Abstract
Borassus flabellifer L. is a tall palm traditionally used for its stimulating, diuretic and anti-inflammatory activities; it is rich in fibers and various pharmacologically important secondary metabolites. This study was undertaken to evaluate the antidiabetic effects of Borassus flabellifer fruit methanol extract (BF-M) on diabetic rats induced with High Fat Diet (HFD)/streptozotocin (STZ). When BF-M (100 or 200 mg/kg) was administered for 21 days orally it led to a sharp decline in triglycerides, total cholesterol, free unsaturated fat, glucose-6-phosphate, fasting blood glucose and fructose 1,6 bisphosphatase in contrast to diabetic control. BF-M also downregulated Protein Tyrosine Phosphatase 1B. In vitro study showed the IC50 value to be 23.98 μg/mL. BF-M significantly increased serum insulin, glycogen content, and body weight. Western blot analysis exhibited significant inhibition of PTP1B in pancreatic tissue which was confirmed by histology and immunohistological studies. GC-MS analysis revelaled that the presence of major compounds such as 5-hydroxymethylfurfural (47.56%), Guanosine (21.01%) and n-hecxadeconoic acid (25.14%) in BF-M. In short, BF-M exerted antidiabetic property by down regulating PTP1B expression, and eventually enhancing glucose stimulated insulin release; it also exhibited favorable effects in diabetes and its secondary complications.
Keywords: Borassus flabellifer, Insulin secretion, Protein Tyrosine Phosphatase 1B, Diabetes mellitus, β-Cells regeneration, GC-MS
1. Introduction
Diabetic complications develop at a young age, with persistent hyperglycemia and improper metabolism of protein, lipid, and carbohydrate, thus resulting in defective insulin action and its secretion (Sirasanagandla et al., 2013). Its prevalence among populace might be due to diet and lifestyle (Olokoba et al., 2012). Protein Tyrosine Phosphatase 1B (PTP1B) has been objectivized as a mode of treatment for Diabetes Mellitus (DM) (Popov, 2011) by revising glucose tolerance, resistance to insulin sensitivity and diet – induced obesity (Klaman et al., 2000, Elchebly et al., 1999).
Currently herbal remedies are highly preferred for DM due to expensive and serious side effects of synthetic antidiabetic drugs (Gupta et al., 2009, Sendrayaperumal et al., 2014). Borassus flabellifer L. (Arecaceae) is a palm tree; it is generally utilized as a stimulant, diuretic, laxative, aphrodisiac; it also possesses antioxidant property (Bayton, 2007, Pramod et al., 2013a, Pramod et al., 2013b, Mohite et al., 2012, Paschapur et al., 2009). Serum glucose levels in sucrose-loaded normal rats decreased gradually upon treatment with methanol extract of Borassus flabellifer flower (Yoshikawa et al., 2007).
Different parts of B. flabellifer are used by tribal people for various purposes. The male flowers of B. flabellifer (Masayuki, 2007, Saravanan et al., 2012) reported that benzene, chloroform, acetone, methanol and ethanol extracts inhibited the growth of pathogenic bacteria.
The juice obtained from the flower stalk was used to treat diabetes (Shamala et al., 1985). The fruits of B. flabellifer showed antioxidant, antihelmintic, diuretic, antibacterial properties, immunomodulatory and antimalarial properties (Sahni et al., 2014). Palm fruit has antioxidant and anti-inflammatory properties. The plant contains different types of phytoconstituents such as vitamins, minerals and polyphenols (Jerry, 2018).
The present work was carried out to assess the antidiabetic potential of BF-M by downregulating PTP1B expression in pancreas and its effect on glucose and lipid metabolism in diabetic rats.
2. Materials and methods
2.1. Preparation of crude extract
Borassus flabellifer L. (Arecaceae) fruits were gathered from Sirupaniyur Thakka village, Villupuram district, Tamil Nadu, India. The fruits of B. flabellifer were authenticated by botanist Dr. C. Muthukumar, Assistant Professor, National College, Trichy. The fresh fruits were chopped into small pieces (800 g) after shade drying and subsequently soaked in 90% methanol. This set up was kept at room temperature (25 ± 2 °C) for 3 days with intermittent shaking. After 3 days the methanol extract was filtered through filter paper. The extract was condensed utilizing rotary vacuum evaporator at 40 °C (Handa et al., 2008). Finally the crude extract (BF-M) was obtained. The collected crude extract yield was around -20g.
2.2. GC-MS analysis of BF-M
The BF-M extract was studied the gas chromatography (GC-MS-QP 2010 [SHIMADZU). The instrument was equipped with a CPB-capillary column (30.mx 0.25 mm i.d) coated with 5% phynyl with 95% dimethyl siloxane, film thickness 0.2 µm). The temperature program was 70 to 300 ˚with 5˚ per minute. The Injector temperature was 200°; carrier gas was He (20 psi), flow rate was 1.51 ml/min. 1 μl of test sample was injected into the sample receiver with the help of hot needle; split ratio was 10. The instrument was attached with GCMS library, NIST, Wiley. The experiment was carried out at Sargam Laboratory Service, Private Ltd, Chennai-600 089, India (Al-Dhabi et al., 2016).
2.3. Experimental animals
Healthy adult wistar rats were grown (weighing about 170–190 g) in Central animal house, Entomology Research Institute, Loyola College in suitable environmental condition. The room temperature was (22 ± 2 °C), with relative humidity (45 ± 5 °C) and 12/12 h day/night cycle. All the animals were maintained for seven days fed with standard pellet diet supplied from Sai Durga Feeds and Foods, Bangalore. The animal experiments were approved by the Institutional Animal Ethics Committee (IAEC- ERI-LC-04/10).
