Abstract
In the current study; insecticidal, growth regulation, oviposition deterrence and repellency of petroleum ether extracts of Azadirachta indica, Penganum harmala, Datura stramonium, Tribulus terrestris and Chenopodium murale against 2nd instar larvae of housefly was investigated. Five different concentrations (5%, 10%, 15%, 20% and 25%) were used through larval feeding and the mortality data was recorded after 24, 48 and 72 hrs. Highest mortality was induced by P. harmala (63.87%) followed by D. stramonium (62.78%), A. indica (53.84%), T. terrestris (41.86%) and C. murale (4.09%) after 72 h at 25% concentration, respectively. Increased mortality was observed with increased time duration and concentration. Longest larval duration (9.33 ± 0.33 days) and pupal duration (7.33 ± 0.33 days) days) was recorded in larvae treated with 25% concentration of P. harmala which also caused a decrease in the activity of AChE, ACP, AKP, α-Carboxyl, and β-Carboxyl enzymes. However, at 25% concentration, C. murale showed highest oviposition deterrence activity (81.88%) followed by D. stramonium (79.58%). In comet assay test, at highest concentration (25%) the mean comet tail lengths represented by Penganum harmala, Datura stramonium and Azadirachta indica (Reference plant) were 10.20 ± 0.49, 9.20 ± 0.37 and 7.80 ± 0.49 μm while percent DNA damage was 10.56 ± 0.77, 10.67 ± 1.62 and 8.11 ± 0.85% respectively compared to controls cells. Phytochemical analysis indicated the presence of flavonoids, steroids, saponins, cardiac glycosides, tannins, alkaloids, terpenoids and anthraquinones. Fourier Transform Infrared spectroscopy (FTIR) analysis revealed the presence of phenolic flavonoids, saponins, tannins as major functional groups. Further studies are needed to explore and thus, to incorporate weed plant extracts for the management of house flies.
Keywords: Mortality, repellency, genotoxicity; Enzyme inhibition; FTIR; Musca domestica
1. Introduction
House fly (Musca domestica Linn.) is an important ubiquitous insect pest of both homes and farms as well. It acts as vector of more than hundred diseases in humans and animals caused by protozoans, bacteria, viruses, pathogens. These pathogens are transported by means of feces, mouthparts, mouth saliva, gut, and through surface of their body. It, usually disseminates harmful pathogens when get in touch with human and/or animals (Malik et al., 2007).
For the management of house fly population, chemical insecticides are commonly used. However, resistance has been developed against nearly all types of insecticides used for its control and now become a global issue. Additionally, these flies are well-known for their capability to develop metabolic and behavioral adaptations to abstain and for the detoxification of synthetic insecticides. Resistance of flies to DDT was reported after some years of its utilization. New synthetic insecticides are being launched consistently from years, and the result was the development of resistance of flies to carbamate, organophosphate, and pyrethroid classes of insecticides. Such type of challenging circumstances demands such management strategies which restrict the development of insect in order to sustain adult populations via controlling larval populations. The huge costs of chemical insecticides and concerned environmental harmful impacts encouraged scientists to search less toxic, ecofriendly and cheaper biopesticides as alternative way for the pest management in future (Sultana et al., 2016).
Plant-derived products have emerged as a leading source of alternatives for pest control. Plant extracts have bioactive compounds (polyphenols, alkaloids, terpenoids, essential oils, steroids, lignins, fatty acids, and sugars) which are basically secondary metabolites of plants and show broad spectrum of actions against variety of insect pests. Therefore, these products are considered as good substitutes to chemical insecticides for insect pest control and have little effect on human health, environment and predators, parasites and pollinator insects due to minimum residual activity (Regnault-Roger et al., 2012). Sometimes, plants that are being utilized as pest control agents are insect static (growth regulators) more than being insecticides as they are associated with the inhibition of insect development (Pavela, 2008). Most of the plants act as feeding inhibitors in various aspects: feeding repellents, or feeding suppressors and others are involved in the inhibition of growth, production of eggs and development.
P. harmala L. is a herbaceous plant growing in semi-arid lands has multiple potentials and used in curing of various human ailments, such as asthma, lumbago, gastrointestinal problems, jaundice, abortifacient agent and as a stimulant. The most commonly found phytochemical constituents in P. harmala include flavonoids, alkaloids, and anthraquinones (Bukhari et al., 2008).
D. stramonium is recognized as Jimson weed or Devil’s trumpet and efficacy of this plant has been recognized as useful remedy in traditional medicine (Lee, 2007). D. stramonium is also commonly used in numerous human diseases including wounds, ulcers, inflammation, gout, rheumatism, sciatica, swellings, bruises, bronchitis asthma, fever, and toothache etc (Gaire and Subedi, 2013).
Recently, weed plants have been reported against various insect pests. The compounds present in weed plants exhibit toxicological effects on insects (Riaz et al., 2018). Therefore, the present research work was designed to assess the insecticidal activity of P. harmala, D. stramonium, A. indica, T. terrestris and C. murale against 2nd insrtar larvae of M. domestica.
2. Materials and methods
2.1. Rearing of Musca domestica L.
The study was conducted in the Entomology Lab, Department of Zoology, Government College University Faisalabad. Susceptible lab strain was used for bioassays at 28 ± 2 °C temperature and 65–70% relative humidity. Cages of 40 cm × 40 cm × 40 cm size were used for the rearing of house flies. The cages were enclosed with mesh screens and have cloth sleeves at front and back sides. The front sleeve used for the introduction of milk solution, sugar solution and oviposition trays. A cotton pad soaked in the sugar solution (3%) was housed in the cage to provide sugar and water. An adult diet which is comprised of glucose (50%) and powder of MacConkey agar (50%) was prepared. Sugar solution and food was provided daily. Fresh milk soaked cotton wool was furnished to newly emerged flies for three days to augment production of eggs and later they were given milk sugar solution in the same way mentioned above. For rearing of larvae, a mixture of sterilized wheat bran (38 g), milk powder (2 g) and 60 ml water was employed as described by Pavela (2008). The temperature was maintained at 27 ± 2 °C with relative humidity (RH) of 70 ± 5%.
2.2. Collection and extraction of weed plants
All weeds were collected from different rural areas of district Faisalabad and then identified from Department of Botany, Government College University Faisalabad. To remove dust weed plants were then thoroughly cleaned using running tap water and then dried under shade for fifteen days. Crushed dried plants were again oven dried at 60 °C for 20 min. Grinded the oven dried crushed weeds in electrical grinder by using the mesh sieve#2 to obtain the plants in powder form. For extraction, 100 g of sample was mixed with 300 ml of petroleum ether and then the mixture was subjected to Rotary Shaker for 24 h at 220 rpm. Then filtration was made with the help of Whatman filter paper. The filtrate were kept in sterilized grey color air tight screwed glass bottles and stored in refrigerator at 4 °C for use. Following concentrations viz.5, 10, 15, 20 and 25% were prepared using petroleum ether as solvent from the stock solution of each weed.
2.3. Mortality bioassay
Different concentrations (5%, 10%, 15%, 20% and 25%) of botanical extracts prepared in petroleum ether were used for 5 g wheat bran while the control group was only treated with petroleum ether. Then treated food material was air dried for 15–30 min to evaporate the petroleum ether. Twenty 2nd instar larvae were exposed to each concentration of extract in 250 ml glass beakers labeled for different concentrations. The beakers were covered with muslin cloth. Each experiment was replicated three times along with the control and completely randomized design was followed. Those larvae were also considered dead that showed no feedback to sharp pin probing. Larval mortality was recorded after 24 h, 48 h and 72 h of time duration (Sultana et al., 2016).
2.4. Growth regulatory impact of weed plant extracts on M. domestica
To check the growth regulatory impact, treated the wheat bran with different botanical extracts concentration (5, 10, 15, 20, and 25%) while the control was treated with petroleum ether after the evaporation of petroleum ether (15–30 min.). Twenty 2nd instar larvae were released to complete their life cycle in each concentration of extract in 150 ml glass beakers labeled for different concentrations. The beakers were wrapped with muslin cloth with the help of rubber band. Each experiment was carried out with three replication with the control and completely randomized design was monitored (Sultana et al., 2016, Regnault-Roger et al., 2012). Data was collected for survival, pupation and adult emergence from treated larval media after 6–11 days.
2.5. Enzyme assay
2.5.1. Preparation of whole body homogenate
For enzymatic estimation, the larvae of M. domestica were washed thoroughly with distilled water and the extra water adhering to larvae was cleared by using bloating paper. Then, larvae were subjected to homogenization utilizing ice-cold sodium phosphate buffer (pH 7.0, 20 mM) with the help of Teflon hand homogenizer. Then, centrifugation was done at 8000g and 4 °C for 20 min and resultant supernatant was used for the estimation of Esterases or Phosphatases. Solutions and glassware used for homogenization were kept at 4 °C before use, and homogenates were also placed on ice until utilized for enzyme assays (Huang et al., 2008)
2.5.2. Quantitative estimation of Esterases and Phosphatases
2.5.2.1. Estimation of acetylcholinesterase activity
In the 50 µl of enzyme solution, 50 µl of acetylcholine chloride (2.6 mM) as a substrate and 1 ml of SBP (sodium phosphate buffer, pH 7.0, 20 mM) were added. It was placed in incubator at 25 °C for 5 mins. Then 400 µl of 0.3% Fast blue B salt was mixed to stop the reaction. Blank and sample were run through spectrophotometer. Optical density (OD) was checked at 405 nm (Younes et al., 2011).
