Abstract
Objective
Immunotherapy of tuberculosis (TB) to shorten treatment duration represents an unmet medical need. Orally delivered, tableted TB vaccine (V7) containing heat-killed Mycobacterium vaccae (NCTC 11659) has been demonstrated in prior clinical studies to be safe and fast-acting immune adjunct.
Methods
The outcome of Phase III trial of V7 containing 10 µg of hydrolyzed M. vaccae was evaluated in 152 patients randomized at 2:1 ratio: V7 (N = 100), placebo (N = 52). Both arms received conventional 1st or 2nd line TB drugs co-administered with daily pill of V7 or placebo.
Results
After one month mycobacterial clearance was observed in 68% (P < 0.0001) and 23.1% (P = 0.04) of patients on V7 and placebo. Stratified conversion rates in V7 recipients with drug-sensitive and multidrug-resistant TB were 86.7% and 55.6% vs 27.2% and 15% in placebo. Patients on V7 gained on average 2.4 kg (P < 0.0001) vs 0.3 kg (P = 0.18) in placebo. Improvements in hemoglobin levels, erythrocyte sedimentation rate and leukocyte counts were significantly better than in controls. Liver function tests revealed that V7 can prevent chemotherapy-induced hepatic damage.
Conclusion
Oral M. vaccae is safe, can overcome TB-associated weight loss and inflammation, reduce hepatotoxicity of TB drugs, improve sputum conversion three-fold OR 3.15; 95%CI (2.3,4.6), and cut treatment length by at least six-fold. Longer follow-up studies might be needed to further substantiate our findings (Clinicaltrials.gov: NCT01977768).
Keywords: MDR, XDR, Immunotherapy, Therapeutic vaccine, Mycobacterium vaccae
1. Introduction
Tuberculosis (TB) is a global health problem. Ukraine and Mongolia have the largest TB prevalence rates in Europe and Asia. The incidence of pulmonary TB, including HIV co-infection, remained largely unchanged in Ukraine, being 85 per 100,000 in 2005 [1] and 84 in 2017 [2]. The incidence of multidrug-resistant TB (MDR-TB) has doubled from 15.3 in 2012 to 30 in 2017. The proportion of MDR-TB, often with extensively resistant form of TB (XDR-TB), among new and previously treated TB cases was reported to be 24.1% and 58.1% respectively [3]. The favorable treatment outcome for drug-sensitive (DS-TB) and drug-resistant TB in Ukraine was 76% and 51% [2]. On the other hand, Mongolia is one of countries with highest TB burden in the Western Pacific region. In 2010 the estimated incidence of TB was 331 per 100,000 [4]. The rate of MDR-TB in Mongolia has been reported to be 27.5% and 1.4% among retreated and newly diagnosed TB cases, respectively [5]. The treatment success rates were apparently 84% and 60% for new and drug-resistant cases [4].
In general, it is accepted that DS-TB is curable with the first line of anti-tuberculosis treatment (ATT) in 85% of cases within 6 months. Nevertheless, despite the availability of effective treatment, the threat of TB does not seem to go away. Among main challenges posed by Mycobacterium tuberculosis infection are the difficult-to-treat forms of TB, such as MDR-TB, XDR-TB, and TB with HIV. It takes as long as 24 months to treat a patient with MDR-TB and the typical success rate is around 50% [6]. TB refractory to conventional ATT requires the deployment of 2nd line drugs. However, the cost of such a therapy increases by orders of magnitude without substantial benefit of better safety or efficacy. Improved treatment options are urgently needed.
Significant efforts are directed at finding new TB drugs. For example, the Advisory Committee to the US FDA has recently voted to approve pretomanid in combination with bedaquiline and linezolid to treat XDR or MDR tuberculosis. Apart from finding new chemical entities effective against TB, immune-based interventions, like therapeutic vaccines, are sought as an adjunct therapy to conventional ATT [7]. The longest and most studied TB immunotherapeutic consists of killed Mycobacterium vaccae, which was originally discovered in 1972 by John and Cynthia Stanford on shores of lake Kyoga in Uganda [7,8]. They had a hint that M. vaccae may possess anti-TB properties during their sabbatical in Africa in the midst of Buruli ulcer outbreak they were investigating. They noted that some environmental factors may be influencing the pathogenicity of Mycobacterium ulcerans – an infectious agent, which causes skin ulcerations. This prompted them to collect soil and water samples across the country to see if any local microbial species may be involved in this phenomenon. Incidentally, various mycobacteria, including M. vaccae, as related to the genus of TB-causing pathogen M. tuberculosis were discovered in 1964 by Bönicke and Juhasz in milk and dung from cattle [9].
All previous formulations of M. vaccae designed to treat TB were made as injectable preparations producing seemingly consistent success rates [10], but occasionally results were reported as having no clear clinical benefit. As a rule, trials that were declared unsuccessful had TB patients who received just one single shot of M. vaccae [11]. Then, ten years ago, a small trial in Argentina was carried out, indicating that repeated doses of orally delivered capsules with 1 mg of M. vaccae may be more effective than parenteral formulation [12]. Inspired by that report we have made a tableted formulation of M. vaccae (V7) at a hundred-fold smaller dose and have then conducted two Phase II randomized clinical trials, which confirmed safety and efficacy of this non-pathogenic form of mycobacteria [13,14]. The aim of the present Phase III study was to substantiate the clinical benefit of V7 versus placebo in an ethnically diverse population of Ukrainian and Mongolian TB patients.
2. Material and methods
2.1. Ethics consideration
The conduct of the study was approved by the internal review board of the lead hospital in respective countries in accordance with the Helsinki Declaration. As per IRB #00010097 resolution the participants in the trial have provided informed consent, participated in this study voluntarily and were free to withdraw from this study at any time. Treatment was provided free-of-charge. This study is registered with the ClinicalTrials.gov ID: NCT01977768.
2.2. Clinical setting and baseline patients’ characteristics
The study was conducted at four Mongolian and five Ukrainian TB hospitals and dispensaries between 2014 and 2018. The main eligibility criteria were an informed consent and positive sputum smear at study entry. All patients had one or more symptoms of pulmonary TB such as low-grade fever, cough, chest pain, dyspnea, hemoptysis, and weight loss. Patients in each country were randomly allocated by computer-generated sequence into two groups at 2:1 ratio, to receive in double-blinded fashion either V7 pills or identically appearing placebo pills without M. vaccae. The randomization resulted in equal distribution of baseline characteristics; ethnicity, age, gender, height, body weight, blood cell counts, serum biochemistry parameters, severity and various manifestations of the disease. Thus, the statistical bias due to population heterogeneity was unlikely. The distribution of patients with: DS-TB; MDR-TB; XDR-TB and TB-HIV was 45:45:1:9 and 22:20:1:9 in V7 and placebo arms respectively, which did not differ statistically (Chi-square, P = 0.13). Some of the patients, especially those with drug-resistant TB, required individualized treatment rather than standard 1st line TB drugs; the proportion of such patients was dependent from their baseline diagnosis in V7 and placebo arms; the inter-group difference was not statistically significant (Fisher's two-tailed, P = 0.85). Remaining patients were prescribed conventional 2HREZ/4HR regimen, occasionally supplemented with streptomycin. In Mongolia all patients - except one re-treated case in each arm - were conditionally categorized as DS-TB since information on their drug resistance status and HIV infection was not available at the time of treatment initiation.
