Abstract
Tramadol is an analgesic and psychoactive drug that acts primarily upon the central nervous system where it alters brain function, resulting in temporary changes in perception, mood, consciousness and behavior. The aim of present study was to analyze the genotoxicity and repair capability of DNA after Tramadol exposure in albino mice (Mus musculus). For this purpose, forty mice were divided equally into four groups as; a control group (without drug) and three treatment groups that were treated with three doses of Tramadol as minimum dose group, Intermediate dose group and maximum dose group, corresponding to 25 mg/kg, 50 mg/kg and 75 mg/kg of body weight respectively. The dose was given orally for 15 days. After 15 days peripheral blood was drawn from half mice of each group and subjected to comet assay. While the remaining half mice were given a recovery period of 15 days and same procedure was used for blood collection and comet assay. Significant difference in various comet parameters was observed among control and exposed groups. Maximum damage was observed at highest concentration 75 mg/kg of Tramadol and minimum damage was observed at dose 25 mg/kg of Tramadol, while results of repaired mice group showed that repair capability of Tramadol was minor and recovery of Tramadol required a lot of time. It can be concluded that Tramadol cause genotoxicity that is dose dependent and has low repair capability.
Keywords: Tramadol, Mus musculus, Genotoxicity, Comet assay
1. Introduction
Drugs are the substances that influence the biological function by mimicking the action of neurotransmitters upon neurons when taken, and the drugs that alters the brain function by altering the mood, behavior and consciousness are known as psychoactive drugs. Psychoactive drugs are used by humans in all societies that affect them as a little dose can change the thinking and feeling of user and are mostly used for the treatment of common medical conditions for example pain, sleep disorders and anxiety but their unauthorized use is also common (Tjäderborn et al., 2016, Wadley, 2016).
Tramadol is a psychoactive drug and affects mainly CNS. It is a synthetic opioid analgesic that binds to the specific opioid receptors and is used as a first line drug for the treatment of postoperative injury induced acute pain. It has been in use for over 46 years and first used in therapeutic analgesis in Germany in 1977. Tramadol is not used for pregnancy and lactation due to limited clinical research on its use. Tramadol has been used for the treatment of different pain conditions i.e., post thoracotomy pain, abdominal surgery, somatic pain, visceral pain, neuropathic pain, acute dento-alveolar surgical pain (oral), day-case laparoscopic sterilization, orthopaedic surgery, paediatric surgery, obstetrics, acute ureteric colic, acute trauma and myocardial ischaemic pain (Bloor et al., 2012, Li et al., 2017, Miranda et al., 2012).
Tramadol can be used orally, intramuscularly and intravenously for the treatment of moderate to severe pain that may include postoperative, gynecological, obstetric and cancer pain. Tramadol is available in many forms as immediate release tablets or capsule that can be taken orally with the dose 50–100 mg after 4–6 h and extended release capsule (150 mg) or tablets (100–300 mg) that can be taken once daily and also injection (100 mg ampoules) and suppositories (100 mg Anadol). In humans, analgesia by Tramadol is induced about one hour after oral administration and peaks after two to three hours (Mabrouk et al., 2018).
Tramadol act by three different mechanisms as mu-opioid receptor in that it caused the metabolism of O-desmethyltramadol. The 2nd is serotonin reuptake inhibition by (+) – Tramadol and 3rd is norepinephrine reuptake inhibition by (−) –Tramadol. O-desmethyltramadol formed by O-demethylation from Tramadol and catalyzed by CYP2D6. This O-desmethyltramaadol caused the opiate type effects in Tramadol. Tramadol also has anatagonist effects that are similar to NMDA and for that reason it used for neuropathic pain. Tramadol also have anti-inflammatory effect as it impaired immune system by increasing the serotonin and norepinephrine that caused a reduction in inflammatory cytokines that released during stress conditions (Rabei, 2011).
Tramadol cause neural cells disorganization, apoptotic cell formation, chromatolysis, formation of multinuclear cells and congested blood capillary formation in albino rats. Tramadol increases reactive oxygen level in brain mitochondria and mitoc-comhondrial swelling in rat. Chronic Tramadol cause impaired learning and memory by introducing dysfunction of brain mitochondria. Exposure of Tramadol before physical exercise caused brain mitochondrial dysfunction and impairment and is less severe (Mehdizadeh et al., 2017). The LD50 of Tramadol is 300–350 mg/kg body weight in rat and mouse for oral administration. While in case of intravenous administration these values are 50–100 mg/kg body weight. Signs of intoxication in case of chronic toxicity include convulsions, and behavioral problems at dose of 25 mg/kg and Tramadol cause toxicity at reproductive level (Matthiesen et al., 1998). Tramadol caused hepatotoxicity that is major problem. Because metabolism and excretion of opioids takes place in liver and kidney so it caused nephrotoxicity and hepatotoxicity. Kidney is considered as primary target of Tramadol toxicity because its metabolites go through the kidney. Tramadol also caused toxicity at cellular level that is associated with lipid peroxidation. An increase in lipid peroxidation was reported in rats that have received cocaine. Lipid peroxidation was also increase due to heroin use. Another study reported decrease in glutathione level in hapatcytes because of morphine that leads to cell death (Awadalla and Salah-Eldin, 2016).