2.3.1. PTPB1 inhibition assay
Phosphatase activity was analyzed using a substrate namely Para-Nitro Phenyl Phosphate (P-NPP) (Lund et al., 2004). This assay buffer, comprising of glutathione (5 mM), 3, 3-dimethyl glutarate (50 mM) and 1 mM Ethylene Diamine Tetra Acetic Acid (EDTA) was changed in accordance with an ionic nature of 0.15 M by NaCl. The reaction lasted for 60 min. Once the reaction was completed, the ELISA reader (405 nm) was used to measure the enzyme activity.
2.4. Low dose STZ with HFD induced type 2 diabetes
Healthy adult wistar animals of either sex weighing 180 ± 10 g were fed to standard nourishment feeds and water for 1 week with 12-hour light/dark cycle. After 1 week, the rodents to be subjected to experiment were given a diet (60% fat) except normal control. After 2 weeks on high fat diet, overnight fasted adult albino wistar strain male rats were given freshly prepared STZ (40 mg/kg) (Sigma-Aldrich, Bangalore) through intraperitoneal mode. On the seventh day, those rats which had blood glucose level over 288 mg/dL were considered as diabetic and incorporated into the investigation (Triender, 1969).
2.5. Experimental design
There were five groups and each group had six rats (n = 6 per group). Ordinary faucet water was utilized as a vehicle. A single dosage of BF-M (100 and 200 mg/kg) was mixed with vehicle and given orally for 21 days. The BF-M dose was selected based on preliminary tests of a variety of doses.
Group I consisted of normal control rats with access to tap water. Group II was diabetic control; Group 3 was diabetic rats treated with BF-M (100 mg/kg) administered orally; Group 4 was diabetic rats treated orally with BF-M (200 mg/kg); Diabetic rats of group 5 were treated with glibenclamide (5 mg/kg) in aqueous solution.
Fasting glucose level was measured once in a week starting from basal level (on the day prior to STZ injection), then on 7th, 14th, 21st and 28th days after the injection of STZ. Plasma insulin and body weight were measured at basal level, 7th and 28th days of the treatment. Anesthetization of rats with 400 mg/kg Chloral hydrate was done on the final day and their blood was collected.
2.6. Biochemical tests
2.6.1. Serum glucose level and plasma insulin measurement
The ELISA kit (Life Technologies, India) and glucose oxidase-peroxidase approach had been used to assess the plasma insulin level and blood glucose level respectively (Triender, 1969).
2.6.2. Estimation of carbohydrate metabolism enzymes
Protocols of Gancedo and Gancedo, 1971, Koide, 1959 were applied to estimate Fructose 1, 6 bisphosphatase and Glucose-6-phosphatase (G-6-P) enzymes.
2.6.3. Measurement of liver and muscle glycogen contents
Liver and skeletal muscles were used for the measurement of glycogen level using Anthrone method.
2.6.4. Lipid profile measurement
Serum lipid profiles such as total cholesterol (TC), triglycerides (TG), and free fatty acid were measured based on the manufacturer’s instructions (Merck, Mumbai, India; Wako Pure Chemicals, Japan) (Friedewald et al., 1972).
2.6.5. Preparation of histological specimens
The pancreas was sliced up and soaked in 10% formalin. The organs were prepared in an elevated arrangement of liquid and inserted in the prepared wax. Serial segments (5 μm) of this organ were taken utilizing a microtome and then mounted on glass slides, recolored with hematoxylin and eosin (H&E) and subjected to microscopic examination.
2.6.6. Immunohistochemistry study
For the immunohistochemistry study, pancreas was cleansed away using saline, retained in the preferred percentage of formalin (10%) and then it was embedded with paraffin (Yin et al., 2006). Running water was used to wash the specimen overnight and afterwards separated for sectioning (5 μm in thickness). The processes of de-waxing and incubation on the prepared sample were done at room temperature for 1 h. Phosphate Buffered Saline (PBS) (having 0.5% triton X-100 and 2% ordinary goat serum) was used to protect the samples and kept for 16 h at 4 °C. Again, they were cleansed with PBS. The essential monoclonal antibodies (Mouse anti-EMA, USA) were used for incubating the samples at 4 °C throughout the night. Primary antibody and anti-mouse secondary antibody were bound together and incubated for a period of time. Segments were recolored with H&E. The software, Morphometry image analysis (Bethesda, USA) played an important role in measuring the insulin positive area.
2.6.7. Western blot
The expression of PTP1B in pancreatic tissue was analyzed using western blot. The dissected pancreatic tissue was submerged in cold hypotonic lysis buffer solution containing 1 M NaHCO3, 1 M NaN3,0.1 M PMSF, and Protease Inhibitor Cocktail (Calbiochem, SanDiego, CA) and allowed to stand for 30 min. Afterwards, the specimens were subjected to homogenization with high-speed rotational tissue homogenizer (Glas-Col, Terre Hute, IN) and centrifuged at 12,000 RPM for 5 min). Finally the supernatant containing protein was collected and studied further.
The protein (40 mg) was run in 10% (w/v) SDS-PAGE followed by exposure to nitrocellulose membrane. Tween (0.05%) along with Tris buffered saline (TBS) containing 3% skimmed milk powder was used to dip the membrane. Then, primary antibody for PTP1B (Santa Cruz Biochemical, USA) was added and kept at room temperature nearly for 1 h. Then, TBS was used to wash the membrane. After adding the secondary antibody, it was kept at room temperature for 1 h. The counter acting agent receptive groups were exposed using the Amersham product of upgraded chemiluminescence pack and Bio-rad product of ChemiDoc XRS Gel Imager.