2.5.2.2. Estimation of carboxylesterase activity
The activity of α-carboxylesterase and β-carboxylesterase was measured as devised by Van Asperen (1962). In 50 µl enzyme solution (homogenates), SBP (1 ml, pH 7.0, 20 mM,) and 50 µl of each β-naphthyl acetate and α-naphthyl acetate (substrate) were added separately to determine the activities of α-carboxylesterase and β-carboxylesterase respectively. The solutions were incubated at 30 °C for 20mins. After incubation, 400 µl of 0.3% fast blue B which was prepared freshly in 3.3% SDS was mixed in each reaction mixture to stop the reaction and after 15 min at 20 °C the color was developed. Blank and sample were run on spectrophotometer. Optical density (OD) was recorded at 430 and 590 nm for α-carboxylesterase and β-carboxylesterase, respectively ((Younes et al., 2011).
2.5.2.3. Estimation of acid- and alkaline phosphatase activity
Acid phosphatase (ACP) and alkaline phosphatase (AKP) level was assessed in larval homogenates following the protocol as already described by Asakura (1978). ACP activity was measured by adding 50 µl larval homogenate with 50 µl sodium phosphate buffer (50 mM, pH 7.0) and 100 µl of 20 mM p-nitrophenyl phosphate (substrate). To assess AKP activity, 50 µl larval homogenate was added to 50 µl Tris HCl buffer (50 mM, pH 9.0) and 100 µl of 20 mM p-nitrophenyl phosphate (substrate). After that both solution of ACP and AKP were incubated in water bath at 37 °C for 15 min, the enzymatic reaction was stop by mixing 0.5 N NaOH solution. The absorbance (OD) of the resulting clear supernatants of sample and blank was recorded at 440 nm (Younes et al., 2011).
The percentage inhibition of the enzyme activity by the test extracts was calculated as follows:
2.5.3. Oviposition attraction/deterrence
Five pairs of newly emerged (1–2 days old) adult house flies (males and females) were enclosed in the cage. Soaked cotton swab with 1% plant extract plus milk was provided to the flies. The experiments were performed in three replicates. To control group, swab of cotton immersed in milk and carrier solvent was provided only. After the duration of 24 h, eggs were counted and oviposition percentage was evaluated to estimate deterrent activity using protocol of Arivoli and Tennyson (2013) with some minor amendments.
2.6. Repellency bioassay
2.6.1. Double choice area-preference test
The bioassay for the evaluation of repellent efficacy of the plant extracts was evaluated against larvae of M. domestica. It was tested by applying area preference procedure as documented by Mohan and Fields (2002) using 9 cm diameter Whitman No. 1 filter paper. Three different test concentrations of plant extracts in petroleum ether were prepared (5%, 15% and 25%). Half part of the filter paper was treated with respective concentration while control part was poured with only petroleum ether. The treated half discs of filter papers were air dried for 15 min. Then treated and untreated halves were reattached with thin cello tape and complete discs of filter papers were adjusted in Petri dishes (9 cm diameter). Ten 2nd instar larvae of house fly were released in the center of filter paper (both halves) in each petridish and covered.
The experiment was carried out in control condition as dark, 27 ± 1 °C and 50–70% relative humidity. Each experiment was replicated thrice for each plant extract and observations were taken after 15, 24 and 48 h. A little amount of feed was provided on each halve to prevent mortality stress due to hunger.
Percentage repellency (PR) data was calculated by applying this formula (Abbott, 1925).
where,
NC = no. of larvae on control part
NT = no. of larvae on treated part
2.6.2. Comet assay protocol
Comet assay was performed to assess DNA damage following the protocol of Singh et al. (1988). The images were captured and examined at 400× magnification with Komet 5.5 Image Analysis System (Kinetic Imaging, UK) fitted with Olympus BX50 fluorescence microscope equipped with 590 nm barrier filter and 480–550 nm wide band excitation filter. Randomly selected 100 cells per treatment were analyzed. The percentage of DNA in tail (% DNA tail) was used to measure the DNA damage. Percent DNA tail was computed using Komet software version 5.5.
Total comet score was calculated using formula ranging from 0 to 400 arbitrary units.
where,
0–4 is number of comet classes
n0–n4 is the number of comet score in each class
2.6.3. Phytochemical screening of the weed extracts
Qualitative phytochemical analysis of weed extracts (petroleum ether) was carried out by employing standard protocols as prescribed by Harborne, 1973, Trease and Evans, 1989 and Sofowara (1993).
2.6.4. Test for alkaloids
One ml of 1% HCl was mixed with 3 ml of plant extract in a test tube and then treated with few drops of Meyer’s reagent (Potassium mercuric iodine solution). Formation of white yellowish turbidity or precipitate indicated the presence of alkaloids (Siddiqui and Ali, 1997).
2.6.5. Test for terpenoids (Salkowski Test)
Five ml of plant extract was taken in test tube, 2 ml of chloroform (CHCl3) and 3 ml of Conc. H2SO4 was carefully added to form a layer. Reddish brown color at interface indicated the presence of terpenoids (Harborne, 1973).
2.6.6. Test for saponins (Foam test)
To perform test for saponins, 0.5 ml of plant extract was shaken vigorously with 2 ml of distilled water to obtain a stable persistent froth. Retention of foamy lather for 10 min. indicated the presence of Saponins (Sofowara, 1993; Siddiqui and Ali, 1997).
2.6.7. Test for flavonoids
Few drops of sodium hydroxide (NaOH) solution were added to the extract in a test tube. Yellow color becoming colorless when dilute acid added which indicated the presence of flavonoids (Khandelwal, 2004).
2.6.8. Test for tannins
(a) 0.5 ml of crude extract, 1 ml of distilled water in test tube and 1–2 drops of ferric chloride solution were added into it. Appearance of blue and greenish black coloration was an indication of gallic tannins and catecholic tannins respectively.
(b) The test solution was mixed with 1 ml of 1% gelatin solution and 1 ml of 10% NaCl. White precipitate of gelatin indicated the presence of tannins (Trease and Evans, 1989, Sofowara, 1993).
2.6.9. Test for cardiac glycosides (Keller-Killami Test)
Five ml of plant extract, 2 ml of glacial acetic acid and few drop of ferric chloride solution and 2 ml of conc. H2SO4 was added along the side of test tube. Formation of a brown ring at the interface showed the presence of glycosides (Trease and Evans, 1989).
2.6.10. Test for phenols
Ferric Chloride Test: 3–4 drops of ferric chloride solution added to crude extract in test tube and shake well. Formation of bluish black color indicated the presence of phenol
2.6.11. Test for quinones
Added 2 ml of test substance (plant extract) conc. H2SO4 and shacked well for 5 min, appearance of red color indicated the presence of Quinone.
2.6.12. Test for steroids (Libermann Test)
For this test was added 10 ml of chloroform in the test solution and then filtered. In a test tube containing 2 ml filtrate, 2 ml of acetic anhydride and few drops of conc. H2SO4 were added. Formation of blue green ring specified the presence of steroids (Khandelwal, 2004).
2.6.13. Fourier Transform Infrared Spectroscopy (FTIR) analysis
FTIR spectrophotometer apparatus was used to detect the characteristic functional groups of the active constituent present in the plant extract based on their vibrational frequencies between the bonds of atoms. The plant extracts prepared in petroleum ether were frozen at −80 °C followed by lyophilization. The IR spectrum of lyophilized extract was obtained by using the FTIR spectrophotometer (Alpha, Bruker, California, USA). The sample was scanned to measure the FTIR spectra in the frequency ranges of 400–4000 cm−1.
2.7. Data analysis
The mortality data of Musca domestica obtained from bioassay tests was subjected to Abbot’s formula (Abbott, 1925) to obtain corrected mortality. The data of corrected mortality, growth regulatory impact and enzyme assay was analyzed through ANOVA. For oviposition deterrence, methods of Arivoli and Tennyson (2013) were followed with some amendments. Data obtained was subjected to statistical analysis.
where NC = total number of eggs laid in control group, NT = number of eggs laid in treated group and E = number of eggs laid in treated.
The scoring of comet assay was done by arbitrary units and computer image analysis (CaspR software). Results statistically analyzed by one factor ANOVA. Mean comet scores of all groups was also analyzed by LSD to isolate significant differences.
3. Results
3.1. Mortality of larvae of M. domestica (L.) exposed to weed plant extracts
This experiment was conducted to evaluate insecticidal bioactivities of Peganum harmala, Datura stramonium, Tribulus terrestris and Chenopodium murale. A. indica (Neem plant) was used as a reference plant against 2nd instar larvae of Musca domestica Linn. All the tested weed plant extracts were found substantially fatal at all exposure times and concentrations. The mortality was increased with increase in concentration. Longest exposure time of 72 h showed highest mortality followed by 48 and 24 h, respectively. At the highest concentration (25%), P. harmala showed highest mean mortality (63.87%) of all the exposure periods followed by Datura stramonium, (62.78%), A. indica (53.84%), Tribulus terrestris (41.86%) and C. murale (4.09%), respectively. At 5% concentration, highest mean mortality was shown by P. harmala (24.24%) followed by D. stramonium, (14.72%), A. indica (8.03%), T. terrestris (3.00%) and C. murale (0.00%), respectively. All the plant extracts were found active against Musca domestica and their order of toxicity observed was 25% > 20% > 15% > 10% > 5%. The order of toxicity at highest concentration (25%) was as follows: Peganum harmala > D. stramonium > A. indica > T. terrestris > C. murale. Results suggested that the insecticidal activity of the weed plants against M. domestica was time and dose dependent (Table 1).
