Abstract
Background
Integrase inhibitors (INI) induce a rapid decline of HIV-RNA in plasma and CD4+ T-cell recovery in blood. Both characteristics are also associated with immune reconstitution inflammatory syndrome (IRIS). Whether the use of INI-containing combination antiretroviral therapy (cART) increases the risk of IRIS is being questioned.
Methods
Study within the Dutch ATHENA HIV observational cohort. HIV-1 infected late presenters initiating cART after March 2009 were included if they had <200 CD4+ T-cells per μL and were diagnosed with an opportunistic infection. IRIS was defined either according to the criteria by French et al. (IRISFRENCH) or by a clinical IRIS diagnosis of the physician (IRISCLINICAL). The primary outcomes were the association between INI and the occurrence of IRISFRENCH and IRISFRENCH+CLINICAL in multivariable logistic regression.
Findings
672 patients with a median CD4+ T-cell count of 35 cells per μL were included. Treatment with INI was independently associated with IRISFRENCH as well as IRISFRENCH+CLINICAL (OR 2·43, 95%CI:1·45–4·07, and OR 2·17, 95%CI:1·45–3·25). When investigating INI separately, raltegravir (RAL) remained significantly associated with IRISFRENCH (OR 4·04 (95%CI:1·99-8·19) as well as IRISFRENCH+CLINICAL (OR 3·07, 95%CI:1·66-5·69), while dolutegravir (DTG) became associated with IRISFRENCH+CLINICAL after it replaced RAL as preferred INI in the cohort after 2015 (OR 4·08, 95%CI:0·99-16·82, p=0·052). Too few patients used elvitegravir to draw meaningful conclusions. Steroid initiation for IRIS was more likely in those who initiated INI versus in those who did not, but no increased hospital (re)admission or mortality rates were observed.
Interpretation
In HIV late presenters from a resource rich setting, INI based treatment initiation increased the risk of IRIS. This was observed for RAL and DTG when being initiated as preferential INI in the presence of specific AIDS-conditions, indicative of channeling bias. Although we controlled for all relevant measured confounders, we cannot exclude that the observed association is partially explained by residual confounding. INI use was not associated with mortality nor hospitalization. Therefore, our observation is no reason to avoid INI in late presenters.
Funding
The ATHENA database is maintained by Stichting HIV Monitoring and supported by a grant from the Dutch Ministry of Health, Welfare and Sport through the Centre for Infectious Disease Control of the National Institute for Public Health and the Environment.
Keywords: HIV, cART, Opportunistic infection, Integrase strand transfer inhibitor, Immune reconstitution inflammatory syndrome
Research in context.
Evidence before this study
HIV-1 infected patients initiating cART which includes an integrase inhibitor (INI) experience a faster plasma HIV-RNA decay and in most randomized studies also a faster CD4 T-cell recovery compared to protease inhibitor or non-nucleoside reverse transcriptase inhibitor containing cART. These factors have previously been associated with the development of immune reconstitution inflammatory syndrome (IRIS). Thus, the use of INI may theoretically increase the incidence of IRIS. We performed a search in PubMed with the keywords ‘integrase inhibitor’ AND ‘immune reconstitution.' We found 18 papers, including one randomized clinical trial and two observational studies. The randomized REALITY trial evaluated an efavirenz based cART regimen with the same regimen to which raltegravir was added in N =1805 HIV infected late presenters in Kenia, Zimbabwe, Uganda and Malawi. Adding raltegravir to the cART regimen did not increase the incidence of IRIS. In a French observational study a total of N =2287 HIV-infected patients were included of which N =274 started an INI based cART regimen. In this study an assocation between INI use and a higher risk of IRIS was observed. However, the incidence of IRIS in this study was much lower than previously described.
Added value of this study
In our study, we hypothesized that the initiation of INI-containing cART in HIV-1 infected late presenters is an independent risk factor for IRIS. Our study confirmed the hypothesis, but only for the integrase inhibitor raltegravir but not for dolutegravir or elvitegravir. Corticosteroid therapy for IRIS was administered more often to INI users. However, the increased incidence of IRIS did not result in more days in the hospital nor in an increase in mortality. Although we controlled for all relevant measured confounders, we cannot exclude that the observed association is partially explained by residual confounding.
