Abstract
In order to study the relationship between ovarian endometriosis and clinical pregnancy, explore the correlation between endometriosis (EMT) and abortion rate and its mechanism, and provide a new theoretical basis for clinical diagnosis as well as treatment of endometriosis, in this study, pelvic endometriosis under 40 years old and have in vitro fertilization-embryo transfer (IVF-ET) operation will be selected as subjects of study. SPSS20.0 statistical software is used to analyze the data. When the measurement data between groups are compared, it is necessary to use t-test. It is necessary to use mean ± standard deviation () to expressed the results. When the counting data between groups is compared, it is necessary to use Chi-square test. Finally, the binomial classification logistic regression model is established by stepwise regression method to screen out the significant factors. The results show that the infertility duration of ovarian endometriosis cyst is (3.1 ± 1.9). The infertility years of other pelvic endometriosis are (3.9 ± 2.2). The infertility years of ovarian endometriosis cyst group are shorter than those of other pelvic endometriosis groups. Significant difference cannot be seen in follicle stimulating hormone (FSH) between the two groups of patients, and the basal FSH of other pelvic endometriosis groups is obviously lower in the two groups between the ages of 29 and 40 years. In the ovarian endometriotic cyst group, the significant difference can be seen (P < 0.05). Moreover, in the comparison of ovulation induction, the Gn dosage of fresh-cycle ovarian endometriosis patients is obviously higher than that of patients with ovarian endometriosis during the freezing cycle. The fertilization rate of patients with fresh cycle ovarian endometriosis is higher than that of patients with ovarian endometriosis during the freezing cycle. The two groups of patients with the factor of ovarian endometriosis after fresh embryo transplantation and the factor of fallopian tube are compared, and the abortion rate of the ovarian endometriosis group is lower than that of the fallopian tube group. Therefore, controlling the development of ovarian endometriosis can help to improve the pregnancy rate, reduce the abortion rate and improve the pregnancy outcome of patients with ovarian endometriosis.
Keywords: Ovarian endometriosis, Infertility, Clinical pregnancy, Abortion, Cyst
1. Introduction
Endometriosis is a common disease due to the implantation of active endometrial cells outside the endometrium (Yuan et al., 2016). Endometriosis is the most important cause of female infertility, and can greatly affect patients. For example, if it is not cured, it will influence their survival status as well as quality of life (Ma et al., 2017). Endocrine dysfunction, immune defense deficiency and genetic and physical factors are the main causes of endometriosis (Bu et al., 2016). There are many clinical manifestations of endometriosis, such as dysmenorrhea, abnormal menstruation, infertility, sexual intercourse pain and so on (Fang et al., 2016). In recent years, in the statistics of patients with endometriosis, it has been found that the infertility rate of patients with endometriosis can reach 35%-48%, while in these patients, the incidence of endometriosis is about 30%-45%. Compared with women with normal fertility, infertile women are far more likely to suffer from endometriosis than women with normal fertility (Vaegter et al., 2017). In vitro fertilization and embryo transfer (IVF-ET) can effectively improve the pregnancy rate of patients who has infertility and endometriosis. However, as far as early studies are concerned, the rate of pregnant in vitro fertilization in patients with endometriosis is lower than that of infertility caused by other factors (Senapati et al., 2016). It is considered that the low rate of pregnant has a close relationship with the decrease of oocyte quality and the change of endometrial receptivity. There are also literatures comparing endometriosis with non-endometriosis patients receiving donated eggs. Ovarian endometriosis can form cysts, known as endometriosis cysts, habitually known as chocolate cysts (Parazzini et al., 2016). Ovarian endometriosis cyst is the most common form of endometriosis, up to 25–35% of patients with endometriosis.
Abortion is a common complication of pregnancy (Saraswat et al., 2017), which means that the pregnancy is terminated when the pregnancy is less than 28 weeks and the fetus weighs less than 1000 g, or the fetus ceases to develop or spontaneously flows out. According to the time, abortion can be divided into early abortion and late abortion. Abortion can be divided into spontaneous abortion and artificial abortion. In early abortion, the incidence of spontaneous abortion accounts for about 20% of all pregnancies, and more than 80% of abortions occur in the early stage. With the increase of time, the abortion rate begins to decline rapidly (Sterling et al., 2016). There are many reasons for spontaneous abortion, including chromosome number abnormality, chromosome structure abnormality, uterine malformation, reproductive tract infection and environmental factors (Eftekhar et al., 2019).