2.3. V7
The therapeutic modality (V7) tested in this trial is a specially formulated oral tablet containing 10 µg/pill of hydrolyzed and heat-inactivated Mycobacterium vaccae (NCTC 11659) supplied by BioEos company (Marden, Kent, UK). Tableted formulation of V7 is manufactured at GMP-compliant plant according to a patented process developed by Immunitor company (US2011/0081382). Placebo pills are made using same excipients, but without M. vaccae. At the time when this trial was initiated V7 has already been approved either as a health supplement (No. 05.03.02-03/89960) or biologically active product (No. F20151113PH00837) by the Ministries of Health of Ukraine and Mongolia respectively. V7 (Tubivac) has also received orphan drug designation request status from the U.S. FDA (No. DRU-2019-7145) for TB indication. The preparation is stable at ambient temperature with a shelf life of three years.
2.4. Treatment regimen
The TB drugs were provided free-of-charge from the national distribution system administered by the respective Ministries of Health. Standard TB therapy consisted of daily doses of isoniazid (H) 300 mg; rifampicin (R) 600 mg; ethambutol (E) 1200 mg; pyrazinamide (Z) 2000 mg; and when needed streptomycin (S) 1000 mg. Individualized therapy for drug-resistant patients comprised 1st and 2nd line TB drugs as decided empirically by a physician. The choice of 2nd line drugs comprised: aminoglycosides such as kanamycin (K) and amikacin (A); thioamides e.g., ethionamide (T); fluoroquinolones e.g., levofloxacin (L), sparfloxacin (F), and gatifloxacin (G); cycloserine (Cs); para-aminosalicylic acid (P); and clofazimine (C). In the immunotherapy group, in addition to ATT, patients received once-daily pill of V7 which was given 30 min before or after breakfast. In the control group, patients received once-daily placebo pill made of same excipients as V7, but without M. vaccae. All patients, either treatment-naïve or those who had a relapse, were enrolled at the beginning of their TB treatment. No other immune-modulating or anti-infective drugs besides ATT were used during this study.
2.5. Laboratory assays
Light microscopy examination of sputum smear with Ziehl-Neelsen staining or acid-fast bacteria (AFB) was conducted prior to study entry and post-treatment time. Sputum smears were scored by a blinded laboratory technician from 0 to 3. Drug resistance profile to 1st and 2nd line TB drugs was done in Ukraine only, using commercially available kit (Tulip Diagnostics, Goa, India) with ready-to-use tubes containing TB drugs incorporated at manufacturer-predetermined concentrations into standard Lowenstein-Jensen agar slants. The MDR-TB was diagnosed as such when resistance to both isoniazid and rifampicin, with or without resistance to additional drugs, was present. When there was an additional resistance to any fluoroquinolones and amikacin, kanamycin, or capreomycin, then it was classified as XDR-TB. Only one patient with XDR-TB was identified in each treatment arm. Other tests such as complete blood count (CBC) and biochemistry analysis were carried out by routine clinical laboratory methods.
2.6. Statistical analysis
Obtained data were analyzed with statistical software available online (GraphPad Software Inc., La Jolla, CA). All statistical analyses were done on intent-to-treat basis, involving the total number of enrolled patients. Where necessary the analysis of subgroups within responder and non-responder populations was carried out. Parametric baseline values relative to the end of study results were evaluated by paired Student's t-test and intergroup differences were evaluated by unpaired t-test, by comparing observed means ± SD (standard deviations). The comparison of categorical values was performed by Fisher's exact and Chi-square two-tailed tests. Odds ratios were calculated to reveal the association between various outcomes. The probability or P values, commonly from two-tailed tests, were considered as significant at P ≤ 0.05 cut-off value.
3. Results
This study comprised 152 patients with pulmonary TB, including 100 recruited in Ukraine and the remaining 52 in Mongolia. In each country all enrolled patients were randomized at 2:1 ratio to receive either V7 or placebo. The calculation of sample size has taken into consideration the outcome from two prior Phase II studies, which demonstrated that P values were reliable and statistically significant at as little as 20 patients enrolled per treatment arm [13,14]. As expected, the baseline characteristics of patients in the two treatment groups were not statistically different, indicating that the outcome was not biased by sample heterogeneity. For example, the difference of female to male ratio between treatment and placebo groups 26/74 and 7/45 was not statistically different (Chi-square, P = 0.1), so was the average age of participants, i.e., 39.3±11 vs 39.6±9.9 (unpaired t-test, P = 0.96).
3.1. Lack of adverse reactions
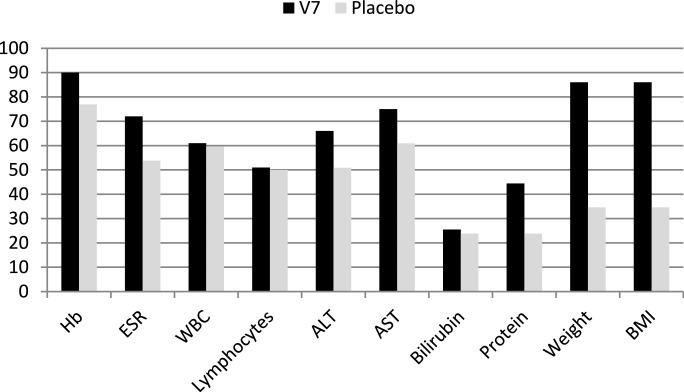
Unlike injectable formulations of M. vaccae that usually cause a local inflammatory skin reaction resulting in permanent scar formation at the site of injection, no adverse side effects attributable to orally administered V7 were seen during the entire study duration. No reactivation of TB, malaise, intolerance or allergic reactions were evident at any time during the study period nor after a decade of commercial use for TB and unrelated clinical indications, e.g., asthma, allergy, COPD, lung cancer, etc. After one month, self-reported and physician-observed clinical symptoms appeared to improve among V7 recipients, while the proportion of patients with satisfactory results was considerably smaller in placebo arm. This subjective impression is supported by analysis of secondary endpoints such as changes in body weight, BMI, erythrocyte sedimentation rate, leukocyte and lymphocyte counts, hemoglobin and total protein concentrations as well as select liver function parameters as detailed below (Table 2 and Fig. 2).
Table 2.