Genotoxicity may be defined as destructive effects on a cell’s genetic material that affect its integrity. A substance that can cause genotoxicity is known as genotoxin. This genotoxin may be a radiation or a chemical. A genotoxin can affect in three ways as it may be carcinogen, mutagen or teratogen. Genotoxicity may cause mutation that lead to cancer. All mutagens are genotoxic but all genotoxins are not mutagenic and these mutations affect the DNA. Researcher used to assess DNA damage in order to assess the genotoxicity and the DNA damage have various conditions as may be of single and double strand breaks, cross linking, point mutations, chromosomal aberrations and loss of excision repair (Chai et al., 2017, Hussain et al., 2018, Nagarathna et al., 2013, Qureshi et al., 2017, Shah, 2012).
A lot of information is available in literature about toxicity of various psychoactive drugs i.e., cocaine, nicotine, mephedrone, methylone amphetamine, morphine, alcohol and benzoylecgonine etc. (Attia, 2007, Steinmetz et al., 2018). However, genotoxicity of Tramadol in albino mice has been neglected. So, the present study was conducted to study the genotoxicity and repair capability of Tramadol in Mus musculus and comet assay was used to observe the DNA damage. Comet assay is a sensitive method in which after lysing, electrophoretic migrated DNA obtained in the thin layer of agarose and the tail is formed by extended DNA loops. These DNA loops are similar to chromatin loops as in the cell nuclei and represent the extent of DNA damage (Solmaz and Kovalak, 2018). Comet assay has been used widely for environmental monitoring, to test newly developed drugs, food additives and also used for the monitoring of DNA repair by living organisms (Ali et al., 2018, Bankoglu et al., 2018, Bastaki et al., 2017). The benefits of comet assay include its applicability to various tissues and/or special cell types, its sensitivity for detecting low levels of DNA damage, its requirement for small numbers of cells per sample, general ease of test performance, the short time needed to complete a study and its relatively low cost.
2. Materials and methods
2.1. Animals and treatment
Albino mice Mus musculus (n = 40) were kept at Animal House, Department of Pharmacy, Government College University. A 30 days trial was conducted to access the genotoxicity and repair capability of Tramadol in mice. 4–6 weeks old mice with an average weight of 29 ± 3.8 g were housed in 4 groups (Control, group 1, 2 and 3) in different cages with 10 mice in each group. Mice were given free access to food and water. Mice were carefully observed for seven days prior to the start of trial. Behavior of mice as well as the amount of water and feed intake was also monitored. Proper hygienic and environmental conditions were maintained to get the best results. Three different concentrations of Tramadol i.e., 25 mg/kg, 50 mg/kg and 75 mg/kg were prepared by mixing them in distilled water to make a solution and administered orally at the same time of the day for 15 days. Drug was administered very carefully to avoid any injury to mice. After 15 days, half mice from each group were dissected, peripheral blood was drawn by puncturing jugular vein then organs were removed and transported immediately to the Research Lab., Department of Zoology, Government College University. Recovery period of 15 days was given to remaining half mice to check the repair capability of Tramadol in them. After 15 days remaining mice were dissected and same procedure was repeated for organs and blood removal.
2.2. Comet assay
Peripheral blood was used to perform the comet assay and followed the procedure of Tice et al. (2000). Layering of slides was performed using 1% NMP agarose. Then slides were placed overnight in a covered box to avoid any dust. When the first layer was completely dried, a second layer of LMP agarose with sample was applied on slide having LMP agarose and sample in 1:3 ratio. After sample loading, lysing was performed by placing the slides in a slide jar having 89 mL lysing buffer. This lysing buffer was placed in dark box at 4 °C for 1 h and 30 min. Then unwinding step was performed by placing these slides in electrophoresis solution for 20 min. Electrophoresis was done in electrophoresis buffer for 20 min, 300 mA and 25 V in an electrophoresis container. As electrophoresis completed neutralization of slides was performed. For neutralization, slides were placed in slide stand and neutralization was performed by cold neutralizing solution having pH of neutralizing solution 7.5. Each slide was washed three times for 5 min with neutralizing solution and 5 mL solution was poured every time. As slides dried completely, staining was performed by pouring 75 μl (2 μg/mL) of Ethidium Bromide on each slide. Staining was performed very carefully by wearing double gloves and disposed the used tips immediately wrapped in paper to avoid in contact with body because ethidium bromide is carcinogenic.