2.6.8. Statistical evaluation
All the data were calculated using mean ± Standard Error of Mean (SEM). The significant differences between two groups (Version 11.5; SPSS program) were evaluated by T-test. The set values were significant at p ≤ 0.05. Each group carried six replications (n = 6).
3. Results
3.1. GC-MS analysis of BF-M
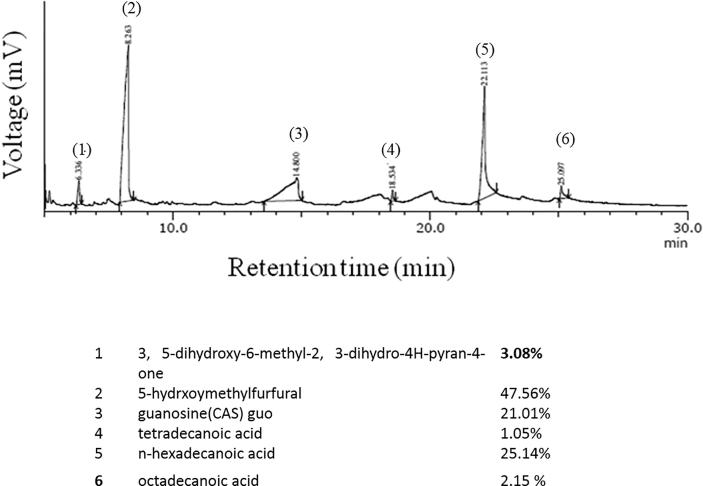
BF-methanol extract was subjected to GC-MS chromatography to assess the chemical constituents. The following compounds were found in the extract; (1) 3, 5-dihydroxy-6-methyl-2, 3-dihydro-4H-pyran-4-one (3.08%)], (2) 5-hydrxoymethylfurfural (47.56%), (3) guanosine (CAS) guo (21.01%), (4) tetradecanoic acid (1.05%), (5) n-hexadecanoic acid (25.14%), (6) and octadecanoic acid (2.15%) (Fig. 1). The major compound was 5-hydrxoymethylfurfural.
Fig. 1.
(a) GC-MS analysis of Borassus flabellifer fruit methanol extract.
3.2. In vitro PTP1B assay
Table 1 shows the PTP1B inhibition in the extract of BF-M and agarose. BF-M and Agarose demonstrated noteworthy dosage reliant inhibition of PTP1B catalyst movement; the IC50 value was observed to be 23.98 μg/mL and 0.362 μg/mL respectively.
Table 1.
Effect of BF-M on Protein Tyrosine Phosphatase-1B (PTP1B) enzyme activity in in vitro.
| Sample | Concentration (μg/mL) | (%) Inhibition | IC 50 |
|---|---|---|---|
| BF-M | 5 | 20.53 ± 0.29 | 23.98 |
| 10 | 37.77 ± 0.34 | ||
| 25 | 56.92 ± 0.28 | ||
| 50 | 77.09 ± 0.63 | ||
| 100 | 98.72 ± 0.32 | ||
| Agarbose | 0.1 | 32.14 ± 0.57 | 0.362 |
| 0.5 | 61.30 ± 0.84 | ||
| 1 | 75.34 ± 0.21 | ||
| 2 | 99.50 ± 0.25 |
Values are expressed as mean ± SEM (% inhibition) and mean (IC50) n = 6.
B-F: Borassus flabellifer Methanol extract Agarbose: Standard.
3.3. Fasting blood glucose and BF-M
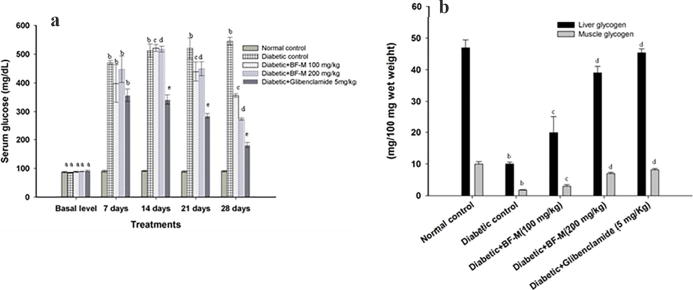
Fig. 2(a) and Table 2 demonstrate the impacts of BF-M on body weight and fasting blood glucose in STZ-induced diabetic rodents. The fasting blood glucose level significantly increased in STZ-induced diabetic rodents and body weight of the animals in the same group was decreased. Oral administration of BF-M (100 or 200 mg/kg) for 21 days to the diabetic animals increased the body weight and fundamentally diminished fasting blood glucose. After 21 days Glibenclamide treated group showed reduction in glucose level compared to disease control group.
Fig. 2.
(a) Effect of BF-M (100 and 200 mg/kg) on Fasting plasma glucose level in HFD fed-STZ induced diabetic rats. (b) Effect of BF-M on liver and muscle glycogen content in HFD fed-STZ induced diabetic rats. Results are expressed as the mean ± SEM (n = 6). Significant difference between two groups were analysed by Student’s t-test. a Significantly no different (P > 0.05) from normal control; b Significantly different (P < 0.01) from normal control; c Significantly different (P < 0.05) from diabetic control; d Significantly different (P < 0.05) from diabetic control; e Significantly different (P < 0.01) from diabetic control.
Table 2.
Effect of BF-M (100 and 200 mg/kg) on body weight in HFD fed- low STZ induced diabetic rats.
| Treatment | Body weight (g) |
|
|---|---|---|
| Initial | Final | |
| Normal control | 184.10 ± 2.01 | 213.40 ± 2.31 |
| Diabetic control | 185.45 ± 3.10 | 189 ± 5.70b |
| Diabetic + BF-M (100 mg/kg) | 191.24 ± 4.21 | 198.50 ± 4.17c |
| Diabetic + BF-M (200 mg/kg) | 189.30 ± 5.10 | 210.50 ± 5.75d |
| Diabetic + glibenclamide (5 mg/kg) | 190.62 ± 3.70 | 214.37 ± 4.12d |
Results are expressed as the mean ± S.E.M in each group (n = 6). Significant difference between two groups were analysed by Student’s t-test.