The results of Phase III one-month trial of daily oral M. vaccae as an immune adjunct to TB therapy.
| Arm | N | Gender F/M |
Age | Height (cm) | Diagnosis* | Sputum conversion (%) | Sputum score |
Weight (kg) |
BMI (kg/cm2) |
Protein (g/L) |
Hb (g/dL) |
Bilirubin (µM/L) |
ESR (mm/h) |
WBC (x109L) |
Lymphocyte (%) |
ALT (mM/h*L) |
AST (mM/h*L) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | |||||||
| V7 | 100 | 26/74 | 39.3 ±11 |
172.1 ±6.6 |
DS = 45 MDR = 45 XDR = 1 TB/HIV = 9 |
DS = 86.7 MDR = 55.6 XDR = 0 TB/HIV = 44.4 |
1.12 ±0.41 |
0.33 ±0.47 |
64.2 ±8.3 |
66.6 ±8.2 |
21.4 ±2.1 |
22.2 ±2.1 |
73.8 ±5.1 |
74.6 ±5.6 |
132 ±17.8 |
136.9 ±14.7 |
11.4 ±2.4 |
13.7 ±3.4 |
18.2 ±12 |
12.4 ±9 |
8.95 ±3.7 |
7.45 ±2.3 |
26.4 ±7.0 |
26.8 ±5.6 |
0.34 ±0.15 |
0.33 ±0.21 |
0.16 ±0.09 |
0.17 ±0.07 |
| P < 0.0001 | P < 0.0001 | P<0.0001 | P=0.043 | P=0.001 | P<0.0001 | P<0.0001 | P<0.0001 | P=0.96 | P=0.65 | P=0.41 | ||||||||||||||||||
| Placebo | 52 | 7/45 | 39.6 ±9.9 |
172.9 ±7.0 |
DS = 22 MDR = 20 XDR = 1 TB/HIV = 9 |
DS = 27.2 MDR = 15 XDR = 0 TB/HIV = 33.3 |
1.23 ±0.55 |
1.04 ±0.77 |
61.5 ±10.7 |
61.8 ±10.5 |
20.5 ±3 |
20.7 ±3 |
74.6 ±6.1 |
74.6 ±5.6 |
128.4 ±15.8 |
126.7 ±17.2 |
11.03 ±3.1 |
13.9 ±4.01 |
16.9 ±11.6 |
17.3 ±13.3 |
8.1 ±2.4 |
7.9 ±2.8 |
27.8 ±5.7 |
29.3 ±5.3 |
0.39 ±0.46 |
0.54 ±0.61 |
0.13 ±0.05 |
0.17 ±0.07 |
| P=0.07 | P = 0.18 | P = 0.1 | P=0.93 | P=0.44 | P=0.0015 | P=0.77 | P=0.56 | P=0.85 | P=0.04 | P=0.005 | ||||||||||||||||||
| This row compares post-treatment data between groups, V7 and placebo; P values are from unpaired t-test; Δ is difference between means and within parentheses are ranges of values at 95%CI confidence intervals separated by coma |
P < 0.0001 Δ = 0.71 95%CI(0.51,0.91) |
P = 0.0025 Δ = 4.8 95%CI(1.7,7.8) |
P = 0.0003 Δ = 1.5 95%CI(0.7,2.4) |
P=0.99 Δ=0.008 95%CI(-2.6,2.6) |
P=0.0002 Δ=10.2 95%CI(4.9,15.5) |
P=0.35 Δ=0.8 95%CI(0.9,2.4) |
P=0.008 Δ=4.9 95%CI(1.3,8.5) |
P=0.33 Δ=0.45 95%CI(-1.3,0.4) |
P=0.01 Δ=2.5 95%CI(0.6,4.6) |
P=0.002 Δ=0.21 95%CI(0.1,0.35) |
P=0.47 Δ=0.01 95%CI(-0.02,0.05) |
|||||||||||||||||
*In TB/HIV category, among those in V7 arm, 4 had DS-TB and 5 MDR-TB; while in placebo all patients had DS-TB. Most of numeric data values are expressed as means ± standard deviations (SD). Continuous data were analyzed by pairwise Student's t-test. Lower row contains data from comparison analysis (inter-group difference) between V7 and placebo groups by unpaired t-test with Δ-difference from respective means and ranges between lower and upper values at 95% confidence intervals (CI).
Fig. 2.
The proportion of patients who responded favorably to treatment. The proportion expressed in % on Y-axis. The P values resulting from comparison of V7 vs. placebo analyzed by Chi-square 2 × 2 contingency table are as follows: HB (P = 0.015); ESR (P = 0.013); WBC (P = 0.43); Lymphocytes (P=0.45); ALT (P = 0.03); AST (P = 0.025); Bilirubin (P = 0.35); Protein (P = 0.006); Weight (P < 0.0001); BMI (P < 0.0001). The statistical analysis of other data relating to each of above shown parameter are described in detail in the Results, see paragraphs 3.3–3.8.
3.2. Effect on mycobacterial clearance
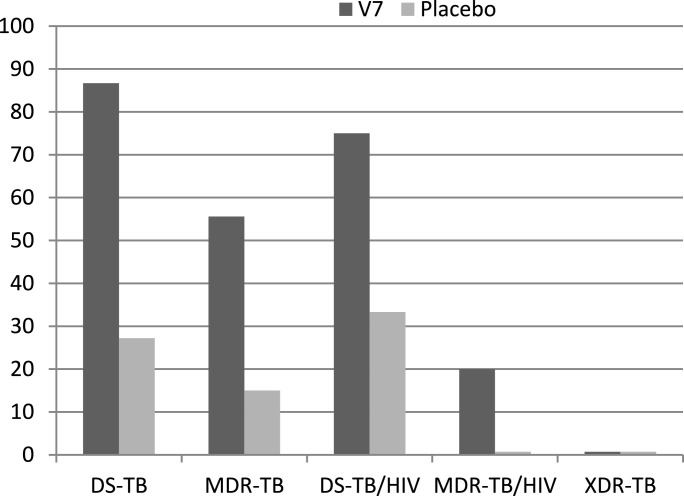
After one month of treatment, mycobacterial clearance in sputum smears was observed in 68 out 100 (68%; P<0.0001) and 12 out 52 (23.1%; P = 0.04) across all patients on V7 and placebo respectively (Fig. 1, Table 2). Stratified conversion rates in V7 recipients with DS-TB and MDR-TB were 86.7% and 55.6% vs 27.2% and 15% in placebo (Fig. 1). The two-tailed Fisher's exact test comparing outcomes within V7 arm across two main categories of TB patients, i.e., DS-TB vs MDR-TB indicates that the difference in sputum conversion rate was dependent on drug resistance status (P < 0.0001). Similarly, the discrepancies in conversion rates between two categories in the placebo arm were also significant (P < 0.0001) supporting the impression that a higher proportion of DS-TB patients responded to ATT and those who had drug-resistant TB were less responsive. As there was only one XDR-TB patient in each arm, neither of whom converted, no statistical analysis was feasible. Fisher's exact test comparing conversion outcome of nine TB-HIV patients in each treatment arm has not produced meaningful results (P = 0.15), due perhaps to population dissimilarity in drug-resistance pattern. In V7 arm, 4 patients had DS-TB and 5 had MDR-TB, while in placebo all nine HIV-positive individuals were diagnosed with drug-sensitive TB. At the baseline TB patients in placebo and V7 arms were similar in terms of their initial mean±SD sputum scores; 1.23±0.55 vs 1.12±0.41 (P = 0.16) but their post-treatment scores differed three-fold; 1.04±0.77 vs 0.33±0.47 (P < 0.0001) for placebo and V7 respectively (Table 1, 2). Paired t-test comparing conversion rate in V7 and placebo arms supports superior efficacy of V7, i.e., 1.12±0.41 vs 0.33±0.47 (P < 0.0001), but not in controls, i.e., 1.23±0.55 vs 1.04±0.77 (P = 0.07).
Fig. 1.
