Abstract
Injectable Platelet rich fibrin (i-PRF) is a platelet concentrate that has been extensively used for multiple medical purposes and is a valuable adjunct for the regeneration of damaged tissues in surgical procedures. The enriched bioactive substances in i-PRF are responsible for speeding the wound healing process. Infection of biofilm producing bacteria in surgical wounds is becoming a serious threat. Research in this field is focused on new strategies to fight infections and to reduce the healing time. The present study was aimed to evaluate the in vitro antimicrobial and antibiofilm effects of i-PRF against oral pathogenic biofilm producing staphylococcus bacteria isolated from patient with dental and oral abscess. The antibacterial activity of i-PRF, was determined through broth microdilution as minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC). i-PRF exhibited bactericidal activity against both non biofilm and biofilm producing bacteria. i-PRF could be potential antimicrobial peptide used to combat postoperative infections caused by biofilm producing staphylococcus.
Keywords: Injectable platelet rich fibrin, Oral pathogen, Antibiofilm, Second generation platelet concentrates, Antimicrobial peptides
1. Introduction
The topical uses of Platelets Rich Fibrin (PRF) have achieved great popularity in various fields of medicine, especially in dentistry, oral maxillofacial surgery, cosmetics and plastic surgery (Massimo et al., 2016). PRF is a second-generation platelet concentrate comprising of complex network of micro fibrins with entrapped platelets and leucocytes. The reason for their application is that enriched platelets and leucocytes release intracellular growth factors, bioactive molecules and bioactive peptides that enhance both hard and soft tissues healing process (Anitua et al., 2004). In addition to this, the physiological architecture of the micro fibrin also favors wound healing (Choukroun et al., 2000). In addition, leucocytes are essential elements of immune system that contain variety of antimicrobial peptides and enzymes. The stimulated leucocytes degranulate and discharge their contents into the phagosomes, thereby killing ingested microorganisms through oxidative and non-oxidative reactions (Levy, 2000). Platelets secondary granules deployed towards the leading edge of neutrophil chemotaxis via distinct antimicrobial proteins and peptides (Agata et al., 2018). In recent years, couple of research studies have explored on regenerative potential of PRF, but only few studies have investigated their antimicrobial effects.
Staphylococcus infections at surgical sites remain one of the widespread postoperative complications (Denis et al., 2002). The staphylococci attached to the wound surface proliferate and produce a biofilm. The biofilm is an extra cellular polymeric matrix produced by bacteria that enable the survival of bacterial network in hostile environment and colonize through dispersal system to form new niches (Mai-Prochnow et al., 2008). Gene expression pattern of biofilm cells differ from their planktonic counterparts (Percival et al., 2015). Biofilms have significantly upregulated genes, resulting in excessive development of degrading enzymes, production of quorum sensing molecules, enhanced microbial proliferation, dissemination and phenotypic protection against antibiotics and antimicrobials (Hurlowet al., 2015, Gilbertet al., 2002). It is recently recognized that biofilm producing bacterial infection is prevalent in postoperative wound and also a causative factor in wound chronicity (Bjarnsholt et al., 2008). In such cases, Immune system of host is less effective against biofilm matrix and these are also more tolerant to antiseptics (Wolcott et al., 2010). The cumulative effect of compromised host defences, tolerance of biofilm to antibiotics, and unresolved tissue damage results in high risk of postoperative surgical wound infections (Phillips and Schultz, 2012). Such infections in surgical sites of oral cavity, face and neck region increase the risk of death by two fold (Deniset al., 2002, Patanwalaet al., 2007). I-PRF is one of the recently introduced platelet concentrate which is available in an injectable form. The enriched level of antimicrobial contents in i-PRF was recently explored (Kour et al., 2018). Hence our study focused on in-vitro antibacterial and antibiofilm activity of i-PRF against pathogenic oral staphylococcus isolates.
2. Materials and methods
2.1. Blood collection and i-PRF preparation
Blood sample was collected from healthy donors. Before enrolment, verbal and written information about the study was communicated to the subjects and signed consent forms were received from them. Patients who had taken antimicrobials or anti-inflammatory medications in the past three months or suffering from other systemic disorders, smokers, and pregnant women were excluded from the study. In addition, subjects with hemoglobin concentration <12 g/dl and platelet count ≤150 × 103/µl were excluded from the study. Blood was collected in test tubes (5 ml) without anticoagulants and i-PRF was prepared centrifuging the blood sample at 1000 rpm for 5 min at 37 °C (Miron et al., 2017a, Miron et al., 2017b). The upper separated liquid portion was collected as i-PRF and analyzed immediately (before gelation).