In this study, the prognosis of young patients with endometriosis is analyzed retrospectively. The study objective is to explore the relationship between endometriosis and abortion rate, and the effect of IVF-ET on ovulation rate, fertilization rate, eugenia rate and pregnancy outcome after transplantation, and to explore the effect of IVF on patients with ovarian endometriosis. The relationship between endometriosis and spontaneous abortion and its mechanism are discussed. It is found that there is a great correlation between ovarian endometriosis and clinical pregnancy and abortion rate. Ovarian endometriosis can lead to adverse pregnancy outcome by affecting the embryo quality of patients. To control the development of ovarian endometriosis can effectively alleviate the abortion rate of patients with ovarian endometriosis, improve the rate of successful pregnancy, and promote the development of a good pregnancy outcome. This study can provide reference and guidance for clinical diagnosis and treatment, and has great significance for improving the pregnancy outcome of ovarian endometriosis.
2. Methodology
2.1. Research subjects
In this study, patients under 40 years old who underwent IVF-ET were selected as subjects. Age plays an essential part in affecting the ovarian function of women. Therefore, in this experiment, women who are less than 40 years old with endometriosis are selected, which can minimize the impact of age on the outcome of IVF-ET. The inclusion criteria are under 40 years old, without malignant or borderline lesions, except for adenomyosis, adenomyoma, hydrosalpinx and other infertility factors, unilateral or bilateral ovarian cyst removal surgery and pathological indication of ovarian endometriosis cyst. Patients with chromosomal abnormality, uterine malformation or other congenital reproductive tract dysplasia and malformation, ovarian benign and malignant tumor, chemotherapy history and serious diseases of heart, liver, brain, lung and other organs were excluded from the experiment. In addition, all IVF-ET patients were treated with ovulation induction. Pregnancy outcomes after the first embryo transfer were calculated, such as multiple ovulations, and the outcomes of the first ovulation in the cycle were studied (Kapfhamer et al., 2018, Chen et al., 2018). Informed consent was signed by all patients as well as their families. Ethics committee approve the study.
2.2. Ovulation induction therapy
The first treatment is to measure blood E2 and P from the middle luteal phase of the previous cycle, and to evaluate the amount of ovarian sinus follicles with B-mode ultrasonography. When P > 10 μg/L, GnRH-a 0.04 mg/d begin to be injected into it. When blood HCG is negative, pregnancy is excluded. On the third day of menstruation in this cycle, B-mode ultrasound is used to reevaluate and measure whether serum hormone levels reach desensitizing state. When the desensitized state is reached, the possibility of pregnancy is excluded and Gn is activated to stimulate ovulation until the follicle matures.
The second treatment is to start Gn ovulation 3 days after the menstrual cycle. The total daily dose is 160-320UL. When the diameter of follicles in uterus reaches 12 mm or LH > 12 IU/1, GnRH-anta is injected into uterus at 0.24 mg/d until follicles mature.
The third treatment is to use 120–220 mg/d Angel tan after the third day of menstruation, and add a certain amount of Gn into it until the follicle matures. If the follicle diameter is greater than 12 mm, or LH is greater than 12 IU/n, GnRH anta of 0.115–0.20 mg/d can be injected into the uterus until the follicle matures (Di Nisio et al., 2018, Thomsen et al., 2018).
2.3. Fertilization and embryo evaluation
The collected oocytes are placed for 3 h before fertilization. During in vitro fertilization, about 35,000 motile sperm are added to each droplet. Before intracytoplasmic sperm microinjection, it is necessary to ensure that there is evidence of intracytoplasmic sperm microinjection and if so continue to fertilize. In this experiment, the embryo culture system is G-1.3 series sequential culture system. After the third day of egg collection, embryo transfer and cryopreservation are initiated. According to the quality and symmetry of blastomeres, the embryo scoring system is divided into four grades. On the third day, when the number of cleavage spheres is 5–10 cells and the cell fragments are less than or equal to 30%, the embryos of grade 2 and above are defined as high-quality embryos. In this experiment, the classical TESTART's slow freezing and rapid embryo thawing techniques are used. Vitrification freezing and thawing is to use a high concentration of cryoprotectant to replace the solution inside and outside the cell and transfer directly to the liquid nitrogen tank for rapid cooling and preservation. More than half of the restored embryos remain intact, i.e. live embryos. After thawing, the embryos are re-rated. Embryo transfer is completed within 6 h after thawing (Thomsen et al., 2018).
2.4. Embryo transfer and pregnancy assessment
After 3 days of egg extraction, the quality of embryos and the patient's condition are evaluated. Three embryos are transferred in each cycle. If the following conditions occur, frozen embryo transfer is used, such as ovarian hyperstimulation syndrome, endometrial factors, early increase of progesterone and specific ovulation stimulation program. Normally, frozen embryo transfer is used 1–3 months after ovulation.
Endometrial preparation in frozen embryo transfer cycle is usually natural cycle if female ovulation is normal. If ovulation is abnormal, hormone replacement cycles or low dose Gn stimulation cycles are used. (Machtinger et al., 2018).