2.3. Scoring and statistical analysis
After staining, slides were observed under Epifluorescent microscope for scoring. Magnification power was adjusted to 40× and 50 cells were counted for each slide. Computer image analysis Casplab® software was used for scoring of cells and various parameters including Length Tail, Tail DNA and Olive Tail Movement. SPSS® (Ver. 17.0) software was used to statistically analyze the results. One Way ANOVA was used for all comparison of comet parameters and Post Hoc Test was used for the comparison of significant values of various parameters within groups and between groups.
3. Results
Genotoxicity and repair capability of Tramadol in Mus musculus was assessed by using comet assay and behavioral patterns of mice were also observed during dose period and recovery period. Various parameters including feeding habits, activeness, sleep time, aggressiveness and body weight were recorded. Feeding and drinking habits were not changed throughout the trial. However, activeness decreased in all treatment groups as compared to control as all the mice gathered at one corner of cage and their body movement was very slow, sleeping time of mice was also increased while their aggressiveness reduced with increase in amount of dose. Body weight of all mice were also recorded before trial, after dose trial and after recovery period and a comparison of these three periods revealed that body weight was reduced after dose period while remain unchanged after recovery period. However, there was gradual decrease in sleeping time during recovery period. Further decrease in body weight was not observed during recovery period with gradual increase in activeness (Table 1).
Table 1.
Behavioral patterns of mice exposed to different concentrations of Tramadol during Dose Period and Recovery Period.
| Behavioral Parameters | Normal Readings | During Dose Period |
During Recovery Period |
||||
|---|---|---|---|---|---|---|---|
| Control Group | 25 mg/kg | 50 mg/kg | 75 mg/kg | 25 mg/kg | 50 mg/kg | 75 mg/kg | |
| Feed Habits | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Activeness | 0 | - | -- | --- | - | -- | - |
| Sleep time | 0 | + | ++ | +++ | + | ++ | ++ |
| Aggressiveness | 0 | - | - - | --- | - | -- | --- |
| Body weight | 0 | - | -- | --- | - | - | -- |
0 = No Change, + = Increase (+ (Low), ++ (Medium), +++ (High)), - = Decrease (- (Low), -- (Medium), --- (High)).
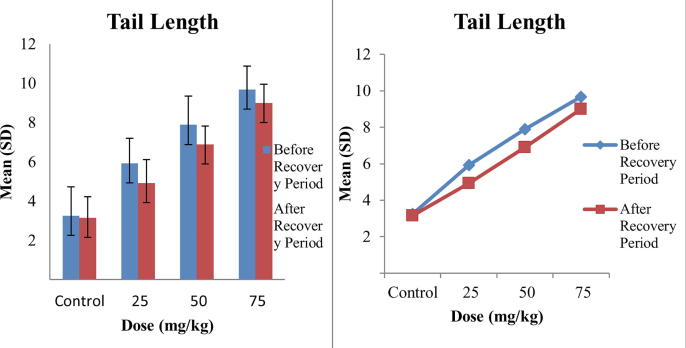
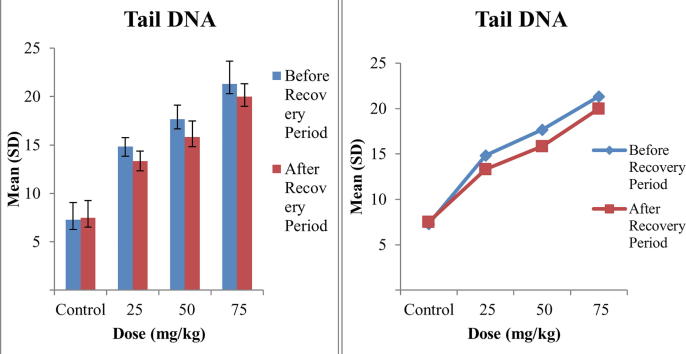
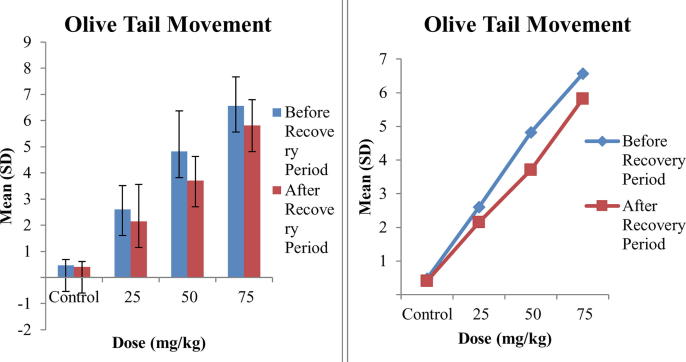
The results of comet assay are shown in Table 2. Three parameters Tail Length, Tail DNA and Olive Tail Movement were used to assess the DNA damage caused by Tramadol and the mean values of these parameters interpret that all the doses caused DNA damage and this DNA damage was dose dependent as by increasing the concentration of Tramadol, DNA damage was increased. The maximum mean values of tail length were 9.68 ± 1.19 at 75 mg/kg before recovery period and minor decrease in tail length was observed after recovery period that is the indication of repair capability of Tramadol. The mean values of tail DNA and olive tail movement also increased with increasing concentration of Tramadol as maximum mean Tail DNA, 21.31 ± 2.36, was observed at maximum dose 75 mg/kg that was reduced to 19.99 ± 1.34 after recovery period. While maximum mean olive tail movement, 6.56 ± 1.11, was also observed at highest dose 75 mg/kg of Tramadol as compared to other doses. A comparison of significance level of all parameters is presented in Table 2. It is clear that the values are significant for Tail Length and Tail DNA while highly significant for Olive Tail Movement before recovery period. After recovery period the values of Tail Length are highly significant, Tail DNA are non-significant and Olive Tail Movement are significant. A comparison in comet values of before and after recovery period revealed that repair capability of Tramadol is non-significant as minor recovery was observed in 15 days trial.
