Abstract
The study was implemented to actuate the qualitative and quantitative phyto constituents of Iresine herbstii extracts and its antiviral efficacy against avian ND virus. Among four tested solvents, the ethanolic extract of Iresine herbstii revealed the presence of highest quantity of all tested phytochemicals while petroleum ether extract showed the least. Folin-Ciocalteu method assessed the range of TPC extended from 81.01 ± 0.67 to 126.35 ± 0.45 µg GAE/mg. Acetonic extract showed the highest amount among all extracts and petroleum ether possessed the lower quantity. TFC ranged from 54.37 ± 0.45 to 88.12 ± 0.26 µg QE/mg followed by colorimetric method. From all extract ethanolic extract showed highest quantity and petroleum ether revealed the lower. HPLC analysis of ethanolic extract of I. herbstii confesses the presence six bioactive components by using the HP5-MS column. To check the antiviral potential of plants, different prepared treatments of plant extract and live virus were inoculated at 9 days old SPF embryonated chicken eggs. Results exposed that all plant extracts produce antiviral activity against NDV in ovo according to their potential and phytochemical profile. The highest survival rate was observed in the ethanolic extract at 400 µg/mL and acetonic extract at 300 µg/mL as it controls the NDV activity completely, evidence of absence of embryo death and HA titre. Dichloromethane and petroleum ether could not inhibit the virus completely. 600 µg/mL concentration was proved as toxic in all extracts except petroleum ether extract which showed a dose dependent pattern.
Keywords: Iresine herbstii, Phytochemicals, Antiviral activity, In ovo, Newcastle disease virus
1. Introduction
Poultry industry was commenced as a cottage industry and play important role in many developing countries’ economies throughout the world (Ismail, 2017). It is developed as the 2nd major industry and one of the affordable and considerable sources of protein as eggs and meat, about 40% meat requirement in Pakistan is bringing with it. But there is a long list of complications linked with the poultry industry (Hussain et al., 2015). Around all entire world, poultry has facing many socio-economic crisis due to viral poultry diseases with every passing year like Newcastle disease (ND) is one of them (Yune and Abdela, 2017). It was reported as a LISTED by Office International des Epizooties [OIE], due to its dreadful concerns (Boynukara et al., 2013).
ND viruses a negative sense, non-segmented, single- stranded encapsulated RNA virus from family paramyxoviridae (Ashraf et al., 2016). ND is prevalent to many regions of world. NDV instigated death rate in high susceptibility. According to International Cooperation in Animal Science (IICAB, 2016) 250 bird species of 27 orders are affected from this virus. It has become stern disease in poultry and killed unvaccinated rural poultry at rate of 70–80% per year. Concerning to pathological index NDV is categorized in three groups; lentogenic, mesogenic and velogenic. In Pakistan incidences of lentogenic, mesogenic and velogenic are 40%, 55% and 5% respectively (Waheed et al., 2013). Disease and death rate extent upto 100% in serious cases.
Presently, many inactivated and live vaccines are present in market, but only few developed medicines proved effective (Xiao et al., 2013). Clinical signs can preclude by vaccination but replication and shedding of virus channot stop, that is main cause of infection (Chukwudi et al., 2012). To discover antiviral agents and new corresponding actions, that are safe, effective, produce less side effects and also overcomes resistance is the basic requirement of time (Nogon et al., 2011). An enormous number of different kinds of plants possessing an antiviral effect is present in rich and still fertile the nature. Each region has its own highly variable and special kind of plants which are not found in other region in world (Waziri, 2015). World Health Organization (WHO) estimated that about 75% of population fulfills their health care necessities by using herbs or plants based medicines (Ravindra et al., 2009). Owing to their availability, effective antimicrobial properties, low cost and enhancement in performance of commercial poultry birds, the medicinal plants are in use since many years in the world (Sajid et al., 2011).
Many Pakistan native plants promise immense properties for the discovery of new compounds with active medicinal functions in immune system. Prior investigation on plants showed different extracted phyto-components which are chief secondary metabolites like lignans, alkaloids, coumarins, triterpenoids, flavonoids, and chromones (Qasim et al., 2014). In accordance of worldwide trend, therefore, aimed at evaluating the antiviral efficacy of Iresine herbstii (chicken gizzard plant) against NDV. Iresine herstii is the member of Amaranthaceae family. Commonly it is called as chicken gizzard, blood leaf, herbst's blood leaf and beef steak. It is intrinsic to South America also found in tropical areas of Asia and India. It has multiple applications in folk medications (Chaudhuri and Sevanan, 2012). Research revealed that this plant used decoction relaxant, fever and kidney disorders also retain anti-allergic, anti-cancer, anti-inflammatory, antipyretic, little antioxidant, apoptotic and cytotoxic abilities (Schmidt et al., 2009). Reason for selecting plant is that plants are easily affordable and accessible and can subscribe to new bioactive constituents that are safe and effective. This make them offered for farmers easily.