2.5. Morphological evaluation index of cleavage embryos
The morphological evaluation indexes of cleavage embryos mainly include the number of cleavage balls, the homogeneity of cleavage balls, the color and cytoplasmic morphology of embryos, the quantity distribution of embryo fragments, the status of zona pellucida and perioval space, etc. The first level refers to the irregular and slightly uneven shape of the cleavage sphere. The refractive index changes slightly, but not significantly, with less than 20% debris. Secondary refers to the spherical cleavage of the same shape as the secondary embryo, with complete zona pellucida and less than 50% fragments. Level 3 means that the survival rate of blastomeres is still more than 50%. High-quality embryos refer to the fusion of primary and secondary embryos and embryos reach 6–10 cells in 72 h.
Endometrial thickness is the maximum diameter of the longitudinal section of the midline of the uterus measured by transvaginal B-mode ultrasonography when injecting HCG. As far as clinical pregnancy is concerned, it refers to the intrauterine or extrauterine pregnancy sac observed by B-mode ultrasonography 30–35 days after embryo transfer, which has the original fetal heart beat. Natural abortion refers to the loss of fetus before 28 weeks of clinical pregnancy, including cessation of embryonic and fetal development or spontaneous outflow (Mastrolia et al., 2018).
2.6. Statistical analysis of experimental data
SPSS20.0 statistical software is used to analyze the data, Kolmogorov-Simirnov test is used to test the normal distribution of a single sample, and t test is used for comparison. It is necessary to use mean ± standard deviation () to expressed the results. When the counting data between groups is compared, it is necessary to use Chi-square test. Fisher's exact probability method is used when the theoretical frequency is less than 1. Spearman rank is used for correlation analysis, and possible pairs are preliminarily screened out. The stepwise regression method is used. Pregnancy is the dependent variable (y = 0.1) and the screening parameters are the independent variable. The significant parameters of the outcome of IVF-ET for ovarian endometriosis cyst are selected, and the significant factors are screened by binomial logistic regression model. The difference has statistical significance. (P < 0.05).
3. Results and discussion
3.1. General situation comparison
Pelvic endometriosis patients under 40 years old who undergo IVF-ET are selected. There are 135 cases of ovarian endometriosis cyst under 28 years old and 205 cases of pelvic endometriosis cyst between 29 and 40 years old. 85 cases of other pelvic endometriosis are younger than or equal to 28 years old. 126 cases of other pelvic endometriosis are between 29 and 40 years old. As shown in the Table 1, the infertility duration of ovarian endometriosis cyst is (3.1 ± 1.9). The infertility years of other pelvic endometriosis are (3.9 ± 2.2). The infertility years of ovarian endometriosis cyst group are shorter than those of other pelvic endometriosis groups. The number of sinus follicles in ovarian endometriotic cysts is (6.9 ± 3.4). The number of sinus follicles in other pelvic endometriosis is (9.3 ± 3.5). The number of sinus follicles in other endometriosis group is higher than that in ovarian endometriosis cyst group (P < 0.01). The basic FSH of ovarian endometriosis cyst group is (7.9 ± 3.2). The basal FSH in other pelvic endometriosis groups is (7.7 ± 2.1). The basic FSH in other pelvic endometriosis group is lower than that in ovarian endometriosis cyst group (see Table 2, Table 3, Table 4, Table 5).
Table 1.
Comparison of the general situation of ovarian endometriotic cyst and other pelvic endometriosis patients.
| Ovarian endometriosis cyst | Other pelvic endometriosis | |
|---|---|---|
| Age | 28.7 ± 2.8 | 30.5 ± 3.1 |
| Infertility years | 3.1 ± 1.9 | 3.9 ± 2.2 |
| Basic FSH (IU/I) | 7.9 ± 3.2 | 7.7 ± 2.1 |
| Number of sinus follicles | 6.9 ± 3.4 | 9.3 ± 3.5 |
| Total Gn (TU) | 2352.5 ± 1358.5 | 1986.3 ± 671.4 |
| Gn days | 9.1 ± 2.4 | 8.9 ± 1.9 |
Table 2.
Comparison of ovulation induction in fresh cycles.
| Ovarian endometriosis factor group | Fallopian tube factor group | |
|---|---|---|
| Gn dosage (branch) | 39.4 ± 13.7 | 35.2 ± 15.8 |
| Number of eggs | 11.5 ± 5.8 | 11.3 ± 8.4 |
| Fertilization rate (%) | 70.2 | 75.2 |
| High quality embryo rate (%) | 62.7 | 79.8 |
Table 3.