Table 1.
Mean mortality of 2nd instar larvae of M. domestica (L) at various concentrations of weed plant extracts.
| Plants | Conc. | Treatment |
Mean | ||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| A. indica (F = 51.07; df = 4; P < 0.05) | 5% | 0.00 ± 0.00f | 6.67 ± 3.33ef | 17.42 ± 3.37def | 8.03 ± 2.88D |
| 10% | 3.33 ± 1.67f | 18.33 ± 4.41def | 27.54 ± 4.46de | 16.40 ± 3.99CD | |
| 15% | 6.67 ± 1.67ef | 18.33 ± 7.26def | 51.13 ± 4.46bc | 25.38 ± 7.11BC | |
| 20% | 8.33 ± 1.67ef | 33.33 ± 4.41 cd | 61.24 ± 4.46b | 34.30 ± 7.87B | |
| 25% | 18.33 ± 1.67def | 56.67 ± 7.26b | 86.52 ± 6.08a | 53.84 ± 10.2A | |
| Mean | 7.33 ± 1.75C | 26.67 ± 5.06B | 48.77 ± 6.80A | ||
| P. harmalia F = 22.4; df. = 4; P < 0.05) | 5% | 6.67 ± 1.67 | 21.67 ± 1.67 | 44.39 ± 2.92 | 24.24 ± 5.59C |
| 10% | 10.00 ± 5.00 | 25.00 ± 7.64 | 52.81 ± 6.08 | 29.27 ± 7.02C | |
| 15% | 20.00 ± 5.00 | 43.33 ± 1.67 | 67.98 ± 9.38 | 43.77 ± 7.59B | |
| 20% | 26.67 ± 4.41 | 46.67 ± 7.26 | 76.41 ± 7.35 | 49.91 ± 7.92B | |
| 25% | 33.33 ± 4.41 | 63.33 ± 9.28 | 94.94 ± 5.06 | 63.87 ± 9.49A | |
| Mean | 19.33 ± 3.12C | 40.00 ± 4.73B | 67.31 ± 5.36A | ||
| D. stramonium (F = 55.53 + df = 4; P < 0.05) | 5% | 15.00 ± 0.00 fg | 5.00 ± 0.00 g | 24.17 ± 0.00efg | 14.72 ± 2.77C |
| 10% | 5.00 ± 2.89 g | 15.00 ± 2.89 fg | 41.02 ± 3.37cde | 20.34 ± 5.58C | |
| 15% | 11.67 ± 1.67 fg | 30.00 ± 5.00def | 61.24 ± 9.38bc | 34.30 ± 7.87B | |
| 20% | 23.33 ± 6.01efg | 50.00 ± 7.64 cd | 81.46 ± 4.46ab | 51.60 ± 8.95A | |
| 25% | 30.00 ± 7.64def | 58.33 ± 4.41bc | 100.00 ± 0.00a | 62.78 ± 10.5A | |
| Mean | 17.00 ± 2.92C | 31.67 ± 5.68B | 61.58 ± 7.50A | ||
| T. terrestris (F = 46.97; df = 4; P < 0.05) | 5% | 0.00 ± 0.00e | 0.00 ± 0.00e | 9.00 ± 2.92de | 3.00 ± 1.72C |
| 10% | 0.00 ± 0.00e | 8.33 ± 3.33de | 17.43 ± 4.46cde | 8.59 ± 2.99C | |
| 15% | 11.67 ± 4.41de | 20.80 ± 3.37 cd | 35.96 ± 4.46bc | 22.81 ± 4.10B | |
| 20% | 18.33 ± 3.33cde | 25.85 ± 6.08bcd | 44.39 ± 5.05b | 29.52 ± 4.60B | |
| 25% | 25.00 ± 5.77bcd | 32.59 ± 4.46bc | 67.98 ± 4.46a | 41.86 ± 7.07A | |
| Mean | 11.00 ± 2.98C | 17.51 ± 3.50B | 34.95 ± 5.79A | ||
| C. murale (F = 9.90; df = 4; P < 0.05) | 5% | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00C |
| 10% | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.63 ± 1.31 | 0.88 ± 0.58BC | |
| 15% | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.63 ± 1.31 | 0.88 ± 0.58BC | |
| 20% | 3.33 ± 1.67 | 3.33 ± 1.67 | 2.63 ± 1.31 | 3.10 ± 0.79AB | |
| 25% | 3.33 ± 1.67 | 5.00 ± 0.00 | 3.94 ± 0.00 | 4.09 ± 0.54A | |
| Mean | 1.33 ± 0.59A | 1.67 ± 0.63A | 2.36 ± 0.52A | ||
3.2. Growth regulatory impact of weed plant extracts on M. domestica
The growth regulatory impact of weed plant extracts was observed for all the tested weed plants and Azadirachta indica (Neem). At 25% concentration, the longest larval duration (9.33 ± 0.33 days) was observed in larvae treated with P. harmala and D. stramonium followed by A. indica (8.67 ± 0.33 days), C. murale (8.33 ± 0.33 days) and the shortest larval duration (8.00 ± 0.58 days) was recorded agaisnt T. terrestris compared to the duration of control (5.33 ± 0.33 days). While in case of pupal duration, a longest pupal duration (7.33 ± 0.33 days) was recorded in both P. harmala and A. indica as compared to D. stramonium (7.00 ± 0.58 days), T. terrestris and C. murale (6.33 ± 0.33 days). However, at 25% concentration, the longest development period (17.00 ± 0.58 days) was shown by P. harmala followed by D. stramonium (16.67 ± 0.33 days), A. indica (15.67 ± 0.33 days), T. terrestris (14.67 ± 0.33 days) and C. murale (14.33 ± 0.33 days) At 5% concentration, no significant difference with P. harmala , D. stramonium and A. indica whereas T. terrestris and C. murale represented no significant difference to each other in development time (12.00 ± 0.58 days) (Table 2).
Table 2.
Growth regulatory impact of weed plant extracts on M. domestica (L.).
| Plant name | Duration (days) | Concentration |
Significance level | |||
|---|---|---|---|---|---|---|
| Control | P=<0.05;df = 3) | |||||
| 5% | 15% | 25% | F value | |||
| A. indica | Larval days | 7.33 ± 0.33B | 7.67 ± 0.33AB | 8.67 ± 0.33A | 5.33 ± 0.33C | 17.58 |
| Pupal days | 5.67 ± 0.33B | 6.00 ± 0.00B | 7.33 ± 0.33A | 4.67 ± 0.33C | 14.56 | |
| Dev. time (days) | 12.67 ± 0.67B | 13.00 ± 0.58B | 15.67 ± 0.33A | 10.00 ± 0.00C | 24.17 | |
| P. harmala | Larval days | 7.33 ± 0.33B | 7.67 ± 0.33B | 9.33 ± 0.33A | 5.33 ± 0.33C | 24.25 |
| Pupal days | 6.33 ± 0.33A | 6.67 ± 0.33A | 7.33 ± 0.33A | 4.67 ± 0.33B | 11.58 | |
| Dev. time (days) | 13.67 ± 0.33B | 14.33 ± 0.33B | 17.00 ± 0.58A | 10.00 ± 0.00C | 59.93 | |
| D. stramonium | Larval days | 7.67 ± 0.33B | 8.33 ± 0.33AB | 9.33 ± 0.33A | 5.33 ± 0.33D | 26 |
| Pupal days | 5.33 ± 0.33BC | 6.33 ± 0.33AB | 7.00 ± 0.58A | 4.67 ± 0.33C | 6.44 | |
| Dev. time (days) | 13.00 ± 0.58B | 14.00 ± 0.00B | 16.67 ± 0.33A | 10.00 ± 0.00C | 68.25 | |
| T. terrestris | Larval days | 7.00 ± 0.58A | 7.67 ± 0.33A | 8.00 ± 0.58A | 5.33 ± 0.33B | 6.33 |
| Pupal days | 5.33 ± 0.33AB | 5.67 ± 0.33AB | 6.33 ± 0.33A | 4.67 ± 0.33B | 4.33 | |
| Dev. time (days) | 12.00 ± 0.58B | 12.67 ± 0.33B | 14.67 ± 0.33A | 10.00 ± 0.00C | 26.67 | |
| C. murale | Larval days | 6.33 ± 0.33B | 6.33 ± 0.33B | 8.33 ± 0.33A | 5.33 ± 0.33B | 14.25 |
| Pupal days | 5.33 ± 0.33AB | 5.33 ± 0.33AB | 6.33 ± 0.33A | 4.67 ± 0.33B | 4.25 | |
| Dev. time (days) | 12.00 ± 0.58B | 12.33 ± 0.67B | 14.33 ± 0.33A | 10.00 ± 0.00C | 14.17 | |
3.3. Oviposition deterrence activity of plant extracts against M. domestica
The oviposition deterrence assay was performed at 5, 15 and 25% concentrations of weed extracts which showed that percent deterrence was increased as the concentration of plant extracts was increased. At 25% concentration, C. murale, showed highest oviposition deterrence activity (81.88%) followed by D. stramonium (79.58%), A. indica (74.10%) P. harmala, (68.14%) and T. terrestris (48.96%). The oviposition deterrence activity of all selected plant extracts was found significant (Table 3).
Table 3.
