Abstract
Arbuscular mycorrhizal fungi (AMF) are one of the most important drivers of soil ecosystem dynamics. AMF have the potential to improve plant growth and development by modulating key hormonal pathways, which result in decreasing the adverse impact of abiotic stress, such as drought. Pot experiments were conducted in this study to investigate the ability of AMF to ameliorate the adverse impact of drought in Ephedra foliate. Non-inoculated AMF E. foliate (Ef) plants, exhibited reduced growth in response to drought stress with a concomitant lowering of chlorophyll pigments, relative to non-stressed and AMF inoculated plant. AMF inoculated E. foliate showed improved nitrogen metabolism by positively regulating nitrate and nitrite reductase activity which results in greater ammonium availability for the synthesis of amino acids. Inoculation with AMF also increased antioxidant enzyme activity, ascorbic acid contents, and reduction in glutathione level. This resulted in significant amelioration of oxidative damage to plant membranes by restricting the excess generation of reactive oxygen species (ROS), such as hydrogen peroxide. Greater content of proline, glucose, and total soluble protein in AMF-inoculated plants provided further benefit to E. foliate plants and their ability to withstand drought stress, and also evident by a greater level of sucrose phosphate synthase activity. AMF significantly enhanced the uptake of essential nutrients like K, Mg, and Ca. Importantly, higher concentrations of plant hormones, including indole acetic acid (IAA), indole butyric acid (IBA), gibberellic acid (GA), and abscisic acid (ABA), were maintained in AMF-inoculated Ef plants. AMF inoculation also boosted phosphorous metabolism by increasing alkaline and acid phosphatase enzyme activity. In summary, AMF-inoculation of Ef plants significantly reduced the deleterious effect of drought stress by up-regulating the antioxidant defense system, synthesis of osmolytes, and maintaining phytohormone levels.
Keywords: Phytohormones, Antioxidants, Nitrogen metabolism, Proline, Drought, Ephedra foliata, AMF
1. Introduction
Drought stress, similar to other abiotic stresses, is a major factor responsible for considerable losses in the yield of crop plants all over the globe. Plants in arid and semi-arid regions experience drought or water deficit stress when the availability of water to roots becomes scarce due to concomitant increase in the rate of transpiration and higher temperatures (Hameed et al., 2014). Drought conditions can significantly reduce the growth and yield of a plants far below its genetic potential (Alwhibi et al., 2017). Global warming and climate change further exacerbated the drought conditions due to lack of suitable irrigation system hence becoming more problematic (Wu et al., 2017).
Drought conditions impede root growth which results in reduction of water and nutrient uptakes by plants (Hameed et al., 2014). The enzyme activities that mediated the metabolism of minerals, like nitrogen, sulphur, phosphate, and the synthesis of metabolites and pigments, are all down-regulated by drought stress (Mirzaee et al., 2013, Punyasheeladevi and Sujatha, 2014). Considerable variation in drought tolerance and its underlying mechanism have been observed in different crop species and genotypes within a species (Egamberdieva et al., 2018). Drought tolerance mechanisms include both up-and down-regulation of several physiological and biochemical traits (Wehner et al., 2015), and the identification and its subsequent modulation of such traits is essential for improving drought stress tolerance (Xu et al., 2014, Wehner et al., 2015). Numerous studies have demonstrated that different abiotic stress trigger the generation of reactive oxygen species (ROS) which resulted in the peroxidation of lipids, proteins, and nucleic acids, as well as altered metabolism (Abd_Allah et al., 2015, Yang et al., 2015, Hashem et al., 2016). ROS can have a negative impact on photosynthesis and mitochondrial electron transport system (ETS), nutrient remobilization, and can initiate an irreversible senescence process (Singh et al., 2016). ROS also mediate redox signalling activity in association with alteration in phytohormone levels and the induction of transcription factors that can regulate senescence-associated genes (Rogers and Munne-Bosch, 2016, Egamberdieva et al., 2018).
ROS play a number of key roles in plant growth regulation and its threshold levels are maintained by the coordinated activity of several antioxidant defense systems (Hashem et al., 2016, Nath et al., 2016). These systems include antioxidants comprising both enzymatic and non-enzymatic components, osmolytes, and the interaction of several phytohormones that regulate the mechanisms for maintaining an optimal concentration of ROS (Rogers and Munne-Bosch, 2016, Singh et al., 2016). Superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), and glutathione reductase (GR) work in an integrated manner with redox and non-enzymatic antioxidants, like ascorbic acid, reduced glutathione (GSH), and tocopherol (Yang et al., 2015, Hashem et al., 2016, Wu et al., 2017) to prevent oxidative stress. Plant cells must eliminate this stress triggered excessive ROS to protect the vital functions of various cellular components of the plant system from oxidative damage. This ability depends upon the proper functioning of an antioxidant system, osmolyte accumulation, and hormone metabolism (Abd_Allah et al., 2015). The accumulation of compatible solutes in response to water deficit conditions greatly contributes to the maintenance of a low tissue water potential in relation to the external soil solution, which allows the continued absorption of water from the surrounding soil environment, thus buffering the deleterious effects of drought (Abd_Allah et al., 2016). Hashem et al., 2015, Abd_Allah et al., 2018 have demonstrated that plants accumulating sufficient concentrations of osmolytes recover quickly from oxidative stress. The mechanisms underlying drought stress tolerance in plants are complex and include maintaining ion homeostasis, osmolyte biosynthesis, and radical neutralisation. Endogenous phytohormone concentrations have also been reported to be directly involved in stress response (Singh et al., 2016, Egamberdieva et al., 2017). The response of phytohormones is very complex and hormones exhibit significant crosstalk during a stress response that ultimately results in increased stress tolerance (Abd_Allah et al., 2018, Egamberdieva et al., 2018).
AMF include beneficial fungal species that form a symbiotic relationship with many plant species and potentially improve plant growth by improving the soil structure of the rhizosphere (Hameed et al., 2014). AMF have also been shown to regulate several plant growth-controlling processes both under normal and stressful environments (Yang et al., 2015, Abd_Allah et al., 2016). Acting as bio-ameliorators in extreme environmental situations, AMF can prevent yield losses under salt stress and other abiotic stress conditions (Nath et al., 2016, Wu et al., 2017). AMF inoculated Acacia gerrardii protects the plants from abiotic stress by increasing the osmotic balance and up-regulating the antioxidant system (Hashem et al., 2016). AMF species have been suggested to be important candidates for neutralizing the adverse impact of high levels of ROS (Nath et al., 2016). They can improve root growth by increasing mineral acquisition leading to increased plant growth and vigor (Hameed et al., 2014, Shafiq et al., 2014, Abd_Allah et al., 2016, Alwhibi et al., 2017). Morphological, nutritional, and physiological changes occur in host plants in response to AMF that can result in increasing yield under both optimal and adverse growth conditions (Hashem et al., 2018).
Ephedra foliate, a member of the Ephedraceae family, is native to North Africa and Southwest Asia. This genus is an important source for the alkaloid, ephedrine, and are used in traditional medicines for the treatment of chronic asthma and associated respiratory problems. In most parts of the world, young stems of Ephedra are usually used in tea or eaten raw. Increased management of Ephedra is needed due to the threat posed by the changing environment, especially given its unique medicinal properties. Powdered plant parts are also boiled and used for the tanning of animal hides. The International Union for Conservation of Nature (IUCN) has noted the need to intensify strategies for protecting Ephedra species (Bell and Bachman, 2011). Therefore, in the current study, we examined the ability of AMF to improve drought stress tolerance in Ephedra foliate and determine the underlying mechanisms by which AMF increases drought tolerance. Information from this study can be used to help develop management strategies to ensure the continued cultivation of Ephedra.
2. Materials and methods
2.1. Seed material and experimental design
Seeds of Ephedra foliata Boiss. ex C.A. Mey were collected from native shrubs growing in the Derab experimental station Riyadh, Saudi Arabia. Seeds were surface disinfected using 0.5% NaOCl (v/v) for 3.0 min. The NaOCl was then washed from the seeds in three successive rinses of sterile, distilled H2O and the seeds were subsequently germinated on wet blotter paper (double layers) in a 9.0 cm Petri dish in the dark at 28 °C for 5 days. Healthy germinated seedlings with uniform growth were used in the pot experiment (one seedling/pot).
The pot experiment was conducted in an environmental growth chamber of Plant Production Department, Collage of Food & Agricultural Sciences, King Saud University, Riyadh, Saudi Arabia. The pots were arranged in a complete randomized block design with seven replicates per treatment. Each pot (diameter 150 mm, height 130 mm) was filled with 2000 g of a 1:1:1 mixture of peat, perlite, and sand (v/v/v).
2.2. Arbuscular mycorrhizal fungi (AMF) and use of the trap culture method
Inoculation was carried out using three AMF species viz; Claroideoglomus etunicatum (syn. Glomus etunicatum), Rhizophagus intraradices (syn. Glomus intraradices), and Funneliformis mosseae (syn. Glomus mosseae) by maintaining the application rate as 25 g of trap soil culture per pot which contained approx. 750 spores/g. Isolation of AMF from the rhizosphere of Acacia gerrardii was done as per the method described in Hashem et al. (2016). Methods described by Daniels and Skipper, 1982, Utobo et al., 2011 were employed for isolation of AMF species. The identification was carried out by employing the properties of asexual spore structures as reported in a manual prepared by the International Culture Collection of Vesicular and Arbuscular Mycorrhizal Fungi (INVAM, 2014). The trap culture method was used to increase the population of AMF which entailed the establishment of a trap plant (Zea mays L.) (Stutz and Morton, 1996). The trap soil was used as AMF inoculum in the pot experiment (approx. 750 spores/g trap soil)/pot. Soil not inoculated with AMF was used for the control plants.
2.3. Treatments and plant growth
Four different treatments were administered to the potted plants:
-
1.
Control treatment: Seedlings were planted in pots containing non-AMF soil. Each pot was watered with a modified Jensen’s nutrient solution (Jensen and Collins, 1985) to maintain 60% field capacity.
-
2.