Comparison of ovulation induction during freezing cycle.
| Ovarian endometriosis factor group | Fallopian tube factor group | |
|---|---|---|
| Gn dosage (branch) | 36.1 ± 12.6 | 33.6 ± 15.6 |
| Number of eggs | 8.5 ± 5.1 | 10.5 ± 6.8 |
| Fertilization rate (%) | 63.1 | 73.2 |
| High quality embryo rate (%) | 60.3 | 78.4 |
Table 4.
Comparison of fresh cycle transplantation.
| EMS factor group | Fallopian tube factor group | |
|---|---|---|
| Intimal thickness (mm) on transplantation day | 11.4 ± 0.8 | 12.4 ± 2.2 |
| High quality embryo transfer rate (%) | 84.1 | 85.9 |
| Embryo implantation rate (%) | 39.5 | 43.1 |
Table 5.
Comparison of cryopreservation cycle transplantation.
| EMS factor group | Fallopian tube factor group | |
|---|---|---|
| Intimal thickness (mm) on transplantation day | 9.4 ± 1.8 | 8.6 ± 1.5 |
| High quality embryo transfer rate (%) | 84.2 | 87.3 |
| Embryo implantation rate (%) | 36.1 | 41.3 |
3.2. Comparison of ovulation induction
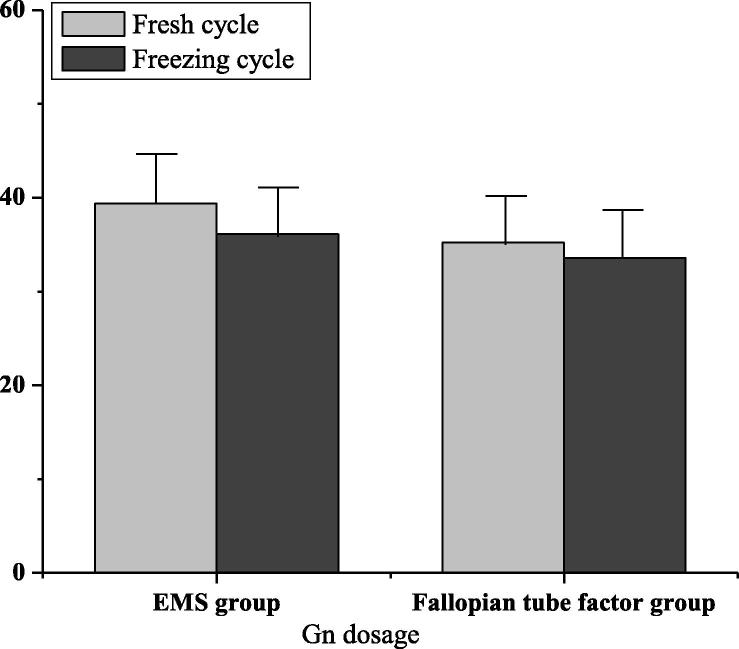
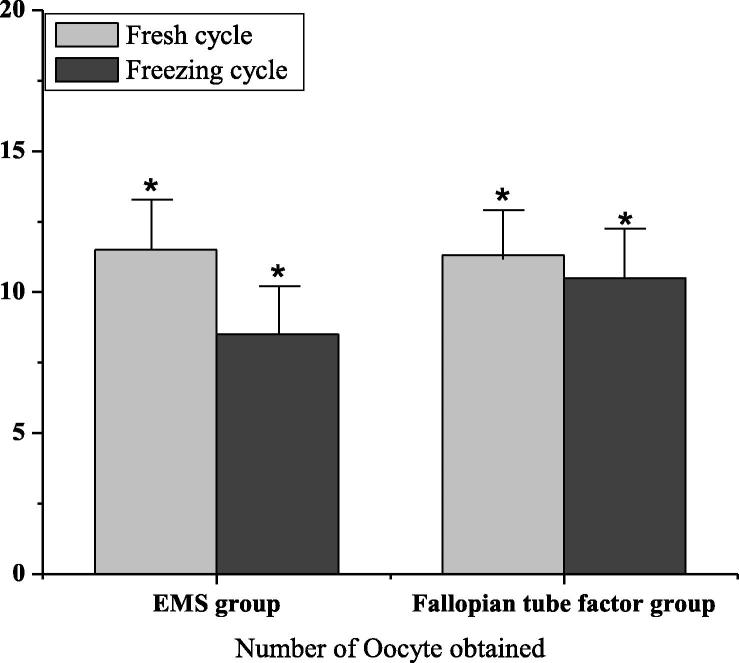
The dosage of Gn was (39.4 ± 13.7) in the factor group of ovarian endometriosis and (35.2 ± 15.8) in the factor group of tubal infertility. As shown in Fig. 1, the amount of Gn in the ovarian endometriosis factor group was higher than that in the tubal infertility factor group. There was no significant difference (P > 0.05). The number of oocytes in the factor group of ovarian endometriosis was (8.5 ± 5.1), and that in the factor group of tubal infertility was (10.5 ± 6.8). The amount of oocytes in the tubal factor group was higher than that in the ovarian endometriosis factor group. There was significant difference (P < 0.05). As shown in Fig. 2, the fertilization rate as well as high-quality embryo rate of the fresh cycle ovarian endometriosis factor group were 70.2 and 62.7 respectively, that of the fallopian tube factor group were 75.2 and 79.8 respectively, and that of the tubal factor group were higher than that in the ovarian endometriosis factor group. There was significant difference (P < 0.05).