Table 2.
Comparison of various concentrations of Tramadol in blood of Mus musculus before and after Recovery Period.
| Parameters | Control | Dose |
||
|---|---|---|---|---|
| 25 mg/kg | 50 mg/kg | 75 mg/kg | ||
| Before Recovery Period | ||||
| Tail Length | 3.25 ± 1.49a | 5.93 ± 1.28ab | 7.89 ± 1.47bc | 9.68 ± 1.19c* (p = 0.03) |
| Tail DNA | 7.28 ± 1.79a | 14.84 ± 0.93b | 17.67 ± 1.42b | 21.31 ± 2.36b* (p = 0.04) |
| Olive Tail Movement | 0.47 ± 0.22a | 2.6 ± 0.91b | 4.82 ± 1.54c | 6.56 ± 1.11c** (p = 0.01) |
| After Recovery Period | ||||
| Tail Length | 3.15 ± 1.07a | 4.93 ± 1.18b | 6.9 ± 0.92c | 9 ± 0.95d** (p = 0.000) |
| Tail DNA | 7.49 ± 1.75a | 13.31 ± 1.06ab | 15.82 ± 1.64b | 19.99 ± 1.34bNS (p = 0.08) |
| Olive Tail Movement | 0.4 ± .21a | 13.31 ± 1.41a | 3.71 ± .92ab | 5.82 ± .98b* (p = 0.014) |
NS = Non-Significant (p > 0.05).
Significant (p ≤ 0.05).
Highly Significant (p < 0.01).
Fig. 1 is representing the comparison of tail length before and after recovery period. A slight increase in bar lengths represent the damage that increase as concentration of tramadol increase. While, comparison of bar lengths before and after recovery period show minor differences that is the indication of repair capability of tramadol. Figs. 2 and 3 are showing the bar lengths for Tail DNA and Olive Tail Movement before and after recovery period. These bar lengths are increased consecutively as concentration of tramadol increase. A slight decrease in bar lengths after recovery period is representing the repair capability of Tramadol and highest recovery observed at lowest concentration i.e., 25 mg/kg of Tramadol.
Fig. 1.
Comparison of means of Tail length in blood of mice exposed to different concentrations of Tramadol before and after Recovery Period.
Fig. 2.
Comparison of means of Tail DNA in blood of mice exposed to different concentrations of Tramadol before and after Recovery Period.
Fig. 3.
Comparison of means of Olive Tail Movement in blood of mice exposed to different concentrations of Tramadol before and after Recovery Period.
4. Discussion
A lot of data is available in literature about toxicity of various psychoactive drugs but very less information is available about the genotoxicity of these drugs. Clinical use of Tramadol has been increased in recent years. In the current study genotoxicity and repair capability of Tramadol was studied in albino mice. Comet assay was performed to check the genotoxic potential of three different concentrations of Tramadol in blood of mice. Results of comet assay revealed that Tramadol is genotoxic and highest genotoxicity of Tramadol was observed at highest concentration, 75 mg/kg, while lowest damage was observed at lowest, 25 mg/kg dose of Tramadol. A minor decrease in mean values of tail length after recovery period indicated the low repair capability of DNA following Tramadol exposure. Tramadol acts by three different mechanisms as mu-opioid receptor caused the metabolism of O-desmethyl tramadol. The 2nd is serotonin reuptake inhibition by (+) – Tramadol and 3rd is norepinephrine reuptake inhibition by (−) –Tramadol. O-desmethyl tramadol formed by O-demethylation from Tramadol and catalyzed by CYP2D6. This O-desmethyl tramaadol caused the opiate type effects in Tramadol. Tramadol also has anatagonist effects that are similar to NMDA and for that reason it is used for neuropathic pain. Tramadol also have anti-inflammatory effects as it impaired immune system by increasing the serotonin and norepinephrine that caused a reduction in inflammatory cytokines that released from the during stress conditions (Rabei, 2011).