Significantly different (P < 0.01) from normal control.
Significantly different (P < 0.05) from diabetic control.
Significantly different (P < 0.01) from diabetic control.
3.4. BF-M effect on plasma insulin
Table 3 elucidates the effects of BF-M on plasma insulin in STZ-treated rats and normal control. Plasma insulin levels in diabetic rats were decreased significantly in comparison with the normal control group. BF-M treated diabetic rats showed increased plasma insulin levels in comparison to diabetic controls.
Table 3.
Effect of BF-M (100 and 200 mg/kg) on plasma insulin level in HFD fed-STZ induced diabetic rats.
| Treatment | Plasma insulin level |
|
|---|---|---|
| 7th day | 28th day | |
| Normal control | 125.11 ± 0.87 | 131.09 ± 1.88 |
| Diabetic control | 54.66 ± 1.89b | 49.49 ± 0.89b |
| Diabetic + BF-M (100 mg/kg) | 52.19 ± 1.96b | 84.85 ± 1.27c |
| Diabetic + BF-M (200 mg/kg) | 51.65 ± 1.73b | 112.29 ± 1.09d |
| Diabetic + glibenclamide (5 mg/kg) | 49.52 ± 1.38b | 117.70 ± 0.98d |
Results are expressed as the mean ± S.E.M in each group (n = 6). Significant difference between two groups were analysed by Student’s t-test.
Significantly different (P < 0.01) from normal control.
Significantly different (P < 0.05) from diabetic control.
Significantly different (P < 0.01) from diabetic control.
3.4.1. Enzymes of carbohydrate metabolism
Table 4 reveals the activities of enzymes associated with carbohydrate metabolism in control and STZ induced diabetic animals. The activities of Fructose 1, 6 bisphosphatase and Glucose-6-Phospatase were increased in diabetic rodents compared to normal control. In animals administered with BF-M (100 or 200 mg/kg) for 21 day, the activities of Fructose 1, 6 bisphosphatase and Glucose-6-phosphatase were significantly reduced when compared to diabetic control.
Table 4.
Effect of BF-M (100 and 200 mg) induced diabetic rats after 21 days study.
| Groups | Glucose 6 phosphatase (μ mole of Pi liberated/min/mg protein) | Fructose 1,6 bisphosphatase (µ mole of Pi liberated/min/mg protein) |
|---|---|---|
| Normal control | 0.15 ± 0.006 | 7.20 ± 0.71 |
| Diabetic control | 0.34 ± 0.122b | 20.36 ± 0.91b |
| Diabetic + BF-M (100 mg/kg) | 0.27 ± 0.007c | 14.67 ± 0.82c |
| Diabetic + BF-M (200 mg/kg) | 0.24 ± 0.006c | 9.28 ± 0.50c |
| Diabetic + Glibenclamide (5 mg/kg) | 0.23 ± 0.014d | 8.94 ± 0.68d |
Results are expressed as the mean ± S.E.M in each group (n = 6). Significant difference between two groups were analysed by Student’s t-test.
Significantly different (P < 0.01) from normal control.
Significantly different (P < 0.05) from diabetic control.
Significantly different (P < 0.01) from diabetic control.
3.5. BF-M effect on liver and muscle glycogen contents
Fig. 2(b) demonstrates the effects of BF-M on liver substance and muscle glycogen in STZ induced diabetic rodents. Diabetic rodents fed with BF-M (100 or 200 mg/kg) for 21 days indicated critical increments in glycogen substance when compared to diabetic control.
3.6. Effects of BF-M on total cholesterol, triglycerides and free fatty acid
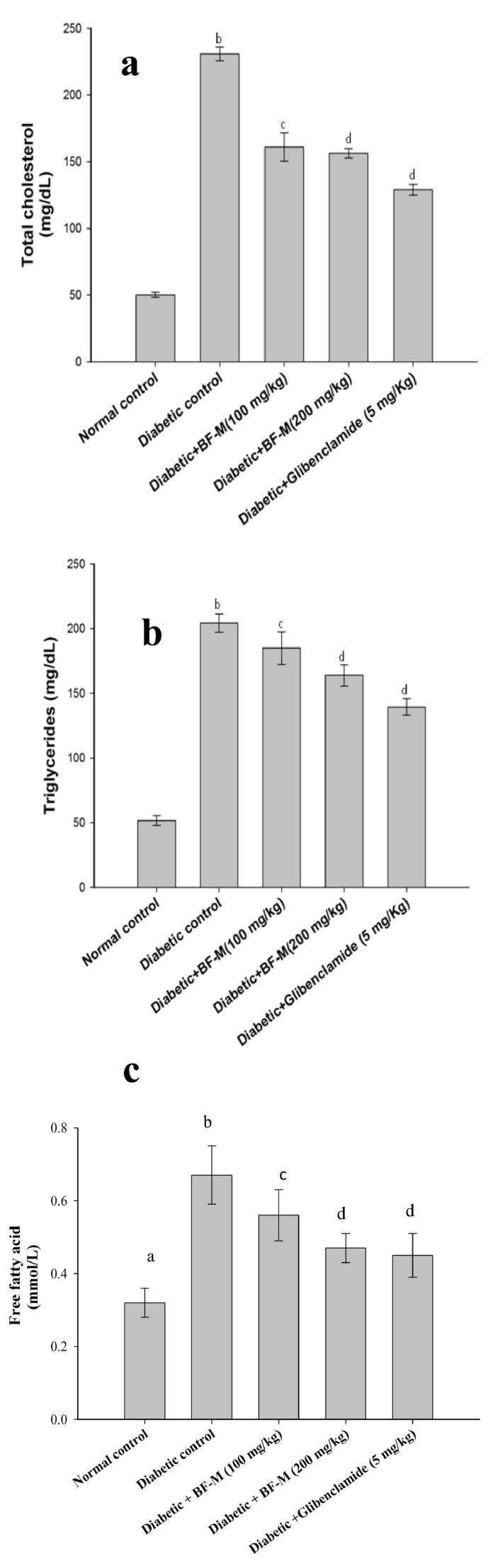
Fig. 3(a), (b) and (c) demonstrate the increased level of triglycerides, free unsaturated fat and serum total cholesterol in diabetic control rodents. On the other hand diabetic rodents treated with 100 or 200 mg/kg of BF-M had essentially low total cholesterol and free unsaturated compared to diabetic control rats.