Fig. 1.
Gn dosage in EMS group and fallopian tube group.
Fig. 2.
Number of Oocyte obtained in EMS group and fallopian tube group (* indicates P < 0.05).
3.3. Comparison of transplantation
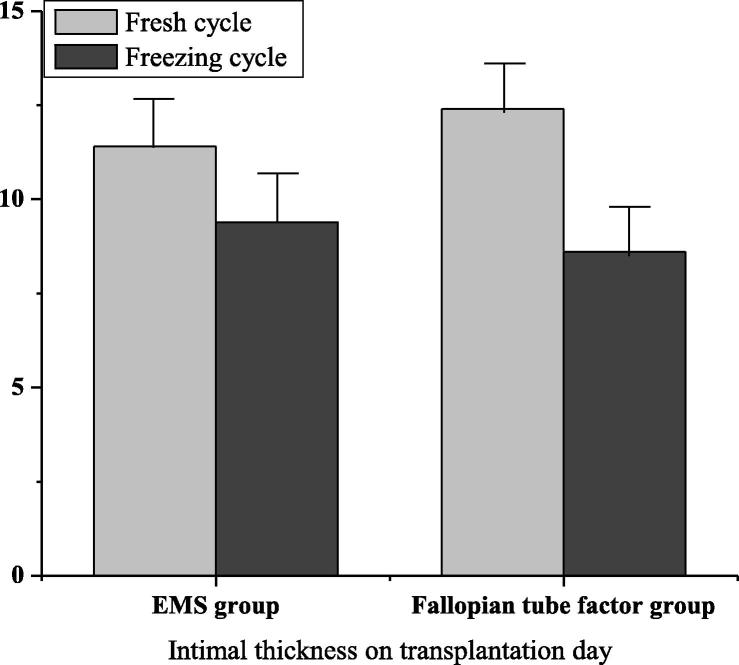
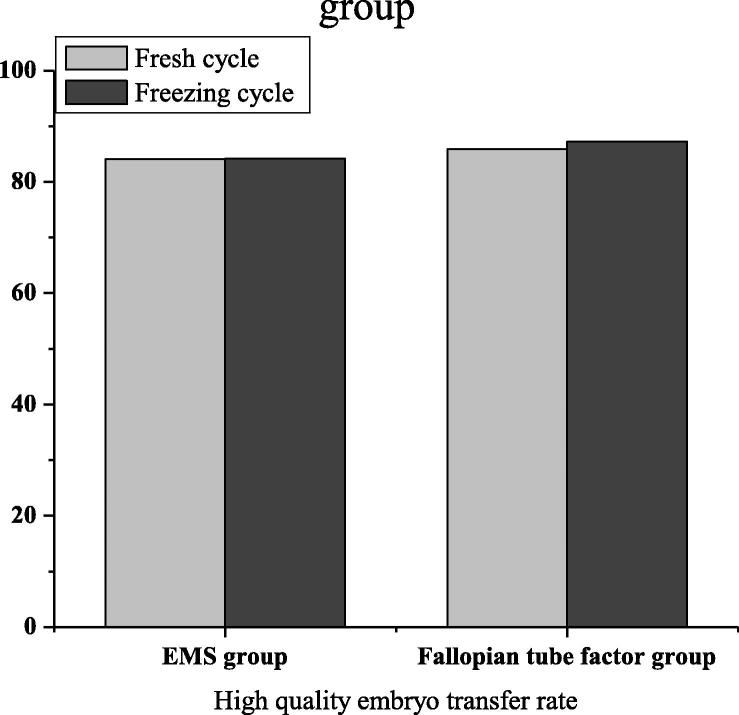
In the factor group of ovarian endometriosis, when transplanting, in terms of the endometrium, its thickness was (11.4 ± 0.8) in fresh cycle and (9.4 ± 1.8) in frozen cycle. That was (12.4 ± 2.2) and (8.6 ± 1.5) in the fallopian tube factor group. After statistical analysis, there was no statistical significance (P > 0.05). In Fig. 3, the high-quality embryo transfer rates of fresh cycle and frozen cycle of ovarian endometriosis factor group were 84.1 and 84.2 respectively, while those of fresh cycle and frozen cycle of fallopian tube factor group were 85.9 and 87.3 respectively. After statistical analysis, there was no statistical significance (P > 0.05). In Fig. 4, the embryo implantation rates of fresh cycle and frozen cycle of ovarian endometriosis factor group are 39.5 and 36.1 respectively, while those of fresh cycle and frozen cycle of fallopian tube factor group are 43.1 and 41.3 respectively. No matter at what time, the embryo implantation rate of the fallopian tube factor group was higher than that of the ovarian endometriosis factor group. There was significant difference (P < 0.05).