Implications of the available evidence
INI based cART has become the preferred first line cART in resource rich settings and more recently has been postulated as the preferred regimen in resource poorer settings as well. This will increase the number of HIV-1 late presenters exposed to INI based cART. Our observation warrants additional studies and most importantly randomized studies in HIV-1 late presenters to investigate whether the observated association is real. The observation is no reason to withold INI based cART in HIV-1 late presenters.
Alt-text: Unlabelled box
1. Introduction
Treatment with an integrase inhibitor (INI)-containing combination antiretroviral therapy (cART) regimen is recommended as the preferred first-line cART in current treatment guidelines for HIV-1 infected patients [1,2]. The initiation of INI-containing cART in treatment-naive patients is associated with a faster decline of plasma HIV-RNA than when a protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI)-containing cART regimen is initiated. In some studies, INI are also associated with a faster recovery of CD4+ T-cells (CD4 count) [3], [4], [5]. However, a steep decline of HIV-RNA and a fast immune recovery are also risk factors for the immune reconstitution inflammatory syndrome (IRIS) [6], [7], [8]. Therefore, HIV-1 infected late presenters are at a particularly high risk for IRIS [9], [10], [11].
IRIS is an excessive, pathological inflammatory response against antigens of opportunistic infections (OI) [12,13]. In view of the abovementioned arguments, the incidence of IRIS might be higher in patients initiating INI-containing cART than in patients starting a non-INI-containing cART regimen. However, most of the randomized phase III trials, comparing INI-containing cART regimens with non-INI containing cART, were not suited to answer this question. Indeed, by excluding patients with an active OI at the start of cART, the number of patients at risk for IRIS in these studies was very limited. In fact, patients with a suspected OI were often explicitly excluded from being enrolled in phase III trials [4,5]. In contrast, large prospective observational HIV cohort studies typically include a significant number of HIV-1 infected late presenters and can therefore be used to determine the IRIS risk in these patients [14]. IRIS can be difficult to diagnose and is associated with significant morbidity and (re)hospitalization. Occasionally IRIS can be lethal, in particular in patients with intracranial infections. We hypothesize that the initiation of INI-containing cART is an independent risk factor for development of IRIS in HIV-1 infected late presenters.
2. Methods
2.1. Study design and participants
This was a retrospective analysis using data from the prospective Dutch nationwide observational HIV cohort maintained by the HIV Monitoring Foundation (Stichting HIV Monitoring, SHM), also known as the “AIDS Therapy Evaluation in the Netherlands” (ATHENA) cohort [15]. The ATHENA cohort comprises all patients in care for HIV in one of the 25 Dutch HIV treatment centers, who consented to have their data collected in ATHENA. IRIS has not been systematically collected in ATHENA. Therefore, we performed chart reviews in 22 of the 25 centers, comprising 90% of all people in care for HIV in the Netherlands, to retrospectively diagnose and collect information on HIV late presenters and IRIS. The chart reviews were limited to patients initiating first-line cART in the INI era who were considered at increased risk for the development of IRIS. We therefore included antiretroviral treatment naive HIV positive adults initiating cART included in the ATHENA cohort as of March 2009 (the date that raltegravir (RAL) became available in the Netherlands) until December 2016. Furthermore, the included patients had to have (1) a CD4 count below 200 cells per μL and (2) a diagnosis of an OI prior to or within 12 months after initiation of cART. To improve case finding of unmasking IRIS we also included all patients for full chart review when they fulfilled criterion 1 and had received corticosteroids within 12 months after cART initiation (as a proxy for severe unmasking IRIS). Finally, we included all patients who had died within 12 months after initiation of cART to ensure reviewing all patients where IRIS might have contributed to their death. Patients without clinical data available after the start of cART were excluded. The patient files of all patients who were identified with this strategy were reviewed on site by one of the investigators as described below.