The percentage of Oviposition deterrence activity of weed plant extracts against M. domestica (L.).
| Plant name | Concentration of extract |
||
|---|---|---|---|
| 5% | 15% | 25% | |
| Azadirechta indica (F = 182.31; d.f. = 3; P < 0.0)5 | 54.59 ± 3.47C | 57.96 ± 2.51C | 74.10 ± 3.42B |
| Datura stramonium (F = 170.55; d.f. = 3; P < 0.05) | 59.33 ± 2.62C | 65.26 ± 1.58C | 79.58 ± 4.23B |
| Penganum harmala (F = 205.55; d.f. = 3; P < 0.05) | 40.25 ± 3.03C | 46.50 ± 3.23C | 68.14 ± 4.05B |
| Tribulus terrestris (F = 502.64; d.f. = 3; P < 0.05) | 40.60 ± 1.86C | 45.45 ± 1.40BC | 48.96 ± 0.86B |
| Chenopodium murale (F = 230.64; d.f. = 3; P < 0.05) | 74.99 ± 1.72B | 80.09 ± 1.22B | 81.88 ± 1.56B |
| Control | 153.67 ± 4.18A | 153.67 ± 4.18A | 153.67 ± 4.18A |
3.4. Repellency induced by weed plant extracts against M. domestica
The mean percent repellency induced by plant extracts against 2nd instar larvae of M. domestica is shown in Table 4. It is evident that percent repellency of first two exposure periods of 1st h and 12 h was considerably different. The repellency potential of weed extracts had direct relation with concentration while indirectly related to exposure time. The highest repellent effect was observed after 1st hour at 25% concentration among all tested plants. The highest mean repellency at 25% concentration was shown by P. harmala (76.67%) followed by D. stromium (73.16%), A. indica (58.55%), T. terrestris (48.89%) and C. murale (25.82%), respectively. The mean repellency observed at all the concentrations was lowest at 48 h than 1st, 12th and 24 h. Overall, results showed that repellency was highest during 1 h and decreased after 12, 24 and 48 h (1st h > 12 h > 24 h > 48 h).
Table 4.
Repellency data regarding different concentration and exposure of time against 2nd instar larvae of M. domestica (L.).
| Weed plants | Conc. | Time |
Mean | |||
|---|---|---|---|---|---|---|
| 1 h | 12 h | 24 h | 48 h | |||
| Azadirachta indica (F = 68.31 ; d.f. = 4; P < 0.05) | 5% | 20.00 ± 7.70 | 15.56 ± 4.44 | 6.67 ± 0.00 | 6.67 ± 0.00 | 12.22 ± 2.57E |
| 10% | 37.78 ± 4.45 | 28.89 ± 4.44 | 15.56 ± 4.44 | 15.56 ± 4.44 | 24.44 ± 3.42D | |
| 15% | 55.67 ± 0.00 | 46.56 ± 4.44 | 24.44 ± 4.44 | 20.00 ± 7.70 | 36.67 ± 4.96C | |
| 20% | 64.56 ± 4.44 | 55.44 ± 4.44 | 33.89 ± 4.44 | 28.33 ± 1.93 | 45.56 ± 4.79B | |
| 25% | 68.44 ± 4.44 | 64.89 ± 4.44 | 58.63 ± 1.01 | 42.22 ± 4.45 | 58.55 ± 3.47A | |
| Mean | 49.29 ± 4.48A | 42.26 ± 5.82A | 27.83 ± 4.89B | 22.55 ± 3.80B | ||
| Penganum harmala (F = 50.70; d.f. = 4; P < 0.05) | 5% | 20.00 ± 7.70 | 15.56 ± 4.44 | 12.22 ± 4.45 | 11.11 ± 4.44 | 14.22 ± 3.05D |
| 10% | 37.78 ± 4.45 | 28.89 ± 4.44 | 24.00 ± 7.70 | 20.44 ± 4.44 | 27.78 ± 3.05C | |
| 15% | 64.44 ± 16.0 | 60.00 ± 15.4 | 37.78 ± 8.89 | 37.78 ± 4.45 | 50.00 ± 6.38B | |
| 20% | 91.11 ± 4.44 | 77.78 ± 4.45 | 51.11 ± 8.89 | 46.67 ± 7.70 | 66.67 ± 6.25A | |
| 25% | 95.56 ± 4.44 | 86.67 ± 7.70 | 68.89 ± 4.44 | 55.56 ± 4.44 | 76.67 ± 5.22A | |
| Mean | 61.78 ± 8.52A | 53.78 ± 8.01A | 38.80 ± 6.79B | 34.31 ± 4.67B | ||
| D. stramonium (F = 110.40 d.f. = 4; P < 0.05) | 5% | 37.78 ± 2.26f | 33.33 ± 0.00f | 12.22 ± 4.45 h | 6.67 ± 0.00gh | 22.50 ± 4.86E |
| 10% | 55.56 ± 3.13 cd | 42.22 ± 4.45ef | 15.56 ± 2.94 g | 15.56 ± 2.94 g | 32.22 ± 5.42D | |
| 15% | 68.89 ± 4.44b | 64.44 ± 4.44bc | 33.33 ± 1.93f | 33.33 ± 0.00f | 50.00 ± 5.24C | |
| 20% | 91.11 ± 4.44a | 68.89 ± 4.44b | 44.78 ± 4.45f | 37.22 ± 2.45def | 60.50 ± 6.60B | |
| 25% | 100.00 ± 0.00a | 73.33 ± 7.70b | 68.21 ± 8.21b | 51.11 ± 4.84de | 73.16 ± 5.90A | |
| Mean | 70.67 ± 6.21A | 56.44 ± 4.59B | 34.82 ± 6.26C | 28.77 ± 4.61C | ||
| Tribulus terrestris (F = 38.90; d.f. = 4; P < 0.05) | 5% | 15.56 ± 4.44efg | 11.11 ± 4.44 fg | 6.67 ± 0.00 g | 6.67 ± 0.00 g | 10.00 ± 1.74D |
| 10% | 24.44 ± 4.44def | 15.56 ± 4.44efg | 11.11 ± 4.44 fg | 11.11 ± 4.44 fg | 15.56 ± 2.51D | |
| 15% | 37.78 ± 4.45 cd | 24.44 ± 4.44def | 20.00 ± 0.00efg | 15.56 ± 4.44efg | 24.44 ± 3.00C | |
| 20% | 60.00 ± 7.70b | 28.89 ± 4.44cde | 24.44 ± 4.44def | 24.44 ± 4.44def | 34.44 ± 5.05B | |
| 25% | 82.22 ± 4.45a | 42.22 ± 4.45c | 42.89 ± 4.44cde | 28.22 ± 11.76c | 48.89 ± 6.73A | |
| Mean | 44.00 ± 6.79A | 24.44 ± 3.36B | 21.02 ± 2.56B | 17.20 ± 4.11B | ||
| Chenopodium murale (F = 16.68; d.f. = 4; P < 0.05) | 5% | 11.11 ± 4.44 | 11.11 ± 4.44 | 6.67 ± 4.45 | 2.22 ± 0.00 | 7.78 ± 1.98C |
| 10% | 24.44 ± 4.44 | 15.56 ± 4.44 | 11.11 ± 4.44 | 11.11 ± 4.44 | 15.56 ± 2.51B | |
| 15% | 28.89 ± 4.44 | 20.00 ± 7.70 | 15.06 ± 3.86 | 13.56 ± 4.44 | 19.38 ± 2.91AB | |
| 20% | 33.33 ± 0.00 | 24.44 ± 4.44 | 20.00 ± 2.50 | 15.00 ± 0.00 | 23.19 ± 2.30A | |
| 25% | 37.78 ± 4.45 | 28.89 ± 4.44 | 20.62 ± 1.74 | 16.00 ± 0.00 | 25.82 ± 3.64A | |
| Mean | 27.11 ± 2.87A | 20.00 ± 2.60B | 14.69 ± 1.81C | 11.57 ± 1.75C | ||
3.5. Enzyme assay
The effect of the tested weed plants on the enzymatic activity (AChE, AcP, AkP, α-Carboxyl, and β-Carboxyl) in 2nd instar larvae of M. dometica was observed at 5, 15 and 25% of concentrations. Maximum decrease was observed at 25% concentration of extracts. D. stramonium induced maximum decrease (59.07%) in acetylcholine esterase followed by P. harmala (48.10%). Maximum decrease in ACP was induced by P. harmala (53.95%), than D. stramonium (48.64%) followed by A. indica (41.61%), T. terrestris (24.20 ± 5.02) and C. murale (17.66%), respectively. However, D. stramonium, induced maximum percent inhibition activity of AKP followed by A. indica, P. harmala, T. terrestris and C. murale, respectively. The decreasing order was observed as 46.72% <44.06% < 35.35% < 20.71% < 12.88%, respectively. In addition, maximum decrease in the activity of α-Carboxyl, and β-Carboxyl was also observed with D. stramonium followed by P. harmala (Table 5).
Table 5.