Sputum clearance percentage in V7 and placebo arms depending on TB form. Post-treatment sputum conversion rates, expressed in % on the Y-axis, among patients with DS-TB; MDR-TB; DS-TB/HIV; MDR-TB/HIV and XDR-TB after one month on V7 vs. placebo had the following P values by Fisher's exact test in 2 × 2 contingency table: P < 0.0001; P = 0.0021; P = 0.077; P = 1.0 and P = 1.0 respectively.
Table 1.
Baseline characteristics of patients with pulmonary TB randomized at 2:1 ratio into V7 and placebo arms.
| Characteristics | V7 N = 100 (%) |
Placebo N = 52 (%) |
P value |
|---|---|---|---|
| Asian | 33 (33%) | 19 (36.5%) | 0.72 |
| Caucasian | 67 (67%) | 33 (63.5%) | |
| Females | 26 (26%) | 7 (13.5%) | 0.1 |
| Males | 74 (74%) | 45 (86.5%) | |
| Age (years) | 39.7±11 | 39.6±10 | 0.96 |
| Height (cm) | 173.1±7 | 172.9±7 | 0.85 |
| Weight (kg) | 64.2±8.3 | 61.5±10.7 | 0.09 |
| BMI (kg/m2) | 21.4±2.1 | 20.5±2.9 | 0.05 |
| AFP-positive smears | 100 (100) | 52 (100) | 1.0 |
| Mean sputum score | 1.23±0.55 | 1.12±0.41 | 0.16 |
| DS-TB | 45 (45%) | 22 (42.3%) | 0.86 |
| MDR-TB | 45 (45%) | 19 (36.5%) | 0.39 |
| XDR-TB | 1 (1%) | 1 (1.9%) | 1.0 |
| TB-HIV | 9 (9%) | 9 (17.3%) | 0.18 |
| DS-TB/HIV | 4 (4%) | 9 (17.3%) | 0.03 |
| MDR-TB/HIV | 5 (5%) | 0 (0%) | 0.17 |
| Leukocytes (x109L) | 8.95±3.7 | 8.1±2.4 | 0.13 |
| Lymphocytes (%) | 26.4±7 | 27.8±5.7 | 0.23 |
| Hemoglobin (g/dL) | 132±17.8 | 128.4±15.8 | 0.22 |
| Total protein (g/L) | 73.8±5.1 | 74.6±6.1 | 0.56 |
| ESR (mm/h) | 18.18±11.9 | 16.88±11.6 | 0.52 |
| ALT (mM/h*L) | 0.34±0.15 | 0.39±0.46 | 0.32 |
| AST (mM/h*L) | 0.16±0.09 | 0.13±0.05 | 0.41 |
| Total bilirubin (µM/L) | 11.4±2.4 | 11.4±2.5 | 0.97 |
3.3. Effect on body weight
Wasting is a prominent feature of TB. The body mass index (BMI), can unequivocally distinguish those who are normal or cachexic based on 18.5 kg/m2 cut-off threshold. At study entry both arms, V7 and placebo, had a substantial proportion of individuals with wasting; i.e., 11/100 (11%) and 15/52 (28.8%), respectively. While the inter-group difference in proportions was statistically significant (P=0.04), the mean absolute body weight and BMI values at baseline were not statistically significant for V7 vs placebo, i.e., 64.2±8.3 vs 61.5±10.7 kg (unpaired t-test; P = 0.09) and 21.4±2.1 vs 20.5±3 kg/m2 (P = 0.05) respectively. After one month the average body weight gain in V7 arm was 2.4 kg: from 64.2±8.3 to 66.6±8.2 (P < 0.0001), but placebo recipients gained only 0.3 kg, which was not significant: 61.5±10.7 to 61.8±10.5 (P = 0.18). The changes in BMI mirrored absolute weight values; in V7 and placebo the BMI increased from 21.4±2.1 to 22.19±2.1 (P < 0.0001) and 20.5±3 to 20.7±3 (P = 0.1), respectively. The proportions of patients who responded favorably, by either parameter, i.e., absolute weight and BMI, were 86% and 34.6% for V7 and placebo respectively, which was highly significant by Fisher's 2 × 2 contingency table (P < 0.0001). Those who ended up with reduced weight or maintained the same body mass were 3% and 11% vs 17.3% and 48.1% in the V7 and placebo arms respectively, indicating that V7 intake resulted in better overall outcome in larger population of patients.
3.4. Effect on TB-associated inflammation
The patients with TB are known to have elevated erythrocyte sedimentation rate (ESR) and higher leukocyte counts, both of which are indicative of TB-associated inflammation. At baseline, the patients who had ESR levels above 20 mm/h normal threshold were not significantly different, i.e., 34% vs 25% (Fisher's test; P = 0.27) in adjunct and placebo groups. After one month, 72% of patients on V7 had ESR levels decreased, while among those on TB drugs alone the proportion of responders became 53.8%. The difference in trend indicating better resolution of inflammatory status in V7 recipients vs placebo was statistically significant (P = 0.03). In absolute numbers, V7 reduced ESR levels from mean 18.2±12 to 12.4±9 mm/h (paired t-test; P < 0.0001), whereas in placebo arm ESR has increased, albeit insignificantly, i.e., 16.9±11.6 to 17.3±13.3 (P = 0.77).
In the immunotherapy arm, absolute numbers of leukocytes decreased from mean 8.95±3.7 to 7.45±2.3 × 109 cells/L (P < 0.0001), while among those on placebo this reduction was not significant; 8.1±2.4 to 7.9±2.8 × 109 cells/L (P = 0.56). This drastic difference was not evident when ratios of responders vs non-responders were compared in V7 and placebo arms prior to and after treatment. The proportion of patients who experienced decline in WBC among subjects receiving V7 and placebo was similar, i.e., 61% vs 59.6% (P = 0.86). However, the decline in average leukocyte counts was more than double in V7 than in placebo arm, i.e., 3.6±2.5 (range: 12.4–0.1) vs 1.6±1.5 (range: 6.5-0.1) x109 cells/L (P < 0.0001).
3.5. Effect on anemia
Many TB patients are characterized by anemia, which is usually associated with poor prognosis. About a quarter of patients in both groups, V7 and control, were anemic with hemoglobin content below normal 120 g/dL level; 24% and 25% (P = 0.32). After one month V7 recipients experienced an increase in hemoglobin from 132±17.8 to 136.9±14.7 g/dL (P = 0.0011), but in placebo the opposite trend was observed, albeit insignificant, i.e.,128.4±15.8 to 126.7±17.2 g/dL (P = 0.44). The unpaired t-test of the outcome shows that the inter-group difference 136.9±14.7 vs 126.7±17.2 g/dL is significant (P = 0.0002), which is also attributable to favorable change in the proportion of non-anemic patients after one month; 90% vs 76.9%, respectively.
3.6. Effect on liver function
TB drugs are known to adversely affect liver function. We have evaluated whether V7 influences in any way the drug-induced hepatotoxicity by measuring alanine transaminase (ALT), aspartate transaminase (AST) and total bilirubin levels. In V7 arm, the difference between baseline and post-treatment mean ALT values expressed in mM/h*L was 0.34±0.15 vs 0.33±0.21, which by paired t-test was not significant (P=0.23). In placebo arm, where participants were treated with TB drugs only, the mean ALT values have increased significantly within one month, i.e., from 0.39±0.46 to 0.54±0.61 (P = 0.04). Similarly, AST levels in placebo group have risen from 0.13±0.05 to 0.17±0.07 (P = 0.005), whereas in V7 recipients no liver damaging effect was observed, i.e., 0.16±0.09 vs 0.17±0.07 (P = 0.41). The total bilirubin values (µM/L) in V7 arm increased from 11.4±2.4 to 13.7±3.4 (P < 0.0001), but placebo patients also experienced rising bilirubin levels from 11.03 ± 3.1 to 13.9±4 (P = 0.0015).