Fig. 3.
(a,b,c). Effect of BF-M on Total cholesterol, Triglycerides and Free fatty acids in HFD fed-STZ induced diabetic rats. Results are expressed as the mean ± SEM (n = 6). Significant difference between two groups were analysed by Student’s t-test. b Significantly different (P < 0.01) from normal control; c Significantly different (P < 0.05) from diabetic control; d Significantly different (P < 0.05) from diabetic control.
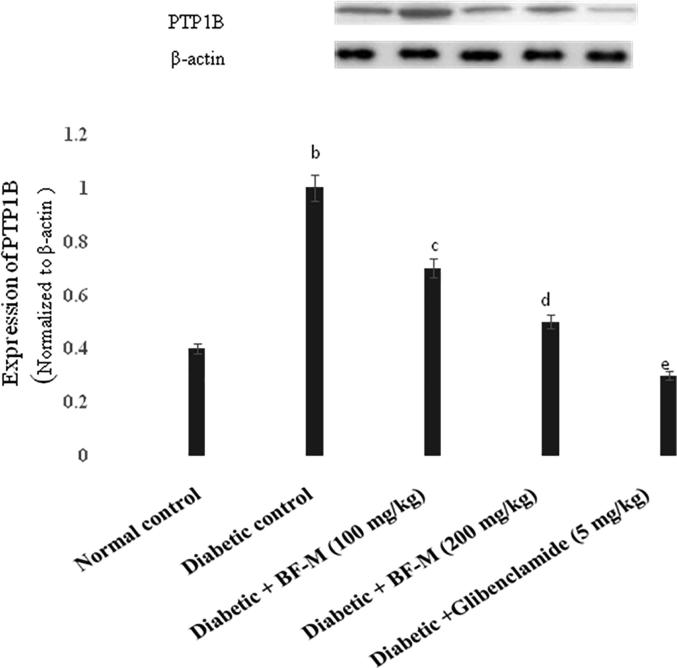
3.7. PTP1B expression in pancreatic tissue
The impact of BF-M on PTP1B in pancreatic tissue was analyzed using western blot (Fig. 4). There was a high expression of PTP1B in the pancreatic tissues of the diabetic rats; however the BF-M and glibenclamide treated groups showed decreased levels of PTP1B.
Fig. 4.
Effect of BF-M on PTP1B expression on normal and HFD fed-STZ induced diabetic rats. Results are expressed as the mean ± SEM (n = 6). Significant differences between two groups were analyzed by Student’s t-test. b Significantly different (P < 0.01) from normal control; c Significantly different (P < 0.05) from diabetic control; d Significantly different (P < 0.05) from diabetic control; e Significantly different (P < 0.01) from diabetic control.
3.8. H&E staining
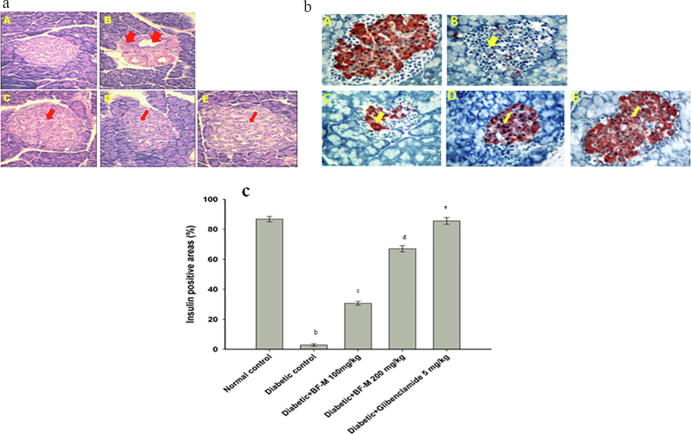
The light microscopy photographs of normal control group rats showed normal architecture of pancreatic islets Fig. 5a (A). Diabetic controls exhibited degenerative changes, necrotic changes, infiltration of acute inflammatory cells, interstitial edema, and vacuoles Fig. 5a (B). BF-M (100 or 200 mg/kg) treated diabetic rats showed markedly less pancreatic vascular necrosis compared to diabetic controls Fig. 5a (C-D). Glibenclamide (5 mg/kg) treated diabetic rats exhibited similar patterns as those of normal controls Fig. 5a (E)
Fig. 5.