Effect of different concentrations of plant extracts on the percent inhibition of enzymatic activity in M. domestica (L.).
| Plants | Enzyme | Concentration |
Mean | ||
|---|---|---|---|---|---|
| 5% | 15% | 25% | |||
| A. indica (F = 9.70; d.f. = 4; P < 0.05) | ACh E | 13.93 ± 1.26f | 27.52 ± 2.42def | 58.51 ± 3.12ab | 33.32 ± 6.70B |
| ACP | 20.74 ± 2.59ef | 34.08 ± 2.06cde | 70.00 ± 1.28a | 41.61 ± 7.43A | |
| AKP | 16.10 ± 1.33f | 46.97 ± 1.89bc | 69.13 ± 1.68a | 44.06 ± 7.73A | |
| α-Carboxy. | 26.74 ± 3.31ef | 40.63 ± 3.01 cd | 57.64 ± 5.12ab | 41.67 ± 4.88A | |
| β-Carboxy. | 23.92 ± 3.49ef | 34.51 ± 2.83cde | 45.49 ± 1.04bc | 34.64 ± 3.39B | |
| Mean | 20.28 ± 1.60C | 36.74 ± 1.99B | 60.15 ± 2.63A | ||
| P.harmala (F = 28.21; df = 4; P < 0.05) | ACh E | 35.15 ± 1.45ef | 46.35 ± 2.58cde | 62.80 ± 1.43b | 48.10 ± 4.13B |
| ACP | 29.63 ± 2.96fgh | 52.96 ± 3.23bcd | 79.26 ± 0.37a | 53.95 ± 7.28A | |
| AKP | 17.23 ± 1.68 h | 30.11 ± 3.70 fg | 58.71 ± 1.55bc | 35.35 ± 6.26D | |
| α-Carboxy. | 21.18 ± 2.43gh | 36.46 ± 3.01ef | 59.38 ± 1.80b | 39.01 ± 5.68CD | |
| β-Carboxy. | 30.59 ± 4.08 fg | 40.78 ± 0.78def | 57.65 ± 1.36bc | 43.01 ± 4.14BC | |
| Mean | 26.76 ± 2.03C | 41.33 ± 2.36B | 63.56 ± 2.21A | ||
| D. stramonium (F = 10.33, df = 4; P < 0.05) | ACh E | 48.74 ± 1.04bcd | 61.13 ± 1.67abc | 67.34 ± 1.67ab | 59.07 ± 2.83A |
| ACP | 24.07 ± 1.62f | 48.15 ± 4.07 cd | 73.70 ± 2.67a | 48.64 ± 7.32B | |
| AKP | 25.38 ± 0.76f | 37.50 ± 8.68def | 77.27 ± 3.28a | 46.72 ± 8.28B | |
| α-Carboxy. | 25.69 ± 4.00f | 45.83 ± 1.80cde | 59.72 ± 3.52abc | 43.75 ± 5.20B | |
| β-Carboxy. | 27.84 ± 3.06ef | 45.10 ± 3.42cde | 52.94 ± 4.75bcd | 41.96 ± 4.17B | |
| Mean | 30.35 ± 2.64C | 47.54 ± 2.71B | 66.20 ± 2.70A | ||
| T. terrestris (F = 10.17, df = 4, P < 0.05) | ACh E | 16.31 ± 0.83cde | 21.55 ± 1.26bcd | 30.62 ± 2.19ab | 22.83 ± 2.23A |
| ACP | 8.52 ± 1.96de | 22.96 ± 4.55bc | 41.11 ± 3.40a | 24.20 ± 5.02A | |
| AKP | 9.47 ± 3.12cde | 22.16 ± 1.43bcd | 30.49 ± 1.37ab | 20.71 ± 3.24A | |
| α-Carboxy. | 8.68 ± 2.11de | 15.28 ± 1.25cde | 18.06 ± 3.82b-e | 14.01 ± 1.91B | |
| β-Carboxy. | 5.10 ± 1.96e | 14.90 ± 3.74cde | 21.96 ± 3.06bcd | 13.99 ± 2.87B | |
| Mean | 9.62 ± 1.27C | 19.37 ± 1.42B | 28.45 ± 2.40A | ||
| C. murale (F = 6.39; d.f. = 4; P < 0.05) | AChE | 5.82 ± 2.27ef | 12.73 ± 1.49c-f | 29.43 ± 4.38ab | 15.99 ± 3.81AB |
| ACP | 3.33 ± 1.70f | 15.19 ± 3.16cde | 34.45 ± 3.39a | 17.66 ± 4.75A | |
| AKP | 5.49 ± 1.55ef | 12.12 ± 1.36c-f | 21.02 ± 1.18bc | 12.88 ± 2.35ABC | |
| α-Carboxy. | 5.56 ± 0.92ef | 11.46 ± 2.08c-f | 18.06 ± 0.69 cd | 11.69 ± 1.93BC | |
| β-Carboxy. | 3.14 ± 0.39f | 9.02 ± 1.41def | 18.04 ± 1.96 cd | 10.07 ± 2.28C | |
| Mean | 4.67 ± 0.65C | 12.10 ± 0.93B | 24.20 ± 2.04A | ||
3.6. Comet assay
House fly larvae were exposed to extracts of P. harmala, D. stramonium and A. indica to evaluate the genotoxic effects in individual cells using comet assay. Three different concentrations (5%, 15%, 25%) were used to analyze the migration of DNA fragments by agarose gel-electrophoreses (Table 6).
Table 6.
DNA damage and comet parameters of M. domestica (L.) larvae at different concentrations of weed plant extracts.
| Weed name | Comet parameter | Control |
5% |
15% |
25% |
|---|---|---|---|---|---|
| Mean ± S.E | |||||
| P. hermala | L. Tail (µm) (F = 23.96 + d.f. = 3;P < 0.05) | 3.60 ± 0.24c | 7.60 ± 0.93b | 8.60 ± 0.40ab | 10.20 ± 0.49a |
| Tail DNA % (F = 41.43;df. = 3;P < 0.05) | 1.14 ± 0.56c | 6.32 ± 0.65b | 9.05 ± 0.56a | 10.56 ± 0.77a | |
| T.M (µm) (F = 34.42; .f. = 3;P < 0.05) | 0.10 ± 0.05c | 0.47 ± 0.04c | 0.78 ± 0.08b | 1.07 ± 0.10a | |
| D. stromium | L. Tail (µm) (F = 22.55 + f. = 3;P < 0.05) | 3.60 ± 0.24c | 6.20 ± 0.73b | 7.60 ± 0.51b | 9.20 ± 0.37a |
| Tail DNA% (F = 21.74;df. = 3;P < 0.05) | 1.14 ± 0.56c | 5.48 ± 0.34b | 8.71 ± 0.37a | 10.67 ± 1.62a | |
| T.M (µm) (F = 11.29; .f. = 3;P < 0.05) | 0.10 ± 0.05c | 0.33 ± 0.04b | 0.65 ± 0.04a | 0.90 ± 0.19a | |
| A. indica | L. Tail (µm) (F = 8.7 + df. = 3; P < 0.05) | 3.60 ± 0.24c | 4.60 ± 0.68bc | 5.80 ± 0.86b | 7.80 ± 0.49a |
| Tail DNA (F = 22.29;d.f. = 3;P < 0.05) | 1.14 ± 0.56c | 5.45 ± 0.38b | 6.40 ± 0.64ab | 8.11 ± 0.85a | |
| T.M µm (F = 17.12; df. = 3;P < 0.05) | 0.10 ± 0.05c | 0.25 ± 0.04bc | 0.36 ± 0.05b | 0.62 ± 0.07a | |
Tail length (TL): The highest mean value of tail length was induced by P. hermala (7.60 μm) at 5% concentration followed by D. stromium (6.20 μm) and A. indica (4.60 μm). The highest mean value of tail length (10.20 μm) was observed at 25% concentration by P. hermala followed by D. stromium (9.20 μm) and A. indica (7.80 μm), respectively.
Tail DNA%: At 5% concentration, P. hermala was found most active (6.32%) amongst all other tested weed extracts compared to D. stromium (5.48%) and A. indica (5.45%) Tail DNA. However, at 25%, D. stromium, P. hermala and A. indica forced to move DNA as percentage ratios of 10.67%, 10.56% and 8.11%, respectively. The tail DNA values show that both D. stromium, and P. hermala have almost similar results.
Tail moment: The mean T.M values compared to control (0.10) at 5% concentration were observed in P. hermala (0.47) followed by D. stromium (0.33), and A. indica (0.25), respectively. While at 25% concentration, highest mean TM value was found in P. hermala (1.07) followed by D. stromium (0.90) while the lowest mean value was observed in A. indica (0.62) (Table 6).
The overall genotoxic trend of TL, Tail DNA% and TM of tested weeds at three concentrations were as followed: P. hermala > D. stromium > A. indica, 25% > 15% > 5% respectively. It was found that with the increase of concentration the value of TM also increases.
3.7. Phytochemical analysis of weed plant extracts
The qualitative tests for phytochemical components of petroleum ether extract of five weed plants viz: Azadirechta indica (Reference plant), Penganu harmala, Datura stramonium, Tribulus terrestris and Chenopodium murale revealed positive result for the constituents i.e. flavonoids, saponins, tannins, steroids, cardiac glycosides, alkaloids, anthrequinones and terpenoid (Table 7). Flavonoids and Alkaloids were found present in extracts of all tested weed plants. Saponins, Tanins and cardiac glycosides were absent in the extract of P. hermala, while steroids were not detected in D. stromium. Cardiac Glycosides were not found in P. hermala and T. terrestris extracts respectively. Only C. murale showed negative test for anthrequinones. The terpenoids were not detected in P. hermala and C. murale.
Table 7.
Phytochemical constituents of petroleum ether extract of selected weed plants.
| Sr. No. | Weed Plants | Chemical Constituents |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Fl | Sa | Tn | St | CG | Al | Anth | Ter | ||
| 1 | A. indica | + | + | + | + | + | + | + | + |
| 2 | P. hermala | + | – | – | + | – | + | + | – |
| 3 | D. stromium | + | + | + | – | + | + | + | + |
| 4 | T. terrestris | + | + | + | + | – | + | + | + |
| 5 | C. murale | + | + | + | + | + | + | – | – |
Fl = flavinoids, Sa = saponins, Tn = tannins, St = steroids, CG = Cardiac glycosides, Al = alkaloids, Anth = anthrequinones, Ter = terpenoids, + = Present and – = Absent.