2.2. Bacterial strains
The bacterial strains of Staphylococcus aureus and Staphylococcus epidermis were isolated from patients with oral and dental abscess. The isolated pathogenic strains were identified using a biochemical kit (HiMedia, Mumbai, India). Further, bacterial strains were identified by 16 s rRNA sequencing (Genbank accession numbers: MK054200, MK054201). Biofilm produced by the isolates at 24 h were assessed using 96-well polystyrene microtiter plates. Overnight culture of each isolate (20 µl) on tryptone soya broth (TSB) (HiMedia, Mumbai, India) along with fresh 230 µl of fresh TSB was inoculated on 96 -well and incubated at 37 °C for 24 h. After incubation, planktonic cells were removed by washing with PBS buffer (pH 7.4) and stained with 0.2% crystal violet The absorbance was then read in a microplate reader at 600 nm. S.epidermis ATCC 35,984 (biofilm forming strain) and S.epidermis ATCC 12,228 (biofilm negative strain) were used as positive and negative controls respectively.
2.3. Minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC)
The anti-bacterial activity was determined as MIC and MBC using the broth microdilution method. The bacterial strains have been grown in TSB overnight. The overnight cultures washed twice with 10 mM Tris HCl (containing 5 mM glucose, pH 7.4) and then were diluted 1:100 and the final inoculum size adjusted to 1 × 104 cfu/ml. A 96 well microtiter plate containing 10, 20, 40, 60, 80, 100, 120 and 140 μl/well of i-PRF innoculated with 10μl of the inoculum. To prevent peptide aggregation and release of platelet contents 0.01% acetic acid was added along with fresh TSB medium. Positive control maintained with Chloramphenicol (30 µg/ml) and negative control maintained with growth medium alone and all the plates were incubated at 37 °C for 24 h. After incubation, no visible growth considered as MIC and then sub cultured on Muller Hinton agar (MHA – Himedia, Mumbai, India) for each strain and no visible growth on MHA was recorded as MBC. The assay was conducted in triplicates.
2.4. Live/Dead bacterial assay (LD)
LD bacterial assay was performed for bacterial cells by double fluorescent staining that consists of acridine orange (AO) and ethidium bromide (EB). The stain was prepared by mixing 200 µl of AOEB from the stock solution (stock solution contained equal quantity of AO and EB dissolved in 10 ml of PBS). The determined MIC and MBC levels of i-PRF were treated with bacterial cells. The cells were harvested, after the treatment by centrifugation at 5000 rpm for 5 min. The pellets were washed twice with PBS and cells were suspended in 300 µl of fluorescent dye and incubated for 15 min. The sample was placed on a glass slide with coverslip, after removing the unbounded dyes by rinsing with PBS. The presence of Live and dead cells were observed under an inverted fluorescent microscope (ZEISS, Germany).
2.5. Biofilm inhibitory assay
Biofilm inhibitory effects were evaluated by semi-quantitative plate method described by Rajapandiyan et al. (2018), with few modifications. Briefly, overnight bacterial cultures were prepared in TSB medium at 37 °C. Inoculum with an OD600- 0.7 (2 × 103 CFU ml−1) density was incubated in 96 well-polystyrene plates with a fresh TSB medium at 37 °C for 6 h in static conditions. Then, the planktonic cells were removed with PBS (pH 7.4) and i-PRF was added to each well along with 0.01% acetic acid and fresh TSB medium and the plates were further incubated for 24 h at 37 °C. After incubation, the non-adherent cells were removed by washing the wells with PBS and adherent cells in the biofilm were fixed by adding 200 μl of 100% methanol before staining with crystal violet for 20 min. Excess stains were washed with PBS twice and the plates were air-dried. The crystal violet bounded in the biofilm of air dried plates was eluted by 200 µl of 33% acetic acid. The quantity of biofilm was determined by measuring the absorbance in a microplate reader at OD600 nm (i-mark, Bio-Rad, Japan).