Fig. 3.
Endometrial thickness on transplantation day in EMS group and fallopian tube group.
Fig. 4.
High-quality embryo transfer rate in EMS group and fallopian tube group.
3.4. Comparisons of pregnancy outcomes
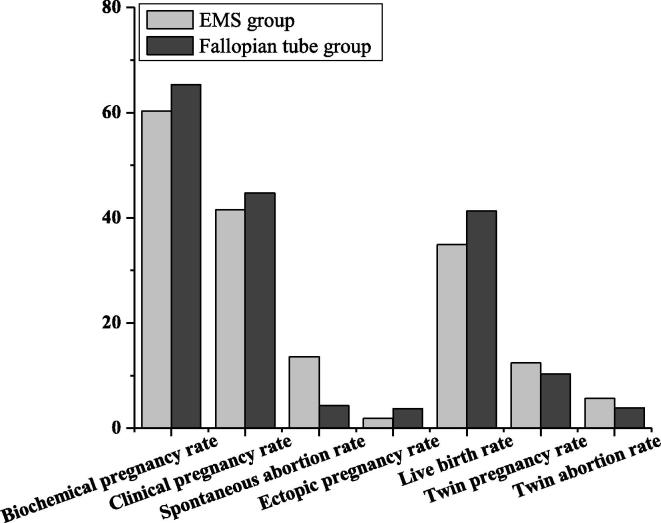
As shown in Fig. 5 and Table 6, the biochemical pregnancy rate (BPR), clinical pregnancy rate (CPR), twin pregnancy rate (TPR), twin abortion rate (TAR) and ectopic pregnancy rate (EPR) of the ovarian endometriosis factor group of fresh embryo transfer were 60.3, 41.5, 12.4, 5.7 and 1.9 respectively, while the BPR, CPR, TPR, TAR and EPR of the fallopian tube factor group of fresh embryo transfer were 65.3, 44.7, 10.3, 3.9 and 3.7 respectively. There was no significant difference (P > 0.05). The spontaneous abortion and live rate of fresh embryo transplantation in the factor group of ovarian endometriosis were lower than those in the factor group of fallopian tube. There was significant difference (P < 0.05). There was no significant difference in BPR, CPR, spontaneous abortion, TPR, TAR and ectopic pregnancy between the two groups, while the live birth rate of the fallopian tube factor group was higher than that of the ovarian endometriosis factor group. There was significant difference (P < 0.05) (see Table 7)
Fig. 5.
Fresh embryo cycle pregnancy outcome.
Table 6.
Comparison of pregnancy outcomes in fresh embryo cycle.
| EMS factor group | Fallopian tube factor group | |
|---|---|---|
| Biochemical pregnancy rate (%) | 60.3 | 65.3 |
| Clinical pregnancy rate (%) | 41.5 | 44.7 |
| Natural abortion rate (%) | 13.6* | 4.3 |
| Ectopic pregnancy rate (%) | 1.9 | 3.7 |
| Live birth rate (%) | 34.9* | 41.3 |
| Twin pregnancy rate (%) | 12.4 | 10.3 |
| Twin abortion rate (%) | 5.7 | 3.9 |
Table 7.
Area under ROC curve.
| Area under curve | Standard error | P value | lower limit | Upper limit | Specificity | Sensitivity | Truncated value | |
|---|---|---|---|---|---|---|---|---|
| Model Prediction Probability | 0.742 | 0.027 | 0 | 0.673 | 0.794 | 0.652 | 0.768 | 0.45 |
| Number of sinus follicles | 0.614 | 0.029 | 0 | 0.582 | 0.652 | 0.607 | 0.631 | 6.7 |
| 2PN Preferential Embryo Number | 0.732 | 0.027 | 0 | 0.659 | 0.798 | 0.674 | 0.746 | 1.3 |
3.5. Establishment of predictive model of IVF-ET treatment outcome in patients with ovarian endometriosis cyst
Pearson correlation analysis is performed between the parameters and the success of treatment. The number of infertility years, sinus follicles, eggs harvested, 2PN embryos and 2PN pregnant embryos are all related to the success of treatment. Except that the infertility years are only related to the number of 2PN eugenic embryos, there are correlations among other factors. These five factors are used to establish binomial classification logistic regression model by stepwise regression method. The number of sinus follicles and 2PN pregnant embryos are the key factors for the success of IVF-ET in ovarian endometriosis cyst patients.