3.8. Fourier Transform Infrared Spectroscopy (FTIR)
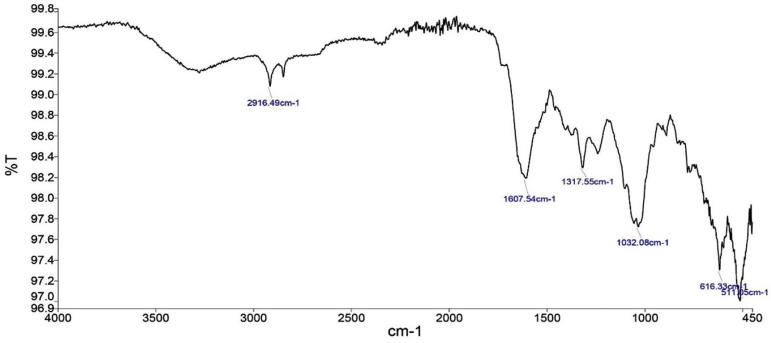
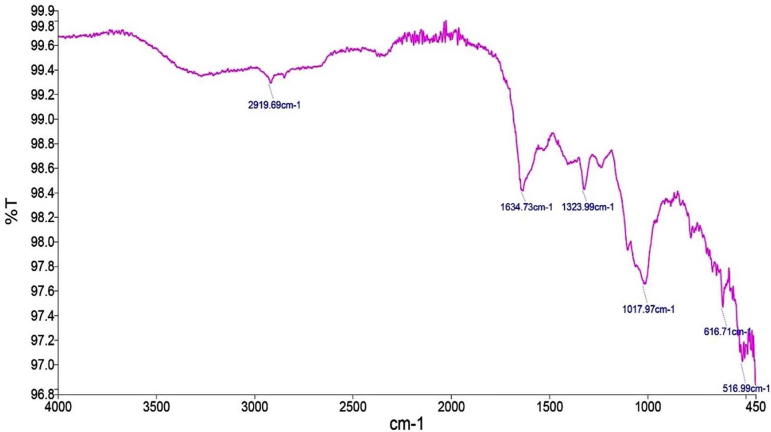
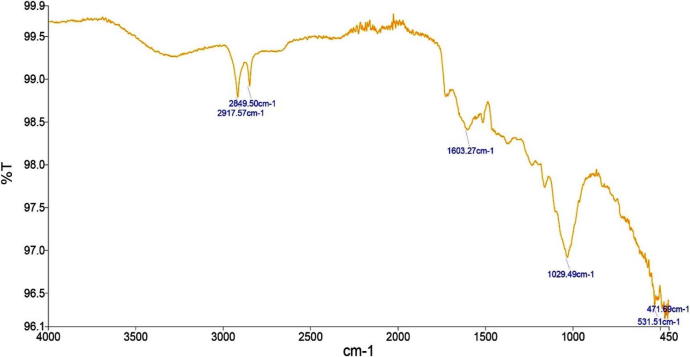
The crude extract of the weed plants are used to determine organic and inorganic compounds by Fourier transform infrared spectroscopy (FTIR). Generally, the FTIR analysis looks at the vibration of functional groups present in organic molecules and searches the structural alterations as the function of shifts in wave number. So, the resultant peaks specify the functional groups present in organic and inorganic compounds. The FTIR spectroscopic analysis of P. hermala extract revealed the presence of various chemical constituents (Fig. 1). The intense absorption bands at 2916.49 cm−1 corresponds to CH group stretching due to CH3 and 1607.54 cm−1 represents C O stretching. The absorption band at 1317.55 cm−1 denotes the presence of C—N bond vibration possibly due to NH2, NH, or N absorption. Similarly, for Datura stromium, the peaks at 2919.69 cm−1 merged O—H stretching correspond to C—H stretching, 1634.73 cm−1 represents carbonyl (C O) group, 1323.99 cm−1 assigned to C H group and 1017.97 cm−1 to aromatic in-plane C—H bending (Fig. 2). FTIR profile of Azadirachta indica is also shown in Fig. 3.
Fig. 1.
Full FTIR spectrum of Peganum harmala.
Fig. 2.
Full FTIR spectrum of Datura stromium.
Fig. 3.
Full FTIR spectrum of Azadirachta indica.
4. Discussion
The synthetic pesticides have been considered better option for the management of insect pests; but however, employment of pesticides poses serious concerns to the environment. The selection of new pesticides that fulfill the requirements of economy, safety, and efficacy highlights the objectivity of farmers. Therefore, now-a-days biopesticides are being used to control different insect pests (Needham et al., 2004: Anjum et al., 2010a, Anjum et al., 2010b, Alves et al., 2014). Bio-insecticides, in fact, are pesticides originated from natural resources like plants, bacteria, viruses, animals, and certain minerals. Subsequently, the botanicals has been accepted as the most effective source of chemical compounds and/or products that are used to protect plants against various pests (Isman and Akhtar, 2007).
Neem, A. indica was used as a reference to observe their efficacy against house fly. The present study identifies that the weed plants are rich source of various bioactive metabolites that can be degraded into nontoxic compounds which are potentially suitable for integrated pest management (IPM). Similarly, the study also indicates the sensible use of surplus weeds that are commonly eradicated by employing the herbicides because they are regarded as pests in crops (Alves et al., 2014). Since, many scientists as well as entomologists proved the efficacy of plant extracts and essential oils derived from A.indica. So, Neem plant was taken as a reference plant for the current study (Sultana et al., 2016).
Mortality: The insecticidal activities of extracts obtained from P. harmala, D. stramonium, T. terrestris and C. murale were evaluated against 2nd instar larvae of M. domestica. P. harmala was proved most efficient toxicant with high mortality (63.87%) followed by D. stramonium (62.78%), A. indica (53.84) and T. terrestris (41.86%). Recently, Riaz et al. (2018) investigated the toxicity of some weed plants against D. melanogaster. In accordance, the current results showed that mortality of 2nd instar larvae of Musca domestica was significantly increased with the increase in dose rate and exposure time. Similar results were obtained by Caballero-Gallardo et al. (2012) who investigated the toxicity of Cymbopogon flexuosus, C. martini, and Lippia origanoides against T. castaneum. High mortality rate was recorded at high concentration of extract. Subsequently, the findings are also consistent with the studies of Lu and Wu (2010). They demonstrated that toxicity of Ailanthus altissima (Swingle) (Sapindales: Simaroubaceae) bark essential oil against S. oryzae adults was increased with exposure time. Zia et al. (2013) described efficacy of citrus peel extracts and revealed that the vapors of essential oil displayed varying toxicity to test insects relying on concentration and exposure duration.
Mansour et al. (2011) compared the toxicity of commercial insecticides with 11 plant extracts against the larval stage of the housefly, M. domestica. They described a range of toxicity attributes of the tested plant extracts against the larvae of M. domestica. Crude ethanol extracts of Annona squamosa and Calotropis procera leaves were found toxic and could be used as bio-insecticides against Musca domestica. While petroleum-ether extracts of seeds of Griffonia simplicifolia and root extract of Zanthoxylum xanthoxyloides was found effective repellents and toxicants against the fly with LD50 of 0.28 and 0.35ug/mg, respectively (Begum et al., 2011).
The current results also showed consistency with the results of Mamun et al. (2009) who evaluated the toxic potential of six botanicals Ghora-neem (Melia sempervirens), Bazna (Zanthoxylum rhetsa), Hijal (Barringtonia acutangula), Mahogoni (Swietenia mahagoni), Karanja (Pongamia pinnatd), and Neem (Azadirachta indica) against Tribolium castaneum Herbs. They found that A. indica seed extract showed highest toxicity (mortality, 52.50%). Tripathi et al. (2002) investigated the toxic potential of essential oils extracted from turmeric leaves Curcuma longa L., against three beetles Sitophilus oryzae L., Rhyzopertha dominica F., and Tribolium castaneum Herbst. It was found that R. dominica adults were significantly susceptible to action of C. longa leaf oil, having LD50 value of 36.71 ug/mg weight of insect.
The result of current study were lined with Ahmad et al. (2015) who represented the activity of extracts of neem (Azadirachta indica), tobacco (Nicotiana tabacum), ginger (Zingiber officinale), holy basil (Ocimum sanctum), jatropha (Jatropha podagrica) and turmeric (Curcuma longa) on house fly larvae. Their results revealed that the effect of extracts increased with concentration and significantly influenced the life history parameter so that these extracts could be used for the management of housefly. During the present study, growth regulatory impact of weed plant extracts of all the weed plants was observed. P. harmala showed longest larval and pupal duration The results obtained was strongly supported by the findings of Ayvaz et al., 2010, Alzogaray et al., 2011 they suggested that insecticidal potential of the essential oils vary in accordance to species, stage of insect, and the plant origin of the essential oil that would be attributed to the diversified chemical composition of the oils and the interactions in between the individual constituents of the mixture. The insecticidal activity of N. tabacum, A. indica, C. citrullus, M. azedarachta, and E. camaldulensis was confirmed by Khan and Marwat (2004).