2.2. Study procedures
All relevant data available in the ATHENA database (e.g. demographics, use of cART, CD4 counts, plasma HIV viral loads, diagnosis and treatment of OI, concomitant medication, hospital admissions, mortality) were retrieved. All clinical data required to verify whether a patient fulfilled the predefined definitions of IRIS (see below) were collected or verified on site from the individual patient files by IEAW, AMP, VCMB and GB using a standardized case report form. If based on the predefined IRIS definitions the suspicion of a potential case of IRIS arose, the case was discussed with IEAW and BJAR or CR until a unanimous decision on the presence of IRIS was made. By design, blinding the investigators for the cART regimen was not always possible.
2.3. Definitions of IRIS
Two definitions of IRIS were used: IRIS according to the criteria described by French et al. [16] (IRISFRENCH) and a broader clinical definition (IRISCLINICAL). IRISCLINICAL included all patients with IRIS documented as the most likely diagnosis in the patient file by the treating physician or if IRIS was mentioned in the differential diagnosis and immunosuppressive therapy for IRIS was initiated. For a more detailed description of the IRIS definitions see supplementary appendix, page 4 and table S1. Data on all OIs which were diagnosed before or after the start of cART were collected. Detailed information on OI and what was considered appropriate therapy in relation to the diagnosis of IRIS is described in the supplementary appendix, page 4.
2.4. Objectives
The primary objective of this study was to evaluate whether the use of INI-containing cART is an independent risk factor for a combined endpoint of both types of IRIS (IRISFRENCH+CLINICAL) as well as for IRISFRENCH. Secondary objectives were to evaluate whether the use of INI-containing cART is associated with an increased risk of the use of corticosteroids for IRIS, hospital (re)admission after initiation of cART and death. Endpoints were assessed within 12 months of cART initiation. The occurrence of all endpoints together up to 12 months after cART initiation was evaluated as composite endpoint.
By initial study design, we had planned to also investigate whether time from initiation of cART to reach a plasma viral load below 1000 and 50 copies per mL, and time from initiation of cART to reach a CD4 count above 100 and 200 cells per μL were independent risk factors for IRIS, but as CD4 count and especially plasma viral loads were not systematically measured at uniform time-points by clinicians at the time IRIS was diagnosed, we were unable to look into these endpoints.
2.5. Statistical analyses
The risk of IRIS was compared between the patients using INI containing cART and non-INI containing cART by Kaplan Meier analysis and by calculating odds ratios (OR) with 95% confidence intervals (CI) by univariable logistic regression analysis. We performed multivariable logistic regression to identify independent risk factors for IRIS. We tested for interactions of INI-use with risk factors that may also be associated with IRIS. Patients were censored when any of the following occurred: a switch from an INI to a non-INI-containing cART regimen or vice versa, death, or loss to follow up of the patient. Potential risk factors for IRIS that could confound the association between the use of INI and risk of IRIS were investigated in the multivariable models. These factors were demographical, immunological and virological parameters, cART, and OI-characteristics including the use of corticosteroids as part of OI-treatment. A full list of these variables can be found in the supplementary appendix, table S2. In an attempt to adjust the analysis for confounding by indication, the final multivariable regression models were repeated using inverse probability of treatment weighting. The analyses were done using SAS statistical software version 9.4 (SAS Institute, Cary, NC, USA).
2.6. Ethical considerations
All patients were enrolled in the ATHENA cohort and had consented to have their data used by the SHM. The study protocol was approved by the scientific review board of the SHM.