AMF treatment: Seedlings were planted in AMF soil, added to the pots as 25 g trap soil/pot (approx. 750 spores/g trap soil). These non-stressed plants were also watered with Jensen’s nutrient solution.
-
3.
Drought treatment: Seedlings planted in non-AMF soil were subjected to a drought stress by regulating the amount of Jensen’s nutrient solution (withholding water supply) added to each pot according to Fatokun et al. (2012). The procedure was described in detail in our previous study (Jemo et al., 2017).
-
4.
AMF + Drought treatment: Seedlings were planted in pots amended with AMF (25 g trap soil/pot) and subjected to a drought stress by regulating the amount of Jensen’s nutrient solution provided to each pot.
There were seven replicates (n = 7) for each treatment with each pot representing a replicate. The pots were arranged in a completely randomized design and their positions were re-arranged at weekly intervals. Plants were grown in an environmental growth chamber under controlled conditions (25 ± 2 °C temperature, 14 h/10 h of day/night cycle with 350 μmol photon m−2 s−1 light intensity and 65–70% humidity). The plants were harvested at fifteen weeks after planting to evaluate and record morphological criteria, mycorrhizal status, and to conduct a variety of biochemical and physiological analyses.
2.4. Determination of arbuscular mycorrhizal colonization
Roots from each treatment were thoroughly washed using distilled water for removing the adhering soil and debris. Thereafter, root segments of a length of 1 cm were chopped and subsequently stained using trypan blue-lactophenol (Phillips and Hayman, 1970, Zubek et al., 2012). Stained root segments (50 per replicate) were observed under digital computerized microscope (model DP-72, Olympus) at 20× magnification in order to assess the AMF colonization of roots. Level of AMF colonization was recorded in terms of arbuscules, vesicles and mycelia as described by Hashem et al. (2016).
Percent colonization was calculated as follows:
2.5. Photosynthetic pigments
For measuring the photosynthetic pigments, fresh leaves (100 mg) were crushed in acetone and the absorbance of supernatant was recorded at 622, 664, and 440 nm using spectrophotometer (Lichtenthaler and Wellburn, 1983).
2.6. Determination of nitrogen assimilation
Nitrate and nitrite reductase were extracted in phosphate buffer (pH 7.5) containing 0.1 M potassium nitrate and n-propanol (5%). Methods described by Hageman and Hucklesby, 1971, Finka et al., 1977 were employed for determination of nitrate and nitrite reductase activities respectively and expressed as μmNO2 released/h/g FW and μmNO2 disappeared/h/g fresh wt. described in details by Hashem et al. (2016).
Soluble nitrogen fractions were extracted according to the method adopted by Said and El-Shishiny (1944). Nitrate content was determined calorimetrically (410 nm) according to the method described by Cataldo et al. (1975). Nitrate content was expressed in µM NO3 g−1 DW (calibration curve of NO3 used as reference). Ammonium content was measured calorimetrically (630 nm) using indophenol blue according to (Zanini, 2001). Ammonium content was expressed in µM NH4 g−1 DW (calibration curve of NH4Cl used as reference).
2.7. Determination of lipid peroxidation (MDA) and hydrogen peroxide (H2O2)
Formation of malonaldehyde (MDA) content was taken as a measure of lipid peroxidation. 0.2 g of fresh tissue was extracted in 0.1% tri-chloro-acetic acid (TCA) followed by centrifugation for 5 min at 10,000g. Thereafter one mL supernatant was mixed with 4 mL of TBA (5%, prepared in 20% TCA) and incubated at 100 °C for 30 min in a water bath. After cooling, the samples were again centrifuged at 10,000g for 10 min and the absorbance of supernatant was read at 532 and 600 nm (Hodges et al., 1999). For calculation of MDA extinction coefficient of 155 mM−1 cm−1 was used and expressed as µmol MDA g−1 FW.
For determination of hydrogen peroxide (H2O2), fresh leaf tissue was extracted in 0.1% TCA (w/v) and homogenate was centrifuged at 12,000g for 15 min. 0.5 mL of potassium phosphate buffer (10 mM, pH 7.0) and 1 mL of 1 M potassium iodide to 0.5 mL of supernatant. Absorbance was taken at 390 nm and the content of H2O2 was calculated using standard curve of H2O2 and expressed as µmol g−1FW (Sergiev et al., 1997).
2.8. Determination of sucrose phosphate synthase, alkaline and acid phosphatase activity
Sucrose phosphate Synthase (EC 2.4.1.14, SPS) activity was measured according to Hubbard et al. (1989). For measuring the activity of aid (EC 3.1.3.2) and alkaline phosphatase (EC 3.1.3.1) method described by Gianinazzi-Pearson and Gianinazzi (1976) was employed. For assaying the acid phosphatase, 0.2 mL of enzyme extract was added to 1.0 mL of 5.5 mM p-nitrophenol phosphate in 5.5 mM citrate buffer (pH 4.8). While as for determining the alkaline phosphatase activity, 0.05 M tris-citrate (pH 8.5) was used instead of citrate buffer (pH 4.8). Reaction mixture was subsequently incubated for 30 min at 37 0C and reaction was terminated by adding 200 mM NaOH. Absorbance was recorded at 410 nm and activity was expressed as μmol p-nitrophenol released min−1 mg protein−1.
2.9. Estimation of proline
0.5 g of leaves were extracted using 3% sulphosalicylic acid (5 mL) and the extract was centrifuged for 10 min at 10,000g. To 2 mL of supernatant, acid ninhydrin and glacial acetic acid was added and reaction mixture was incubated at 100 °C for 1 h. Thereafter reaction was terminated on an ice bath and proline was separated using toluene. Intensity of color was measured at 520 nm (Bates et al., 1973).
2.10. Determination of antioxidant enzyme activities
For extraction of antioxidant enzymes, frozen (0.4 g) leaves were macerated in pre-chilled pestle and mortar using 4 mL of potassium phosphate buffer (50 mM; pH 7.0) containing 4% PVP. Homogenate was subjected to centrifugation for 30 min at 14,000g at 4 °C and supernatant was collected and used for assaying the activity of antioxidant enzymes. Bradford method (1976) was used for determination of soluble protein.
The activity of superoxide dismutase (SOD, EC1.15.1.1) was determined by monitoring the enzyme mediated inhibition of photochemical reduction of nitroblue tetrazolium (NBT). Briefly 1 mL reaction mixture has 50 mM potassium phosphate buffer (pH 7.4), methionine (13 mM), 75 µM NBT, EDTA (0.1 mM), riboflavin (2 µM) and 100 µL enzyme extract. For reaction initiation, samples were first incubated for 15 min under fluorescent lights and then stopped by switching off the light. Absorbance was read at 560 nm and one SOD unit was considered as the amount of protein causing 50% inhibition of photochemical reduction of NBT and was expressed as U mg−1 protein (Beauchamp and Fridovich, 1971).
Catalase (CAT, EC1.11.1.6) activity was measured by monitoring the decomposition of hydrogen peroxide (H2O2) at 240 nm. Assay mixture (1 mL) was containing potassium phosphate buffer (100 mM; pH 7.0), 0.1 mM EDTA, H2O2 (0.1%) and 100 µL enzyme extract. For calculation, molar extinction coefficient (ε) of 39.4 mM−1 cm−1 was used and activity was expressed as U mg−1 protein (Aebi, 1984).
Method of Nakano and Asada (1981) was used for assaying the activity of ascorbate peroxidase (APX, EC1.11.1.1) and the disappearance of hydrogen peroxide was followed at 290 nm. Activity was expressed as U mg−1 protein and molar extinction coefficient of 2.8 mM−1 cm−1 was used for calculation.
Glutathione reductase (GR, EC1.6.4.2) activity was assayed by following the formation of GSH at 412 nm for 3 min. Reaction mixture contained 100 mM potassium phosphate buffer (pH 7.5), 0.75 mM 5, 5-dithiobis-2-nitrobenzoic acid (DTNB), 1.0 mM EDTA, 0.1 mM NADPH, 1 mM GSSG and enzyme extract (1.0 mL) (Smith et al., 1988). GR activity was expressed as was EU mg−1 protein.
2.11. Determination of reduced glutathione and ascorbic acid
Reduced glutathione (GSH) content was measured by extracting 500 mg fresh tissue in phosphate buffer and centrifuging the homogenate at 3000g for 15 min. Supernatant (500 µL) was mixed with DTNB and absorbance was recorded at 412 nm after 10 min (Ellman, 1959). Content of GSH was calculated from standard curve of GSH and expressed as µmol g−1 FW.
For determination of ascorbic acid, leaf samples (0.5 g) were extracted in 6% TCA and the homogenate was mixed with 2% dinitrophenyl-hydrazine (prepared in 50% H2SO4) and 10% thiourea (prepared in 70% ethanol). Thereafter the resultant mixture was boiled in water bath for 15 min and centrifuged at 1000g for 10 min after cooling. 80% H2SO4 was added to dissolve the pellet and the absorbance was read at 530 nm using a spectrophotometer (Mukherjee and Choudhuri, 1983). Calculations were done using a calibration curve for ascorbic acid and expressed as µmol g−1 FW.
2.12. Extraction and quantification of plant growth regulators
Plant growth regulators were extracted in 80% aqueous acetone (4:1, v/v) containing 10 mg/L butylated hydroxyl toluene. Resulting extract was purified in EtOAc and NaHCO3 (Kusaba et al., 1998). Gas chromatograph–mass spectrometer (GC–MS) adjusted in a selected ion monitoring (SIM) mode was used for quantification of gibberellic acid (GA) and standard GA was used as a reference (Lee et al., 1998). Method of Qi et al. (1998) was used for extraction of abscisic acid (ABA) and calculation was done using ABA as reference (Kamboj et al., 1999). Quantification of indole acetic acid (IAA) and indole butyric acid (IBA) was carried in accordance with the method of Kelen et al. (2004). Purified extract residue was subjected to HPLC on a column of PEGASIL ODS (6 mm i.d. × 150 mm, Senshu Kagaku, Tokyo, Japan) and quantifications were derived from standard curves of IBA ranging from 10 to 200 ng/ml.