3.7. Effect on total protein levels
Compared to healthy controls patients with pulmonary TB have either decreased or increased levels of total protein in serum. We have evaluated the effect of TB therapy on this parameter. Placebo patients had 74.6±6.1 g/L of total protein at baseline, which has not changed after one month, i.e., 74.6±5.6 (P = 0.93). Patients in the V7 arm had a similar baseline content of protein, 73.8±5.1 g/L, which had slightly increased to 74.6±5.6 (P = 0.043). The proportion of patients who had either increased or decreased serum protein were about same in V7 and placebo arms, i.e., 44.4% vs 23.8% and 33.3% vs 19% respectively (P = 1.0).
3.8. Effect on lymphocyte counts
In our patients, the average baseline figures were 26.4±7% and 27.8±5.7% (P = 0.22) and the proportion of patients with lower than normal lymphocyte percentage (≤20%) was 21% vs 11.5% (P = 0.18) for V7 and placebo, respectively. After one month, V7 patients had retained their lymphocytes at the same level 26.8±5.6% (paired t-test P = 0.96), but in controls they have slightly increased to 29.3±5.3% (P = 0.85). Both outcomes were not statistically significant. The break-down analysis of those who gained lymphocytes vs those who had their counts declined reveals that 51% of V7 recipients vs 50% in placebo arm were gaining lymphocytes (P = 0.81), while 44% vs 34.6% (P = 0.06) were losing and a negligible proportion 5% vs 13.5% (P = 0.55) had stable lymphocytes counts.
4. Discussion
TB remains the deadliest infectious disease, which is fueled by malnutrition, drug-resistance and HIV infection [15]. Many previously-reported immune interventions including, IFN-gamma, IL-2, IL-12, corticosteroids, vitamin D, thalidomide and various anti-cytokine regimens showed some improvements, but results were inconsistent and occasionally associated with adverse effects [7]. Clearly, safer and better immune therapies are needed. The results of this Phase III study in a representative population of patients in two countries with distinct ethnic background, indicate that when TB drugs are combined with V7, this combination produces faster clearance of M. tuberculosis at a significantly higher rate than in the placebo arm receiving TB chemotherapy only.
After one month, 68% of V7 recipients became sputum smear negative, whereas in placebo only 23.1% sputum converted. When patients were stratified by their TB diagnosis, the following conversion rates were observed among DS-TB; MDR-TB, TB/HIV; DS-TB/HIV and MDR-TB/HIV: 86.7%; 55.6%; 44.4%; 75%; 20% vs placebo 22.1%; 15%; and 33.3% for DS-TB; MDR-TB; and DS-TB/HIV accordingly (Fig. 2). Superior response rate resulting from immune intervention, occurring within such a short period of time, i.e., one month, has never been reported in any of TB treatment trials up to date. There was a three-fold difference in mean study-end sputum score, i.e., 1.04 vs 0.33, i.e., OR = 3.15 with 95%CI [2.3 to 4.6], indicating that V7 helped to reduce mycobacterial burden even among those who had not yet fully converted. Our findings support two prior Phase II clinical trials of V7 involving individuals with diverse forms of TB, i.e., DS, MDR, XDR and TB/HIV using two unrelated strains of M. vaccae, i.e., ATCC 95051 and NCTC 11659 [13,14]. In this study, we have used strain of M. vaccae provided by BioEos, which is the same NCTC 11659 strain we have received from Immodulon for our Phase II trial [13]. While today the identity of this strain, also known as SRL172 or DAR-901, is being questioned and proposed to be either M. obuense [16] or M. kyogaense [17], in our hands it has, nevertheless, better efficacy than M. vaccae strain deployed in China (VaccaeTM) as the therapeutic vaccine [10,18], and which is also being tested in Phase III as a prophylactic TB vaccine [10,19]. Statistically, sputum conversion rates resulting from the use of two British strains, were identical, i.e., 68% vs 72.2% (P = 1.0), respectively, but different from Chinese strain (presumably ATCC 95051), courtesy of Anhui Longcom company, i.e., 68% vs 31.8% (P = 0.003).
Consumption is a term synonymous with TB and represents poorly manageable wasting syndrome associated with malnutrition and disease severity [20]. The remarkable feature of our immunotherapy is the beneficial effect on body weight gain, which was eight-fold higher than in controls, i.e., 2.4 kg vs 0.3 kg. None of our patients received weight-boosting supplements, thus ruling out the role of nutritional intervention [21]. Lack of weight gain during TB treatment has been associated with treatment failure and poor immune status [22]. On average, our patients on V7 regimen had gained 3.7% from their baseline mean weight, whereas those on placebo gained 0.47%. While this appear as a modest accrual, it is double the reported 1.4% weight gain after two months of TB chemotherapy among TB patients in the USA, indicating that our immune adjunct contributes to superior clinical benefit [22]. In general, our experience with V7 and two unrelated TB immunotherapies developed by us, i.e., V5 and Immunoxel, indicates that a strong relationship exists between reversal of cachexia by immune intervention and sputum conversion [23,24]. Another indicator of malnutrition – low level of serum total protein – has also been improved in V7, but not in the placebo arm (P = 0.04 vs P = 0.93).
It is well known that cachexia, leukocytosis, elevated ESR, and low hemoglobin content are requisite parameters to gauge the severity of TB. The return of these indices to normal levels is an indication of disease control and a good correlation has been found with sputum conversion. The notion that weight loss in TB is associated with inflammation is supported by the potent anti-inflammatory activity of V7. Two simple biomarkers associated with inflammation and common in TB are high WBC counts and elevated ESR [25]. The effective TB therapy has been shown to normalize these markers. V7 brought down ESR and leukocyte counts more efficiently than ATT alone. Anemia is another common manifestation of pulmonary TB and HIV. The precise cause of anemia is not clear, but inflammation and iron deficiency are thought to be the main culprits [26]. As our patients were not receiving iron supplementation, we believe that the increase in hemoglobin content relates to the anti-inflammatory property of V7. The fact that V7 has not influenced the lymphocyte counts in an adverse manner is another beneficial outcome seen in this and previous V7 trials. Finally, as judged by ALT and AST assays, V7 counteracts TB drug-induced hepatotoxicity - an important factor that affects negatively compliance and success of TB chemotherapy [27]. We are not sure why bilirubin has not followed the same trend as ALT and AST, but this perhaps is due to disparate pathways between hemoglobin-driven, catabolic production of bilirubin and subsequent processing by liver [28].