The objective of study is to preliminary phytochemical profiling of shoot part of four extracts of Iresine herbstii qualitatively, total phenolic and total flavonoid contents (quanitative), detect bioactive composite by HPLC and antiviral activity of its ethanolic, acetonic, dichloromethane and petroleum ether extracts against NDV in embryonated chicken eggs in ovo.
2. Materials & methods
2.1. Plant collection and identification
Clean, hygienic and disease free shoot part Iresine herbstii was collected from Botanical garden of Government College University Faisalabad (GCUF). According to standard descriptions and keys plant was identified in field (Dalziel, 1937). Botanical identity was authenticating by Department of Botany, GCUF. After collection the leaves and stem of plant were dried and then pulverized them in the grinder.
2.2. Maceration
Following the method designated by Dipankar and Murugan (2012) extraction of solvent was carried out. Plant was macerate at room temperature for 3 days by assorted separately in four different solvents as Acetone, Dichloromethane, Ethanol and Petroleum ether 500 g/1 L for each concentration. Then filter the macerated solution by What Mann’s paper. Now the filtrate was concentrated in rotary evaporator to viscous the extract until all the ethanol (or other solvents) was cleared. Stock solutions were prepared by dissolving the crude extracts in Dimethyl sulphoxide (DMSO) solution. Then different experimental concentrations of plant extracts were prepared to estimate its antiviral efficacy. The crude extracts can be stored at 4 °C in airtight bags for future use.
2.3. Sterility test
Poured 0.1 mL of all extract on a nutrient agar plate and left for 2 min. Incubated overnight at 37 °C to observe the antibacterial activity (Ashraf et al., 2017).
2.4. Phytochemical screening
Phytochemical profiling of different extract of I. herbstii was executed by following standard assays (Ayoola et al., 2008, Joshi et al., 2013).
2.5. Total phenolic quantitation
Method adopted by Chlopicka et al. (2012) was followed for determination of total phenolic contents of all extracts of I. herbstii. 1500 µL of 10 fold diluted Folin-Ciocalteu reagent mixed with 300 µL of extract. 1200 µL of Na2CO3 was added after 5 min and kept the mixture in dark room for 2 h. Gallic acid was used as standard for calibration curve. Then observe the absorbance at 765 nm by spectrophotometer and results were expressed as Gallic acid equivalent (µg GAE/mg).
2.6. Total flavonoids quantitation
Procedure described by Stankovic (2011) was adopted to estimate TFC of I. herbstii extracts. One mL extract was mixed with 1 mL of 2% AlCl3. Incubate it for 10 min at 37 °C. Quercetin was used as a standard for calibration. Checked the absorbance was determined at 415 nm by spectrophotometer (Thermo, Waltham, MA, USA). Results were expressed as Quercetin equivalents (µg QE/mg).
2.7. High-performance liquid chromatography (HPLC)
2.7.1. Sample preparation
Reverse phase chromatographic analyses were carried out under gradient conditions using C18 column (4.6 mm × 250 mm) packed with 5 μm diameter particles. The extract was prepared in HPLC grade solvent. Then, the sample was sonicated using ultra sonicator for 10 min. The extract was filtered and injected into the HPLC column using mobile phase of 30:70 (acetonitrile:0.1% phosphoric acid).
2.8. In ovo antiviral assay
The Lasota strain (least virulent/avirulent strain of NDV) was purchased from Qadri market, Gol bazar, Faisalabad, Pakistan. Specific pathogen free embryonated chicken eggs (ECE) were incubated for 9 days in incubator at 37 °C. Median Embryo infectious dose (EID50) of the virus was determined as documented by (Young et al., 2002). From this, 100EID50/0.1 mL of the virus stock was prepare for the trial. The index calculated by Reed and Muench formula.