2.13. Estimation of the concentration of mineral elements
For estimation of Na+, K+, Mg2+ and Ca2+ method described by Wolf (1982) was followed. Dried plant samples were digested in acid and content of Na+, K+, Mg2+ and Ca2+ was determined using a flame photometer Jenway Flame Photometer, Bibby Scientific Ltd-Stone-Staffs-St15 0SA–UK. Standard curves for each mineral (10–100 μg ml−1) were used to calculate ion concentrations in the plant samples.
2.14. Statistical analysis
Data was analysed in a randomized block design with each pot being the replicate for all the treatments. Analysis of variance (ANOVA) was performed using SPSS software (version 15) to determine the significance of the treatments and Least Significant Difference (LSD) test at P < 0.05 was used to determine differences between means. The SAS version (9.3) was used to analyse the degree of correspondence (Pearson’s coefficient).
3. Results
Exposure of non-AMF inoculated Ef plants to drought stress resulted in a significant inhibition of growth as measured by morphological parameters like shoot and root length, biomass content of shoot and root system(Fig. 1, Table 1). Drought reduced the shoot and root length by 51.4 and 33.1%, respectively, relative to nonstressed controlled Ef plants. In contrast, AMF inoculation of non-stressed Ef plants increased its shoot length, shoot fresh and dry weight by 19.8, 13.4, and 14.5%, respectively, compared to non-stressed control Ef plants; and root length, root fresh and dry weight by 26.41, 26.27, and 38.54%, respectively. AMF-inoculation of Ef plants subjected to drought stress mitigated the deleterious effect of drought on shoot length, shoot fresh and dry weight by 1.0, 28.3, and 29.1%, respectively, and on root length, root fresh and dry weight by 21.8, 15.9, and 18.9%, respectively. Compared to non-stressed control, the shoot and root fresh weight of AMF-inoculated Ef plants subjected to drought stress was reduced by 39.8 and 44.4%, respectively, and dry weight of shoot and root was reduced by 33.9 and 32.9%, respectively (Table 1).
Fig. 1.
Effect of drought stress and arbuscular mycorrhizal fungi (AMF) on the morphological appearance (shoot and root) of Ephedra foliate.
Table 1.
Effect of drought and arbuscular mycorrhizal fungi (AMF) on the length, fresh weight, dry weight, and relative water content (RWC) of shoots and roots of Ephedra foliata. Data represent the mean of seven replicates and data followed by different letters are statistically different at P < 0.05.
| Treatment | Shoot |
Root |
Shoot length/root length | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Length (cm) | Fresh weight (g) | Dry weight (g) | RWC (%) | Length (cm) | Fresh weight (g) | Dry weight (g) | RWC (%) | ||
| Control | 160.2b | 2.31b | 1.534b | 78.1b | 82.2b | 1.672b | 0.7916b | 88.0b | 1.93 |
| AMF | 199.8a | 2.67a | 1.796a | 87.7a | 111.7a | 2.268a | 1.288a | 97.9a | 1.39 |
| Drought | 77.9c | 1.39d | 0.853d | 54.0d | 55.0d | 1.104d | 0.5306d | 57.4d | 1.61 |
| Drought + AMF | 78.7cd | 1.94c | 1.203c | 74.1c | 70.4c | 1.314c | 0.6546c | 65.9c | 1.83 |
| LSD at: 0.05 | 6.18 | 0.07 | 0.14 | 2.11 | 6.25 | 0.13 | 0.03 | 0.02 | 0.07 |
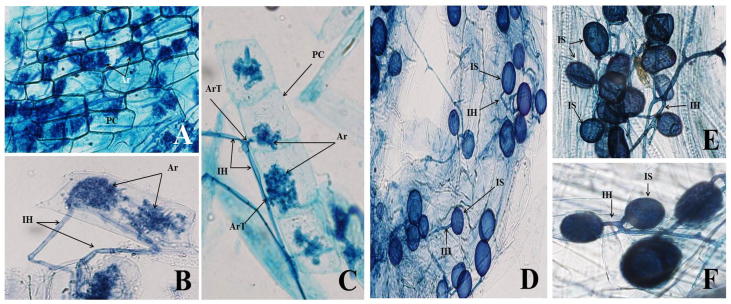
The level of mycorrhizal colonization in Ef plants is presented in Table 2 and Fig. 2A–F. showing the presence of different AMF structures. The total number of spores was >250 spore/50 g soil and mycelium and arbuscules were present in >85% of the samples. However, the presence of vesicles was >50% (Fig. 2A–F). Drought stress significantly reduced the AMF colonization of Ef roots by decreasing the total spore percentage (spores/50 g of soil) to 71.9%, arbuscules to 33.3%, vesicles to 32.8%, and that of mycelia to 79.7%, relative to levels in non-stressed, AMF-inoculated Ef plants (Table 2). The colonisation potential in AMF-inoculated Ef plants subjected to drought stress was significantly lower compared to AMF-inoculated, non-stressed plants and significantly reduced the percentage of all AMF structures (mycelium, vesicles, and arbuscules).
Table 2.
Effect of drought on the colonisation potential of arbuscular mycorrhizal fungi (AMF) in E. foliata. Data represent the mean of seven replicates and data followed by different letters are statistically different at P < 0.05.
| Treatment | Total colonisation (%) |
Intensity of structural colonization (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total* Spores | Mycelium | Vesicles | Arbuscules | Mycelium |
Vesicles |
Arbuscules |
|||||||
| P | M | A | P | M | A | P | M | A | |||||
| AMF | 253.1a | 87.0a | 56.0a | 86.0a | 20.6b | 28.0a | 53.6a | 14.3b | 27.0b | 69.0a | 11.3b | 74.0a | 84.0a |
| Drought + AMF | 71.0b | 17.6b | 37.6bb | 57.3b | 51.3a | 11.6b | 6.66b | 48.0a | 34.3a | 17.6b | 33.6a | 53.6b | 21.3b |
| LSD at: 0.05 | 19.465 | 14.456 | 9.794 | 8.068 | 17.681 | 16.452 | 29.543 | 15.976 | 18.717 | 25.005 | 9.838 | 18.37 | 18.017 |
P = poor; M = moderate; A = abundant.
Total spore population was recorded as number/50 g dry soils.
Fig. 2.
A–F: Photomicrographs of root colonization of Ephedra foliate illustrating different AMF structural components. Ar = Arbuscule, IH = intraradical Hyphea, ArT = Arbuscular Trunck, IS = intact spore.
Drought stress also considerably reduced the level of photosynthetic pigments in Ef leaves, relative to non-stressed Ef plants, while as AMF inoculation Ef plant leaves significantly ameliorated the negative impact of drought conditions. The observed reduction in the decline of chlorophyll a, chlorophyll b, carotenoids, and total chlorophyll contents was 30.0, 40.7, 84.7, and 36.5% respectively, in non-AMF inoculated Ef plants. While as, its reduction declined by 20.3, 22.5, 91.97, and 34.9%, respectively in AMF inoculated drought subjected Ef plants (Table 3). Under non-stress conditions, Ef plants with AMF inoculation significantly increased the level of photosynthetic pigments relative to non-stressed non-AMF Ef plants. The percent increase was 27.9% for chlorophyll a, 30.1% for chlorophyll b, 30.2% for total chlorophylls, and 63.8% for carotenoids (Table 3).
Table 3.
Effect of drought and arbuscular mycorrhizal fungi (AMF) on the concentration of photosynthetic pigments (mg/g FW) of Ephedra foliata. Data presented represent the mean of seven replicates and data followed by different letters are statistically different at P < 0.05.
| Treatment | Photosynthetic pigments (mg/g fresh weight) |
|||||
|---|---|---|---|---|---|---|
| Chlorophyll (a) | Chlorophyll (b) | Chlorophyll (a + b) | Chlorophyll (a/b) | Carotenoid | Total Chlorophyll | |
| Control | 0.7163b | 0.2786b | 0.995 | 2.58 | 0.072c | 1.067b |
| AMF | 0.9943a | 0.3990a | 1.393 | 2.49 | 0.199a | 1.530a |
| Drought | 0.5013d | 0.1650d | 0.666 | 3.03 | 0.011d | 0.677d |
| Drought + AMF | 0.6293c | 0.2130c | 0.842 | 2.96 | 0.137b | 1.041bc |
| LSD at: 0.05 | 0.0273 | 0.0215 | 0.0357 | 0.2513 | 0.0074 | 0.1685 |
The Pearson’s correlation between spores, mycelium, arbuscules, vesicles, chlorophyll a, chlorophyll b, carotenoids and total chlorophyll is presented in Table 4. A significant positive correlation was observed between the percentage of mycelia and the spores. This indicates that with the increase in the growth of mycelia, the production of spores also increased. A positive and high significant correlation was also evident between the percentage of arbuscules and spore formation, as well as between the percentage of vesicles and spore formation. The level of chlorophyll a and chlorophyll b, also exhibited a significant positive correlation with the percentage of mycelia. The correlation coefficients were 99,142** and 99,246**, respectively. In contrast, carotenoids content decreased with the increase in mycelial growth and spore formation, hence indicated a negative and a non-significant correlation (−0.20629) was observed between carotenoid content and spore formation and mycelial growth. Total chlorophyll content exhibited a significant and positive correlation with spore formation. Mycelia displayed a significant and positive correlation (0.965) with the percentage of arbuscular mycorrhiza and vesicles. As the mycelial growth increased, the percentage of vesicles also increased. Chlorophyll a and b content also exhibited a significant positive correlation with increased growth of the mycelia, while carotenoid content exhibited a negative and non-significant correlation with AMF structures. Total chlorophyll content, however, increased with the increase in mycelial growth, exhibiting a significant positive correlation. The percentage of vesicles exhibited a significant positive correlation with spore production (0.9259), mycelial growth (0.9677), the percentage of arbuscules (0.9266), and chlorophyll a content. A non-significant correlation was observed between chlorophyll b and carotenoid content. Lastly, total chlorophyll levels exhibited a significant positive correlation with vesicle production. Chlorophyll a and b content exhibited a significant positive correlation with spore formation, mycelial growth, and the percentage of arbuscules. In contrast, a significant negative correlation was observed between carotenoid content and chlorophyll a and b content (Table 4).