2.6. Visualization of biofilm inhibition by confocal laser scanning microscopy (CLSM)
To visualize the biofilm inhibitory activity, we followed the same procedure as described in biofilm inhibition assay section 2.5. For this experiment, cell culture dishes (Hi-Media, Mumbai, India) were employed instead of polystyrene 96-well plates. The treated culture dishes were incubated at 37 °C for 24 h. Biofilms were stained using AOEB after removing the non-adherent cells by PBS wash and were subsequently analyzed with a ZEISS fluorescence microscope (ZEISS, Germany). A series of images was obtained to measure the biofilm thickness in microns. ZEISS ZEN lite software has been used to create a 2D view of the formed biofilms. Six optical fields were selected and observed randomly for each specimen and the thickness of the biofilm was calculated and reported as mean with standard deviation.
3. Results
3.1. Biofilm formation assay
In the current study, biofilm formations of oral isolates were determined using microtiter plate assay with S. epidermis ATCC 35,984 as a positive control strain which gave a OD570 0.46 ± 0.04 (weak biofilm formation) and non-biofilm producing S. epidermis ATCC 12,228 as a negative control strain which gave an OD570 0.19 ± 0.01. The isolated strains were classified into moderate biofilm producing S. epidermis (OD570 0.65 ± 0.01) and strong biofilm producing S. aureus (OD570 1.06 ± 0.09).
3.2. Determination of antimicrobial activity
The i-PRF was able to inhibit the growth of non-biofilm producing bacteria at the concentration of 80 µl/ml (MIC) and at the concentration of 160 µl/ml, recorded as MBC, no growth was found on MHA. Weak, moderate and strong biofilm producing oral pathogenic strains exhibited no visible growth at the concentration of 160 µl/ml (MIC) and there is no growth found at the concentration of 240 µl/ml (MBC) and the values are summarized in Table 1.
Table 1.
MIC and MBC values of i-PRF against Staphylococcal isolates.
| S.NO | Name of the organism | MIC (µl/ml) | MBC (µl/ml) |
|---|---|---|---|
| 1 | S. aureus | 160 | 240 |
| 2 | S. epidermis | 160 | 240 |
| 3 | S. epidermis ATCC 35984 | 160 | 240 |
| 4 | S. epidermis ATCC 12228 | 80 | 160 |
3.3. Inhibition of biofilm formation
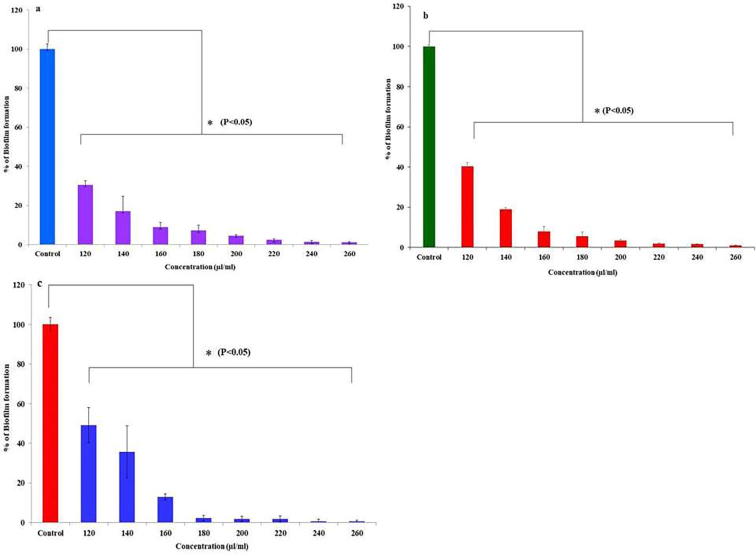
I-PRF actively inhibited the biofilm formations of tested bacterial strains at 24 h. Dose dependent activities were observed against all biofilm producers. A significant reduction (P < 0.05) in percentage of biofilm formation were observed at MIC against weak biofilm producer strain S. epidermis (30.4 ± 2.17%, Fig. 1a), moderate biofilm producer strain S. epidermis (36.2 ± 2.52%, Fig. 1b) and strong biofilm producer strain S. aureus (49.1 ± 8.79%, Fig. 1c). However, at 240 µl/ml concentration, all biofilm producers were unable to produce the biofilm; ATCC S. epidermis showed biofilm formation of 9.8 ± 2.65%, S. epidermis showed 7.16 ± 1.86% and S. aureus 12.8 ± 1.45%.