(a) Histopathological study of normal and HFD fed-STZ induced diabetic rat pancreas. A-Normal pancreas; B- Diabetic control; C- Diabetic +BF-M (100 mg/kg); D- Diabetic + 66 BF-M (200 mg/kg); E- Diabetic + Glibenclamide (5 mg/kg). (b). Immunohistochemical analysis of normal and .HFD fed-STZ induced diabetic rat pancreas. A-Healthy β-cells were seen Langerhans in the islets of normal control group, B – Weak insulin immuno reactivity can be seen in a few β-cells in the islet of Langerhans in Diabetic control, C- few β-cells in some islets displaying insulin immunopositive in very small granules of diabetic rats treated with BF-M 100 mg/kg; Diabetic animal treated with BF-M 200 mg/kg and glibenclamide 5 mg/kg protected the majority of β-cells in the islet of Langerhans and gave strong straining with anti-insulin antibody, immunoperoxidase and hemotoxylin. (c) Relationship between insulin immunopositive and positive islets area (%) of HFD fed-STZ Induced diabetic rats. Results are expressed as the mean ± SEM of the number β-cells detected in each islet section. a Significantly different (P < 0.01) from normal control; b Significantly different (P < 0.05) from diabetic control; c Significantly different (P < 0.01) from diabetic control.
3.9. Immunohistological study
In control rats normal β-cells were found in the islets of Langerhans upon immunohistochemical staining [Fig. 5b (A)]. In contrast, diabetic rats exhibited β-cells with weak insulin immunoreactivity [Fig. 5b (B)]. BF-M (100 mg/kg) treated diabetic rodents showed some active β-cells [Fig. 5b (C)]. BF-M (200 mg/kg) and glibenclamide (5 mg/kg) treated diabetic rodents showed more active β-cells [(Fig. 5b (D, E)]. Image analysis revealed less than 3% of active islets area in diabetic control, whereas rats treated with 100 and 200 mg/kg BF-M, exhibited 30.73 ± 12.0 and 55.08 ± 7.21 active β-cell respectively. We found that rats treated with 5 mg/kg glibenclamide exhibited more active (80.00 ± 8.20) β-cells than BF-M (200 mg/kg) treated rats (Fig. 5c).
4. Discussion
Glucose storage and insulin levels are the major factors with respect to Type 2 diabetes. Further, glucose storage is increased in response to insulin resistance, prompting hyperinsulinemia and hyperglycemia (Bhandari et al., 2013). PTP1B influences glucose homeostasis regulation and several physiological functions, where its expression has direct connection to hyperglycemia (Fernandez-Ruiz et al., 2014, Popov et al., 2009). PTP1B is the main insulin sensitivity regulator and it has been confirmed by whole-body knockout in mice. The target regulator PTP1B has used for the treatment of diabetes and obesity (Delibegovic et al., 2009). Dose dependency study showed that BF-M inhibited PTP1B effectively with IC50 value of 23.98 μg/mL; more over GC-MS analysis revealed the presence of n-hexadecanoic acid (25.14%), octadecanoic acid (2.15%) and tetradecanoic acid (1.05%) whose PTP1B inhibiting effects are well documented. This proves that BF-M is an effective PTP1B inhibitor (Huerxidan et al., 2012). A couple of studies have shown that the HFD fed rats developed insulin protection; meanwhile streptozotocin has been known to devastate the pancreatic β-cells specifically (Zheng et al., 2011). Hence, using low dosage of STZ with high-fat diet nearly mimics the diabetes metabolic qualities in rodents; this model was used to assess the impact of BF-M on diabetes.
HFD fed STZ induced diabetic rats showed increased levels of blood glucose, decreased levels of insulin and amplified expression of PTP1B. Insulin signaling is highly controlled by phosphorylation status of several components and pathways. The most important phosphatase is PTP1B which regulates the insulin signaling cascade and inhibits insulin receptors (IR and IRS 1) by direct phosphorylation affecting the insulin secretion which results in the increased glucose level during diabetic state (Xue et al., 2007). In the current study, BF-M showed a substantial rise in insulin level and significant reduction in blood glucose level. Furthermore, Western blot analysis of BF-M treated diabetic rats revealed the reduction of PTP1B expression significantly. From this result we speculated that decreased PTP1B expression by BF-M in pancreas could lead to enhanced beta cell mass which eventually enhanced glucose stimulated insulin release which brought down the blood glucose level significantly (Lu et al., 2012). It is well known that the extracellular signal-regulated protein kinase 1/2 (ERK1/2) is activated by PTP1B. In addition, AKT phosphorylation helps proapoptic protein degradation and regulates the β cell survival (Ogawara et al., 2002). Our histopathological analysis of pancreas strongly correlated with this concept, where BF-M treated rats’ pancreas exhibited increased β cell mass. Furthermore, the glycemic index of HFD fed STZ treated diabetic rats gradually came back to the normal range due to treatment with BF-M.
In our study, BF-M was administered orally to the diabetic rats and it significantly increased the glycogen level both in liver and muscle clearly showing that it can act through the modulation of PTP1B. Interestingly, a study has shown that diminished PTP1B expression enhanced the insulin stimulated glycogen synthesis in both muscle and tissue (Egawa et al., 2001).
Diabetes is linked with significant changes in the lipoprotein profile and plasma lipid. Increased levels of TC, TG and FFAs are seen in uncontrolled type 2 diabetes. TC, TG and FFAs contribute to coronary artery disease and assume a noteworthy role in the pathogenesis of insulin protection. Treatment with BF-M significantly decreased serum TG, FFAs and TC probably through inhibition of PTP1B expression; since PTP1B regulates lipogenesis and hypertriglyceridemia, its inhibition might reduce TG, FFAs and TC (Veerapur et al., 2010).