2.7. Role of the funding source
The ATHENA cohort is maintained by SHM and supported by a grant from the Ministry of Health, Welfare, and Sport through the Center for Infectious Disease Control of the National Institute for Public Health and the Environment. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
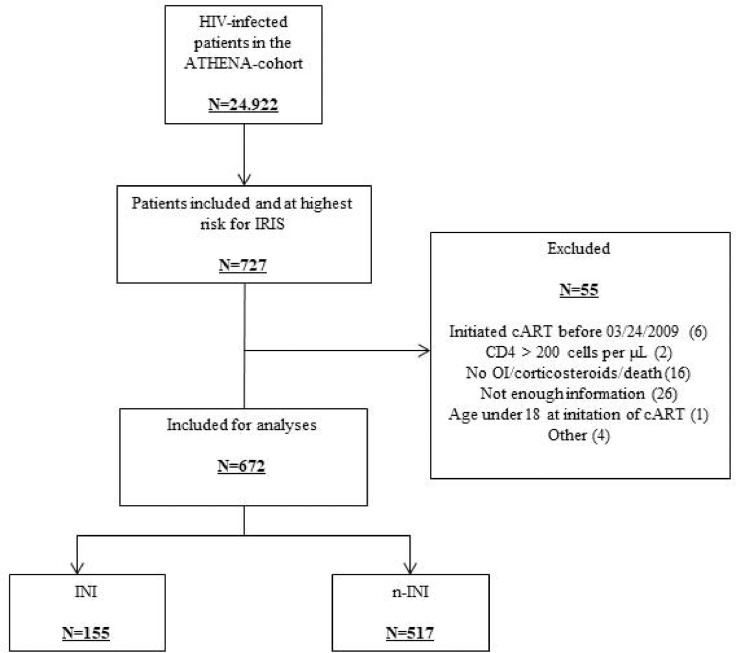
Of 24,922 patients registered in the ATHENA-cohort by December 2016, 24,684 were adults and consented to data collection [15]. Of them, 727 were included in the study based on the selection criteria. A total of 55 patients was subsequently excluded for various reasons identified during the chart review; therefore, 672 patients were included in the analyses (Fig. 1). Of those, 155 initiated an INI-containing cART-regimen and 517 initiated a cART-regimen containing a NNRTI and/or a PI. Baseline characteristics of patients who initiated an INI-containing (‘INI’) and a non-INI-containing (‘non-INI’) first-line cART-regimen are listed in Table 1. The two groups were well balanced for most of the baseline characteristics. For obvious reasons, patients starting an INI-containing cART entered HIV-care in later years than patients from the non-INI group (2014 versus 2011, p < 0·001). In the INI-group, 60 (38·7%), 21 (13·6%), and 74 (47·7%) initiated a RAL, elvitegravir-(EVG), and dolutegravir-(DTG) containing cART-regimen, respectively, whereas in the non-INI group a comparable number of patients initiated a PI-(276/517, 53·4%) or an NNRTI-containing (250/517, 48·4%) regimen (nine patients used both a PI and a NNRTI). The baseline characteristics of the patients starting RAL, EVG, or DTG are listed in Table 2. Differences were observed with more female patients starting RAL than DTG, (p < 0·001) and patients on RAL starting cART in earlier calendar years than patients on EVG or DTG (available in the Netherlands since 2013 and 2014 respectively, p < 0·001).
Fig. 1.
Patient disposition in the study. IRIS = immune reconstitution inflammatory syndrome, cART = combination antiretroviral therapy, OI = opportunistic infection, INI = integrase strand transfer inhibitor.
Table 1.
Baseline characteristics of patients who initiated INI-containing cART versus patients who initiated non-INI-containing cART. INI = INtegrase Inhibitor (containing cART), non-INI = non-INtegrase Inhibitor (containing cART), HSX = HeteroSeXual, MSM = Men having Sex with Men, NL = the Netherlands, RAL = Raltegravir, EVG = Elvitegravir, DTG = Dolutegravir, CS = Chi square test, UT = Unpaired T-test, WRS = Wilcoxon Rank Sum test, * = not applicable.