Fig. 1.
Antibiofilm activity of i-PRF, a. Weak biofilm producer, b. moderate biofilm producer, c. Strong biofilm producer.
3.4. Live/dead bacterial staining
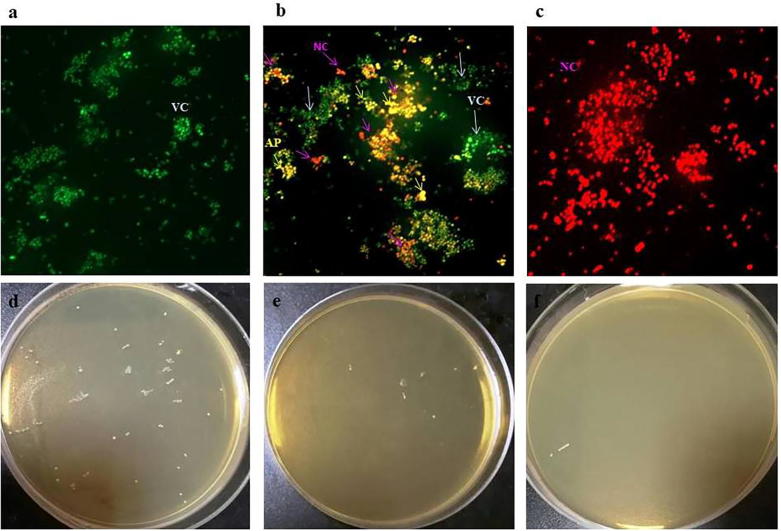
The action of i-PRF on bacterial cells was visualized by Live/Dead (L/D) bacterial staining assay. Fig. 2a shows that in the untreated control, 100% viable cells appear green in color. The cells treated with i-PRF at MIC exhibit >50% dead cells which appear pale red and yellow color. Yellow color indicates the last stage of apoptosis and necrotic/dead cells appears dark red in color (Fig. 2b). At MBC cells exhibit 100% necrosis (Fig. 2c) and subculture on MHA plates confirms the reduction on colony formation (Fig. 2 d, e & f) compared to control, due to bactericidal action of i-PRF.
Fig. 2.
Fluorescence microscopic image of AOEB Live/Dead staining assay of i-PRF, (a) Untreated cells (100% viable cells), (b) Cells treated at MIC, (c) Cells treated at MBC, S. aureus colony formation on MHA - (d) untreated control (e) i-PRF treated at 80 µl/ml, (d) i-PRF treated at 160 µl/ml. VC – viable cells (green color), AP – Last stage of apoptosis (yellow color), NC - Necrosis or dead cells (red color).
3.5. Confocal laser scanning microscopic analysis
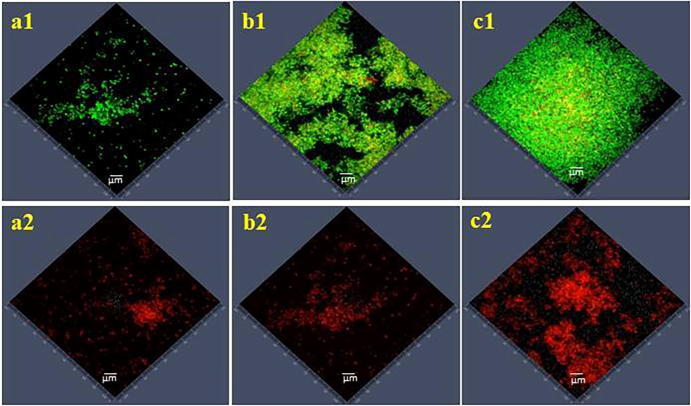
Biofilm formation of staphylococcus isolates and biofilm inhibition potential of i-PRF were analyzed by confocal laser scanning microscopy (CLSM). The standard strain S. epidermis produced weak biofilm ranging from 2 to 4 ± 0.75 µm thickness (Fig. 3.a1). The isolated strains of S. epidermis produced moderate biofilm ranging from 5 to 7 ± 0.83 µm thickness (Fig. 3.b1) and S. aureus produced strong, dense biofilm with 15–18 ± 1.16 µm thickness (Fig. 3.c1). The inhibitory activity of i-PRF against biofilm formation was measured. At a concentration of 240 µl/ml, notable reduction of biofilm was observed; 3 ± 0.92 µm for weak biofilm producers (Fig. 3.a2), 5 ± 0.75 µm for moderate biofilm producer (Fig. 3.b2) and 13 ± 0.53 µm for strong biofilm producer (Fig. 3.c2).