In conclusion, this study highlighted the ability of BF-M in successfully reducing the effect of diabetes in HFD fed STZ induced diabetic rats by inhibiting the PTP1B expression in vivo and in vitro. GC-MS analysis of BF-M revealed that several secondary metabolites were responsible for PTP1B inhibition. BF-M, a potent PTP1B inhibitor, enhanced the insulin secretion leading to noteworthy lessening in blood glucose level, through which BF-M normalized altered lipid and glucose metabolism. Furthermore improved glycemic control positively influenced food intake and body weight. Immunohistopathological studies also correlated with the findings. Hence, diabetes and its respective complications can be treated using BF-M. Further investigation is necessary to reveal the clear mechanism of action of BF-M.
Declaration of Competing Interest
The authors have nothing to disclose
Acknowledgement
The authors extend their sincere gratefulness to the Deanship of Scientific Research at King Saud University, Saudi Arabia for its funding of this research through the Research Group project No RGP-213. The authors pay their condolences to one of the author Dr. R. Balamurugan who passed away on June 17, 2018.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Dhabi N.A., Esmail G.A., Duraipandiyan V., Valan Arasu M., Salem-Bekhit M.M. Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp.Al-Dhabi-1 isolated from Tharbanhot spring, Saudi Arabia. Extremophiles. 2016;20:79–90. doi: 10.1007/s00792-015-0799-1. [DOI] [PubMed] [Google Scholar]
- Bayton R.P. A revision of Borassus L. (Arecaceae) Kew Bull. 2007;62:561–585. [Google Scholar]
- Bhandari U., Chaudhari H.S., Bisnoi A.N., Kumar V., Khanna G., Javed K. Anti-obesity effect of standardized ethanol extract of Embelia ribes in murine model of high fat diet-induced obesity. Pharm. Nut. 2013;1:50–57. [Google Scholar]
- Delibegovic M., Zimmer D., Kauffman C., Rak K., Hong E.G., Cho Y.R., Kim J.K., Kahn B.B., Neel B.G., Bence K.K. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes. 2009;58:590–599. doi: 10.2337/db08-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa K., Maegawa H., Shimizu S., Morino K., Nishio Y., Bryer-Ash M., Cheung A.T., Kolls J.K., Kikkawa R., Kashiwagi A. Protein Tyrosine Phosphatase-1B negatively regulates insulin signaling in L6 myocytes and Fao hepatoma cells. J. Biol. Chem. 2001;276:10207–10211. doi: 10.1074/jbc.M009489200. [DOI] [PubMed] [Google Scholar]
- Elchebly M., Payette P., Michaliszyn E., Cromlish W., Collins S., Loy A.L., Normandin D., Cheng A., Himms-Hagen J., Chan C.C., Ramachandran C. Increased insulin sensitivity and obesity resistance in mice lacking the Protein Tyrosine Phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz R., Vieira E., Garcia-Roves P.M., Gomis R. Protein Tyrosine Phosphatase-1B modulates pancreatic β-cell mass. PLoS ONE. 2014;9:90344. doi: 10.1371/journal.pone.0090344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.T., Frederickson D.S. Estimation of the concentration oflow-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Gancedo J.M., Gancedo C. Fructose-1, 6-diphosphatase, phosphofructokinase and glucose-6-phosphate dehydrogenase from fermenting and non-fermenting yeasts. Archiv. Für. Mikrobiologie. 1971;76:132–138. doi: 10.1007/BF00411787. [DOI] [PubMed] [Google Scholar]
- Gupta S., Sharma S.B., Bansal S.K., Prabhu K.M. Antihyperglycemic and hypolipidemic activity of aqueous extract of Cassia auriculata L. leaves in experimental diabetes. J. Ethnopharmacol. 2009;123:499–503. doi: 10.1016/j.jep.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Handa, S.S., Khanuja. S.P.S., Longo, G., 2008. Extraction technologies for medicinal and aromatic plants. International Centre for Science and High Technology. Trieste.
- Huerxidan Y., Qing-ling M.A., Abulimiti Y. Component and PTP1B inhibition of fatty acid from seeds of Ocimum basilicum L. Nat. Prod. Res. Dev. 2012;24 [Google Scholar]
- Jerry A. Comprehensive review on the medicinal properties of Borassus flabellifer. J. Acad. Ind. Res. (JAIR) 2018;7(7):93–97. [Google Scholar]
- Klaman L.D., Boss O., Peroni O.D., Kim J.K., Martino J.L., Zabolotny J.M., Moghal N., Lubkin M., Kim Y.B., Sharpe A.H., Stricker-Krongrad A. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol. Cell. Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide H. Pathological occurrence of glucose-6-phosphatase in serum in liver disease. Clin. Chim. Acta. 1959;4:554–561. doi: 10.1016/0009-8981(59)90165-2. [DOI] [PubMed] [Google Scholar]
- Lu B., Wu H., Gu P., Du H., Shao J., Wang J., Zou D. Improved glucose-stimulated insulin secretion by intra-islet inhibition of protein-tyrosine phosphatase 1B expression in rats fed a high-fat diet. J. Endocrinol. Invest. 2012;35:63–70. doi: 10.3275/7766. [DOI] [PubMed] [Google Scholar]
- Lund I.K., Andersen H.S., Iversen L.F., Olsen O.H., Møller K.B., Pedersen A.K., Ge Y., Holsworth D.D., Newman M.J., Axe F.U., Møller N.P.H. Structure-based design of selective and potent inhibitors of protein-tyrosine phosphatase β. J. Biol. Chem. 2004;279:24226–24235. doi: 10.1074/jbc.M313027200. [DOI] [PubMed] [Google Scholar]