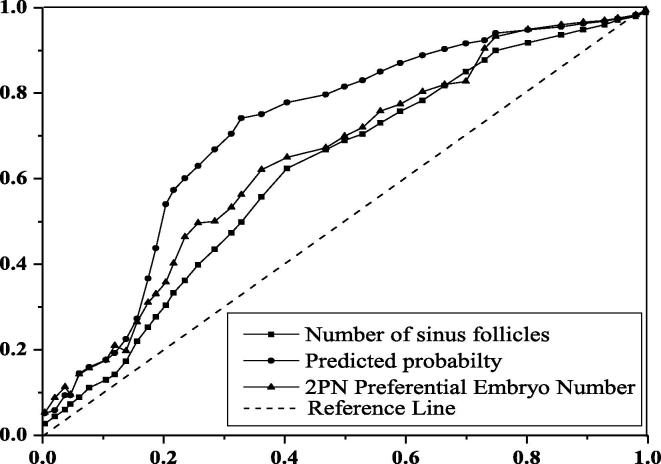
Two key factors and their regression coefficients were used to establish a probability model for predicting the outcome of IVF-ET in ovarian endometriosis cyst patients: P = 1/1 + exp (0.870–0.088 × (number of antral follicles) − 0.432 × (number of 2PN excellent embryos)), draw the ROC curve of the prediction rate of this model, and draw the ROC curve of single factor for the prediction rate of clinical outcome. As shown in Fig. 6, the area under ROC curve of the model was 0.742, with statistical significance (P < 0.001). The 95% confidence interval was (0.673–0.794), indicating that the predictive value was higher than any other parameter. The truncation value of euembryo 2PN is 1.3. The cutoff value of the amount of antral follicles was 6.7. The sensitivity of prediction probability, amount of antral follicles and amount of 2pn embryos were 0.768, 0.631 and 0.746, respectively.
Fig. 6.
Model prediction of subject working characteristic curves.
The data shows that there is no significant difference in the average age of ovarian endometriotic cysts between the operation group and the non-operation group, and the size of the preoperative cysts in the operation group is larger than that in the non-operation group (P < 0.001), while the number of sinus follicles in the operation group is better than that in the non-operation group (P < 0.05). The clinical pregnancy rate in the operation group is higher than that in the non-operation group, but there is no significant difference between the two groups.
4. Conclusion
In this study, by comparing the indexes of ovarian endometriosis cyst patients with other pelvic endometriosis patients and ovarian endometriosis factor group patients with fallopian tube factor group patients, it is found that there are significant differences in the number of antral follicles, basic FSH, number of oocytes obtained, 2PN embryo number and 2PN superior embryo number, and there is correlation among most factors. Through this study, it is found that ovarian function as well as embryo quality are the key factors that affect the final outcome of IVF-ET, which is also consistent with the results of many researchers.
The incidence of IVF assisted reproductive technology, implantation rate, high-quality embryo rate and live birth rate of infertile patients with ovarian endometriosis alone are lower than those of infertile patients with fallopian tube alone, and the rate of spontaneous abortion is higher than that of infertile patients with fallopian tube alone. This may be because ovarian endometriosis affects the quality of embryo through immune environment and other factors, leading to poor pregnancy outcome. Investigating whether ovarian endometriosis is related to spontaneous abortion is the objective of the study. Embryo quality may be the main factor affecting spontaneous abortion. Low embryo quality may eventually lead to high spontaneous abortion rate and low live rate of ovarian endometriosis. In conclusion, the following conclusions can be drawn:
First, ovarian endometriosis can affect the embryo quality of patients and other aspects, resulting in adverse pregnancy outcomes. To control the development of ovarian endometriosis can help to improve the rate of pregnant, reduce the abortion rate, and effectively improve the pregnancy outcome of patients with ovarian endometriosis.
Second, whether or not there is ovarian endometriosis cyst in endometriosis patients, the effect on ovum, embryo quality as well as endometrial receptivity is similar.
In this study, the innovative stepwise regression method is used to establish a two category logistic regression model. It is found that the key factors for the success of IVF-ET in patients with ovarian endometriosis cyst are the number of antral follicles as well as the 2PN embryos. However, the data of this study is only clinical pathological data, lack of relevant substantive evidence to show the correctness and reliability of the research results. In the future research, the experimental data in many aspects should be applied to enhance the reliability of the experimental results.