Fig. 3.
Confocal laser scanning microscopic analysis of biofilm inhibition by i-PRF, weak biofilm producer (a1) Untreated & (a2) Treated, moderate biofilm producer (b1) Untreated & (b2) Treated, strong biofilm producer (c1) Untreated & (c2) Treated.
4. Discussion
The increasing prevalence of postoperative staphylococcus infection is a complication that prolongs hospital stay and affects the quality of life (Barasch et al., 2008). Notably, biofilm matrix in staphylococci infection at surgical sites is a source of phenotypic resistance and their ability to impaired host immunity that often results in antibiotic treatment failure (Liduma et al., 2012). Treatment of such infections depends on the bacterial growth rate, quantity of biofilm density and affinity of antibiotics bindings to the biofilm matrix. It is important to eliminate such infections and risk factors associated with it as early as possible. In this context, we analyzed in vitro antimicrobial and antibiofilm activity of i-PRF against oral pathogenic staphylococcus isolates. In the past few decades, the use of platelet concentrates has been explored and several types have been developed including i-PRF. I-PRF, an injectable form of PRF (Ghanaati et al., 2014), contains several factors such as antimicrobial proteins, complement binding proteins and antimicrobial peptides (Dragoet al., 2013, Levy, 2000, Blair and Flaumenhaft, 2009, Tohidnezhadet al., 2012), In the present study, i-PRF exhibited wide spectrum of activity against weak, moderate and strong biofilm producing staphylococcus strains. Notably, there was significant reduction of biofilm formation by all oral biofilm producers in the presence of i-PRF. In order to assure the reliability of the experiment, we used standard ATCC biofilm and non-biofilm producing S. epidermis strains. Interestingly, in the case of non-biofilm producing bacteria a greater antimicrobial effect was found at lower concentration of i-PRF. According to Paharik and Horswill, (2016), biofilm phenotype allows sessile bacteria to resist antimicrobial agents due to upregulation of large number of genes which is not observed in its planktonic phenotypes. The wide range of inhibitory and bactericidal activity of i-PRF is due to its composition of platelets, fibrin, fibronectin, thrombin, HBD-3 peptide (antimicrobial peptide) myeloperoxidase, and inclusion of white blood cells (Radek and Gallo, 2007, Moojenet al., 2008, Blair and Flaumenhaft, 2009). Tohidnezhad et al. (2012), identified platelet concentrates to be active against E.coli and P. mirabilis owing to the presence of these molecules. The wide spectrum of antimicrobial and antibiofilm activity of i-PRF is probably related to permeability proteins, lactoferin, defensins, heparin binding protein, cathelicidines, and phospholipase A2. These molecules interfere with metabolic activity of bacterial cells, which leads to apoptosis and necrotic stages and were observed as yellow and red colored cells in L/D staining (Fig. 2b and c) (Anituaet al., 2012, Intraviaet al., 2014). Therefore, oral biofilm producing S. epidermis and S. aureus were unable to form biofilms (Cieslik Bielecka et al., 2008). The finding of our study highlights the promising role of i-PRF as antibacterial and antibiofilm agent. Further, investigation is necessary to analyze the mechanism of broad spectrum bactericidal activity in more detail.
5. Conclusion
It can be concluded that i-PRF can be easily prepared during surgery, and it possesses, bactericidal and antibiofilm activity. This could act as an antimicrobial peptide and potential bioactive agent to prevent post-operative infections at surgical sites. Further research is necessary to evaluate the broad-spectrum antimicrobial properties of i-PRF in-depth using an in vivo model.