Growth regulation: Vinuela et al. (2000) also investigated that azadirachtin, one of the active metabolite of A. indica has disturbing effects on insect development including prolongation of larval or pupal stages and inhibition of moulting. These biochemical metabolites induce elongation of pupation period and adult emergence due to the interference with bioformation of ecdysone hormone by flavonoids which affect cytochrome- P450 involved in control of moulting process in insects. Similar findings were also observed during present study, the longest larval duration (9.33 ± 0.33 days) of M. domestica treated with P. harmala and D. stramonium followed by A. indica (8.67 ± 0.33 days). On the other hand, prolonged pupal duration (7.33 ± 0.33 days) was noted in P. harmala and A. indica as compared to D. stramonium (7.00 ± 0.58 days). Whereas P. harmala induced maximum development time (17.00 ± 0.58 days) than D. stramonium (16.67 ± 0.33 days), A. indica (15.67 ± 0.33 days), T. terrestris (14.67 ± 0.33 days) and C. murale (14.33 ± 0.33 days.
Oviposition deterrence: The results of oviposition deterrence in present work showed that percent deterrence was increased with the increase in concentration of plant extracts. C. murale showed highest oviposition deterrence activity (81.88%) followed by D. stramonium (79.58%), A. indica (74.10%), P. harmala, (68.14%) and T. terrestris (48.96%). Inhibition of egg-laying and hatchability (99.2% effectiveness at a 0.01% concentration) due to compound extracted from Guarea kunthiana against cattle tick Rhipicephalus (Boophilus) microplus is reported by Miguita et al. (2014). Lantana camara extract caused significant reduction in number of eggs of M. domestica as reported by Elkattan et al. (2011). All of these studies revealed that plant extracts showed oviposition deterrent effect.
Repellency: Of repellent activity, it was found that the repellency potential of extracts was increased as the test concentration was increased. The highest repellent effect was observed after 1st hour at 25% concentration among all used plants. The highest mean repellency at the concentration of 25% after all exposure times was showed by P. harmala (76.67%) followed by D. stromium (73.16%), A. indica (58.55%), T. terrestris (48.89%) and C. murale (25.82%). The repellent, larvicidal and pupicidal effect of essential oils of plants against Musca domestica has already been reported by Kumar et al. (2011). These results were also supported by Sagheer et al. (2013) they investigated acetone extracts of four indigenous plants, Nicotiana tabacum, Pegnum hermala, Saussurea costus and Salsola baryosma in different concentrations viz. 2.5, 5, 7.5 and 10% for their repellent and insecticidal effects against Tribolium castaneum (Herbst). P. hermala imposed significant repellency and mortality of T. castaneum (Herbst). Sidra-Tul-Muntaha et al. (2017) also evauated the repellent and growth regulatory efficiency of five plant extracts (Pegnum hermala, Melia azadirach, Azadirachta indica, baryosma and Zingiber officinale) and three synthetic insecticides on Callosobruchus chinensis populations. A. indica was found to be most efficient among plant extracts and showed higher than 90% repellency and upto 80% progeny inhibition at high concentration (20%) level. Current weed plant extracts showed significant repellency and mortality against M. domestica larvae and these findings are parallel to the results reported.
Enzyme Activity: These results are related to Riaz et al. (2018) that plant extracts caused reduction in the activities AChE, ACP, AKP, α-Carboxyl, and β-Carboxyl enzymes. The tested weed plant extracts revealed substantial inhibitory impact on target enzymes which was enhanced with increased concentration. Similarly, the enzymes inhibition activity of plant extracts have already reported by Zibaee and Bandani (2010) and enhancing the plant extract concentrations enhanced the enzyme inhibition. The present results of enzymes inhibition activity of plant extracts are supported by AChE inhibition activity of Periplaneta americana L., at 4 ppm in the cockroach, observed by Shafeek et al. (2004).
Comet assay: The studied weed plants also represented significant DNA damage that are supported by the findings of Dua et al. (2013), reported genotoxicity induced by essential oil of Psoralea corylifolia Linn. against Culex quinquefasciatus. The observed mean comet tail lengths were 6.2548 ± 0.754 μm and 8.47 ± 0.931 μm and their respective DNA damages were 6.713% and 8.864% compared to control at 0.034 and 0.069 mg/cm2. Kumar et al. (2015) also observed genotoxicity of adult flies Drosophila melanogaster at 30 and 55 mg/L ethanolic extract of Acorus calamus. The mean comet tail length was 4.24 ± 0.653 μm and 6.13 ± 0.721 μm and the respective DNA damage was 5.1% and 7.3% with reference to controls. These findings are parallel to the of present study which showed the mean comet tail lengths of Penganum harmala, Datura stramonium and Azadirachta indica at highest concentration as 10.20 ± 0.49, 9.20 ± 0.37 and 7.80 ± 0.49 μm while percent DNA damage was 10.56 ± 0.77, 10.67 ± 1.62 and 8.11 ± 0.85% respectively. It is also reported that alkaloids are known to cause death of susceptible organisms by their ability to bind to the DNA of the organisms therefore affecting replication and subsequent synthesis.
4.1. Phytochemical screening
The phytochemical current results of petroleum ether extracts of weeds and A.indica (Reference plant) revealed the presence of flavonoids, alkaloids, cardiac glycosides, saponins, steroids, terpenoids and tannins. Kaur and kalia (2012) evaluated the presence of flavonoids, tannins, saponins and glycosides in phytochemical analysis of petroleum ether extract of arial parts of C. arvensis. The concentration of phytochemical compounds in decreasing order was as alcohols > CHCl3 > petroleum ether. Similarly, Abbas et al. (2012) studied 15 weeds and reported steroids, alkaloids, saponins, flavonoids, glycosides, terpenoids, anthraquinine and tannins from the weed seeds during qualitative phytochemical screening.
FTIR analysis: FTIR analysis was performed for those plants displaying most prominent pesticide potential for the analysis of major secondary bioactive compounds. Mahendra et al. (2016) described that ethanolic extract of O. corniculata possess secondary metabolites such as steroids, phenolic, saponins, groups, tannin, flavonoids, coumarins, carbohydrates, alkaloids and terpenoids which were further supported by FTIR analysis. The present results of IR spectra of P. harmala are related to the previously reported FTIR analysis. The extract revealed major peaks and possible chemical bonds indicating C—H bending assigned to C H group, carbonyl (C O) signifying the ester linkage, C—H group stretching due to alkyl group and aromatic ring having phosphate group. Furthermore, the phytochemical analysis indicated the occurrence of flavonoids, alkaloids and anthraquinones. And these bioactive ingredients can be potentially employed for the management of insect pest (Isman et al., 2007). Based on the current results and supported work reported in the literature; it is suggested that plant-derived bioactive compounds have shown high biological activity against life history attributes of house fly, Musca domestica. Thus, the results revealed that weed plants extract can be used as an effective alternative of synthetic pesticides for its control.
Author contributions
MKZ designed and supervised the work. Attaullah performed the experiments. MZ, KR, KS did collection, extraction and helped in chemical as well as data analyses. SQ, MAZ, MSM, HNM helped to perform biochemical assays as well as gave their input during write up. MI gave suggestions and helped during write-up. All authors read and approved the final version of the manuscript
Acknowledgments
The facilities and support provided by the Department of Zoology, Government College University Faisalabad are highly acknowledged.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbas M., Rana S., Shahid M., Rana N., Mahmood-ul-Hassan M., Hussain M. Chemical evaluation of weed seeds mixed with wheat grains at harvest. J Anim Plant Sci. 2012;22:283–288. [Google Scholar]
- Anjum S.I., Yousaf M.J., Ayaz S., Siddiqui B.S. Toxicological evaluation of chlorpyrifos and neem extract (Biosal b) against 3rd instars larvae of Drosophila melanogaster. J. Anim. Plant Sci. 2010;20(1):9–12. [Google Scholar]
- Abbott W.S. A method of computing the effectiveness, of an insecticide. J. Econ. Entomol. 1925;18:265–267. [Google Scholar]
- Alves A.P.C., Corr̂ea A.D., Alves D.S., Saczk A.A., Lino J.B.R., Carvalho G.A. Toxicity of the phenolic extract from jabuticabeira (Myrciaria cauliflora (Mart.) O. Berg) fruit skins on Spodoptera frugiperda. Chil. J. Agric. Res. 2014;74:200–204. [Google Scholar]
- Alzogaray R.A., Lucia A., Zerba E.N., Masuh H.M. Insecticidal activity of essential oils from eleven Eucalyptus spp. and two hybrids: lethal and sublethal effects of their major components on Blattella germanica. J. Econ. Entomol. 2011;104:595–600. doi: 10.1603/EC10045. [DOI] [PubMed] [Google Scholar]
- Anjum S.I., Yousf M.J., Ayaz S., Siddiqui B.S. Toxicological evaluation of chlorpyrifos and Neem extract (Biosal B) against 3rd instars larvae of Drosophila melanogaster. J. Anim. Plant. Sci. 2010;20:9–12. [Google Scholar]
- Arivoli S., Tennyson S. Screening of plant extracts for oviposition activity against Spodoptera litura (Fab) (Lepidoptera: Noctuidae) Int. J. Fauna Biol. Stud. 2013;1:20–24. [Google Scholar]
- Ayvaz A., Sagdic O., Karaborklu S., Ozturk I. Insecticidal activity of the essential oils from different plants against three stored-product insects. J. Insect Sci. 2010;10:21. doi: 10.1673/031.010.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura K. Phosphatase activity in the larva of the euryhaline mosquito, Aedes togoi Theobald, with special reference to sea-water adaptation. J. Exp. Mar. Biol. Ecol. 1978;31(3):325–337. [Google Scholar]
- Begum N., Sharma B., Pandey R.S. Evaluation of insecticidal efficacy of Calotropis procera and Annona squamosa ethanol extracts against Musca domestica. J Biofertil Biopestic. 2011;1 [Google Scholar]
- Bukhari N., Choi J.H., Jeon C.W., Park H.W., Kim W.H., Khan M.A., Leet S.H. Phytochemical studies of the Alkaloids from Peganum Harmala. Appl. Chem. 2008;12:101–104. [Google Scholar]
- Caballero-Gallardo K., Olivero-Verbel J., Stashenko E.E. Repellency and toxicity of essential oils from Cymbopogon martinii, Cymbopogon flexuosus and Lippia origanoides cultivated in Colombia against Tribolium castaneum. J. Stored. Prod. Res. 2012;50:62–65. [Google Scholar]
- Elkattan N.A., Ahmed K.S., Elbermawy S.M., El-Gawad R.M.A. Effect of some botanical materials on certain biological aspects of the house fly Musca domestica. Egypt J Hosp Med. 2011;42:33–48. [Google Scholar]
- Gaire B.P., Subedi L. A review on the pharmacological and toxicological aspects of Datura stramonium L. Chin J. Integr. Med. 2013;11:73–79. doi: 10.3736/jintegrmed2013016. [DOI] [PubMed] [Google Scholar]
- Harborne, J.B., 1973. Phytochemical methods, London. Chapman and Hall, Ltd. 49, 188.