Acknowledgement
This work was supported by Natural Science Foundation of Guangxi (2018JJB140171); Development and Application of Appropriate Medical and Health Technologies in Guangxi (S2018111); The excellent Medical Talents Training Program of The First Affiliated Hospital of Guangxi Medical University (2018-12-9).
Footnotes
Peer review under responsibility of King Saud University.
References
- Bu Z., Wang K., Dai W. Endometrial thickness significantly affects clinical pregnancy and live birth rates in frozen-thawed embryo transfer cycles. Gynecol. Endocrinol. 2016;32(7):524–528. doi: 10.3109/09513590.2015.1136616. [DOI] [PubMed] [Google Scholar]
- Chen I., Lalani S., Xie R. Association between surgically diagnosed endometriosis and adverse pregnancy outcomes. Fertility Sterility. 2018;109(1):142–147. doi: 10.1016/j.fertnstert.2017.09.028. [DOI] [PubMed] [Google Scholar]
- Di Nisio M., Ponzano A., Tiboni G.M. Effects of multiple inherited and acquired thrombophilia on outcomes of in-vitro fertilization. Thrombosis Res. 2018;167:26–31. doi: 10.1016/j.thromres.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Eftekhar M., Mirhashemi E.S., Tabibnejad N. Assisted reproductive outcomes in women with different polycystic ovary syndrome phenotypes. Int. J. Gynecol. Obstetr. 2019;144(2):147–152. doi: 10.1002/ijgo.12707. [DOI] [PubMed] [Google Scholar]
- Fang R., Cai L., Xiong F. The effect of endometrial thickness on the day of hCG administration on pregnancy outcome in the first fresh IVF/ICSI cycle. Gynecol. Endocrinol. 2016;32(6):473–476. doi: 10.3109/09513590.2015.1132304. [DOI] [PubMed] [Google Scholar]
- Kapfhamer J.D., Palaniappan S., Summers K. Difference between mean gestational sac diameter and crown-rump length as a marker of first-trimester pregnancy loss after in vitro fertilization. Fertility Sterility. 2018;109(1):130–136. doi: 10.1016/j.fertnstert.2017.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N.Z., Chen L., Dai W. Influence of endometrial thickness on treatment outcomes following in vitro fertilization/intracytoplasmic sperm injection. Reprod. Biol. Endocrinol. 2017;15(1):5. doi: 10.1186/s12958-016-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R., Gaskins A.J., Racowsky C. Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes. Environ. Int. 2018;111:23–31. doi: 10.1016/j.envint.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrolia S.A., Baumfeld Y., Hershkovitz R. Independent association between uterine malformations and cervical insufficiency: a retrospective population-based cohort study. Arch. Gynecol. Obstetr. 2018;297(4):919–926. doi: 10.1007/s00404-018-4663-2. [DOI] [PubMed] [Google Scholar]
- Parazzini F., Tozzi L., Bianchi S. Pregnancy outcome and uterine fibroids. Best Practice Res. Clin. Obstetr. Gynaecol. 2016;34:74–84. doi: 10.1016/j.bpobgyn.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Saraswat L., Ayansina D.T., Cooper K.G. Pregnancy outcomes in women with endometriosis: a national record linkage study. BJOG: Int. J. Obstetr. Gynaecol. 2017;124(3):444–452. doi: 10.1111/1471-0528.13920. [DOI] [PubMed] [Google Scholar]
- Senapati S., Sammel M.D., Morse C. Impact of endometriosis on in vitro fertilization outcomes: an evaluation of the Society for Assisted Reproductive Technologies Database. Fertility Sterility. 2016;106(1):164–171. doi: 10.1016/j.fertnstert.2016.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling L., Liu J., Okun N. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertility Sterility. 2016;105(3):791–797. doi: 10.1016/j.fertnstert.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Thomsen L.H., Kesmodel U.S., Erb K. The impact of luteal serum progesterone levels on live birth rates—a prospective study of 602 IVF/ICSI cycles. Human Reprod. 2018;33(8):1506–1516. doi: 10.1093/humrep/dey226. [DOI] [PubMed] [Google Scholar]
- Vaegter K.K., Lakic T.G., Olovsson M. Which factors are most predictive for live birth after in vitro fertilization and intracytoplasmic sperm injection (IVF/ICSI) treatments? Analysis of 100 prospectively recorded variables in 8,400 IVF/ICSI single-embryo transfers. Fertility Sterility. 2017;107(3):641–648. doi: 10.1016/j.fertnstert.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Yuan X., Saravelos S.H., Wang Q. Endometrial thickness as a predictor of pregnancy outcomes in 10787 fresh IVF–ICSI cycles. Reprod. Biomed. Online. 2016;33(2):197–205. doi: 10.1016/j.rbmo.2016.05.002. [DOI] [PubMed] [Google Scholar]