Table 4.
Pearson’s correlation matrix between mycorrhizal structures and photosynthetic pigments.
| Spores | Mycelium | Arbuscules | Vesicles | Ch a | Ch b | Carot. | Total PP | |
|---|---|---|---|---|---|---|---|---|
| Spores | 1.00000 | 0.97910 | 0.98973 | 0.92598 | 0.99142 | 0.99246 | −0.20629 | 0.85912 |
| Mycelium | 1.00000 | 0.96507 | 0.96778 | 0.97669 | 0.97027 | −0.15041 | 0.87153 | |
| Arbuscules | 1.00000 | 0.92666 | 0.96493 | 0.96792 | −0.17911 | 0.84815 | ||
| Vesicles | 1.00000 | 0.92661 | 0.90258 | 0.01793 | 0.90492 | |||
| Ch a | 1.00000 | 0.99393 | −0.18296 | 0.87734 | ||||
| Ch b | 1.00000 | −0.28436 | 0.82241 | |||||
| Carot. | 1.00000 | 0.31111 | ||||||
| Total PP | 1.00000 |
Ch a: Chlorophyll a, Ch b: Chlorophyll b, Carot.: carotenoids, Total PP: Total photosynthetic pigments.
Subjecting Ef plants to drought stress significantly increased sucrose phosphate synthase (SPS), acid and alkaline phosphatase activity by 46.8%, 20.9% and 39.0%, respectively, relative to the non-stressed control. Both drought stress and AMF inoculation induced higher activity of these enzymes. In the activity of these three enzymes, an increase of 19.1%, 6.4%, and 14.3% was observed, respectively, in non-stressed plants inoculated with AMF and 62.1%, 25.9%, and 50.6% in the drought + AMF treatment group. The increases were all relative to non-stressed, non-inoculated Ef plants (Fig. 3A–C).
Fig. 3.
A–C: Effect of drought and arbuscular mycorrhizal fungi (AMF) on the activity of (A) sucrose phosphate synthase, (B) acid phosphatase, and (C) alkaline phosphatase in Ephedra foliata. Data represent the mean of seven replicates and data followed by different letters are statistically different at P < 0.05.
The Pearson’s correlation of AMF colonization with SPS, acid phosphatase and alkaline phosphataseis was presented in Table 5. Spore formation exhibited a significant negative correlation with SPS (−0.995), acid phosphatase (−0.995) and alkaline phosphatase (−0.9896) activity. The percentage of arbuscules exhibit significantly negative correlation with SPS (−0.978), acid phosphatase (−0.9872), and alkaline phosphatase (−0.941) activity. SPS activity showed a significant negative correlation with spore formation, mycelial growth, percentage of arbuscules, and the percentage of vesicles; while a significantly positive correlation was observed between acid phosphatase and alkaline phosphatase activity (Table 5). Both enzymes had a significant negative correlation with spore formation (−0.999, −0.989), mycelial growth (−0.984, −0.994), and the percentage of arbuscules (−0.987, −0.968) and vesicles (−0.935, −0.941). The effects of drought and AMF on nitrogen assimilation shown in Fig. 4A–D had a negative impact on nitrate reductase (NR) and nitrite reductase (NiR) activity and dramatically reduced the levels of NH4 and NO3. Non-stressed Ef plants inoculated with AMF, however, exhibit increased NR (39.64%) and NiR (29.38%) activity with a concomitant increase in the level of NH4 (26.66%) and NO3 (33.11%) relative to non-stressed, control plants. When inoculated to drought stressed plants, AMF ameliorated the negative effect of drought on NR and NiR activity, NH4 and NO3 levels were significantly reduced by 33.2%, 25.2%, 33.8, and 33.6% respectively in Ef plants inoculated with AMF, relative to non-inoculated, drought stressed Ef plants (Fig. 4A–D).
Table 5.
Pearson’s correlation matrix between mycorrhizal structures (spores, mycelium, arbuscules, and vesicles) and phosphatase enzyme activity (SPS, acid phosphatase, and alkaline phosphatase).
| Spores | Mycelium | Arbuscules | Vesicles | SPS | AcidPase | AlkPase | |
|---|---|---|---|---|---|---|---|
| Spores | 1.00000 | 0.97910 | 0.98973 | 0.92598 | −0.99529 | −0.99921 | −0.98965 |
| Mycelium | 1.00000 | 0.96507 | 0.96778 | −0.98342 | −0.98431 | −0.99460 | |
| Arbuscules | 1.00000 | 0.92666 | −0.97897 | −0.98721 | −0.96823 | ||
| Vesicles | 1.00000 | −0.91517 | −0.93575 | −0.94157 | |||
| SPS | 1.00000 | 0.99517 | 0.99503 | ||||
| AcidPase | 1.00000 | 0.99258 | |||||
| AlkPase | 1.00000 |
SPS: Sucrose phosphate synthase, Acid Pase: Acid phosphatase, Alk Pase: Alkaline phosphatase.
Fig. 4.
A–D: Effect of drought and arbuscular mycorrhizal fungi (AMF) on the content of (A) ammonium, (B) nitrate and (C) nitrate reductase activity and (D) nitrite reductase activity in Ephedra foliata. Data represent the mean of seven replicates and data followed by different letters are statistically different at P < 0.05.
Proline, glucose, and soluble protein increased in AMF inoculated Ef plants both under non-stressed and drought-stressed conditions. In non-inoculated Ef plants, drought induced a significant decline in the soluble protein content While as in AMF inoculated plants, induced an increase of 36.2 and 26.5% in proline and glucose, respectively which is an increase of 37.4% and 26.9% of proline and glucose, respectively. AMF reduced the negative effect of drought on the soluble protein content by 18.97%. Drought stress increased the level of proline (73.55%) and glucose (78.78%) but reduced the level of total soluble protein by 34.88% (Fig. 5A–C).
Fig. 5.
A–C: Effect of drought and arbuscular mycorrhizal fungi (AMF) on the content of (A) glucose (B) proline, and (C) soluble protein in Ephedra foliata. Data represent the mean of seven replicates and data followed by different letters is statistically different at P < 0.05.
Drought stressed Ef plants also increased the generation of ROS, such as H2O2; causing considerable oxidative injury as evidenced by lipid peroxidation, measured as malonaldehyde content. Non-inoculated, drought-stressed plants exhibited a 70.1% & 53.76% increase in H2O2 and lipid peroxidation compared to non-stressed control plants. However, AMF-inoculated drought stressed Ef plants (drought + AMF) exhibited only 55.7% and 41.3% increase in H2O2 and lipid peroxidation, respectively (Fig. 6A–B).
Fig. 6.
A–B: Effect of drought and arbuscular mycorrhizal fungi (AMF) on (A) lipid peroxidation, and (B) hydrogen peroxide in Ephedra foliata. Data represent the mean of seven replicates and data followed by different letters are statistically different at P < 0.05.
AMF also significantly increased antioxidant enzyme activity and the level of non-enzymatic antioxidants relative to control plants (Fig. 7A–F). AMF stimulated the activity of SOD, CAT, APX, and GR by 12.1%, 8.8%, 36.2% and 38.9% respectively relative to non-stress control plants. In Drought stress non-inoculated plants the activity of SOD, CAT, APX and GR is increased by 37.81%, 30.30%, 64.26% and 72.96% respectively. Also GSH and AsA increased by 54.55 and 50.45%, relative to the non-stressed control plants. However, AMF-inoculated plants subjected to a drought stress exhibited an additional boost in the activity of SOD (17.31%), CAT (20.22%), APX (27.04%), and GR (30.43%), and increased the accumulation of GSH (49.80%) and AsA (17.84%) relative to non-inoculated, drought-stressed Ef plants. These results indicate that AMF decrease the potential of oxidative damage through the upregulation of the antioxidant system in Ef plants (Figs. 6A–B and 7A–F).
Fig. 7.
A–F: Effect of drought and arbuscular mycorrhizal fungi (AMF) on the activity of (A) superoxide dismutase (B) catalase (C) ascorbate peroxidase (D) glutathione reductase, and (E) the content of reduced glutathione and (F) ascorbic acid in Ephedra foliata. Data presented represent the mean of seven replicates and data followed by different letters are statistically different at P < 0.05.
The current study was conducted to examine the effect of AMF colonization on the activity of antioxidant enzymes such as catalase (CAT), peroxidase (APX), superoxide dismutase (SOD) and glutathione reductase (GR). A Pearson’s correlation matrix was constructed to examine the relationship of colonization structures (spore, mycelium, arbuscules, and vesicles) with antioxidant activity and results were presented in Table 6. A significant negative correlation was observed between spore formation and antioxidant enzyme activity, A similar relationship was observed between the other AMF structures and antioxidant levels (Table 6). The general results of the study indicated that the increased appearance of AMF structures improves the growth of Ef plants, however, increases in antioxidant enzyme activity (SOD, CAT, APX, and GR) inhibits AMF colonization (Table 6). Also, the results indicated that AM symbiosis can improve the anti-oxidant defense system in Ef plants, especially with respect to SOD activity. The increase in antioxidant enzyme activity allows the rapid conversion of O2-to H2O2 and this subsequent build-up of H2O2 is prevented by the increased activity of CAT, APX, and PX (Table 6).
Table 6.
