to produce more SA as a defense mechanism to reduce population of nematode (Molinari et al., 2014). On contrast, a significant reduction in SA level was observed in dual inoculated plants (RKN + PI) as compared to plants infested with RKN alone. This reduction might indicate the significant effect of P. indica to mitigate the M. incognita population density and consequently injures severity due to RKN + PI interaction. Moreover, other studies confirmed that P. indica stimulated the production of salicylic acid that greatly decreased the phytoparasitic nematodes (Heterodera schachtii) development in plant roots (Daneshkhah et al., 2013).
Indeed, SA is a controller of plant resistance to biotrophic and hemibiotrophic pathogens. Molinari and Baser (2010), also confirmed that SA application delayed root knot nematode development and impaired egg masses production. Similarly, inducing systemic resistance against M. incognita (Kofoid & White) Chitwood, by SA addition caused a significant reduction in the reproductive parameters of the root nematodes in root (Mostafanezhad et al., 2014). These results agree with other previous investigations (Molinaria and Offredo, 2006, Mukherjee et al., 2012, Pu et al., 2014, Szitenberg et al., 2017, Tomalova et al., 2012).
In fact, one of the most distinctive defense gene dependent SA-signaling involved in plant immunity pathway is NPR1. So, this gene is necessary for the activation of pathogenesis-related PR genes such as PR1-a in cucumber during infection plants against nematodes in term of leading to hypersensitive response (HR) and systemic acquired resistance (SAR) in plants against biotrophic pathogens (Sahebani et al., 2011). Our result revealed that P. indica stimulated expression of defense genes related to salicylic acid in cucumber plants, in agreement with an earlier reports in which a genes of SA was strongly elevated in roots of nematode-attacked (Wu et al., 2012, Molinari et al., 2014, Hajipour et al., 2015). However, the level of SA reduced in (RKN + PI) plants, but the level was not reached to neither control (CT) nor plant treated with P. indica alone (PI). Many studies clearly reported that SA is an essential regulator of plant immunity as a plan to persuade the good resistance responses (Vlot et al., 2009, Thaler et al., 2012). Our study indicated that higher levels of expression of SA genes putatively linked to improvement of stress responses as induced by both M. incognita and P. indica in cucumber plants.
Acknowledgments
Acknowledgement
Authors like to thank the administration of Agricultural Genetic Engineering Research Institute (AGERI) - ARC, as well as Faculty of Agriculture – Cairo University for their continued support. Also, would like to thank Sabra Ali for providing P. indica fungus and its related valuable microbiological comments.
Author Contributions
MEA, MAMA, EAA, GO and MTA designed the experimental. MEA and EAA carried out the greenhouse experiments and agronomical analysis. DSI, IS and EAA performed the nematological analysis. MA and IA performed the physiological analysis. MAMA and MTA conduct the genes expression work. MAMA and EAA wrote the manuscript. MEA coordinated the project.
Funding
The author(s) received no specific funding for this work.
Declaration of Competing Interest
None declared.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2019.09.007.
Contributor Information
Mohamed A.M. Atia, Email: matia@ageri.sci.eg.
Emad A. Abdeldaym, Email: emad.abdeldaym@agr.cu.edu.eg.
Mohamed Abdelsattar, Email: mteima@ageri.sci.eg.
Dina S.S. Ibrahim, Email: mecky@gmail.com.
Mohamed Abd Elwahab, Email: mohamed.mahmoud@agr.cu.edu.eg.
Gamal H. Osman, Email: geosman@uqu.edu.sa.
Ibrahim A. Arif, Email: iaarif@ksu.edu.sa.