What is the mechanism of V7 action? It is unlikely that V7 affects directly the replication of mycobacterial bacilli. V7 targets host's immunity since it has shown its utility in a variety of etiologically unrelated diseases, i.e., various cancers, psoriasis, dermatitis, colitis, asthma and a range of brain disorders such as anxiety, stress, depression, etc [29]. TB is a disease whereby pulmonary tissues harboring mycobacteria are constantly assaulted by the host's immune system, creating chronic inflammation and ensuing pulmonary damage [7]. Therefore, immune therapies should be aimed at downplaying rather than exacerbating an already intense immune response. It has been described that M. vaccae can efficiently reduce pro-inflammatory cytokines [16,18,19]. However, it is not enough to just suppress inflammation since some anti-inflammatory agents produced contradictory outcomes [30,31]. It is well known that an orally delivered antigen produces the immune tolerance reaction to it, thus M. vaccae, which mimics M. tuberculosis, is likely to induce specific anti-inflammatory response in a host that results in clinical benefit [32]. We do not know yet how the down-regulation of inflammation correlates with bacterial clearance; the precise mechanism of action of M. vaccae is being investigated and further studies addressing this question need to be carried out [29]. Clearly, there is a synergy when a regulator of immunity against TB, e.g., Mycobacterium bovis or BCG vaccine is combined with TB drugs – this leads to enhanced inhibition of mycobacterial growth in an ex vivo setting [33].
Our study has several limitations. First is that our trial concluded after one month without further information, e.g., relapse rate in V7 arm beyond this time-point. By now we have extensive long-term follow-up experience encompassing hundreds of patients, indicating that such relapses are rare, i.e., no more than one-or-two in 100 over 12 months. Our budget was not large enough to carry out the longitudinal study; our priority was to confirm that treatment can be shortened. The choice of study duration was deliberate as we knew a priori that the difference between immunotherapy and placebo will become apparent within one month. Our one-month conversion rates for DS-TB and MDR-TB are on a par with rates, i.e., 85% and 50%, achieved by conventional 1st and 2nd line TB drugs after 6 and 12–24 months of laborious treatment. Second, we have relied on sputum smear conversion by microscopy rather than culture-based method. Again, based on our own experience of using either of the methods and published evidence, both criteria are equally valid in assessing treatment efficacy, difference being only 1–2%, but the former method is certainly easier, faster and less costly, making it more suitable in countries with limited infrastructure [34]. Third, the assortment of measured parameters was limited as complete lab analysis was not feasible at every site and budget restraints prevented us establishing a centralized analytical facility. Nevertheless, whatever was available, was sufficient in every instance to reach reliable P values to support our conclusions. Finally, it may appear at first glance that we had very relaxed inclusion criteria, but again it was our conscious intention to have a real-life population sample without variance from the natural occurrence of TB in its various manifestations.
The current thinking is geared toward developing TB drugs to suppress mycobacteria, but interventions targeting the host have been somewhat neglected until recent times [35]. We believe strongly that V7 holds a promise in improving and shortening conventional TB treatment regimens. V7 is available over-the-counter in Ukraine and Mongolia as an affordable immune supplement, but immunomodulators, especially those that do not require a prescription, are dismissed and not taken seriously despite superior performance. The global emergence of drug-resistant TB and TB-HIV - a problem that is unlikely to go away by itself - concerns greatly TB caregivers and policymakers [15]. This ongoing public-health threat may prompt a shift from the current paradigm and may result in a wider acceptance of immunotherapeutic approaches as an adjunct to TB treatment regimens and as preventive therapy directed against latent TB [36].
5. Conclusion
This Phase III trial culminates our effort started in 2009, which nevertheless has a long history in the making, originating from serendipitous discovery of the properties of M. vaccae by John and Cynthia Stanford back in 1972 [7,8]. Our trial reveals not only the clinical benefit, but also drastically reduced treatment duration to as short as one month. Oral delivery of M. vaccae, first unveiled by John Stanford's team in Argentina [12], has obvious advantages over parenteral preparations. V7 is stable and can be kept for three years at ambient temperature without costly cold-chain requirements. Our findings derived from an easy-to-use once-daily tableted formulation support previous clinical trials of M. vaccae showing shortened treatment duration with beneficial effect on well-being, body weight, inflammation, anemia and hepatotoxicity due to TB drugs. These favorable outcomes were invariably statistically more significant than in ATT alone arm [10,19,37]. We hope that adequate policy changes will be implemented to embrace V7 and unrelated TB immunotherapeutic interventions, which have been brought to the marketing stage [23,24]. In addition to VACCAETM sold by Anhui Longcom, there is another therapeutic TB vaccine consisting of heat-killed Mycobacterium indicus pranii manufactured in India by Cadila Pharmaceuticals [38]. Lesser known immune adjuvants are being developed by other companies [39] or are already commercialized as supplements as reviewed elsewhere [7].
Ethical Statement
The conduct of the study was approved by the internal review board of the lead hospital in respective countries in accordance with the Helsinki Declaration. The participants in the trial have provided informed consent, participated in this study voluntarily and were free to withdraw from this study at any time. Treatment was provided free-of-charge. This study is registered with the ClinicalTrials.gov under identifier NCT01977768.
CRediT authorship contribution statement
Aldar S. Bourinbaiar: Supervision, Writing - review & editing. Uyanga Batbold: Investigation. Yuri Efremenko: Investigation. Munkhburam Sanjagdorj: Investigation. Dmytro Butov: Investigation. Narantsetseg Damdinpurev: Investigation. Elena Grinishina: Investigation. Otgonbayar Mijiddorj: Investigation. Mikola Kovolev: Investigation. Khaliunaa Baasanjav: Investigation. Tetyana Butova: Investigation. Natalia Prihoda: Investigation. Ochirbat Batbold: Investigation. Larisa Yurchenko: Investigation. Ariungerel Tseveendorj: Investigation. Olga Arzhanova: Investigation. Erkhemtsetseg Chunt: Resources. Hanna Stepanenko: Investigation. Nina Sokolenko: Investigation. Natalia Makeeva: Resources. Marina Tarakanovskaya: Investigation. Vika Borisova: Project administration. Alan Reid: Writing - review & editing. Valeryi Kalashnikov: Visualization. Peter Nyasulu: Data curation. Satria A. Prabowo: Writing - review & editing. Vichai Jirathitikal: Investigation. Allen I. Bain: Funding acquisition. Cynthia Stanford: Conceptualization. John Stanford: Conceptualization.
Declaration of Competing Interest
ASB, VB, MT, VK, VJ, AR and AIB are officers of Immunitor and affiliated companies. Late John Stanford and his colleague and spouse Cynthia Stanford are founders and owners of BioEos company. Remaining authors declare no conflict of interest.
Acknowledgments
This study is dedicated to the memory and pioneering contribution of Professor John Stanford, who unfortunately passed away on June 11, 2018 at the age of 79. We are missing him dearly and deeply regret that he has not lived long enough to see his last paper published. We hope that one day in the future his tireless, life-long work will reach the global community and make radical changes in lives of millions, if not billions of people who otherwise may succumb to TB. Described trial was supported by Grant #0532-2014 “Oral therapeutic TB vaccine” under Stars in Global Health program initiated by Bill and Melinda Gates Foundation and administered by Grand Challenges Canada. The preliminary data from this study were presented at the 5th Global Forum on TB Vaccines, 20-23 February 2018, New Delhi, India [40].