2.8.1. Inoculation of eggs
The SPF embryonated eggs for every extract (ethanolic, acetonic, dichloromethane and petroleum ether) were divided into seven groups (five eggs in each group) and labeled according to concentration of extract used (Table 1). Pinch a hole in shell just above the air sac to let vertical inoculation of 0.1 mL of the inoculum. In all different extract groups, Group 1 (G1) to G4 was inoculated with 0.2 mL of virus/extract mixtures at final concentration with 300 µg/mL, 400 µg/mL, 500 µg/mL, 600 µg/mL for I. herbstii ethanolic extract. G5 inoculated with only 0.2 mL of virus and took as positive control. G6 has eggs inoculated with extract only (no virus). In G7 all eggs were uninoculated (negative control). Same concentrations were taken for other extracts. Inoculated sites were closed with paraffin then incubated at 37 °C for 96 h. Tested Eggs were perceived daily for death of embryo till 72 h post inoculation. After 96 h, cooled the eggs and embryos were perceived for survival & growth. Collect allantoic fluid from treated eggs haemagglutination test to detect NDV.
Table 1.
Grouping and treatment allocation for different concentration of four extracts of Iresine herbstii in ovo.
| Group (G) n = 7 | Treatment |
|---|---|
| G1 | Ethanol/Acetone/DCM/PE extract of Iresine herbstii 300 µg/mL + 0.2 mL 4HA Virus |
| G2 | Ethanol/Acetone/DCM/PE ether extract of Iresine herbstii 400 µg/mL + 0.2 mL 4HA Virus |
| G3 | Ethanol/Acetone/DCM/PE ether extract of Iresine herbstii 500 µg/mL + 0.2 mL 4HA Virus |
| G4 | Ethanol/Acetone/DCM/PE ether extract of Iresine herbstii 600 µg/mL + 0.2 mL 4HA Virus |
| G5 | Virus control |
| G6 | Extract control |
| G7 | Untreated embryonated chicken egg |
DCM = Dichloromethane; PE = Petroleum Ether.
2.8.2. Hemagglutination test
To quantify the sum of virus, HA titre test was used by using two fold dilutions. In sterile plastic bottom allantoic fluid was put by harvesting it from surviving embryos eggs. From harvested allontoic 50 µL was serially diluted by 50 µL of normal saline. In 96 well V bottom designed microtiter plate. Then, to each well 50 µL of RBCs 1% (freshly collected from chicken) was added and mixed gently. Allow them to stand for 25 min at room temperature. After sometime virus titre was noted as reciprocal of highest dilution that triggered agglutination of chicken RBCs (Khaldoun, 2016).
2.9. Statistics analysis
The results were evaluated using Minitab statistics- 17. The differences were analyzed by analysis of variance (ANOVA) and significance was reported at P ≤ 0.05. The square root values of HA titre were converted to standardize the data before put in it into regression analysis.
3. Results
3.1. Qualitative phytochemical screening
The preliminary qualitative phytochemical screening for all sequential of Iresine herbstii extracts reveals the incidence of alkaloids, anthraquinones, glycosides, flavonoids, saponins, phenols, terpenoids, sugar bearing compound, protein, thiols and inferences are shown in Table 2.
Table 2.
Qualitative phytochemical analysis of ethanolic, acetonic, dichloromethane & petroleum ether extracts of Iresine herbstii.
| Phytochemicals | Test Type | Inferences |
|||
|---|---|---|---|---|---|
| Ethanol | Acetone | DCM | PE | ||
| Alkaloids | i.Dragendroff’s test | + | + | + | + |
| ii.Mayer’s test | + | + | + | + | |
| iii.Wagner’s test | + | + | + | + | |
| Flavonoids | NaOH Test | + | + | + | + |
| Glycosides | Keller–Kiliani’s Test | + | + | + | + |
| Phenols | Ferric chloride Test | + | + | + | + |
| Protein | Ninhydrin Test | + | + | + | − |
| Reducing sugar | Fehling Test | + | + | + | − |
| Tannins | Ferric chloride Test | + | − | − | − |
| Terpenoids | Salkowski’s Test | + | + | − | − |
| Thiol | + | + | + | − | |
| Saponins | Froth Test | + | + | + | − |
| Anthraquinones | Borntrager’s Test | + | + | + | − |
(+) = present, (−) = absent.
3.2. Total phenolic quantitation
The total phenolic contents intended against Gallic acid Equivalent (GAE) are shown in Table 3. The phenol contents range from 81.01 ± 0.67 µg GAE/mg to 126.35 ± 0.45 µg GAE/mg. Acetonic extract showed the highest amount among all four extracts and petroleum ether possessed the lower quantity of TPC. Phenols are considered as central compound due to their reducing/scavening capacity. The interest on phenols and polyphenolic compounds such as flavonoids which possess antioxidant ability is increases in food industry because they slow down the oxidative degeneration of lipids thus improving the quality and nutritive value of food. Therefore, it is justifiable to determine phenolic content in plant extract.
Table 3.