Mohamed E. Abdelaziz, Email: mohamed.ewis@agr.cu.edu.eg.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Abad P., Williamson V.M. Plant nematode interaction, a sophisticated dialogue. Adv. Bot. Res. 2010;53:147–192. [Google Scholar]
- Abdelaziz M.E., Abdelsattar M., Abdeldaym E.A., Atia M.A., Mahmoud A.W.M., Saad M.M., Hirt H. Piriformospora indica alters Na+/K+ homeostasis, antioxidant enzymes and LeNHX1 expression of greenhouse tomato grown under salt stress. Sci. Hortic. 2019;256 [Google Scholar]
- Abdel-Dayem E.A., Erriquens F., Verrastro V., Sasanelli N., Mondelli D., Cocozza C. Nematicidal and fertilizing effects of chicken manure, fresh and com-posted olive mill wastes on organic melon. Helminthologia. 2012;49:259–269. [Google Scholar]
- Abdeldaym E.A., Erriquens F., Sasanelli N., Ceglie F.G., Zaccone C., Miano T., Cocozza C. Effects of several amendments on organic melon growth and production, Meloidogyne incognita population and soil properties. Sci. Hort. 2014;180:156–160. [Google Scholar]
- Abouseadaa H.H., Osman G.H., Ramadan A.M., Hassanein S.E., Abdelsattar M.T., Morsy Y.B., Alameldin H.F., El-Ghareeb D.K., Nour-Eldin H.A., Salem R., Gad A.A., Elkhodary S.E., Shehata M.M., Mahfouz H.M., Eissa H.F., Bahieldin Development of transgenic wheat (Triticum aestivum L.) expressing avidin gene conferring resistance to stored product insects. BMC Plant Biol. 2015;15:183–190. doi: 10.1186/s12870-015-0570-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achatz B., Ruden S., Andrade D., Neumann E., PonsKuhnemann J., Kogel K.H., Franken P., Waller F. Root colonization by Piriformospora indica enhances grain yield in barley under diverse nutrient regimes by accelerating plant development. Plant Soil. 2010;333:59–70. [Google Scholar]
- Ali M., Anjam M., Nawaz M., Lam H.M., Chung G. Signal transduction in plant-nematode interactions. Int. J. Mol. Sci. 2018;19:1648. doi: 10.3390/ijms19061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anith K.N., Aswini S., Varkey S., Radhakrishnan N.V., Nair D.S. Root colonization by the endophytic fungus Piriformospora indica improves growth, yield and piperine content in black pepper (Piper nigurm L.) Biocatal. Agric. Biotechnol. 2018;14:215–220. [Google Scholar]
- Ansari M.W., Bains G., Shukla A., Pant R.C., Tuteja N. A critical review on fungi mediated plant responses with special emphasis to Piriformospora indica on improved production and protection of crops. Plant Physiol. Biochem. 2013;70:403–410. doi: 10.1016/j.plaphy.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Arora M., Saxena P., Abdin M.Z., Varma A. Interaction between Piriformospora indica and Azotobacter chroococcum governs better plant physiological and biochemical parameters in Artemisia annua L. plants grown under in vitro conditions. Symbiosis. 2018;75:103–112. [Google Scholar]
- Arora M., Saxena P., Choudhary D.K., Abdin M.Z., Varma A. Dual symbiosis between Piriformospora indica and Azotobacter chroococcum enhances the artemisinin content in Artemisia annua L. World J. Microbiol. Biotechnol. 2016;32:19. doi: 10.1007/s11274-015-1972-5. [DOI] [PubMed] [Google Scholar]
- Atia M.A., Osman G.H., Elmenofy W.H. Genome-wide in silico analysis, characterization and identification of microsatellites in Spodoptera littoralis multiple nucleopolyhedrovirus (SpliMNPV) Sci. Rep. 2016;6:33741. doi: 10.1038/srep33741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj R., Hu W., Huang Y., Chen S., Prasad R., Varma A., Bushley K.E. The beneficial root endophyte Piriformospora indica reduces egg density of the soybean cyst nematode. Biol. Control. 2015;90:193–199. [Google Scholar]
- Barker K.R. Nematode extraction and bioassays. In: Barker K.R., Sasser J.N., Carter C.C., editors. Vol. II. North Carolina State University Graphics; Raleigh, NC: 1985. pp. 19–35. (An advanced treatise on Meloidogyne, Methodology). [Google Scholar]
- Bartlem D.G., Jones M.G.K., Hammes U.Z. Vascularization and nutrient delivery at root-knot nematode feeding sites in host roots. J. Exp. Bot. 2014;65:1789–1798. doi: 10.1093/jxb/ert415. [DOI] [PubMed] [Google Scholar]