Acknowledgement
The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support. The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for supporting the work through college of food and Agriculture Science Research Centre.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agata C.B., Paweł R., Rafał S., Aleksandra K., Tomasz B. A new aspect of in vitro antimicrobial leukocyte- and platelet-rich plasma activity based on flow cytometry assessment. Platelets. 2018:1–9. doi: 10.1080/09537104.2018.1513472. 10. 1080/09537104.2018.1513472. [DOI] [PubMed] [Google Scholar]
- Barasch A., Safford M.M., Litaker M.S., Gilbert G.H. Risk factors for oral postoperative infection in patients with diabetes. Spec Care Dentist. 2008;28(4):159–166. doi: 10.1111/j.1754-4505.2008.00035.x. [DOI] [PubMed] [Google Scholar]
- Anitua E., Andia I., Ardanza B., Nurden P., Nurden A.T. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost Stuttgart. 2004;91(1):4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- Anitua E., Alonso R., Girbau C., Aguirre J.J., Murozabal F., Orive G. Antibacterial effect of plasma rich in growth factors (PRGFEndoret) against Staphylococcus aureus and Staphylococcus epidermidis strains. Clin Exp Dermatol. 2012;37:652–657. doi: 10.1111/j.1365-2230.2011.04303.x. [DOI] [PubMed] [Google Scholar]
- Bjarnsholt T., Kirketerp-Møller K., Jensen P.Ø., Madsen K.G., Phipps R., Krogfelt K., Høiby N., Givskov M. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 2008;16:2–10. doi: 10.1111/j.1524-475X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Blair P., Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choukroun J., Adda F., Schoeffer C., Vervelle A. PRF: An opportunity in perio-implantology. Implantodontie. 2000;42:55–62. [Google Scholar]
- Cieslik-Bielecka A., Bielecki T., Gazdzik T.S., Cieslik T., Szczepanski T. Improved treatment of mandibular odontogenic cysts with platelet- rich gel. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(4):423–429. doi: 10.1016/j.tripleo.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Denis O., Nonhoff C., Byl B., Knoop C., Bobin-Dubreux S., Struelens M.J. Emergence of vancomycin-intermediate Staphylococcus aureus in Belgian hospital: microbiological and clinical features. JAC. 2002;50:383–391. doi: 10.1093/jac/dkf142. [DOI] [PubMed] [Google Scholar]
- Ghanaati S., Booms P., Orlowska A., Kubesch A., Lorenz J., Rutkowski J., Landes C., Sader R., Kirkpatrick C.J., Choukroun J. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40:679–689. doi: 10.1563/aaid-joi-D-14-00138. [DOI] [PubMed] [Google Scholar]
- Gilbert P., Allison D.G., McBain A.J. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J. Appl. Microb. 2002;92:98–110. [PubMed] [Google Scholar]
- Hurlow J., Couch K., Laforet K., Bolton L., Metcalf D., Bowler P. Clinical biofilms: a challenging frontier in wound care. Adv Wound Care (New Rochelle) 2015;4(5):295–301. doi: 10.1089/wound.2014.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intravia J., Allen D.A., Durant T.J., McCarthy M.B., Russell R., Beitzel K., Cote M.P., Dias F., Mazzocca A.D. In vitro evaluation of the antibacterial effect of two preparations of platelet rich plasma compared with cefazolin and whole blood. Muscles Ligaments Tendons J. 2014;4:79–84. [PMC free article] [PubMed] [Google Scholar]
- Levy O. Antimicrobial proteins and peptides of blood: templates for novel antimicrobial agents. Blood. 2000;96:2664–2672. [PubMed] [Google Scholar]
- Liduma I., Tracevska T., Bers U., Zilevica A. Phenotypic and genetic analysis of biofilm formation by Staphylococcus epidermidis. Medicina (Kaunas) 2012;48:305–309. [PubMed] [Google Scholar]