Pearson’s correlation matrix between mycorrhizal structures (spores, mycelium, arbuscules, and vesicles) and antioxidant enzyme activity (SOD, CAT, APX, GR).
| Spores | Mycelium | Arbuscules | Vesicles | SOD | CAT | APX | GR | |
|---|---|---|---|---|---|---|---|---|
| Spores | 1.00000 | 0.97910 | 0.98973 | 0.92598 | −0.99670 | −0.99698 | −0.99539 | −0.98883 |
| Mycelium | 1.00000 | 0.96507 | 0.96778 | −0.98777 | −0.98718 | −0.98588 | −0.99221 | |
| Arbuscules | 1.00000 | 0.92666 | −0.97838 | −0.97857 | −0.97939 | −0.96499 | ||
| Vesicles | 1.00000 | −0.93228 | −0.92956 | −0.92160 | −0.94798 | |||
| SOD | 1.00000 | 0.99936 | 0.99723 | 0.99674 | ||||
| CAT | 1.00000 | 0.99874 | 0.99535 | |||||
| APX | 1.00000 | 0.99076 | ||||||
| GR | 1.00000 |
APX: Ascorbate peroxidase, CAT: Catalase, SOD: Superoxide dismutase, GR: Glutathione reductase.
Drought stressed Ef plants inoculated with AMF resulted in differential changes in the endogenous concentrations of growth hormones. AMF increased ABA, IAA, IBA and GA by 46.3%, 26.6%, 54.5% and 33.8%, respectively, compared to non-inoculated, non-stressed, control plants, while as it decreased IAA/IBA concentration. (Fig. 8A–E). ABA in AMF inoculated drought stressed plants increased by 74.3% and 80.2% while as, IAA/IBA increased in non-inoculated drought stressed plants. When drought stressed was compared to the non-stressed, controlled plants, IAA, IBA, and GA decreased by 81.4%, 54.4%, and 68.4%, respectively. AMF inoculation significantly reduced the negative impact of drought on the concentration of IAA (58.57%), IBA (46.20%), and GA (58.75%), relative to non-inoculated, drought-stress Ef plants (Fig. 8A–E). AMF and host roots requires a continuous exchange of signals to ensure the proper development of mycorrhizae. Plant hormones play an important role in plant development and are also involved in the regulation of the host/mycorrhizae symbiotic relationship. The relationship (Pearson’s correlation) between hormone levels and AMF colonization is presented in Table 7. Spore formation exhibited a significant positive correlation with the level of IAA, IBA, and GA. In contrast to the other hormones, the level of ABA exhibited a significant negative correlation with AMF colonization structures. The other AMF structures (mycelium, arbuscules, and vesicles) also exhibited a significant positive correlation with the IAA, IBA, and GA but a negative correlation with ABA. AMF colonization and the level of growth-promoting hormones was positively correlated (Table 7). The production of ABA also increased with AMF colonization (Table 7). In summary, all of the AMF colonization structures were positively correlated with the level of growth promoting hormones (IAA, IBA, and GA).
Fig. 8.
A–E: Effect of drought and arbuscular mycorrhizal fungi (AMF) on the endogenous concentration of (A) abscisic acid, (B) indole acetic acid [IAA], (C) indole butyric acid [IBA], (D) gibberellic acid, and (E) the ratio of IAA/IBA in Ephedra foliata. Data represent the mean of three replicates and data followed by different letters are statistically different at P < 0.05.
Table 7.
Pearson’s correlation matrix between mycorrhizal structures (spores, mycelium, arbuscules, and vesicles) and the endogenous concentration of phytohormones (GA/BA, IAA, IBA, and GA).
| Spores | Mycelium | Arbuscules | Vesicles | ABA | IAA | IBA | GA | |
|---|---|---|---|---|---|---|---|---|
| Spores | 1.00000 | 0.97910 | 0.98973 | 0.92598 | −0.99632 | 0.99840 | 0.99556 | 0.99250 |
| Mycelium | 1.00000 | 0.96507 | 0.96778 | −0.98626 | 0.98621 | 0.99065 | 0.99334 | |
| Arbuscules | 1.00000 | 0.92666 | −0.97920 | 0.98212 | 0.97946 | 0.98278 | ||
| Vesicles | 1.00000 | −0.92355 | 0.93426 | 0.93674 | 0.94585 | |||
| FABA | 1.00000 | −0.99825 | −0.99908 | −0.99616 | ||||
| IAA | 1.00000 | 0.99838 | 0.99470 | |||||
| IBA | 1.00000 | 0.99829 | ||||||
| GA | 1.00000 |
ABA: Abscisic acid, IAA: Indole acetic acid, IBA: Iindole butyric acid, GA: Gibberellic acid.
Drought stress resulted in a significant decline in the uptake of essential mineral elements such as K, Mg, and Ca, as well as Na (Table 8). The uptake of these mineral elements, however, was significantly increased in AMF-inoculated plants. AMF inoculation increased the content of K, Mg, and Ca by 9.2%, 18.8%, and 20.4%, respectively, in non-stressed plants and also reduced the negative impact of drought on the uptake of these mineral elements. The decline in Na, K, Mg, and Ca in non-inoculated, drought stressed plants was 43.7%, 36.4%, 36.4%, and 48.0%, respectively, however, this reduction was considerably reduced in AMF-inoculated, drought-stressed plants (Table 8).
Table 8.
Effect of drought and arbuscular mycorrhizal fungi (AMF) on the concentration (mg/g DW) of sodium (Na), potassium (K), magnesium (Mg) and calcium (Ca) in Ephedra foliata. Data presented represent the mean of three replicates and data followed by different letters are statistically different at P < 0.05.
| Treatment | Elements uptake (mg/g dry weight) |
|||
|---|---|---|---|---|
| Na | K | Mg | Ca | |
| Control | 17.96b | 24.69b | 3.906b | 1.169b |
| AMF | 22.48a | 27.18a | 4.813a | 1.468a |
| Drought | 10.11d | 15.70d | 2.484d | 0.608d |
| Drought + AMF | 12.06c | 21.13c | 2.937c | 0.891c |
| LSD at: 0.05 | 0.177 | 0.4239 | 0.1831 | 0.062 |
4. Discussion
Climate change globally increases the occurrences of drought stress. Therefore, new management strategies will be required to deal with this problem (Hameed et al., 2014, Wehner et al., 2015, Wu et al., 2017). Based on the results of the current study, we propose that the inoculation of plants with AMF in areas prone to frequent occurrences of drought stress can help to ameliorate potential plant growth and injury under non-stressed conditions. Our results indicate that AMF boost the level of osmolytes and antioxidant enzymes, both of which are beneficial for plants subjected to abiotic stresses which otherwise exhibited a significant decline in growth. In contrast, drought stress had a lower impact on AMF inoculated Ef plants inoculated also boosted growth under non-stressed conditions, relative to non-inoculated plants. Species of AMF are known for their ability to promote plant growth under stressful conditions through their beneficial effect on root growth, which allows plants to increase both mineral and water uptake (Hameed et al., 2014, Wu et al., 2017, Hashem et al., 2018). As reported earlier, (Sochacki et al., 2013, Hashem et al., 2018), our results indicated that drought stress reduced the ability of AMF to colonize plants as evident by the reduction in the number of AMF structural components (spores, mycelia, arbuscule, and vesicles). Drought reduces the germination and growth of AMF spores, resulting in a considerable decline in the formation of additional endogenous fungal structures (Huang et al., 2017).
The reduced chlorophyll content observed in drought-stressed Ef plants and the ability of AMF to inhibit that loss corroborates with the results of Sochacki et al. (2013) in Piriqueta morphotypes. AMF has the potential to improve the production of photoassimilates by increasing the rate of photosynthesis and reducing formation of ROS (Hashem et al., 2016). In the medicinal plants Hypericum perforatum (Zubek et al., 2012) and Piriqueta morphotypes (Sochacki et al., 2013), inoculation of AMF increased photochemical efficiency resulting in a significant increase in plant growth. In the present study, drought stress reduced the uptake of Mg, an important component of chlorophyll, while uptake of this mineral increased in AMF inoculated plants; making it available for incorporation into chlorophyll. The increase in Mg uptake and the reduced loss of chlorophyll pigment proteins in AMF-inoculated, drought stressed and non-stressed plants is also in agreement with earlier studies (Yang et al., 2015, Huang et al., 2017, Hashem et al., 2018). Abiotic stresses induce a down-regulation of enzymes responsible for regulating chlorophyll biosynthesis with a concomitant increase in chlorophyllase activity, triggering chlorophyll degradation (Hameed et al., 2014, Zhu et al., 2017). It is reasonable to consider that AMF inoculation may have down-regulated chlorophyllase activity and up-regulated the expression of chlorophyll biosynthetic genes, resulting in greater pigment synthesis. Shahabivand et al., 2012, Hashem et al., 2016, and Zhu et al. (2017) reported a similar increase in chlorophyll synthesis due to AMF inoculation. A drought-mediated decline of chlorophyll pigments has also been reported by others as well (Punyasheeladevi and Sujatha, 2014, Alwhibi et al., 2017). The higher carotenoid content observed in AMF-inoculated Ef plants may have also protected the photosynthetic system from the accumulation of ROS and their toxic effects, allowing optimum levels of photosynthesis (Abd_Allah et al., 2016). The findings of Abd_Allah et al. (2016) are in agreement with the present study results in which a Pearson’s correlation analysis determined a highly significant, positive correlation between AMF and photosynthetic pigments.
Increased sucrose phosphate synthase activity in AMF-inoculated contributes to the maintenance of sufficient carbon pool for several important pathways, such as secondary metabolites etc. (Mohanta et al., 2017). Wu et al. (2017) demonstrated that increased sucrose phosphate synthase activity in trifoliate orange plants inoculated with AMF resulted in a greater accumulation of carbon metabolites, such as sucrose, fructose, and glucose, and greater drought stress tolerance. Increases in sucrose phosphate synthase activity have been shown to thrive better under stressful conditions (Peng et al., 2016). In the current study, up-regulation of sucrose phosphate synthase activity in AMF-inoculated plants indicates its functional involvement in the maintenance of the carbon pool. We have assumed here that greater synthesis of glucose can lead to the optimisation of signalling events mediated by kinases and phytohormones in the regulation of developmental events in plants subjected to abiotic stress (O’Hara et al., 2013). Therefore, we suggest that AMF provide a holistic integration of stress tolerance strategies to support continued growth under stressful conditions. Pearson’s correlation analysis indicated a significant positive correlation between the AMF structural component, mycelium, and phosphatase enzymes. A similar result was reported by Kebrabadi et al. (2014) and is most likely due to an increase in the accumulation of phosphate in the host plant, as indicated by Jemo et al., 2017.