References
- 1.Dubrovina I, Miskinis K, Lyepshina S, Yann Y, Hoffmann H, Zaleskis R, Nunn P, Zignol M. Drug-resistant tuberculosis and HIV in Ukraine: a threatening convergence of two epidemics? Int J Tuberc Lung Dis. 2008;12(7):756–762. [PubMed] [Google Scholar]
- 2.WHO Country Report: TB situation in Ukraine 2017 https://extranet.who.int/sree/Reports?oP=Replet&name=/WHO_HQ_Reports/G2/PROD/EXT/TBCountryProfile&ISO2=UA&outtype=html.
- 3.Pavlenko E, Barbova A, Hovhannesyan A, Tsenilova Z, Slavuckij A, Shcherbak-Verlan B, Zhurilo A, Vitek E, Skenders G, Sela I, Cabibbe AM, Cirillo DM, de Colombani P, Dara M, Dean A, Zignol M, Dadu A. Alarming levels of multidrug-resistant tuberculosis in Ukraine: results from the first national survey. Int J Tuberc Lung Dis. 2018;22(2):197–205. doi: 10.5588/ijtld.17.0254. [DOI] [PubMed] [Google Scholar]
- 4.WPRO Tuberculosishttp://www.wpro.who.int/mongolia/topics/tuberculosis/en/.
- 5.Buyankhishig B, Naranbat N, Mitarai S, Rieder HL. Nationwide survey of anti-tuberculosis drug resistance in Mongolia. Int J Tuberc Lung Dis. 2011;15(9):1201–1205. doi: 10.5588/ijtld.10.0594. [DOI] [PubMed] [Google Scholar]
- 6.Nunn AJ, Phillips PPJ, Meredith SK, Chiang CY, Conradie F, Dalai D, van Deun A, Dat PT, Lan N, Master I, Mebrahtu T, Meressa D, Moodliar R, Ngubane N, Sanders K, Squire SB, Torrea G, Tsogt B, Rusen ID, STREAM Study Collaborators A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. 2019;380(13):1201–1213. doi: 10.1056/NEJMoa1811867. [DOI] [PubMed] [Google Scholar]
- 7.Bourinbaiar AS, Mezentseva MV, Butov DA, Nyasulu PS, Efremenko YV, Jirathitikal V, Mishchenko VV, Kutsyna GA. Immune approaches in tuberculosis therapy: a brief overview. Expert Rev Anti Infect Ther. 2012;10(3):381–389. doi: 10.1586/eri.12.1. [DOI] [PubMed] [Google Scholar]
- 8.Gröschel MI, Prabowo SA, Cardona PJ, Stanford JL, van der Werf TS. Therapeutic vaccines for tuberculosis–a systematic review. Vaccine. 2014;32(26):3162–3168. doi: 10.1016/j.vaccine.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 9.Bönicke R, Juhasz SE. Beschreibung der neuen species Mycobacterium vaccae n. sp. Zentralblatt fur Bakteriologie Parasitenkunde Infektionskrankheiten und Hygiene. Abteilung I. 1964;192:133–135. [PubMed] [Google Scholar]
- 10.Huang CY, Hsieh WY. Efficacy of Mycobacterium vaccae immunotherapy for patients with tuberculosis: a systematic review and meta-analysis. Hum Vaccin Immunother. 2017;13(9):1960–1971. doi: 10.1080/21645515.2017.1335374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Bruyn G, Garner P. Mycobacterium vaccae immunotherapy for treating tuberculosis. Cochrane Database Syst Rev. 2003;(1) doi: 10.1002/14651858.CD001166. [DOI] [PubMed] [Google Scholar]
- 12.Dlugovitzky D, Notario R, Martinel-Lamas D, Fiorenza G, Farroni M, Bogue C, Stanford C, Stanford J. Immunotherapy with oral, heat-killed, Mycobacterium vaccae in patients with moderate to advanced pulmonary tuberculosis. Immunotherapy. 2010;2(2):159–169. doi: 10.2217/imt.09.90. [DOI] [PubMed] [Google Scholar]
- 13.Butov DA, Efremenko YV, Prihoda ND, Zaitzeva SI, Yurchenko LV, Sokolenko NI, Butova TS, Stepanenko AL, Kutsyna GA, Jirathitikal V, Bourinbaiar AS. Randomized, placebo-controlled Phase II trial of heat-killed Mycobacterium vaccae (Immodulon batch) formulated as an oral pill (V7) Immunotherapy. 2013;5(10):1047–1054. doi: 10.2217/imt.13.110. [DOI] [PubMed] [Google Scholar]
- 14.Efremenko YV, Butov DA, Prihoda ND, Zaitzeva SI, Yurchenko LV, Sokolenko NI, Butova TS, Stepanenko AL, Kutsyna GA, Jirathitikal V, Bourinbaiar AS. Randomized, placebo-controlled phase II trial of heat-killed Mycobacterium vaccae (Longcom batch) formulated as an oral pill (V7) Hum Vaccin Immunother. 2013;9(9):1852–1856. doi: 10.4161/hv.25280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bloom BR, Atun R, Cohen T, Dye C, Fraser H, Gomez GB, Knight G, Murray M, Nardell E, Rubin E, Salomon J, Vassall A, Volchenkov G, White R, Wilson D, Yadav P. Tuberculosis. In: Holmes KK, Bertozzi S, Bloom BR, Jha P, editors. Major infectious diseases. 3rd edition. The International Bank for Reconstruction and Development / The World Bank; Washington (DC): 2017. pp. 233–311. Chapter 11PMID: 30212088. [PubMed] [Google Scholar]
- 16.Masonou T, Hokey DA, Lahey T, Halliday A, Berrocal-Almanza LC, Wieland-Alter WF, Arbeit RD, Lalvani A, von Reyn CF. CD4+ T cell cytokine responses to the DAR-901 booster vaccine in BCG-primed adults: a randomized, placebo-controlled trial. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0217091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nouioui I, Brunet LR, Simpson D, Klenk HP, Goodfellow M. Description of a novel species of fast growing mycobacterium: Mycobacterium kyogaense sp. nov., a scotochromogenic strain received as Mycobacterium vaccae. Int J Syst Evol Microbiol. 2018;68(12):3726–3734. doi: 10.1099/ijsem.0.003039. [DOI] [PubMed] [Google Scholar]
- 18.Weng H, Huang JY, Meng XY, Li S, Zhang GQ. Adjunctive therapy of Mycobacterium vaccae vaccine in the treatment of multidrug-resistant tuberculosis: a systematic review and meta-analysis. Biomed Rep. 2016;4(5):595–600. doi: 10.3892/br.2016.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng J, Chen L, Liu L, Li H, Liu B, Zheng D, Liu T, Dong J, Sun L, Zhu Y, Yang J, Zhang X, Jin Q. Proteogenomic analysis and discovery of immune antigens in Mycobacterium vaccae. Mol Cell Proteom. 2017;16(9):1578–1590. doi: 10.1074/mcp.M116.065813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoyt KJ, Sarkar S, White L, Joseph NM, Salgame P, Lakshminarayanan S, Muthaiah M, Vinod Kumar S, Ellner JJ, Roy G, Jr Horsburgh CR, Hochberg NS. Effect of malnutrition on radiographic findings and mycobacterial burden in pulmonary tuberculosis. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0214011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandra RK. Nutrient supplementation as adjunct therapy in pulmonary tuberculosis. Int J Vitam Nutr Res. 2004;74(2):144–146. doi: 10.1024/0300-9831.74.2.144. [DOI] [PubMed] [Google Scholar]