Behavioral responses of mice under various concentrations of Tramadol were observed during the trial. The normal behavior was observed in control group while severity of all responses was observed in maximum dose group that was administered with 75 mg/kg dose of Tramadol. Increasing the Tramadol concentration caused a reduction in aggressiveness, body weight and activeness. Behavioral changes caused by Tramadol was also observed by Tayal et al. (2008) who investigated the antidepressant activity of Tramadol and stated that Tramadol produce antidepressant effects and immobility that is dose dependent and increase by increasing concentration of tramadol. In another study, conducted by Azmy et al. (2018) it was found that Tramadol also cause disturbance in the brain of mice. They concluded that chronic co administration of Tramadol and nicotine improves the social interaction and decrease anxiety. However, that combination was extremely neurotoxic and has adverse effects on memory. Sehonova et al. (2017) evaluated the toxic effects of Tramadol and mixture of Tramadol and naproxen sodium on early life stages of fish. Tramadol, Tramadol and naproxen sodium mixture and naproxen sodium alone was given to fish for 32 days. Theses exposures showed that mixture of both drugs affected the early stages of fish, caused oxidative stress, and mortality. Bodera et al. (2013) stated that Tramadol caused a decrease in antioxidant capacity of blood in rats.
Although different groups have explored different toxicological parameters of Tramadol however, its genotoxicity was not studied before. Genotoxicity is the ability of a toxic substance to induce damage to genetic material, so it carries the probability of mutations and certain type of mutations may lead to cancer. It is concluded that genotoxicity of Tramadol in mice was increased by increasing the concentration of Tramadol. Highest concentration of Tramadol, 75 mg/kg had caused maximum DNA damage as compared to other doses i.e., 25 mg/kg and 50 mg/kg. Our findings are nearly similar to Alvarenga et al. (2010) findings who investigated the genotoxic effects of cocaine in multiple mice organs by using comet assay. They concluded that cocaine is genotoxic and genotoxicity increased by increasing the concentration of drug. Parolini et al. (2016) studied the genotoxicity of illicit drugs including cocaine, morphine and some others on zebra mussel. Results showed that this mixture caused DNA fragmentation, micronuclei formation, genotoxicity and triggered apoptotic process. Suzuki et al. (2018) stated that nicotine cause genotoxicity, carcinogenicity and hyperplasia in kidney and bladder of rat that is dose dependent.
In the current study Tail Length, Tail DNA and Olive Tail Movement were recorded to analyze genotoxic potential of the Tramadol. These are extremely important and sensitive parameters that have been widely used by different groups to observe the extent of DNA damage after exposure to different environmental chemicals. Ginzkey et al. (2009) investigated the genotoxic and carcinogenic potential of nicotine in human salivary glands by using comet assay. A gradual increase in tail length, tail DNA and olive tail movement revealed that nicotine cause dose dependent DNA damage. Similar parameters were used by Soria et al. (2015) who investigated the effects of acrylnitrile and amine acridine in rats and found that former is a genotoxic and carcinogen and latter is genotoxic non carcinogen in rat. Danadevi et al. (2004) used similar parameters and observed a gradual increase in mean tail length in leucocytes of mice following the exposure to nickel chloride is genotoxic and this DNA damage is dose dependent. Results of present study are also similar to the findings of Maranho et al. (2017) who evaluated the toxic effects of cocaine on various life stages of mussel Perna. They assessed the fertilization rate, DNA strand breaks, embryo-larval development and lysosomal membrane stability. Their results showed that cocaine caused genotoxicity in mussels. De Sousa Coelho et al. (2018) studied the genotoxic effects of alcohol on embryonic and fetal development in rats and their offspring. Their results demonstrated that alcohol cause DNA damage in blood and liver of rats, offspring and neonates brain. A similar study was conducted by Cristóbal-Luna et al. (2018) who studied the genotoxic and mutagenic effects of Kramecyne. They observed the potential developmental toxicity in different groups of female rats and genotoxicity in different groups of male mice. Naseri et al. (2018) investigated the toxic effects of mephedrone during gestation period in mice. Their results showed that mephedrone affect the birthrate by stillbirth and lower percent of weight gain. They also observed cell apoptosis, cell proliferation and impairment in learning and memory process. It was concluded that during gestation, regular or repeated exposure to mephedrone had increased the low birth rate and stillbirth and repeated use lead to impairment of learning process and effect memory by hippocampal damaging.