- Beaubois E., Girard S., Lallechere S., Davies E., Paladian F., Bonnet P., Ledoigt G., Vian A. Intercellular communication in plants: evidence for two rapidly transmitted systemic signals generated in response to electromagnetic field stimulation in tomato. Plant Cell Environ. 2007;30:834–844. doi: 10.1111/j.1365-3040.2007.01669.x. [DOI] [PubMed] [Google Scholar]
- Bowden R.L., Rouse D.I. Chronology of gas exchange effects and growth effects of infection by Verticillium dahliae in potato. Phytopathology. 1991;81:301–310. [Google Scholar]
- Branch C., Hwang C.F., Navarre D.A., Williamson V.M. Salicylic acid is part of the Mi-1-mediated defense responses to root-knot nematode in tomato. Mol. Plant Microbe. Interact. 2004;17:351–356. doi: 10.1094/MPMI.2004.17.4.351. [DOI] [PubMed] [Google Scholar]
- Chen P., Tsay T. Effect of crop rotation on Meloidogyne spp. and Pratylenchus spp. populations in strawberry fields in Taiwan. J. Nematol. 2006;38:339–344. [PMC free article] [PubMed] [Google Scholar]
- Collange B., Navarrete M., Peyre G., Mateille T., Tchamitchian M. Root-knot nematode (Meloidogyne) management in vegetable crop 2 production: the challenge of an agronomic system analysis. Crop. prot. 2011;30:1251–1262. [Google Scholar]
- Dababat A.A., Sikora R.A. Influence of Fusarium oxysporum 162, a non-pathogenic fungus, induced systemic resistance toward Meloidogyne incognita on tomato. Nematology. 2007;9:771–776. [Google Scholar]
- Daneshkhah R., Cabello S., Rozanska E., Sobczak M., Grundler F., Wieczorek K., Hofmann J. Piriformospora indica antagonizes cyst nematode infection and development in Arabidopsis roots. J. Exp. Bot. 2013;64:3763–3774. doi: 10.1093/jxb/ert213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Medeiros H.A., de Araújo Filho J.V., De Freitas L.G., Castillo P., Rubio M.B., Hermosa R., Monte E. Tomato progeny inherit resistance to the nematode Meloidogyne javanica linked to plant growth induced by the biocontrol fungus Trichoderma atroviride. Sci. Rep. 2017;7:40216. doi: 10.1038/srep40216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deau F., Esquibet M., Flutre T., Goldstone J.V., Hamamouch N., Hewezi T., Jaillon O., Jubin C., Leonetti P., Magliano M., Maier T.R., Markov G.V., McVeigh P., Pesole G., Poulain J., Robinson-Rechavi M., Sallet E., Segurens B., Steinbach D., Tytgat T., Ugarte E., van Ghelder C., Veronico P., Baum T.J., Blaxter M., Bleve-Zacheo T., Davis E.L., Ewbank J.J., Favery B., Grenier E., Henrissat B., Jones J.T., Laudet V., Maule A.G., Quesneville H., Rosso M.N., Schiex T., Smant G., Weissenbach J., Wincke P. Genome sequence of the metazoan plant parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008;26:909–915. doi: 10.1038/nbt.1482. [DOI] [PubMed] [Google Scholar]
- Dutta J., Thakur D. Evaluation of multifarious plant growth promoting traits, antagonistic potential and phylogenetic affiliation of rhizobacteria associated with commercial tea plants grown in Darjeeling. PloS one. 2017 doi: 10.1371/journal.pone.0182302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar C., Barcala M., Cabrera J., Fenoll C. Overview of Root-knot nematodes and giant cells. In: Escobar C., Fenoll C., editors. Vol. 73. Elsevier Academic Press; Oxford: 2015. p. 132. (Adv. Bot. Res.). [Google Scholar]
- Escobar C., García A., Aristizábal F., Portillo M., Herreros E., Muñoz-Martín M.A., Grundler F., Mullineaux P.M., Fenoll C. Activation of geminivirus V-sense promoters in roots is restricted to nematode feeding sites. Mol. Plant Pathol. 2010;11:409–417. doi: 10.1111/j.1364-3703.2010.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhro A., Andrade-Linares D.R., von Bargen S., Bandte M., Buttner C., Grosch R., Schwarz D., Franken P. Impact of Piriformospora indica on tomato growth and on interaction with fungal and viral pathogens. Mycorrhiza. 2010;20:191–200. doi: 10.1007/s00572-009-0279-5. [DOI] [PubMed] [Google Scholar]