Drought exposed Ef plants exhibited lower levels of nitrate and nitrite reductase activity which indicated a reduction in nitrogen metabolism. Our data showed that AMF regulated nitrogen metabolism by enhancing nitrate and nitrite reductase activity in drought subjected plants by maintaining a higher content of nitrate. AMF induced greater ammonium synthesis increased availability of precursors that could be used for the incorporation of nitrogen into amino acids. Punyasheeladevi and Sujatha (2014) reported the reduced activity of nitrogen metabolising enzymes in Cajanus cajan subjected to drought stress. In contrast, increased amino acid synthesis resulting from the up-regulation of nitrogen metabolism is correlated with increased stress tolerance (Zarea et al., 2011). In the present study, AMF-inoculated plants exhibited greater ammonium availability, thereby decreased the negative impact of drought stress by maintaining a higher availability of amino acids, such as glutamine, for the production of proline. Greater production of ammonium due to AMF and improved amino acid synthesis and salt stress tolerance has also been reported by Abd_Allah et al. (2016) in Sesbania sesban. In addition to its effect on nitrogen metabolism, AMF inoculated plants also impacts phosphate nutrition by increasing phosphatase enzyme activity. Increased alkaline and acidic phosphatase activity in AMF-inoculated plants has also been previously reported by Kebrabadi et al. (2014). The enzyme activity of phosphatases regulates the processes associated with phosphorous nutrition in order to maintain efficient absorption, assimilation and metabolism of phosphorus (Abd_Allah et al., 2015). The increased activity of phosphatases in AMF-inoculated plants could potentially enhance the release of organically bound phosphorous, thereby making it available for transport and uptake (Egamberdieva et al., 2017). Abd_Allah et al. (2015) previously reported greater cadmium tolerance in AMF-inoculated Helianthus annus plants that also exhibited greater phosphorous content and activity of phosphatases.
In addition to the up-regulation of the antioxidant system in AMF-inoculated plants, an increase in the synthesis of osmolytes, including proline and glucose, was also observed. Drought stress triggered the synthesis of proline and glucose in non-inoculated plants but their synthesis was further enhanced by AMF inoculation. Plant species with high accumulation of free proline exhibit increased stress (Alwhibi et al., 2017, Wu et al., 2017). Accumulation of proline in response to drought stress corroborates the findings reported by Zhu et al. (2011) for maize and Alwhibi et al. (2017) for tomato. The accumulation of osmolytes, including proline, is the result of an increase in the activity of specific biosynthetic pathways (Wu et al., 2017). In this study, AMF appears to have up-regulated the biosynthetic enzymes involved in proline synthesis. The accumulation of proline protects the plant proteins and membranes from the damage caused due to excess levels of ROS (Hashem et al., 2015). Osmolytes contribute to maintaining tissue water content under water deficit conditions. In our study, AMF induced the accumulation of proline, which supports its beneficial role in the growth of Ephedra foliate subjected to drought stress (Abd_Allah et al., 2016). Proline also influences nitrogen metabolism, which is needed for stress recovery. Our data demonstrated that AMF-inoculated Ef plants exhibit greater nitrogen metabolising enzyme activity, resulting in increased synthesis of energy-rich amino acids (Ahanger et al., 2014, Hameed et al., 2014).
The present study demonstrated that Ef plants inoculated with AMF exhibited lower levels of oxidative stress due to reduced generation of ROS. The reduction in oxidative damage was correlated with the up-regulation of the antioxidant system in AMF-inoculated plants. Increased ROS generation has been associated with lipid peroxidation and altered membrane function in Brassica napus (Mirzaee et al., 2013) and Robinia pseudoacacia (Yang et al., 2015). It is believed that ROS, similar to H2O2, diffuses through membrane aquaporin channels resulting in damage to cells distant from the source of ROS generation (Bienert et al., 2007). The increased antioxidant activity in AMF-inoculated plants allows for the quick neutralisation of ROS, thus reducing or preventing the damage. Drought-induced accumulation of H2O2 damages the functional integrity of membranes and results in reduced growth (Singh et al., 2016). We suggest that the increased antioxidant defence system in AMF-inoculated plants helps to maintain ROS levels that do not cause cellular injury but rather function in integrating various developmental processes, including cell proliferation and differentiation, seed germination, gravitropism, root hair growth and pollen tube development, programmed cell death, and senescence (Abd_Allah et al., 2017). Reduced lipid peroxidation in AMF-inoculated Ef plants supports the beneficial role of AMF in plants subjected to drought stress.
Beneficial microorganisms, like AMF, integrate several tolerance mechanisms by modulating signalling processes and their associated signalling molecules, such as phytohormones (Egamberdieva et al., 2017). The protection of drought stressed Ef plants inoculated with AMF can be ascribed to the optimisation of endogenous levels of phytohormones responsible for plant growth. Hashem et al., 2016, Abd_Allah et al., 2018 have also reported a strong positive effect of beneficial microbes in reducing oxidative stress which is caused due to up-regulation of antioxidant system in Cassia italica and Cicer arietinum. Stress-induced upsurge in ROS can result in lipid peroxidation of the polyunsaturated component of membranes, thereby affecting their fluidity and function. Greater antioxidant enzyme activity in AMF-inoculated plants for SOD, CAT, APX, and GR, together with a higher rate of synthesis of AsA and GSH, contributes to increased cellular stability (Hashem et al., 2015, Hashem et al., 2016). With SOD forming the first line of defence in the prevention of oxidative stress, APX, GR, AsA, and GSH are the key components of the ROS scavenging pathway – AsA-GSH cycle. AMF induced the up-regulation of these antioxidant components that neutralizes the H2O2 by preventing the formation of toxic OH and thus helps in the protection of mitochondrial and chloroplast electron transport (Abd_Allah et al., 2015, Alwhibi et al., 2017). Greater maintenance of the AsA-GSH component makes NADP available for keeping photosynthetic electron transport occurring at a normal rate. AsA and GSH are key redox components involved in regulating the structure and activity of key enzymes (Scheibe and Dietz, 2012, Abd_Allah et al., 2016). Greater antioxidant activity in AMF-inoculated plants supports the quick neutralisation of H2O2 at the site of its generation, thereby preventing its diffusion to other parts of the plant. Further studies of this phenomenon would be very informative. Processes that eliminate excess ROS are elevated in AMF-inoculated plants, thus AMF provides greater stability to cellular metabolism at the whole plant level (Nath et al., 2016). Increased SOD, CAT, APX, and GR has been previously reported in drought-stressed seedlings by Mirzaee et al., 2013, Weng et al., 2015, and Alwhibi et al. (2017). Recently, Yang et al. (2015) demonstrated greater protection of photosynthesis and up-regulation of the antioxidant system in AMF-inoculated plants of Robinia pseudoacacia. An increase in ascorbic acid and glutathione was also reported in AMF-inoculated plants of Brassica napus (Mirzaee et al., 2013, Shafiq et al., 2014). AMF increased AsA and GSH levels in Sesbania sesban (Abd_Allah et al., 2016); resulting in greater salt stress tolerance. Reports showing a combined effect of AMF and drought on antioxidant system of host plants, however, are rare. Generally speaking, increased expression of antioxidants has been observed to impart stress tolerance and yield in a variety of plants (Weng et al., 2015). Correlation matrix analysis indicates that AMF symbiosis improves the anti-oxidative defense system and thus protects plant cells against the oxidative damage due to excess levels of ROS (Mirzaee et al., 2013, Singh et al., 2016, Wu et al., 2017).
Increased growth of plants colonized by AMF could be attributed to the optimisation of the endogenous concentration of growth-promoting phytohormones, such as IAA, IBA, and GA. Endophytic fungi have been shown to enhance the production of IAA and GA (Vishwakarma et al., 2017, Egamberdieva et al., 2017, Hashem et al., 2018). Additionally, increased ABA levels in AMF-inoculated plants can act as anti-transpirant and prevent excess water loss under water deficit conditions by regulating stomatal closure. Enhanced biosynthesis of ABA in drought-stressed and AMF-inoculated plants induces a redistribution and accumulation of ABA within stomatal guard cells, which triggers stomatal closure (Mohanta et al., 2017, Egamberdieva et al., 2018). Recently, Alwhibi et al. (2017) demonstrated drought-induced alterations in the endogenous concentration of growth-related hormones in tomato leading to a significant reduction in growth retardation. Increased levels of ABA helps to integrate different tolerance mechanisms in plants by actively mediating several downstream signalling events (Vishwakarma et al., 2017). Higher level of ABA in AMF-inoculated plants may have modified the expression levels of genes and the cis- and trans-acting regulatory elements of stress responsive promoters, leading to the regulation of growth (Fedaka et al., 2016). Reduced levels of GA and increased levels of ABA are considered to be key determinants of stress tolerance. However, AMF-inoculated plants tended to maintain both growth-promoting and stress-adaptive mechanisms, such as increases in level of ABA, at the same time. The end result appears to be a minimum impact on cell metabolism and increased stress tolerance (Huang et al., 2012). In the current study, the improved phytohormone synthesis and increased nutrient uptake in AMF-inoculated Ef plants have been observed to contribute to a system the acquired resistance by increasing the availability of precursor molecules for the synthesis of metabolites, like phenols and proline. Auxins and GA metabolites have major roles in the regulation of plant growth subjected to abiotic stress conditions (Ahanger et al., 2014, Vishwakarma et al., 2017). In this study, colonization of roots with AMF structural components exhibited a significant positive correlation with plant promoting hormones (IAA, IBA, GA). The accumulation of growth-promoting hormones in AMF-inoculated plants has been reported to be a key indicator of stress tolerance (Hashem et al., 2018). Thus, modulation of endogenous plant growth hormones appears to be a promising approach for protecting plants against the adverse impact of abiotic stresses (Egamberdieva et al., 2018).