- Huang Q., Liu M., Feng J., Liu Y. Effect of dietary benzoxadiazole on larval development, cuticle enzyme and antioxidant defense system in housefly (Musca domestica L.) Pest Biochem. Physiol. 2008;90:119–125. [Google Scholar]
- Isman, M. B., Akhtar, Y., 2007. Plant natural products as a source for developing environmentally acceptable insecticides, in: Insecticides Design Using Advanced Technologies. Springer,Berlin, Germany, I. Shaaya, R. Nauen, A.R. Horowitz (Eds.), pp. 235–248.
- Kaur M., kalia AN. Pharmacognostic parameters and phytochemical screening of Convolvulus arvensis Linn. IRJP. 2012;3(10):162–163. [Google Scholar]
- Khan S.M., Marwat A.A. Effect of Bakain (Melia azadarach) and AK (Calatropis procera) against lesser grain borer Rhyzopertha dominica (F.) J. Res. Sci. 2004;15:319–324. [Google Scholar]
- Khandelwal, K.R., 2004. Practical pharmacognosy techniques & experiments. 20th ed. Nirali Parkashan, Pune, pp. 149–156.
- Kumar A., Sharma S., Verma G. Insecticidal and genotoxic potential of Acorus calamus rhizome extract against Drosophila melanogaster. Asian J. Pharm. Clin. Res. 2015;8:113–116. [Google Scholar]
- Kumar P., Mishra S., Malik A., Satya S. Repellent, larvicidal and pupicidal properties of essential oils and their formulations against the housefly Musca domestica. Med. Vet. Entomol. 2011;25:302–310. doi: 10.1111/j.1365-2915.2011.00945.x. [DOI] [PubMed] [Google Scholar]
- Lee M. Solanaceae IV: Atropa belladonna, deadly nightshade. J. R Coll Physicians Edinb. 2007;37:77–84. [PubMed] [Google Scholar]
- Lu J., Wu S. Bioactivity of essential oil from Ailanthus altissima bark against 4 major stored-grain insects. Afr. J. Microbiol. Res. 2010;4:154–157. [Google Scholar]
- Mahendra S.K., Kambleb S.P., Mohan B.W., Anup D.P., Sangita D.J. Chemical profiling and free radical scavenging potential of Oxalis corniculata. The Explorer. 2016;1(1):88–93. [Google Scholar]
- Malik A., Singh N., Satya S. House fly (Musca domestica): a review of control strategies for a challenging pest. J. Environ. Sci. Health B. 2007;42:453–469. doi: 10.1080/03601230701316481. [DOI] [PubMed] [Google Scholar]
- Mamun M.S.A., Shahjahan M., Ahmad M. Laboratory evaluation of some indigenous plant extracts as toxicants against red flour beetle, Tribolium castaneum Herbst. J. Bangladesh Agric Univ. 2009;7:1–5. [Google Scholar]
- Mansour, S.A., Bakr, R.F., Mohamed, R.I., Hasaneen, N.M., 2011. Larvicidal activity of some botanical extracts, commercial insecticides and their binary mixtures against the housefly, Musca domestica L. The Open Toxinology J 4.
- Miguita C.H., Barbosa C.D.S., Hamerski L., Sarmento U.C., Nascimento J.N.D., Garcez W.S., Garcez F.R. 3β-O-Tigloylmelianol from Guarea kunthiana: a new potential agent to control Rhipicephalus (Boophilus) microplus, a cattle tick of veterinary significance. Molecules. 2014;20:111–126. doi: 10.3390/molecules20010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S., Fields P.G. A simple technique to assess compounds that are repellent or to stored-product insects. J. Stored Prod. Res. 2002;38:23–31. [Google Scholar]
- Needham A.J., Kibart M., Crossley H., Ingham P.W., Foster S.J. Drosophila melanogaster as a model host for Staphylococcus aureus infection. Microbiol. 2004;150:2347–2355. doi: 10.1099/mic.0.27116-0. [DOI] [PubMed] [Google Scholar]
- Pavela R. Insecticidal properties of several essential oils on the house fly (Musca domestica L.) Phytother. Res. 2008;22:274–278. doi: 10.1002/ptr.2300. [DOI] [PubMed] [Google Scholar]
- Regnault-Roger C., Vincent C., Arnason J.T. Essential oils in insect control: low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012:57. doi: 10.1146/annurev-ento-120710-100554. [DOI] [PubMed] [Google Scholar]
- Riaz, B., Zahoor, M.K., Zahoor, M.A., Majeed, H.N., Javed, I., Ahmad, A., Jabeen, F., Zulhussnain, M., Sultana, K., 2018. Toxicity, phytochemical composition, and enzyme inhibitory activities of some indigenous weed plant extracts in fruit fly, Drosophila melanogaster. Evid Based Complement Alternat Med. [DOI] [PMC free article] [PubMed]
- Sagheer M., Ali K., Mansoor-ul-Hasan, Rashid A., Sagheer U., Alvi A. Repellent and Toxicological Impact of Acetone Extracts of Nicotiana tabacum, Pegnum hermala, Saussurea costus and Salsola baryosma against Red Flour Beetle, Tribolium castaneum (Herbst) Pakistan J. Zool. 2013;45:1735–1739. [Google Scholar]
- Siddiqui, A.A., Ali, M., 1997. Practical Pharmaceutical chemistry. 1st ed. CBS Publishers and Distributors, New Delhi, p. 126–131.
- Sidra-Tul-Muntaha, Sagheer M., Mansoor-ul-Hasan, Sahi S.T. Repellent and growth inhibitory impact of plant extracts and synthetic pyrethroids on three strains of Callosobruchus chinensis L. Pakistan J. Zool. 2017;49:581–589. [Google Scholar]
- Sofowara, A., 1993. Medicinal plants and traditional medicine in Africa. Spectrum Books Ltd, Ibadan, Nigeria, p. 289.
- Sultana K., Zahoor M.K., Sagheer M., Nasir S., Zahoor M.A., Jabeen F., Bushra R. Insecticidal activity of weed plants, Euphorbia prostrata and Chenopodiastrum murale against stored grain insect pest Trogoderma granarium Everts, 1898 (Coleoptera: Dermestidae) Turk. Entomol. 2016;40:291–301. [Google Scholar]
- Trease, G.E., Evans, W.C., 1989. Pharmacognsy. 11th ed. Brailliar Tiridel Can. Macmillian Publishers.
- Tripathi A.K., Prajapati V., Verma N., Bahl J.R., Bansal R.P., Khanuja S.P., Kumar S. Bioactivities of the leaf essential oil of Curcuma longa (var. ch-66) on three species of stored-product beetles (Coleoptera) J. Econ. Entomol. 2002;95:183–189. doi: 10.1603/0022-0493-95.1.183. [DOI] [PubMed] [Google Scholar]
- Vinuela E.A., Adan A., Smagghe G., Gonzalez M., Medina M.P., Budia F., Vogt H., Estal P.D. Laboratory effects of ingestion of azadirachtin by two pests (Ceratitis capitata and Spodoptera exigua) and three natural enemies (Chrysoperla carnea, Opius concolor and Podisus maculiventris) Biocontrol. Sci. Technol. 2000;10:165–177. [Google Scholar]
- Van Asperen K. A study of housefly esterases by means of a sensitive colorimetric method. J. Insect. Physiol. 1962;8(4):401–416. [Google Scholar]
- Younes M.W., Othman S.E., Elkersh M.A., Youssef N.S., Omar G.A. Effect of seven plant oils on some biochemical parameters in Khapra beetle Trogoderma granarium Everts (Coleoptera: Dermestidae) Egypt. J. Exp. Bio. 2011;7:53–61. [Google Scholar]
- Zia S., Sagheer M., Razaq A., Mahboob A., Mehmood K., Haider Z. Comparative bioefficacy of different Citrus peel extracts as grain protectant against Callosobruchus chinensis, Trogoderma granarium and Tribolium castaneum. World Appl. Sci. J. 2013;21:1760–1769. [Google Scholar]