- Forcat S., Bennett M.H., Mansfield J.W., Grant M.R. A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress. Plant Methods. 2008;4:16. doi: 10.1186/1746-4811-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraire-Velázquez S., Rodríguez-Guerra R., Sánchez-Calderón L. Abiotic Stress Response in Plants-Physiological, Biochemical and Genetic Perspectives. In Tech; 2011. Abiotic and biotic stress response crosstalk in plants. [Google Scholar]
- Gillet F., Bournaud C., Junior J., Grossi-de-Sa M.F. Plant-parasitic nematodes: towards understanding molecular players in stress responses. Ann Bot. 2017;119:775–789. doi: 10.1093/aob/mcw260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Zhao X., Hassell R., Thies J. Defense mechanisms involved in disease resistance of grafted vegetables. Hort Sci. 2012;47:164–170. [Google Scholar]
- Hajipour A., Sohani M., Babaeizad V., Hassani-Kumleh H. The symbiotic effect of Piriformospora indica on induced resistance against bakanae disease in rice (Oryza sativa L.) J. Plant Mol. Breeding (JPMB) 2015;3:11–19. [Google Scholar]
- Huang S., Li R., Zhang Z., Li L., Gu X., Fan W., Lucas W.J., Wang X., Xie B., Ni P., Ren Y., Zhu H., Li J., Lin K., Jin W., Fei Z., Li G., Staub J., Kilian A., van der Vossen E.A.G., Wu Y., Guo J., He J., Jia Z., Ren Y., Tian G., Lu Y., Ruan J., Qian W., Wang M., Quanfei H., Li B., Xuan Z., Cao J., Asan Wu Z., Zhang J., Cai Q., Bai Y., Zhao B., Han Y., Li Y., Li X., Wang S., Shi Q., Liu S., Cho W., Kim J., Xu Y., Heller-Uszynska K., Miao H., Cheng Z., Zhang S., Wu J., Yang Y., Kang H., Li M., Liang H., Ren X., Shi Z., Wen M., Jian M., Yang H., Zhang G., Yang Z., Chen R., Liu S., Li J., Ma L., Liu H., Zhou Y., Zhao J., Fang X., Li G., Fang L., Li Y., Liu D., Zheng H., Zhang Y., Qin N., Li Z., Yang G., Yang S., Bolund L., Kristiansen K., Zheng H., Li S., Zhang X., Yang H., Wang J., Sun R., Zhang B., Jiang S., Wang J., Du Y., Li S. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 2009;41:1275–1281. doi: 10.1038/ng.475. [DOI] [PubMed] [Google Scholar]
- Hussey R.S., Barker K.R. Comparison of methods for collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Report. 1973;57:1025–1028. [Google Scholar]
- Javed N., Gowen S.R., Inam-ul-Haq M., Anwar S.A. Protective and curative effect of neem (Azadirachta indica) formulations on the development of root-knot nematode Meloidogyne javanica in roots of tomato plants. Crop Prot. 2007;26:530–534. [Google Scholar]
- Kayani M.Z., Mukhtar T., Hussain M.A. Effects of southern root knot nematode population densities and plant age on growth and yield parameters of cucumber. Crop Prot. 2017;92:207–212. [Google Scholar]
- Le T.H., Padgham L.J., Sikora R.A. Biological control of the rice root-knot nematode Meloidogyne graminicola on rice, using endophytic and rhizosphere fungi. Int. J. Pest Manage. 2009;55:31–36. [Google Scholar]
- Li L., Li L., Wang X., Zhu P., Wu H., Qi S. Plant growth-promoting endophyte Piriformospora indicaalleviates salinity stress in Medicago truncatula. Plant Physiol. Biochem. 2017;119:211–223. doi: 10.1016/j.plaphy.2017.08.029. [DOI] [PubMed] [Google Scholar]
- Liu B., Ren J., Zhang Y., An J., Chen M., Chen H., Xu C., Ren H. A new grafted rootstock against root-knot nematode for cucumber, melon, and watermelon. Agron. Sustain. Dev. 2015;35:251–259. [Google Scholar]
- Loveys B.R., Bird A.F. The influence of nematodes on photosynthesis in tomato plants. Physiol. Plant Pathol. 1973;3:525–529. [Google Scholar]
- Melakeberhan H., Webster J.M., Brooke R.C., D’auria J.M., Cackette M. Effect of Meloidogyne incognita on Plant Nutrient Concentration and Its Influence on the Physiology of Beans. J. Nematol. 1987;19:324–330. [PMC free article] [PubMed] [Google Scholar]
- Molinari S., Baser N. Induction of resistance to root-knot nematodes by SAR elicitors in tomato. Crop Prot. 2010;29:1354–1362. [Google Scholar]