| INI (N = 155) | Non-INI (N = 517) | p-value (test) | |
|---|---|---|---|
| Male sex, N (%) | 123 (79·4) | 433 (83·8) | 0·41 (CS) |
| Age, mean (SD) | 44 (11) | 44 (11) | 0·60 (UT) |
| Year of HIV-diagnosis, median (Q1,Q3) | 2014 (2011,2015) | 2011 (2010,2013) | <0·0001 (WRS) |
| HIV-RNA at HIV-diagnosis, log10 copies per mL, median (Q1,Q3) | 5·5 (5·1,6·0) | 5·5 (5·1,5·8) | 0·38 (WRS) |
| CD4+ T-lymphocytes at HIV-diagnosis, cells per μL, median (Q1,Q3) | 39 (13,100) | 33 (18,80) | 0·33 (WRS) |
| Mode of transmission, N (%) | 0·25 (CS) | ||
| HSX | 58 (37·4) | 197 (38·1) | |
| MSM | 54 (34·8) | 205 (39·7) | |
| Unknown | 19 (12·3) | 56 (10·8) | |
| Other | 24 (15·5) | 59 (11·4) | |
| Region of origin, N (%) | 0·76 (CS) | ||
| NL | 84 (54·2) | 297 (57·5) | |
| Europe | 10 (6·5) | 40 (7·7) | |
| Africa | 22 (14·2) | 75 (14·5) | |
| South America and Caribbean | 16 (10·3) | 57 (11·0) | |
| Other | 23 (14·8) | 48 (9·3) | |
| Type of INI, N (%) | |||
| RAL | 60 (38·7) | * | |
| EVG | 21 (13·6) | * | |
| DTG | 74 (47·7) | * | |
| Type of cART initiated, N (%) | |||
| INI + 2 NRTI | 136 (87·7) | * | |
| INI + PI + 2 NRTI | 13 (8·4) | * | |
| INI + NNRTI + 2 NRTI | 6 (3·9) | * | |
| NNRTI + 2 NRTI | * | 241 (46·6) | |
| PI + 2 NRTI | * | 267 (51·6) | |
| NNRTI + PI + 2 NRTI | * | 9 (1·8) |
Table 2.
Baseline characteristics of users of different types of INI. HSX = HeteroSeXual, MSM = Men having Sex with Men, NL = the Netherlands, RAL = Raltegravir, EVG = Elvitegravir, DTG = Dolutegravir, CS = Chi square test, OWA = One way ANOVA, KW = Kruskal Wallis test.
| RAL (N = 60) | EVG (N = 21) | DTG (N = 74) | p-value | |
|---|---|---|---|---|
| Male sex, N (%) | 41 (68·3) | 17 (81·0) | 65 (87·8) | 0·006 (CS) |
| Age, mean (SD) | 42 (11) | 46 (10) | 46 (12) | 0·73 (OWA) |
| Year of HIV-diagnosis, median (Q1,Q3) | 2011 (2009,2013) | 2014 (2014,2015) | 2015 (2015,2016) | <0·0001 (KW) |
| HIV-RNA at HIV-diagnosis, log10 copies per mL, median (Q1,Q3) | 5·6 (5·0,6·1) | 5·6 (5·0,5·9) | 5·4 (5·1,5·8) | 0·55 (KW) |
| CD4+ T-lymphocytes at HIV-diagnosis, cells per μL, median (Q1,Q3) | 30 (10,79) | 50 (20,115) | 40 (12,105) | 0·34 (KW) |
| Mode of transmission, N (%) | 0·36 (CS) | |||
| HSX | 32 (53·3) | 5 (23·8) | 21 (28·4) | |
| MSM | 15 (25·0) | 10 (47·6) | 29 (39·2) | |
| Unknown | 6 (10·0) | 4 (19·0) | 9 (12·2) | |
| Other | 7 (11·7) | 2 (9·5) | 15 (20·3) | |
| Region of origin, N (%) | 0·80 (CS) | |||
| NL | 28 (46·7) | 11 (52·4) | 45 (60·8) | |
| Europe | 6 (10·0) | 0 (0·0) | 4 (5·4) | |
| Africa | 13 (21·7) | 3 (14·3) | 6 (8·1) | |
| South America and Caribbean | 6 (10·0) | 3 (14·3) | 7 (9·5) | |
| Other | 7 (11·7) | 4 (19·0) | 12 (16·2) |
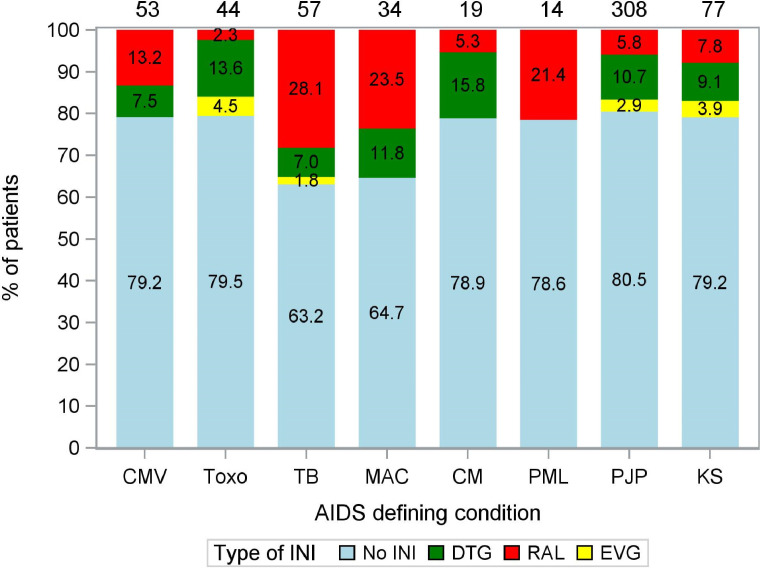