Total phenolic contents and total flavonoid contents of I. herbstii shoot part of different extracts.
| Extracts | Total phenolic contents (µg GAE/mg) | Total flavonoid contents (µg QE/mg) |
|---|---|---|
| Ethanolic | 98.00 ± 0.05 | 88.12 ± 0.26 |
| Acetonic | 126.35 ± 0.45 | 72.01 ± 1.00 |
| Dichloromethane | 89.05 ± 0.15 | 68.19 ± 2.55 |
| Petroleum ether | 81.01 ± 0.67 | 54.37 ± 0.45 |
3.3. Total flavonoids quantitation
Flavonoids are commonly found in natural products and have great importance because they help human body to fight against diseases. Among different extracts of shoot part of I. herbstii flavonoids are raged from 88.12 ± 0.26 µg QE/mg to 54.37 ± 0.45 µg QE/mg. Ethanolic extract showed highest quantity and petroleum ether revealed the lower shown in Table 3.
3.4. HPLC analysis
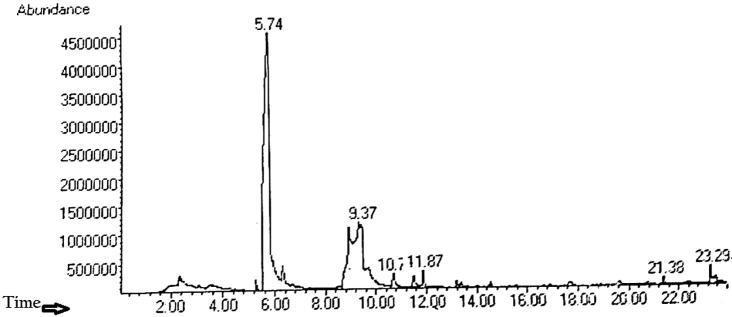
HPLC is an interesting technique to identify the volatile matter, long chain, branched chain hydrocarbons, alcohols acids, esters, etc. with respect to Peak area, retention time and molecular formula in plant compound library (as shown in Fig. 1 and Table 4) which are active bioconstituents in drugs, food, pharmaceutical and cosmetic industry. It is evident from the Table 4 that the ethanolic extract has a complex chemical composition. Six bioactive molecules are identified among these Dimethyl Sulfaxide, 1H-Imidazale, Silicic acid, diethyl bis(trimethylsilyl) ester, Cycloheptasiloxane tetradecamethyl-, cyclononasiloxane octadecamethyl-, cyclodecasiloxane eicosamethy- are present. Some of the HPLC peaks were not identified because of lack of authentic library data to corresponding compounds. A general observation is that the quantity of the aromatic compounds is more than that of aliphatic compounds and these are mainly phenolic and flavonoids (see Table 5).
Fig. 1.
HPLC Chromatogram of shoot part of I. herbstii ethanolic extract.
Table 4.
List of probable phytochemical constituents identified by HPLC spectra.
| Peak No. | Retention Time | Compound Found | Area % | Molecular formula | Molecular weight |
|---|---|---|---|---|---|
| 1 | 5.737 | Dimethyl Sulfaxide | 58.16 | C2H6OS | 78.13 g/mol |
| 2 | 9.368 | 1H-Imidazale | 35.67 | C3H4N2 | 68.079 g/mol |
| 3 | 10.704 | Silicic acid, diethyl bis(trimethylsilyl) ester | 1.89 | C10H28O4Si3 | 296.585 g/mol |
| 4 | 11.867 | Cycloheptasiloxane tetradecamethyl- | 1.99 | C14H42O7Si7 | 519.078 g/mol |
| 5 | 21.383 | cyclononasiloxane octadecamethyl- | 0.47 | C18H54O9Si9 | 667.386 g/mol |
| 6 | 23.292 | cyclodecasiloxane eicosamethy- | 1.79 | C20H60O10Si10 | 741.54 g/mol |
Table 5.
Median percent Embryo Infectious Dose (EID50/mL).
| EID50/mL | No. of eggs | No. of eggs alive | No. of eggs dead | Percentage Mortality |
|---|---|---|---|---|
| Dilutions | ||||
| 10−1 | 5 | 0 | 5 | 100% |
| 10−2 | 5 | 2 | 3 | 60% |
| 10−3 | 5 | 3 | 2 | 40% |
| 10−4 | 5 | 5 | 5 | 60% |
| 10−5 | 5 | 5 | 0 | 0% |
| 10−6 | 5 | 5 | 0 | 0% |
| 10−7 | 5 | 5 | 0 | 0% |
| 10−8 | 5 | 5 | 0 | 0% |
| 10−9 | 5 | 5 | 0 | 0% |
| 10−10 | 5 | 5 | 0 | 0% |
| Virus control | 5 | 0 | 5 | 100% |
| PBS control | 5 | 5 | 0 | 0% |
3.5. Median percent embryo infectious dose
The concentration of NDV in a suspension is expressed as an Infectivity titre. The Infectivity titre is established by performing a titration. Table 3 shows the results of titration values by Reed and Muench formula are as follows:
Apply the index calculated using this formula to the dilution that produced the infection rate immediately above 50 percent = 10−3.5. This dilution of the virus suspension contained one EID50 unit of virus in 0.2 mL. 1 mL of the virus suspension will contain ten times the reciprocal of the calculated dilution.