- Molinari S., Fanelli E., Leonetti P. Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Mol. Plant Pathol. 2014;152:55–64. doi: 10.1111/mpp.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinaria A., Offredo E. The role of salicylic acid in defense response of tomato to root-knot nematodes. Physiol. Mol. Plant Pathol. 2006;68:69–78. [Google Scholar]
- Mostafanezhad H., Sahebani N., Zarghani S.N. Induction of resistance in tomato against root-knot nematode Meloidogyne javanica with salicylic acid. Crop Prot. 2014;3:499–508. [Google Scholar]
- Mukherjee A., Babu S.P.S., Mandal F.B. Potential of salicylic acid activity derived from stress-induced (water) Tomato against Meloidogyne incognita. Arch Phytopathol. Plant Protect. 2012;45:1909–1916. [Google Scholar]
- Mukhtar T., Kayani M.Z., Hussain M.A. Response of selected cucumber cultivars to Meloidogyne incognita. Crop Prot. 2013;44:13–17. [Google Scholar]
- Navarrete M., Djian-Caporalino C., Mateille T., Palloix A., Sage-Palloix A.M., Lefèvre A., Fazari A., Marteu N., Tavoillot J., Dufils A., Furnion C., Pares L., Forest I. A resistant pepper used as a trap cover crop in vegetable production strongly decreases root-knot nematode infestation in soil. Agron. Sustain. Dev. 2016;36:68. [Google Scholar]
- Nicolopoulou-Stamati P., Maipas S., Kotampasi C., Stamatis P., Hens L. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front Public Health. 2016;4:148. doi: 10.3389/fpubh.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei K., Gowen S.R., Pembroke B., Brandenburg R.L., Jordan D.L. Potential of leguminous cover crops in management of a mixed population of root-knot nematodes (Meloidogyne spp.) J. Nematol. 2010;42:173–178. [PMC free article] [PubMed] [Google Scholar]
- Pu X., Bingyan X., Peiqian L., Zhenchuan M., Jian L., Huifang S., Jingxin Z., Ning H., Birun L. Analysis of the defence-related mechanism in cucumber seedlings in relation to root colonization by nonpathogenic Fusarium oxysporum CS-20. Microbiol. Lett. 2014;355:142–151. doi: 10.1111/1574-6968.12461. [DOI] [PubMed] [Google Scholar]
- Qiang X., Zechmann B., Reitz M.U., Kogel K.H., Schäfer P. The mutualistic fungus Piriformospora indica colonizes Arabidopsis roots by inducing an endoplasmic reticulum stress-triggered caspase-dependent cell death. Plant Cell. 2012;24:794–809. doi: 10.1105/tpc.111.093260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai J., Pemmasani J.K., Voronovsky A., Jensen I.S., Manavalan A., Nyengaard J.R., Golas M.M., Sander B. Strep-tag II and Twin-Strep based cassettes for protein tagging by homologous recombination and characterization of endogenous macromolecular assemblies in Saccharomyces cerevisiae. Mol. Biotechnol. 2014;56:992–1003. doi: 10.1007/s12033-014-9778-5. [DOI] [PubMed] [Google Scholar]
- Sahebani N., Hadavi N.S., Zade F.O. The effects of b-aminobutyric acid on resistance of cucumber against root-knot nematode, Meloidogyne javanica. Acta Physiol. Plant. 2011;33:443–450. [Google Scholar]
- Salem R., El-Kholy A.A., Omar Abu el-naga O.A.M.N., Ibrahim M., Osman G. Construction, Expression and Evaluation of Recombinant VP2 Protein for serotype-independent Detection of FMDV Seropositive Animals in Egypt. Sci. Rep. 2019;9:10135. doi: 10.1038/s41598-019-46596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem R., El-Kholy A.A., Ibrahim M. Eight novel single chain antibody fragments recognising VP2 of foot-and-mouth disease virus serotypes A, O, and SAT 2. Virology. 2019;533:145–154. doi: 10.1016/j.virol.2019.05.012. [DOI] [PubMed] [Google Scholar]
- Salem R., Arif A.I., Salama M., Osman G.E.H. Polyclonal antibodies against the recombinantly expressed coat protein of the Citrus psorosis virus. Saudi J. Biol. Sci. 2018;25:733–738. doi: 10.1016/j.sjbs.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma M.V.R.K., Kumar V., Saharan K., Srivastava R., Sharma A.K., Prakash A., Sahai V., Bisaria V.S. Application of inorganic carrier-based formulations of fluorescent pseudomonads and Piriformospora indica on tomato plants and evaluation of their efficacy. J. Appl. Microbiol. 2011;111:456–466. doi: 10.1111/j.1365-2672.2011.05062.x. [DOI] [PubMed] [Google Scholar]