- Masayuki Y. Medicinal flowers. XII (1)) new spirostane-type steroid saponins with antidiabetogenic activity from Borassus flabellifer. Chem Pharm Bull. 2007;55(2):308–316. doi: 10.1248/cpb.55.308. [DOI] [PubMed] [Google Scholar]
- Mohite M., Pramod H.J., Yadav A.V., Raje V.N., Wadkar G.H. Evaluation of antiulcer activity of aqueous extract of Borassus flabellifer (Linn.) fruits. J. Pharm. Res. 2012;5:3782–3786. [Google Scholar]
- Ogawara Y., Kishishita S., Obata T., Isazawa Y., Suzuki T., Tanaka K., Masuyama N., Gotoh Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J. Biol. Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- Olokoba A.B., Obateru O.A., Olokoba L.B. Type 2 diabetes mellitus: a review of current trends. Oman Med. J. 2012;27:269. doi: 10.5001/omj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschapur M.S., Patil M.B., Kumar R., Patil S.R. Evaluation of anti-inflammatory activity of ethanolic extract of Borassus flabellifer L. male flowers (inflorescences) in experimental animals. J. Med. Plants Res. 2009;3:49–54. [Google Scholar]
- Popov D. Novel Protein Tyrosine Phosphatase 1B inhibitors: interaction requirements for improved intracellular efficacy in type 2 diabetes mellitus and obesity control. Biochem. Biophys. Res. Commun. 2011;410:377–381. doi: 10.1016/j.bbrc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Popov D., Nemecz M., Dumitrescu M., Georgescu A., Böhmer F.D. Long-term high glucose concentration influences Akt, ERK1/2, and PTP1B protein expression in human aortic smooth muscle cells. Biochem. Biophy. Res. Comm. 2009;388:51–55. doi: 10.1016/j.bbrc.2009.07.141. [DOI] [PubMed] [Google Scholar]
- Pramod H.J., Yadav A.V., Raje V.N., Mohite M., Wadkar G. Antioxidant activity of Borassus flabellifer (linn.) Fruits. Asian J. Pharm. Tech. 2013;3(1):16–19. [Google Scholar]
- Pramod H.J., Yadav A.V., Raje V.N., Mohite M., Wadker G. Antioxidant activity of Borassus flabellifer (Linn.) fruits. Asian J. Pharm. Tech. 2013;3:16–19. [Google Scholar]
- Sahni C., Najam A.S., Vidyanath J., Rajinder K.G. Screening of nutritional, phytochemical, antioxidant and antibacterial activity of the roots of Borassus flabellifer (Asian Palmyra Palm) J. Pharmacog. A Phytochem. 2014;3(4):58–68. [Google Scholar]
- Saravanan C., Priya B., Asir B.S., Uma S. Preliminary phytochemical screening of antibacterial activity of palmyra palm (Borassus Flabellifer) root extract. Int. J. Pharm. Sci. Res. 2012;3(11):4489–4491. [Google Scholar]
- Sendrayaperumal V., Pillai S.I., Subramanian S. Design, synthesis and characterization of zinc–morin, a metal flavonol complex and evaluation of its antidiabetic potential in HFD–STZ induced type 2 diabetes in rats. Chem. Biol. Interact. 2014;219:9–17. doi: 10.1016/j.cbi.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Shamala D., Arseculeratne S.N., Pathmanathan R., McKenzie I.F.C., Pang Suppression of cell-mediated immunity following oral feeding of mice with palmyrah (Borassus flabellifer L) flour. Aust. J. Exp. Biol. Med. Sci. 1985;63:371–379. doi: 10.1038/icb.1985.43. [DOI] [PubMed] [Google Scholar]
- Sirasanagandla S., Kasetti R.B., Shaik A.N., Natava R., Surtineni V.P., Cirradur S.R., Chippada A. Antihyperglycemic and antihyperlipidemic activities of 2-(4-[(2-hydroxybenzyl) amino]-phenyl amino-methyl)-phenol in STZ induced diabetic rats. Eur. J. Med. Chem. 2013;66:400–406. doi: 10.1016/j.ejmech.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Triender P. Determination of glucose using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969;6:24–27. [Google Scholar]
- Veerapur V.P., Prabhakar K.R., Thippeswamy B.S., Bansal P., Srinivasan K.K., Unnikrishnan M.K. Antidiabetic effect of Dodonaea viscosa (L). Lacq. aerial parts in high fructose-fed insulin resistant rats: a mechanism based study. Indian J. Exp. Biol. 2010;8:800–810. [PubMed] [Google Scholar]
- Xue B., Kim Y.B., Lee A., Toschi E., Bonner-Weir S., Kahn C.R., Neel B.G., Kahn B.B. Protein-tyrosine phosphatase 1B deficiency reduces insulin resistance and the diabetic phenotype in mice with polygenic insulin resistance. J. Biol. Chem. 2007;282:23829–23840. doi: 10.1074/jbc.M609680200. [DOI] [PubMed] [Google Scholar]
- Yin D., Tao J., Lee D.D., Shen J., Hara M., Lopez J., Kuznetsov A., Philipson L.H., Chong A.S. Recovery of islet β-cell function in streptozotocin-induced diabetic mice: an indirect role for the spleen. Diabetes. 2006;55:3256–3263. doi: 10.2337/db05-1275. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Xu F., Morikawa T., Pongpiriyadacha Y., Nakamura S., Asao Y., Kumahara A., Matsuda H. Medicinal flowers. XII. New spirostane-type steroid saponins with antidiabetogenic activity from Borassus flabellifer. Chem. Pharm. Bull. 2007;55:308–316. doi: 10.1248/cpb.55.308. [DOI] [PubMed] [Google Scholar]
- Zheng X.K., Zhang L., Wang W.W., Wu Y.Y., Zhang Q.B., Feng W.S. Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) Spring in rats induced by high fat diet and low dose STZ. J. Ethnopharmacol. 2011;137:662–668. doi: 10.1016/j.jep.2011.06.018. [DOI] [PubMed] [Google Scholar]