Another major finding of proposed study is that after discontinuing the use of Tramadol, the repair capacity of DNA is not very pronounced. Maximum recovery was observed at lowest dose treatment i.e., 25 mg/kg while minimum was observed at highest dose treatment group i.e., 75 mg/kg. Result of Attia, (2007) supported our results, who studied the genotoxic effects of nicotine in mice bone marrow and observed highest toxicity at higher concentration and also observed recovery in one treatment that they observed after 48 h of treatment. Recovery was also observed by Azari et al. (2014) who studied the effects of Tramadol on epididymal sperm quality and testicular tissue in mice. They divided the mice into three groups having 21 mice in each and control group was treated with normal saline while remaining two treated with 10 mg/kg and 20 mg/kg dose of Tramadol respectively. Dissection was performed at 3, 6 and 12th week after treatments. Their results showed a significant decrease in sperm concentration, vitality and motility in second and third group as compared to control group at 3rd and 6th week but slight recovery was observed at 12th week. They concluded that long-term administration of Tramadol caused adverse effects on sperm quality and testicular tissues and these effects are dose dependent.
In the current study we observed minor to non-significant recovery in DNA damage after discontinuing oral administration of Tramadol. One of the possible explanation of this is that exposure to Tramadol decrease the activities of glutathione, catalase and superoxide dismutase and caused to increase malondialdehyde in liver and kidney of treated group. Glutathione are major liver enzymes responsible for detoxification of exogenously administrated substances. Repeated dose of Tramadol for long periods caused histopathological and biochemical changes as effect on of hepatic, renal and sexual function, it also caused norepinephrine and serotonin reuptake inhibition. These actions lead to serotonin syndrome and seizures. Although effects of Tramadol in inducing serotonin syndrome and seizure are modest but if not treated it may lead to serious effects therefore its management is necessary (Awadalla and Salah-Eldin, 2016, Sayed and Zidan, 2016).
5. Conclusion
Tramadol is a genotoxic drug and this genotoxicity is dose dependent that is increased by increasing Tramadol concentration and it also affect the repair capacity of DNA.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Tayyaba Ali, Email: ali.tayyaba@gmail.com.
Samina Qamar, Email: saminabee@gmail.com.
References
- Ali T., Ismail M., Asad F., Ashraf A., Waheed U., Khan Q.M. Pesticide genotoxicity in cotton picking women in Pakistan evaluated using comet assay. Drug Chem. Toxicol. 2018;41:213–220. doi: 10.1080/01480545.2017.1343342. [DOI] [PubMed] [Google Scholar]
- Alvarenga T.A., Andersen M.L., Ribeiro D.A., Araujo P., Hirotsu C., Costa J.L., Battisti M.C., Tufik S. Single exposure to cocaine or ecstasy induces DNA damage in brain and other organs of mice. Addict. Biol. 2010;15:96–99. doi: 10.1111/j.1369-1600.2009.00179.x. [DOI] [PubMed] [Google Scholar]
- Attia A.S. cis- and trans-Bis(pyridine)chromium(III) complexes containing mixed-valence chrysenesemiquinonate-chrysenecatecholate ligands: synthesis, characterization and interpretation of the electronic absorption spectra using ZINDO/S method. Polyhedron. 2007;26:2550–2558. [Google Scholar]
- Awadalla E.A., Salah-Eldin A.E. Molecular and histological changes in cerebral cortex and lung tissues under the effect of tramadol treatment. Biomed. Pharmacother. 2016;82:269–280. doi: 10.1016/j.biopha.2016.04.024. [DOI] [PubMed] [Google Scholar]
- Azari O., Emadi L., Kheirandish R., Shafiei Bafti H., Esmaili Nejad M.R., Faroghi F. The effects of long-term administration of tramadol on epididymal sperm quality and testicular tissue in mice. Iran. J. Veterin. Surg. 2014;9(1):23–30. [Google Scholar]
- Azmy S.M., Abd El Fattah M.A., Abd El-Rahman S.S., Nada S.A., Abdel Salam O.M.E., El-Yamany M.F., Nassar N.N. Does nicotine impact tramadol abuse? Insights from neurochemical and neurobehavioral changes in mice. NeuroToxicology. 2018;67:245–258. doi: 10.1016/j.neuro.2018.06.004. [DOI] [PubMed] [Google Scholar]