- Sasser J.N., Eisenbach J.N., Carter C.C. The international Meloidogyne project – its goals and accomplishments. Ann. Rev. Phytopathol. 1983;21:271–288. [Google Scholar]
- Schmidt B., Gaşpar S., Camen D., Ciobanu I., Sumălan R. Arbuscular mycorrhizal fungi in terms of symbiosis-parasitism continuum. Commun. Agric. Appl. Biol. Sci. 2011;76:653–659. [PubMed] [Google Scholar]
- Southey J.F. Ministry of Agriculture; London: 1986. Laboratory methods for work with plant and soil nematodes; p. 402p. [Google Scholar]
- Strajnar P., Širca S., Urek G., Šircelj H., Železnik P., Vodnik D. Effect of Meloidogyne ethiopica parasitism on water management and physiological stress in tomato. Eur. J. Plant Pathol. 2012;132:49–57. [Google Scholar]
- Sun C., Shao Y., Vahabi K., Lu J., Bhattacharya S., Dong S., Yeh Sherameti K.I., Lou B., Baldwin I., Oelmüller R. The beneficial fungus Piriformospora indica protects Arabidopsis from Verticillium dahliae infection by downregulation plant defense responses. BMC Plant Biol. 2014;14:268. doi: 10.1186/s12870-014-0268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szitenberg A., Salazar-Jaramillo L., Blok V.C., Laetsch D.R., Joseph S., Williamson V.M., Blaxter M.L., Lunt D.H. Comparative genomics of apomictic root-knot nematodes: hybridization, ploidy, and dynamic genome change. Genome Biol. Evol. 2017;9:2844–2861. doi: 10.1093/gbe/evx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler J.S., Humphrey P.T., Whiteman N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012;17:260–270. doi: 10.1016/j.tplants.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Tomalova I., Iachia C., Mulet K., Castagnone-Sereno P. The map-1 gene family in root-knot nematodes, Meloidogyne spp.: a set of taxonomically restricted genes specific to clonal species. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0038656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadassery J., Ranf S., Drzewiecki C., Mithofer A., Mazars C., Scheel D., Lee J., Oelmuuller R. A cell wall extract from the endophytic fungus Piriformospora indica promotes growth of Arabidopsis seedlings and induces intracellular calcium elevation in roots. Plant J. 2009;59:193–206. doi: 10.1111/j.1365-313X.2009.03867.x. [DOI] [PubMed] [Google Scholar]
- Varma A., Verma S., Sahay N.S., But¨ehorn B., Franken P. Piriformospora indica, a cultivable plant growth promoting root endophyte. Appl. Environ. Microbiol. 1999;65:2741–2744. doi: 10.1128/aem.65.6.2741-2744.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman J., Audenaert K., Verwaeren J., Baert G., Boeckx P., Moango A.M. Congolese rhizospheric soils as a rich source of new plant growth-promoting endophytic Piriformospora isolates. Front. Microbiol. 2017;8:212. doi: 10.3389/fmicb.2017.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot A.C., Dempsey D.M.A., Klessig D.F. Salicylic acid, a multifaceted hormone to combat disease. Ann. Rev. Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- Vos C.M., Tesfahun A.N., Panis B., De Waele D., Elsen A. Arbuscular mycorrhizal fungi induce systemic resistance in tomato against the sedentary nematode Meloidogyne incognita and the migratory nematode Pratylenchus penetrans. Appl. Soil Ecol. 2012;61:1–6. [Google Scholar]
- Wu Y., Zhang D., Chu J.Y., Boyle P., Wang Y., Brindle I.D., De Luca V., Després C. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 2012;1:639–647. doi: 10.1016/j.celrep.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Xu L., Wang A., Wang J., Wei Q., Zhang W. Piriformospora indica confers drought tolerance on Zea mays L. through enhancement of antioxidant activity and expression of drought-related genes. Crop J. 2017;5:251–258. [Google Scholar]
- Xu L., Wu C., Oelmüller R., Zhang W. Role of phytohormones in Piriformospora indica-induced growth promotion and stress tolerance in plants: more questions than answers. Front. Microbiol. 2018;9:1646. doi: 10.3389/fmicb.2018.01646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.