Enhancing the uptake of Ca2+ also contributes to the stress signalling system to acquire greater stress tolerance (Abd_Allah et al., 2017). Also, increased K+ levels while playing a key role in the activation of several metabolic enzymes may also contribute to the up-regulation of several defence systems, such as the antioxidant system and osmolyte accumulation (Zhu et al., 2011, Yang et al., 2015, Hashem et al., 2016). AMF-inoculated plants exhibit increased growth under non-stressed conditions and sustained growth under drought stress conditions partly by increasing the uptake of N, Mg, K, and P (Hameed et al., 2014, Wu et al., 2017, Egamberdieva et al., 2017). AMF inoculated Ef plants helps to sustain root and shoot growth under drought stress conditions by increasing the synthesis of phytohormones and the uptake of mineral elements; all of which may contribute to increased cell division and growth. Drought impedes the uptake of minerals by simply making them inaccessible to plant roots due to a lack of root exploration in dry soils (Hameed et al., 2014).
5. Conclusion
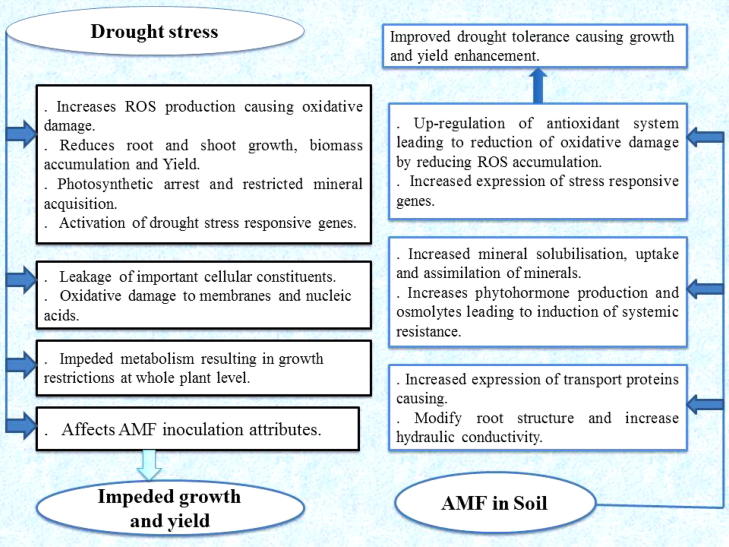
Drought negatively affects growth of Ef plants by its negative impact on chlorophyll synthesis, hormonal balance, and nitrogen metabolism. The deleterious impact of drought stress in AMF-inoculated plants was significantly mitigated by the up-regulation of the host antioxidant defence system which helps in rapid neutralization of ROS; thus preventing oxidative damage to membranes and proteins. The combined up-regulation of antioxidant metabolism, and the synthesis of phytohormones and osmolytes in AMF-inoculated plants could protect the destruction of cellular membranes from excess of ROS generated due to drought. A model summarizing the different roles of AMF in increasing stress tolerance is presented in Fig. 9. It may be concluded that AMF-inoculated Ef plants can be a useful strategy for mitigating the negative impact of drought stress on plant growth.
Fig. 9.
A model illustrating the different mechanisms by which arbuscular mycorrhizal fungi (AMF) increase drought tolerance in Ephedra foliata.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding to the Research Group number (RGP-271).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd_Allah E.F., Alqarawi A.A., Hashem A., Radhakrishnan R., Al-Huqail A.A., Al-Otibi F.O.N., Malik J.A., Alharbi R.I., Egamberdieva D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Int. 2018;13(1):37–44. [Google Scholar]
- Abd_Allah E.F., Hashem A., Alqarawi A.A., Wirth S., Egamberdieva D. Calcium application enhances growth and alleviates the damaging effects induced by Cd stress in sesame (Sesamum indicum L.) J. Plant Int. 2017;12(1):237–243. [Google Scholar]
- Abd_Allah E.F., Hashem A., Alqarawi A.A., Bahkali A.H., Alwhibi M.S. Enhancing growth performance and systemic acquired resistance of medicinal plant Sesbania sesban (L.) Merr using arbuscular mycorrhizal fungi under salt stress. Saudi J. Biolo. Sci. 2016;22:274–283. doi: 10.1016/j.sjbs.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd_Allah E.F., Hashem A., Alqarawi A.A., Alwathnani H.A. Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 2015;47(2):785–795. [Google Scholar]
- Aebi H. Catalase in vitro. In: Colowick S.P., Kaplan N.O., editors. vol. 105. Academic Press; New York: 1984. pp. 121–126. (Methods in Enzymology). [Google Scholar]
- Ahanger, M.A., Hashem, A., Abd Allah, E.F., Ahmad, P., 2014. Arbuscular mycorrhiza in crop improvement under environmental stress. In: Ahmad, P., Rasool, S. (Eds.), Emerging Technologies and Management of Crop Stress Tolerance, vol. 2, pp. 69–95.
- Alwhibi M.S., Hashem A., Abd_Allah E.F., Alqarawi A.A., Soliman D.W.K., Wirth S., Egamberdieva D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integ. Agri. 2017;16(8):1751–1757. [Google Scholar]
- Bates L.S., Waldre R.P., Teare I.D. Rapid determination of free proline for water stress studies. Plant Sci. 1973;39:205–207. [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. Rev. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bell, A., Bachman, S., 2011. Ephedra foliata. The IUCN Red List of Threatened Species 2011: e.T201696A9167394.
- Bienert G.P., Møller A.L., Kristiansen K.A., Schulz A., Møller I.M., Schjoerring J.K., Jahn T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Analyst Biochem. 1976;72:248–259. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cataldo D.A., Haroon M., Schrader L.E., Youngs V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975;6:71–80. [Google Scholar]
- Daniels, B.A., Skipper, H.B., 1982. Methods for recovery and quantitative estimation of propagules from soil. In: Schenck, N.C. (Ed.), Methods and Principles of Alycorrhizal Research, pp. 29–35. Anierican Phytopathological Society, St. Paul, Minnesota, p. 244.
- Egamberdieva D., Wirth S.J., Alqarawi A.A., Abd_Allah E.F., Hashem A. Phytohormones and beneficial microbes: essential components for plants to balance stress and fitness. Front. Microbiol. 2017;8:2104. doi: 10.3389/fmicb.2017.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egamberdieva, D., Wirth, S., Abd_Allah, E.F., 2018. Plant hormones as key regulators in plant-microbe interactions under salt stress. In: Egamberdieva, D., Ahmad, P. (Eds.), Plant Microbiome: Stress Response, Microorganisms for Sustainability 5, doi: 10.1007/978-981-10-5514-0_7.
- Ellman G.L. Tissue sulphydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fatokun C.A., Boukar O., Muranaka S. Evaluation of cowpea (Vigna unguiculata (L.)Walp.) germplasm lines for tolerance to drought. Plant Genet. Resour. 2012;10:171–176. [Google Scholar]
- Fedaka H., Palusinskaa M., Krzyczmonik K., Brzezniak L., Yatusevich R., Pietras Z., Kaczanowski S., Swiezewski S. Control of seed dormancy in Arabidopsis by a cis-acting noncoding antisense transcript. PNAS. 2016;113(48):E7846–E7855. doi: 10.1073/pnas.1608827113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finka R.L., Warner R.L., Muzit T.J. Effect of herbicides on in vivo nitrate and nitrite reduction. Weed Sci. 1977;25:18–22. [Google Scholar]
- Gianinazzi-Pearson V., Gianinazzi S. Enzymatic studies on the metabolism on vesicular arbuscular mycorrhiza. 1. Effect of mycorrhiza formation and phosphorus nutrition on soluble phosphatase activities in onion roots. Physiol. Veg. 1976;14:833–841. [Google Scholar]
- Hageman, R.H., Hucklesby, D.P., 1971. Nitrate reductase from higher plants. In: Pietro, A.S. (Ed.), Methods in Enzymology, Academic Press, New York, NY, pp. 491–503.
- Hameed, A., Wu, Q.S., Abd_Allah, E.F., Hashem, A., Kumar, A., Lone, H.A., Ahmad, P., 2014. Role of am fungi in alleviating drought stress in plants. In: Miransari, M. (Ed.), Use of Microbes for the Alleviation of Soil Stresses, doi: 10.1007/978-1-4939-0721-2_4.
- Hashem, A., Abd_Allah, E.F., Alqarawi, A.A., Egamberdieva, D., 2018. Arbuscular mycorrhizal fungi and plant stress tolerance. In: Egamberdieva, D., Ahmad, P. (Eds.), Plant Microbiome: Stress Response, Microorganisms for Sustainability 5, doi: 10.1007/978-981-10-5514-0_4.
- Hashem A., Abd_Allah E.F., Alqarawi A.A., Al-Huqail A.A., Wirth S., Egamberdieva D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Microbiol. 2016;7:1089. doi: 10.3389/fmicb.2016.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem A., Abd_Allah E.F., Alqarawi A.A., Al Huqail A.A., Egamberdieva D., Wirth S. Alleviation of cadmium stress in Solanum lycopersicum L by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J. Biolo. Sci. 2015;23(2):272–281. doi: 10.1016/j.sjbs.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges D.M., DeLong J.M., Forney C.F., Prange R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207(4):604–611. doi: 10.1007/s00425-017-2699-3. https://link.springer.com/article/10.1007/s004250050524 [DOI] [PubMed] [Google Scholar]
- Huang G.T., Ma S.L., Bai L.P., Zhang L., Ma H., Jia P., Liu J., Zhong M., Guo Z.F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012;39:969–987. doi: 10.1007/s11033-011-0823-1. [DOI] [PubMed] [Google Scholar]
- Huang Y.M., Zou Y.N., Wu Q.S. Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep. 2017;7 doi: 10.1038/srep42335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard N.L., Huber S.C., Pharr D.M. Sucrose phosphate synthase and acid invertase as determinants of sucrose concentration in developing muskmelon (Cucumis melo L.) fruits. Plant Physiolo. 1989;91:1527–1534. doi: 10.1104/pp.91.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INVAM, 2014. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. West Virginia University, Morgantown, West Virginia. URL: <http://invam.wvu.edu/the-fungi/species-descriptions> (accessed March 26).