Therefore infectivity Titre of virus suspension in EID50/mL = 10 × 10−3.5 = 10−4.5 EID50/mL.
3.6. In ovo antiviral activity of different extracts of Iresine herbstii
In this study, all chicken embryos dead within 48 h post inoculation with NDV, it was a strong warning that the virus strain is virulent to eggs. However, addition of any four tested extracts of Iresine herbstii significantly prolonged the time of survival of embryos in dose dependent manner. In ethanolic extract treated group concentration of 400 µg/mL had complete inhibition of NDV replication with 0% mortality (see Table 6). Heamagglutination showed 0 HA titre it means that this concentration controlled virus from beginning. 300 µg/mL and 500 µg/mL had 20% mean mortalities at 48 h and has no mortality till 72 h. 300 µg/mL has 4 HA titre, it control the virus replication earlier than 500 µg/mL which have Heamagglutionation at 8. Highest concentration (600 µg/mL) cause 40% mortality after 72 h post inoculation with HA titre of 32 (Table 7). It may suggest that all the concentrations have ability to inhibit virus multiplication.
Table 6.
Embryo deaths following inoculation of embryonated chicken eggs with Newcastle disease virus (NDV) and different concentrations of different extracts of I. herbstii shoot part.
| Treatment | Concentration (µg/mL) | Mortality with different time intervals |
% Mortality | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||
| Ethanolic Extract | 300 | 0 | 1 | 0 | 20% |
| 400 | 0 | 0 | 0 | 0% | |
| 500 | 0 | 1 | 0 | 20% | |
| 600 | 0 | 1 | 1 | 40% | |
| Acetonic Extract | 300 | 0 | 0 | 0 | 0% |
| 400 | 0 | 1 | 0 | 20% | |
| 500 | 0 | 0 | 1 | 20% | |
| 600 | 0 | 1 | 2 | 60% | |
| DCM Extract | 300 | 0 | 1 | 1 | 40% |
| 400 | 0 | 0 | 1 | 20% | |
| 500 | 0 | 1 | 1 | 40% | |
| 600 | 1 | 1 | 1 | 60% | |
| PE Extract | 300 | 1 | 1 | 1 | 60% |
| 400 | 1 | 2 | 0 | 60% | |
| 500 | 0 | 1 | 1 | 40% | |
| 600 | 1 | 1 | 0 | 40% | |
| VC | _ | 2 | 3 | _ | 100% |
| EC | _ | 0% mortality. | 0% | ||
| Uninoculated Group | _ | 0% mortality. | 0% | ||
DCM = Dichloromethane; PE = Petroleum Ether; VC = Virus control; EC = Extract Control
Table 7.
Mean hemagglutination (HA) titres in embryonated chicken eggs inoculation with NDV and different concentrations of different extracts of Iresine herbstii.
| Treatment | Concentration (µg/mL) | HA titre |
|---|---|---|
| Ethanolic Extract | 300 | 4 |
| 400 | 0 | |
| 500 | 8 | |
| 600 | 32 | |
| Acetonic Extract | 300 | 0 |
| 400 | 4 | |
| 500 | 8 | |
| 600 | 128 | |
| Dichloromethane Extract | 300 | 32 |
| 400 | 8 | |
| 500 | 128 | |
| 600 | 512 | |
| Petroleum Ether Extract | 300 | 1024 |
| 400 | 512 | |
| 500 | 512 | |
| 600 | 128 | |
| Virus Control | _ | 2048 |
| Extract Control | _ | 0 |
| Uninoculated Eggs | _ | 0 |
In acetonic extract treated group eggs, 300 µg/mL inoculated group showed 0% mortality throughout three days (72 h) post inoculation showing HA titres of 0. 400 µg/mL and 500 µg/mL inoculated group have 20% mortality indicating that both have same capability to control the virus. High death rate was observed in 600 µg/mL treated group and showing the HA titre is 128. It can be interpreted that this dose has a lethal effect itself on embryo survival (Table 6, Table 7). Findings are in line with results of Ashraf et al. (2017) who documented that low concentrations 300 µg/mL of methanolic and 400 µg/mL ethanolic extract of Glycyrrhiza glabra produce zero % mortality. High mortality was shown by 600 µg/mL of different extract at 24 h, 48 h and 72 h time intervals.