- 22.Phan MN, Guy ES, Nickson RN, Kao CC. Predictors and patterns of weight gain during treatment for tuberculosis in the United States of America. Int J Infect Dis. 2016;53:1–5. doi: 10.1016/j.ijid.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butov DA, Efremenko YV, Prihoda ND, Yurchenko LI, Sokolenko NI, Arjanova OV, Stepanenko AL, Butova TS, Zaitzeva SS, Jirathitikal V, Bourinbaiar AS, Kutsyna GA. Adjunct immune therapy of first-diagnosed TB, relapsed TB, treatment-failed TB, multidrug-resistant TB and TB/HIV. Immunotherapy. 2012;4(7):687–695. doi: 10.2217/imt.12.59. [DOI] [PubMed] [Google Scholar]
- 24.Batbold U, Butov DO, Kutsyna GA, Damdinpurev N, Grinishina EA, Mijiddorj O, Kovolev ME, Baasanjav K, Butova TS, Sandagdorj M, Batbold O, Tseveendorj A, Chunt E, Zaitzeva SI, Stepanenko HL, Makeeva NI, Mospan IV, Pylypchuk VS, Rowe JL, Nyasulu P, Jirathitikal V, Bain AI, Tarakanovskaya MG, Bourinbaiar AS. Double-blind, placebo-controlled, 1:1 randomized Phase III clinical trial of Immunoxel honey lozenges as an adjunct immunotherapy in 269 patients with pulmonary tuberculosis. Immunotherapy. 2017;9(1):13–24. doi: 10.2217/imt-2016-0079. [DOI] [PubMed] [Google Scholar]
- 25.Rohini K, Surekha Bhat M, Srikumar PS, Mahesh Kumar A. Assessment of hematological parameters in pulmonary tuberculosis patients. Indian J Clin Biochem. 2016;31(3):332–335. doi: 10.1007/s12291-015-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isanaka S, Mugusi F, Urassa W, Willett WC, Bosch RJ, Villamor E, Spiegelman D, Duggan C, Fawzi WW. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr. 2012;142(2):350–357. doi: 10.3945/jn.111.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yew WW, Leung CC. Antituberculosis drugs and hepatotoxicity. Respirology. 2006;11(6):699–707. doi: 10.1111/j.1440-1843.2006.00941.x. [DOI] [PubMed] [Google Scholar]
- 28.Kosmachevskaya OV, Topunov AF. Alternate and additional functions of erythrocyte hemoglobin. Biochemistry (Mosc) 2018;83(12):1575–1593. doi: 10.1134/S0006297918120155. [DOI] [PubMed] [Google Scholar]
- 29.Smith DG, Martinelli R, Besra GS, Illarionov PA, Szatmari I, Brazda P, Allen MA, Xu W, Wang X, Nagy L, Dowell RD, Rook GAW, Rosa Brunet L, Lowry CA. Identification and characterization of a novel anti-inflammatory lipid isolated from Mycobacterium vaccae, a soil-derived bacterium with immunoregulatory and stress resilience properties. Psychopharmacology (Berl) 2019;236(5):1653–1670. doi: 10.1007/s00213-019-05253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conesa-Botella A, Meintjes G, Coussens AK, van der Plas H, Goliath R, Schutz C, Moreno-Reyes R, Mehta M, Martineau AR, Wilkinson RJ, Colebunders R, Wilkinson KA. Corticosteroid therapy, vitamin D status, and inflammatory cytokine profile in the HIV-tuberculosis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2012;55(7):1004–1011. doi: 10.1093/cid/cis577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroesen VM, Gröschel MI, Martinson N, Zumla A, Maeurer M, van der Werf TS, Vilaplana C. Non-steroidal anti-inflammatory drugs as host-directed therapy for tuberculosis: A systematic review. Front Immunol. 2017;8:772. doi: 10.3389/fimmu.2017.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silin DS, Lyubomska OV, Jirathitikal V, Bourinbaiar AS. Oral vaccination: where we are? Expert Opin Drug Deliv. 2007;4(4):323–340. doi: 10.1517/17425247.4.4.323. [DOI] [PubMed] [Google Scholar]
- 33.Prabowo SA, Zelmer A, Stockdale L, Ojha U, Smith SG, Seifert K, Fletcher HA. Historical BCG vaccination combined with drug treatment enhances inhibition of mycobacterial growth ex vivo in human peripheral blood cells. Sci Rep. 2019;9(1):4842. doi: 10.1038/s41598-019-41008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devadatta S, Radhakrishna S, Fox W, Mitchison DA, Rajagopalan S, Sivasubramanian S, Stott H. Comparative value of sputum smear examination and culture examination in assessing the progress of tuberculous patients receiving chemotherapy. Bull World Health Organ. 1966;34(4):573–587. [PMC free article] [PubMed] [Google Scholar]
- 35.Zumla A, Rao M, Wallis RS, Kaufmann SH, Rustomjee R, Mwaba P, Vilaplana C, Yeboah-Manu D, Chakaya J, Ippolito G, Azhar E, Hoelscher M, Maeurer M. Host-Directed Therapies Network consortium. Host-directed therapies for infectious diseases: current status, recent progress, and future prospects. Lancet Infect Dis. 2016;16(4):e47–e63. doi: 10.1016/S1473-3099(16)00078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson BD, Altmann D, Barry C, Bishai B, Cole S, Dick T, Duncan K, Dye C, Ehrt S, Esmail H, Flynn J, Hafner R, Handley G, Hanekom W, van Helden P, Kaplan G, Kaufmann SH, Kim P, Lienhardt C, Mizrahi V, Rubin E, Schnappinger D, Sherman D, Thole J, Vandal O, Walzl G, Warner D, Wilkinson R, Young D. Detection and treatment of subclinical tuberculosis. Tuberculosis (Edinb) 2012;92(6):447–452. doi: 10.1016/j.tube.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Yang XY, Chen QF, Li YP, Wu SM. Mycobacterium vaccae as adjuvant therapy to anti-tuberculosis chemotherapy in never-treated tuberculosis patients: a meta-analysis. PLoS One. 2011;6(9):e23826. doi: 10.1371/journal.pone.0023826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talwar GP, Gupta JC, Mustafa AS, Kar HK, Katoch K, Parida SK, Reddi PP, Ahmed N, Saini V, Gupta S. Development of a potent invigorator of immune responses endowed with both preventive and therapeutic properties. Biologics. 2017;11:55–63. doi: 10.2147/BTT.S128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tukvadze N, Cardona P, Vashakidze S, Shubladze N, Avaliani Z, Vilaplana C, Cardona PJ. Development of the food supplement Nyaditum resae as a new tool to reduce the risk of tuberculosis development. Int J Mycobacteriol. 2016;5(Suppl 1):S101–S102. doi: 10.1016/j.ijmyco.2016.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atmakuri K, Penn-Nicholson A, Tanner R, Dockrell HM. Proceedings of the meeting report: 5th global forum on TB vaccines, 20–23 February 2018; New Delhi, India; 2018. pp. 55–64. Tuberculosis (Edinb) [DOI] [PubMed] [Google Scholar]