- Jemo M., Sulieman S., Bekkaoui F., Olomide O.A.K., Hashem A., Abd_Allah E.F., Alqarawi A.A., Tran L.S.P. Comparative analysis of the combined effects of different water and phosphate levels on growth and biological nitrogen fixation of nine cowpea varieties. Front. Plant Sci. 2017;8:2111. doi: 10.3389/fpls.2017.02111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, M.H., Collins, W.L., 1985. Hydroponic Vegetable Production. Horticultural Reviews, vol. 7, pp. 483–559, ISSN 9780870554926.
- Kamboj J.S., Blake P.S., Quinlan J.D., Baker D.A. Identification and quantitation by GC-MS of zeatin and zeatin riboside in xylem sap from rootstock and scion of grafted apple trees. Plant Growth Reg. 1999;28:199–205. [Google Scholar]
- Kebrabadi B.Z., Matinizadeh M., Daryayi M.G., Salehi A. Changes in acid and alkaline phosphatase enzyme activity in rhizosphere ash Fraxinus rotundifolia and its correlation with soil and plant phosphorus. J. Biodiversity Environ. Sci. 2014;4(5):233–238. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.652.3778&rep=rep1&type=pdf [Google Scholar]
- Kelen M., Çubek Demiralay E., Şen S., Ozkan G. Separation of abscisic acid, indole-3-acetic acid, gibberellic acid in 99 R (Vitis berlandieri × Vitis rupestris) and rose oil (Rosa damascena Mill.) by reversed phase liquid chromatography. Turkish J. Chem. 2004;28:603–610. [Google Scholar]
- Kusaba S., Kano-Murakami Y., Matsuoka M., Tamaoki M., Sakamoto T., Yamaguchi I., Fukumoto M. Alteration of hormone levels in transgenic tobacco plants over expressing a rice homeobox gene OSH1. Plant Physiol. 1998;116:471–476. doi: 10.1104/pp.116.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.J., Foster K.R., Morgan P.W. Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol. 1998;116:1003–1010. doi: 10.1104/pp.116.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler H.K., Wellburn A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983;11:591–592. [Google Scholar]
- Mirzaee M., Moieni A., Ghanati F. Effects of drought stress on the lipid peroxidation and antioxidant enzyme activities in two canola (Brassica napus L.) cultivars. J. Agri. Sci. Tech. 2013;15(3):593–602. [Google Scholar]
- Mohanta T.K., Bashir T., Hashem A., Abd_Allah E.F. Systems biology approach in plant abiotic stresses Author links open overlay panel. Plant Physiolo. Biochem. 2017;121:58–73. doi: 10.1016/j.plaphy.2017.10.019. [DOI] [PubMed] [Google Scholar]
- Mukherjee S.P., Choudhuri M.A. Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant. 1983;58:166–170. [Google Scholar]
- Nakano Y., Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nath M., Bhatt D., Prasad R., Gill S.S., Anjum N.A., Tuteja T. Reactive oxygen species generation-scavenging and signaling during plant-arbuscular mycorrhizal and Piriformospora indica interaction under stress condition. Front. Plant Sci. 2016;7:1574. doi: 10.3389/fpls.2016.01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara L.E., Paul M.J., Wingler A. How do sugars regulate plant growth and development? New insight into the role of trehalose-6-phosphate. Mol. Plant. 2013;6(2):261–274. doi: 10.1093/mp/sss120. [DOI] [PubMed] [Google Scholar]
- Peng J., Liu J., Zhang L., Luo J., Dong H., Ma Y., Zhao X., Chen B., Sui N., Zhou Z., Meng Y. Effects of soil salinity on sucrose metabolism in cotton leaves. PLoS ONE. 2016;11(5) doi: 10.1371/journal.pone.0156241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J.M., Hayman D.S. Improved procedures for clearing and staining parasitic and vesicular±arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Brit. Mycol. Soc. 1970;55:158–161. [Google Scholar]
- Punyasheeladevi S., Sujatha B. Effect of drought stress on chlorophyll content, protein and total free amino acid in Cajanus cajan (L.) Mills paugh. Am. J. Pharm. Tech. Res. 2014;4(4):634–643. [Google Scholar]
- Qi Q.G., Rose P.A., Abrams G.D., Taylor D.C., Abrams S.R., Cutler A.J. Abscisic acid metabolism, 3-ketoacyl-coenzyme A synthase gene expression and very-long-chain monounsaturated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol. 1998;117:979–987. doi: 10.1104/pp.117.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H., Munne-Bosch S. Production and scavenging of reactive oxygen species and redox signaling during leaf and flower senescence: similar but different. Plant Physiol. 2016;171(3):1560–1568. doi: 10.1104/pp.16.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said H., EL Shishiny E.D.H. Researches on plant metabolism. III. The effect of disc thickness on the respiration and the various nitrogen fractions of cut discs of radish roots immersed in water and in sugar solutions. Plant Physiol. 1944;19:660–670. doi: 10.1104/pp.19.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R., Dietz K.J. Reduction–oxidation network for flexible adjustment of cellular metabolism in photoautotrophic cells. Plant, Cell Environ. 2012;35:202–216. doi: 10.1111/j.1365-3040.2011.02319.x. [DOI] [PubMed] [Google Scholar]
- Sergiev I., Alexieva V., Karanov E. Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Comp. Ren. Del. Academie Bul. des Sci. 1997;51:121–124. [Google Scholar]
- Shafiq S., Akram N.A., Ashraf M., Arshad A. Synergistic effects of drought and ascorbic acid on growth, mineral nutrients and oxidative defense system in canola (Brassica napus L.) plants. Acta Physiol. Plant. 2014;36:1539–1553. [Google Scholar]
- Shahabivand S., Maivan H.Z., Goltapeh E.M., Sharfi M., Ali S., Aliloo A. The effects of root endophyte and arbuscular mycorrhizal fungi on growth and cadmium accumulation in wheat under cadmium toxicity. Plant Physiol. Biochem. 2012;60:53–58. doi: 10.1016/j.plaphy.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Singh R., Singh S., Parihar P., Mishra R.K., Tripathi D.K., Singh V.P., Chauhan D.K., Prasad S.M. Reactive oxygen species (ROS): beneficial companions of plants’ developmental processes. Front. Plant Sci. 2016;7:1299. doi: 10.3389/fpls.2016.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I.K., Vierheller T.L., Thorne C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid) Anal. Biochem. 1988;175:408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- Sochacki P., Ward J.R., Cruzan M.B. Consequences of mycorrhizal colonization for Piriqueta morphotypes under drought stress. Int. J. Plant Sci. 2013;174(1):65–73. [Google Scholar]
- Stutz J.C., Morton J.B. Successive pot cultures reveal high species richness of arbuscular endomycorrhizal fungi in arid ecosystems. Can. J. Bot. 1996;74:1883–1889. [Google Scholar]
- Utobo E.B., Ogbodo E.N., Nwogbaga A.C. Techniques for extraction and quantification of arbuscular mycorrhizal fungi. Libyan Agric. Res. Center Int. 2011;2:68–78. [Google Scholar]
- Vishwakarma K., Upadhyay N., Kumar N., Yadav G., Singh J., Mishra R.K., Kumar V., Verma R., Upadhyay R.G., Pandey M., Sharma S. Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front. Plant Sci. 2017;8:161. doi: 10.3389/fpls.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner G.G., Balko C.C., Enders M.M., Humbeck K.K., Ordon F.F. Identification of genomic regions involved in tolerance to drought stress and drought stress induced leaf senescence in juvenile barley. BMC Plant Biol. 2015;15:125. doi: 10.1186/s12870-015-0524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M., Cui L., Liu F., Zhang M., Shan L., Yang S., Deng X. Effects of drought stress on antioxidant enzymes in seedlings of different wheat genotypes. Pak. J. Bot. 2015;47(1):49–56. https://www.pakbs.org/pjbot/PDFs/47(1)/07.pdf [Google Scholar]
- Wolf B. A comprehensive system of leaf analysis and its use for diagnosing crop nutrient status. Comm. Soil Sci. Plant Anal. 1982;13:1035–1059. [Google Scholar]
- Wu H.H., Zou Y.N., Rahman M.M., Ni D., Wu Q.S. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci. Rep. 2017;7:42389. doi: 10.1038/srep42389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Yuan Y., Xu Y., Zhang G., Guo X., Wu F., Wang Q., Rong T., Pan G., Cao M., Tang Q., Gao S., Liu Y., Wang J., Lan H., Lu Y. Identification of candidate genes for drought tolerance by whole-genome re-sequencing in maize. BMC Plant Biol. 2014;14:83. doi: 10.1186/1471-2229-14-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Han X., Liang Y., Ghosh A., Chen J., Tang M. The combined effects of arbuscular mycorrhizal fungi (AMF) and lead (Pb) stress on pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymes in Robinia pseudoacacia L. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0145726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanini E. Indophenol blue colorimetric method for measuring cation exchange capacity in sandy soils. Commun. Soil Sci. Plant Anal. 2001;32:2519–2530. [Google Scholar]
- Zarea M.J., Karimi N., Goltapeh E.M., Ghalavand A. Effect of cropping systems and arbuscular mycorrhizal fungi on soil microbial activity and root nodule nitrogenase. J. Saudi Soc. Agric. Sci. 2011;10:109–120. [Google Scholar]
- Zhu X.Q., Tang M., Zhan H.Q. Arbuscular mycorrhizal fungi enhanced the growth, photosynthesis, and calorific value of black locust under salt stress. Photosynthetica. 2017;55(2):378–385. [Google Scholar]
- Zhu X.C., Song F.B., Liu S.Q. Arbuscular mycorrhiza impacts on drought stress of maize plants by lipid peroxidation, proline content and activity of antioxidant system. J. Food Agric. Environ. 2011;9:583–587. [Google Scholar]
- Zubek S., Mielcarek S., Turnau K. Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza. 2012;22:149–156. doi: 10.1007/s00572-011-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]