The three days record of dichloromethane extract of I. herbstii inoculated group indicated that there is no complete restriction of antiviral potential was noticed using different concentrations of plant extract. There is no mortality at 24 h in first three concentrations. After 48 h there is 1 embryo died in both 300 µg/mL and 500 µg/mL. Total mortality is 40% till 72 h in both groups. HA titre showed different titre values regarding to their aptitude to control the viral effects. 600 µg/mL has 60% mortality 72 h post inoculation. From the results it may be concluded that dichloromethane extract of this plant has less ability to control the virus propagation.
Petroleum ether extract of I. herbstii displayed the dose dependent response by level of virus control. In this extract 300 µg/mL and 400 µg/mL have shown 60% mortality (Table 6). In. HA titre 1024 of 300 µg/mL indicates that this concentration of PE did not control virus replication while 400 µg/mL group showed HA titre 512. 500 µg/mL and 600 µg/L has less mortality rates than first two concentrations i.e., 40%. These outcomes are in line with findings of Al- Hadid (2016), who conducted similar work and stated that leaves extract of Ceratonia siliqua at any of plant concentrations did not inhibit NDV activity completely. High concentration 500 µg/mL showed 20%, 250 µg/mL showed 40% and low concentration of Ceratonia siliqua caused 80% mortality.
4. Discussion
The use of medicinal plants in dealing of ailments has provoked renewed attention in recent years, herbal preparations are progressively being used in human and animals healthcare schemes. Use of the extracts from Iresine herbstii for cure of different maladies has been documented (Chudhuri and Sevanan, 2012). It can be predicted that antiviral potential of plant could be attributed the presences of secondary metabolites compounds (Chadare et al., 2009).
The mechanism conduct by the crude extracts of I. herbstii to control in ovo NDV replication is not known yet. This exposes the inhibitory rather than virucidal potential of the extract on this virus at these doses. However, many traditional medicinal plants that are used to cure viral diseases have been exposed to contain different types of substances. Notable examples of these metabolites comprise; alkaloids, coumarins, terpenes, flavonoids, anthraquinones and naphthoquinones (Sulaiman et al., 2011). These compounds exert their effects by killing the virus and interfering with viral replication (Jassim and Naji, 2003).
Huge number of small phytochemicals such as alkaloids, flavonoids, phenols, sugar bearing molecules and terpenes were found to containing anti-herpetic, anti-inflammatory, anti-viral, anti allergesic, anti-cancer, anti-bacterial, anti-malarial and anti-fungal agents (Viol, 2013). Plant having glycosides and carbohydrates which are considered possessing a helpful immune mechanism by enhancing body power and dietary additives (Yadav et al., 2014). Proteins added structural and functional improvements in plants as well as animal cells and have nutritional values (Tijjani et al., 2013). Glycosides were also predicted to be anti diarrheal effects because of prevention of autocoids and prostaglandin (Tiwari et al., 2011).
Plants have natural antioxidants in the form of phenolic and polyphenolic compounds (flavonoids), which presence provide essential plant biochemistry and physiological effects such as anti-oxidant, anti-inflammatory, cytotoxic efficacy and anti-allergic effects (Kumar and Pandey, 2013), antidiabetic, anti-carcinogenic activity, enzyme inhibitor and precursor of toxic substance (Prabhu et al., 2012).
Acetonic extract and ethanolic extract at 300 µg/mL and 400 µg/mL exhibited the complete inhibition of NDV without prompting mortality of any chicken embryo. The response of viral infected embryo is different depending on extract type and concentration supplied. Some extracts of plant expressed dose dependent relationship with virus while other cause toxicity for embryos. The positive responses provided by extract are believed to be as an effect of phytochemicals present in that extract or combination with other compounds. The belief that medicinal plants don’t have side or toxic effects because they are natural so it is clear that toxicological effects may related with dose concentration. Dose dependent toxicological data should be provided while developing new drugs in future.
5. Conclusion
The compounds acknowledged by qualitative and quantitative investigations with HPLC have many uses in medical field. Every compound recognized has their unique characters to treat different ailments. Further investigations expected to uncover its significance in explicit field to treat the infections legitimately. The antiviral assay of Iresine herbstii reveals the significant antiviral potential of ethanolic and acetonic extracts against experimental Newcastle disease infection in local chickens. Administrations of this extract might be a healthier approach in lessening the effects of Newcastle disease virus infection. The positive responses provided by extract are believed to be as an effect of phytochemicals present in that extract or combination with other compounds. Therefore, Iresine herbstii is recommended as a plant of antiviral importance.