- Bankoglu E.E., Kodandaraman G., Stopper H. A systematic review of the use of the comet assay for genotoxicity studies in human colon-derived cells. Mutat. Res. Toxicol. Environ. Mutagen. 2018 doi: 10.1016/j.mrgentox.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Bastaki M., Farrell T., Bhusari S., Pant K., Kulkarni R. Lack of genotoxicity in vivo for food color additive Tartrazine. Food Chem. Toxicol. 2017;105:278–284. doi: 10.1016/j.fct.2017.04.034. [DOI] [PubMed] [Google Scholar]
- Bloor M., Paech M.J., Kaye R. Tramadol in pregnancy and lactation. Int. J. Obstet. Anesth. 2012;21:163–167. doi: 10.1016/j.ijoa.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Bodera P., Stankiewicz W., Zawada K., Antkowiak B., Paluch M., Kieliszek J., Kalicki B., Bartosinski A., Wawer I. Changes in antioxidant capacity of blood due to mutual action of electromagnetic field (1800MHz) and opioid drug (tramadol) in animal model of persistent inflammatory state. Pharmacol. Rep. 2013;65:421–428. doi: 10.1016/s1734-1140(13)71017-x. [DOI] [PubMed] [Google Scholar]
- Chai Q., Zhang S., Wang X., Yang H., Xie Y.F. Effect of bromide on the transformation and genotoxicity of octyl-dimethyl-p-aminobenzoic acid during chlorination. J. Hazard. Mater. 2017;324:626–633. doi: 10.1016/j.jhazmat.2016.11.035. [DOI] [PubMed] [Google Scholar]
- Cristóbal-Luna J.M., Paniagua-Castro N., Escalona-Cardoso G.N., Pérez-Gutiérrez M.S., Álvarez-González I., Madrigal-Bujaidar E., Chamorro-Cevallos G. Evaluation of teratogenicity and genotoxicity induced by kramecyne (KACY) Saudi Pharm. J. 2018;26:829–838. doi: 10.1016/j.jsps.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danadevi K., Rozati R., Banu B.S., Grover P. In vivo genotoxic effect of nickel chloride in mice leukocytes using comet assay. Food Chem. Toxicol. 2004;42:751–757. doi: 10.1016/j.fct.2003.12.013. [DOI] [PubMed] [Google Scholar]
- de Sousa Coelho I.D.D., Neto C.J.C.L., dos Santos Souza T.G., da Silva M.A., Chagas C.A., dos Santos K.R.P., Teixeira V.W., Teixeira Á.A.C. Protective effect of exogenous melatonin in rats and their offspring on the genotoxic response induced by the chronic consumption of alcohol during pregnancy. Mutat. Res./Gen. Toxicol. Environ. Mutagen. 2018;832:52–60. doi: 10.1016/j.mrgentox.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Ginzkey C., Kampfinger K., Friehs G., Köhler C., Hagen R., Richter E., Kleinsasser N.H. Nicotine induces DNA damage in human salivary glands. Toxicol. Lett. 2009;184:1–4. doi: 10.1016/j.toxlet.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Hussain B., Sultana T., Sultana S., Masoud M.S., Ahmed Z., Mahboob S. Fish eco-genotoxicology: comet and micronucleus assay in fish erythrocytes as in situ biomarker of freshwater pollution. Saudi J. Biol. Sci. 2018;25:393–398. doi: 10.1016/j.sjbs.2017.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-Q., Ye Z.-M., Wang J.-B., Fan C.-R., Pan A.-W., Li C., Zhang R.-B. Mucoadhesive buccal films of tramadol for effective pain management. Braz. J. Anesthesiol. Engl. Ed. 2017;67:231–237. doi: 10.1016/j.bjan.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Mabrouk M., Beherei H.H., ElShebiney S., Tanaka M. Newly developed controlled release subcutaneous formulation for tramadol hydrochloride. Saudi Pharm. J. 2018;26:585–592. doi: 10.1016/j.jsps.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranho L.A., Fontes M.K., Kamimura A.S.S., Nobre C.R., Moreno B.B., Pusceddu F.H., Cortez F.S., Lebre D.T., Marques J.R., Abessa D.M.S., Ribeiro D.A., Pereira C.D.S. Exposure to crack cocaine causes adverse effects on marine mussels Perna perna. Mar. Pollut. Bull. 2017;123:410–414. doi: 10.1016/j.marpolbul.2017.08.043. [DOI] [PubMed] [Google Scholar]
- Matthiesen T., Wöhrmann T., Coogan T.P., Uragg H. The experimental toxicology of tramadol: an overview. Toxicol. Lett. 1998;95:63–71. doi: 10.1016/s0378-4274(98)00023-x. [DOI] [PubMed] [Google Scholar]
- Mehdizadeh H., Pourahmad J., Taghizadeh G., Vousooghi N., Yoonessi A., Naserzadeh P., Behzadfar L., Rouini M.R., Sharifzadeh M. Mitochondrial impairments contribute to spatial learning and memory dysfunction induced by chronic tramadol administration in rat: Protective effect of physical exercise. Prog. Neuropsychopharmacol. Biol. Psych. 2017;79:426–433. doi: 10.1016/j.pnpbp.2017.07.022. [DOI] [PubMed] [Google Scholar]