A total of 231 OIs were diagnosed in the 155 INI users, whereas 780 OIs were diagnosed in the 517 patients in the non-INI group (1·49 versus 1·51 OI per patient, respectively). The most frequently diagnosed OIs were Pneumocystis jirovecii pneumonia (PJP), mycobacterial infections and Kaposi's sarcoma (KS). For an overview of the distribution of the use of INI and non-INI-based cART in patients diagnosed with various OIs commonly associated with IRIS, see Fig. 2. Patients diagnosed with mycobacterial infections were most likely to be treated with INI-based cART.
Fig. 2.
Use of INI- and non-INI-based cART regimens in patients diagnosed with various OI. The numbers in the stacked bars represent the percentage of patients diagnosed with a particular OI using non-INI, DTG, RAL or EVG-based cART. The numbers on top of the stacked bars represent the total number of patients diagnosed with a particular OI. Note that some patients were diagnosed with multiple OI. CMV = cytomegalovirus end organ disease, Toxo = cerebral toxoplasmosis, TB = (extra)pulmonary tuberculosis, MAC = non-tuberculous mycobacterial infections, CM = cryptococcal meningitis, PML = progressive multifocal leukoencephalopathy, PJP = pneumocystis jirovecii pneumonia, KS = Kaposi's sarcoma, INI = integrase inhibitor, DTG = dolutegravir, RAL = raltegravir, EVG = elvitegravir.
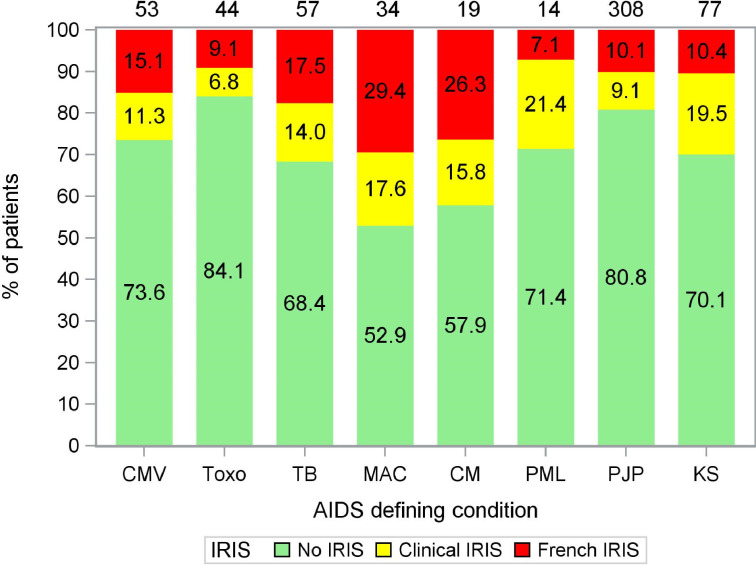
Eight IRIS events were excluded from the analyses because they occurred after patients switched from INI to non-INI cART (N = 1) or vice versa (N = 7). Data from these patients were censored at the time of switch. During the 52 weeks of follow-up IRISFRENCH was diagnosed in 18·1% (28/155) of patients in the INI group, and in 8·3% (43/517) of patients in the non-INI group (OR 2·43, 95%CI 1·45–4·07, p = 0·0010). The majority (84·5%) of the IRISFRENCH cases were classified as “confirmed”, 15.5% were classified as “probable”, with no significant differences between the various INI- and non-INI based cART regimen (Table 3). The overall incidence of IRIS (IRISFRENCH+CLINICAL) was 32·3% (50/155) and 18·0% (93/517) respectively (OR 2·17, 95%CI 1·45–3·25, p = 0·0003). 63·6% of all IRISFRENCH+CLINICAL cases were paradoxical IRIS, 25·2% were unmasking, and for the remaining 11·2% this information was unknown due to limited available data on IRIS-events (Table 3). Patients diagnosed with nontuberculous mycobacterial infections, cryptococcal meningitis, tuberculosis, and KS were most likely to develop IRIS. Fig. 3 shows the distribution of IRISFRENCH and IRISCLINICAL for the various OI. The proportion of patients initiating cART and developing IRISFRENCH or IRISCLINICAL remained stable over the entire observation period, and fluctuated