Declaration of Competing Interest
There were no competing interests among authors.
Acknowledgements
The facilities and support provided by the Department of Zoology, Government College University Faisalabad are highly acknowledged. The authors (SM and KAAG) express their sincere appreciation to the Deanship of Scientific Research at the King Saud University for its funding of this research through the Research Group Project No. RG-1435-012.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Asma Ashraf, Email: asmabinm@gmail.com.
Shahid Mahboob, Email: mushahid@ksu.edu.sa.
References
- Ashraf A., Ashraf M.M., Rafiqe A., Aslam B., Galani S., Zafar S., Asif R. In vivo antiviral potential of Glycyrrhiza glabra extract against Newcastle disease virus. Pak. J. Pharma. Sci. 2017;30(2):567–572. [PubMed] [Google Scholar]
- Al-Hadid J.K. Evaluation of antiviral activity of different medicinal plants against Newcastle Disease Virus. Am. J. Agri. Bio.l Sci. 2016;11(4):157–163. [Google Scholar]
- Ashraf A., Din M.S.U., Habib M., Hussain M., Mahboob S., Al-Ghanim K. Isolation, identification and molecular characterization of highly pathogenic Newcastle Disease Virus from field outbreaks. Braz. J. Arch. Biol. techn. 2016;59:e16160301. [Google Scholar]
- Ayoola G.A., Coker H.A., Adesegun S.A., Adepoju-Bello A.A., Obaweya K., Ezennia E.C., Atangbayila T.O. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharma. Res. 2008;7(3):1019–1024. [Google Scholar]
- Boynukara B., Gülhan T., Çoven F., Kiziroglu I., Durmus A. Determination of Newcastle disease virus among wild bird populations in Lake Van basin, Turkey. Turk. J. Vet. Anim. Sci. 2013;37(1):86–93. [Google Scholar]
- Chadare F.J., Linnemann A.R., Hounhouigan J.D., Nout M.J.R., Van B.M.A.J. Baobab food products. A review on their composition and nutritional value. Critical Rev. Food Sci. Nutr. 2009;49(1):254–274. doi: 10.1080/10408390701856330. [DOI] [PubMed] [Google Scholar]
- Chlopicka J., Pasko P., Gorinstein S., Jedryas A., Zagrodzki P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT Food Sci. Technol. 2012;46:548–555. [Google Scholar]
- Chudhuri D., Sevanan M. Phytochemical composition of the extracts from Iresine herbstii and its therapeutic use via antioxidant and cytotoxic potential by multiple in vitro assays. Int. J. Phytomed. 2012;4(4):477. [Google Scholar]
- Chukwudi O.E., Chukwuemeka E.D., Mary U. Newcastle disease virus shedding among healthy commercial chickens and its epidemiological importance. Pak. Vet. J. 2012;32(3):354–356. [Google Scholar]
- Dalziel, J.M., 1937. The useful plants of west tropical Africa. In: The Crown Agents for the Colonies, London, pp. 52–560.
- Dipankar C., Murugan S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B. Biointerf. 2012;98:112–119. doi: 10.1016/j.colsurfb.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Hussain J., Rabbani I., Aslam S., Ahmad H. An overview of poultry industry in Pakistan. World's Poul. Sci. J. 2015;71(4):689–700. doi: 10.1017/S0043933915002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for International Cooperation in Animal Science, 2016. Newcastle Disease Avian Paramyxovirus-1 Infection, Goose Paramyxovirus Infection, Ranikhet disease. Iowa State University & Institute for International Cooperation in Animal Biologics: Center for Food Security and Public Health. pp 1–9.
- Ismail H.T.H. Biochemical and hematological studies on the effect of neem (Azadirachta indica) leaves aqueous extract on Newcastle Disease vaccine and infection in broiler chickens. Int. J. Recent Sci. Res. 2017;30(3):15876–15884. [Google Scholar]
- Jassim S.A., Naji M.A. Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol. 2003;95(1):412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- Joshi A., Bhobe M., Sattarkar A. Phytochemical investigation of the roots of Grewia microcos Linn. J. Chem. Pharma. Res. 2013;5(1):80–87. [Google Scholar]
- Khaldoun J.H. Evaluation of antiviral activity of different medicinal plants against Newcastle disease virus. Am. J. Agri. Biol. Sci. 2016;11(4):157.163. [Google Scholar]
- Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013;16 doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogon N.R.J., Koanga Mogtomo H.L., Tchinda Tiabou A., Magnifoeut N.H., Motso Chieffo P.R., MballaBounou Z. Ethno-botanical survey of some Cameroonion plants used for the treatment of viral disease. Afr. J. Plant Sci. 2011;5(1):15–21. [Google Scholar]
- Office International des epizooties (OIE), 2012. Manual of diagnostic tests and vaccines for terrestrial animals. Paris, France, pp. 1092–1106.