- Miranda H.F., Romero M.A., Puig M.M. Antinociceptive and anti-exudative synergism between dexketoprofen and tramadol in a model of inflammatory pain in mice. Fundam. Clin. Pharmacol. 2012;26:373–382. doi: 10.1111/j.1472-8206.2010.00922.x. [DOI] [PubMed] [Google Scholar]
- Nagarathna P.K.M., Johnson Wesley M., Sriram Reddy P., Reena K. Review on genotoxicity, its molecular mechanisms and prevention. Int. J. Pharm. Sci. Rev. Res. 2013;22:236–243. [Google Scholar]
- Naseri G., Fazel A., Golalipour M.J., Haghir H., Sadeghian H., Mojarrad M., Hosseini M., Shahrokhi Sabzevar S., Beheshti F., Ghorbani A. Exposure to mephedrone during gestation increases the risk of stillbirth and induces hippocampal neurotoxicity in mice offspring. Neurotoxicol. Teratol. 2018;67:10–17. doi: 10.1016/j.ntt.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Parolini M., Magni S., Castiglioni S., Binelli A. Genotoxic effects induced by the exposure to an environmental mixture of illicit drugs to the zebra mussel. Ecotoxicol. Environ. Saf. 2016;132:26–30. doi: 10.1016/j.ecoenv.2016.05.022. [DOI] [PubMed] [Google Scholar]
- Qureshi S.T., Memon S.A., Abassi A.R., Sial M.A., Bughio F.A. Radiofrequency radiations induced genotoxic and carcinogenic effects on chickpea (Cicer arietinum L.) root tip cells. Saudi J. Biol. Sci. 2017;24:883–891. doi: 10.1016/j.sjbs.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabei, H.M., 2011. <The immunological and histopathological changes of Tramadol,.pdf>, pp. 477–503.
- Sayed H.Y.M., Zidan A.H. Histopathological and biochemical effects of acute and chronic tramadol drug toxicity on liver, kidney, and testicular function in adult male albino rats. J. Foren. 2016;1:41. [Google Scholar]
- Sehonova P., Plhalova L., Blahova J., Doubkova V., Prokes M., Tichy F., Fiorino E., Faggio C., Svobodova Z. Toxicity of naproxen sodium and its mixture with tramadol hydrochloride on fish early life stages. Chemosphere. 2017;188:414–423. doi: 10.1016/j.chemosphere.2017.08.151. [DOI] [PubMed] [Google Scholar]
- Shah S.U. Importance of Genotoxicity & S2A guidelines for genotoxicity testing for pharmaceuticals. IOSR J. Pharm. Biol. Sci. 2012;1:43–54. [Google Scholar]
- Solmaz F.A., Kovalak E. Comparison of tramadol/acetaminophen fixed-dose combination, tramadol, and acetaminophen in patients undergoing ambulatory arthroscopic meniscectomy. Acta Orthop. Traumatol. Turc. 2018;52:222–225. doi: 10.1016/j.aott.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria J.-C., Wu Y.-L., Nakagawa K., Kim S.-W., Yang J.-J., Ahn M.-J., Wang J., Yang J.C.-H., Lu Y., Atagi S. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16:990–998. doi: 10.1016/S1470-2045(15)00121-7. [DOI] [PubMed] [Google Scholar]
- Steinmetz A., Steffens L., Morás A.M., Prezzi F., Braganhol E., Saffi J., Ortiz R.S., Barros H.M.T., Moura D.J. In vitro model to study cocaine and its contaminants. Chem. Biol. Interact. 2018;285:1–7. doi: 10.1016/j.cbi.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Cohen S.M., Arnold L.L., Kato H., Fuji S., Pennington K.L., Nagayasu Y., Naiki-Ito A., Yamashita Y., Takahashi S. Orally administered nicotine effects on rat urinary bladder proliferation and carcinogenesis. Toxicology. 2018;398–399:31–40. doi: 10.1016/j.tox.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Tayal V., Kalra B.S., Chawla S. Evaluation of antidepressant activity of tramadol in mice. Ind. J. Pharmacol. 2008;40:129–130. doi: 10.4103/0253-7613.42307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tice R.R., Agurell E., Anderson D., Burlinson B., Hartmann A., Kobayashi H., Miyamae Y., Rojas E., Ryu J.C., Sasaki Y.F. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Molecul. Mutagen. 2000:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Tjäderborn M., Jönsson A.K., Zverkova T., Ahlner J., Hägg S. Non-prescribed use of psychoactive prescription drugs among drug-impaired drivers in Sweden. Drug Alcohol Depend. 2016;161:77–85. doi: 10.1016/j.drugalcdep.2016.01.031. [DOI] [PubMed] [Google Scholar]
- Wadley G. How psychoactive drugs shape human culture: a multi-disciplinary perspective. Brain Res. Bull. 2016;126:138–151. doi: 10.1016/j.brainresbull.2016.04.008. [DOI] [PubMed] [Google Scholar]