- Drago L., Bortolin M., Vassena C., Taschieri S., Del Fabbro M. Antimicrobial activity of pure platelet rich plasma against microorganisms isolated from oral cavity. BMC Microbiol. 2013;13:47. doi: 10.1186/1471-2180-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai-Prochnow A., Lucas Elio P., Egan S., Thomas T., Webb S.J., Sanchez-Amat A., Kjelleberg S. Hydrogen peroxide linked to lysine oxidase activity facilitates biofilm differentiation and dispersal in several gram-negative bacteria. J. Bact. 2008;190:5493–5501. doi: 10.1128/JB.00549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimo D.F., Monica B., Silvio T., Caterina C., Roberto L., Weinstein R.L. Antimicrobial properties of platelet-rich preparations. A systematic review of the current pre-clinical evidence. Platelets. 2016;27(4):276–285. doi: 10.3109/09537104.2015.1116686. [DOI] [PubMed] [Google Scholar]
- Miron R.J., Fujioka-Kobayashi M., Hernandez M., Kandalam U., Zhang Y., Ghanaati S. Injectable platelet rich fibrin (i-PRF): Opportunities in regenerative dentistry. Clin Oral Investig. 2017;21:2619–2627. doi: 10.1007/s00784-017-2063-9. [DOI] [PubMed] [Google Scholar]
- Moojen D.J., Everts P.A., Schure R.M., Overdevest E.P., van Zundert A., Knape J.T., Castelein R.M., Cremers L.B., Dhert W.J. Antimicrobial activity of plateletleukocyte gel against Staphylococcus aureus. J Orthop Res. 2008;26:404–410. doi: 10.1002/jor.20519. [DOI] [PubMed] [Google Scholar]
- Paharik A.E., Horswill A.R. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol Spectr. 2016;4:0022–2015. doi: 10.1128/microbiolspec.VMBF-0022-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanwala A.E., Erstad B.L., Nix D.E. Costeffectiveness of linezolid and vancomycin In the treatment of surgical site infections. Curr. Med. Res. Opin. 2007;23:185–193. doi: 10.1185/030079906X162700. [DOI] [PubMed] [Google Scholar]
- Percival S.L., McCarty S.M., Lipsky B. Biofilms and wounds: an overview of the evidence. Adv Wound Care (New Rochelle). 2015;4(7):373–381. doi: 10.1089/wound.2014.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P.L., Schultz G.S. Molecular mechanisms of biofilm infection: biofilm virulence factors. Adv Wound Care (New Rochelle). 2012;1:109–114. doi: 10.1089/wound.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radek K., Gallo R. Antimicrobial peptides: natural effectors of the innate immune system. Semin Immunopathol. 2007;29(1):27–43. doi: 10.1007/s00281-007-0064-5. [DOI] [PubMed] [Google Scholar]
- Rajapandiyan K., Jegan A., Periasamy V.S., Abdulraheem R.A., Al-Shuniaber Mohammed A., Gassem Mustafa A., Ali A., Alshatwi A.A. Antimicrobial activity of nanoemulsion on drug-resistant bacterial pathogens. Microb Pathogen. 2018;120:85–96. doi: 10.1016/j.micpath.2018.04.035. [DOI] [PubMed] [Google Scholar]
- Miron R.J., Masako F.K., Maria H., Umadevi K., Yufeng Z., Shahram G., Choukroun J. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry. Clin Oral Invest. 2017;21(8):2619–2627. doi: 10.1007/s00784-017-2063-9. [DOI] [PubMed] [Google Scholar]
- Kour P., Pudakalkatti P.S., Vas A.M., Das S., Padmanabhan S. Comparative Evaluation of Antimicrobial Efficacy of Platelet-rich Plasma, Platelet-rich Fibrin, and Injectable Platelet-rich Fibrin on the Standard Strains of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Contemp Clin Dent. 2018;9:325–330. doi: 10.4103/ccd.ccd_367_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohidnezhad M., Varoga D., Wruck C.J., Podschun R., Sachweh B.H., Bornemann J., Bovi M., Sönmez T.T., Slowik A., Houben A., Seekamp A., Brandenburg L.O., Pufe T., Lippross S. Platelets display potent antimicrobial activity and release human beta-defensin 2. Platelet. 2012;23:217–223. doi: 10.3109/09537104.2011.610908. [DOI] [PubMed] [Google Scholar]
- Wolcott R.D., Rumbaugh K.P., James G. Biofilm maturity studies indicate sharp debridement opens a time-dependent therapeutic window. J Wound Care. 2010;19:320–328. doi: 10.12968/jowc.2010.19.8.77709. [DOI] [PubMed] [Google Scholar]