- Prabhu V.V., Guruvayoorappan C. Phytochemical screening of methanolic extract of mangrove Avicennia marina (Forssk.)Vierh. Der Pharm. Sin. 2012;3(1):64–70. [Google Scholar]
- Qasim M., Abideen Z., Adnan M.Y., Ansari R., Gul B., Khan M. Traditional ethnobotanical uses of medicinal plants from coastal areas. J. Coastal Life Med. 2014;2(1):22–30. [Google Scholar]
- Ravindra P., Tiwari A.K., Sharma B., Chauhan R. Newcastle disease virus as an oncolytic agent. Ind. J. Med. Res. 2009;130:507–513. [PubMed] [Google Scholar]
- Sajid U.R., Durrani F., Chand N., Khan R.U., Rehman F. Comparative efficacy of different schedules of administration of medicinal plants infusion on hematology and serum biochemistry of broiler chicks. Res. Opin. Anim. Vet. Sci. 2011;1(1):8–14. [Google Scholar]
- Schmidt C., Fronza M., Goettert M., Geller F., Luik S., Flores E., Laufer S. Biological studies on Brazilian plants used in wound healing. J. Ethnopharm. 2009;122(3):523–532. doi: 10.1016/j.jep.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Stankovic M.S. Total phenolic content, flavonoid concentration and antioxidant activity of Marrubium peregrinum L. extracts. Kragujevac J. Sci. 2011;33:63–72. [Google Scholar]
- Sulaiman L.K., Oladele O.A., Shittu I.A., Emikpe B.O., Oladokun A.T., Meseko C.A. In ovo evaluation of the antiviral activity of methanolic root-bark extract of the African Baobab (Adansonia digitata Lin) Afr. J. Biotechn. 2011;10(20):4256–4258. [Google Scholar]
- Tijjani M.A., Abdurahaman F., Abba Y.S., Dris M.I., Baburo B.S.I., Mala G.A., Dungus M.H.M., Aji B.M., Abubakar K.I. Evaluation of proximate and phytochemical composition of leaves Annona senegalensis Pers. J. Pharma. Sci. Innov. 2013;2(1):7–9. [Google Scholar]
- Tiwari P., Kumar B., Kaur M., Kaur G., Kaur H. Phytochemical screening and extraction: a review. Int. Pharm. Sci. 2011;1(1):98–106. [Google Scholar]
- Viol, D.I., 2013. Screening of traditional medicinal plants from Zimbabwe for phytochemistry, antioxidant, antimicrobial, antiviral and toxicological activities. http://ir.uz.ac.zw/jspui/bitstream/10646/1105/1/Viol_thesis.pdf.
- Waheed U., Siddique M., Arshad M., Ali M., Saeed A. Preparation of Newcastle disease vaccine from VG/GA strain and its evaluation in commercial broiler chicks. Pak. J. Zool. 2013;45(2):339–344. [Google Scholar]
- Waziri H.M. Plants as antiviral agents. J. Plant Pathol. Microbiol. 2015;6(2):1. [Google Scholar]
- Xiao S., Paldurai A., Nayak B., Mirande A., Collins P.L., Samal S.K. Complete genome sequence of a highly virulent Newcastle disease virus currently circulating in Mexico. Genome Announ. 2013;1(1):e00177–e212. doi: 10.1128/genomeA.00177-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yune N., Abdela N. Update on epidemiology, diagnosis and control technique of Newcastle disease. J. Veterinary Sci. Technol. 2017;8(429):2. [Google Scholar]
- Yadav M., Chatterji S., Gupta S.K., Watal G. Preliminary phytochemical screening of six medicinal plants used in traditional medicine. Int. J. Pharma. Sci. 2014;6(5):539–542. [Google Scholar]
- Young, M.B., Alders, R., Grimes, S., Spradbrow, P.B., Dias, P., Silva, A.D., Lobo, Q., 2002. Controlling Newcastle Disease in Village Chickens-A Laboratory Manual. Monographs. Aust. Centre for International Agri. Res. http://purl.umn.edu/117714.