between 19·9% and 24·4% per calendar year (Supplementary Figure S1). The multivariable logistic regression analyses showed that the use of an INI-containing cART, and a diagnosis of Mycobacterium avium complex before cART initiation were independent risk factors for both IRISFRENCH and IRISFRENCH+CLINICAL, while no significant associations were found for baseline plasma HIV-1 RNA, use of corticosteroids as part of the treatment for the OI, and the time between the start of the OI treatment and the start of cART (Table 4). The latter was evaluated by dichotomizing the number of days between cART initiation and OI therapy initiation as >14 days or not. As a sensitivity analysis, a cut-off of 28 and 42 days was evaluated as well but this did not result in a significant association either. In the model for the combined endpoint of IRISFRENCH+CLINICAL, also a pre-cART diagnosis of cryptococcal meningitis, tuberculosis, and KS were significantly associated with an increased risk for IRIS. The pre-cART CD4 count showed a clinically plausible borderline statistically significant association with lower IRIS risk at higher CD4 counts. Apart from INI, none of the other drug classes or individual antiretroviral agents were significantly associated with IRIS although a trend was observed for efavirenz exposure and a lower risk of IRISFRENCH+CLINICAL. No significant interactions were found between the use of INI and any of the other risk factors tested in the models.
Table 3.
Characteristics of IRIS and clinical conditions underlying the IRIS diagnosis.
| No INI, N (%) | EVG, N (%) | DTG, N (%) | RAL, N (%) | |
|---|---|---|---|---|
| Patients developing IRIS | ||||
| - IRISFRENCH | 43 (8·3) | 1 (4·8) | 10 (13·5) | 17 (28·3) |
| - IRISCLINICAL | 50 (9·7) | 3 (14·3) | 8 (10·8) | 11 (18·3) |
| - No IRIS | 424 (82·0) | 17 (81·0) | 56 (75·7) | 32 (53·3) |
| Diagnostic criteria IRISFRENCH | ||||
| - Confirmed | 36 (83·8) | 4 (100) | 9 (90·0) | 14 (82·4) |
| - Probable | 7 (16·2) | 0 (0) | 1 (10·0) | 3 (17·6) |
| Timing of IRIS | ||||
| - Paradoxical IRIS | 60 (64·5) | 3 (75·0) | 9 (50·0) | 19 (67·9) |
| - Unmasking IRIS | 23 (24·7) | 1 (25·0) | 8 (44·4) | 4 (14·3) |
| - Unknown | 10 (10·8) | – | 1 (5·6) | 5 (17·9) |
| Conditions underlying the IRIS event | ||||
| - Pneumocystis jirovecii pneumonia | 24 (25·8) | 1 (25·0) | 1 (5·6) | 3 (10·7) |
| - Kaposi's sarcoma | 13 (14·0) | 1 (25·0) | 3 (16·7) | 3 (10·7) |
| - Mycobacterial infections, other than TB | 11 (11·8) | – | 6 (33·3) | 6 (21·4) |
| - Tuberculosis | 7 (7·5) | – | 1 (5·6) | 5 (17·9) |
| - Cerebral toxoplasmosis | 5 (5·4) | – | 1 (5·6) | – |
| - Cryptococcal meningitis | 5 (5·4) | – | – | 1 (3·6%) |
| - Progressive multifocal leukoencephalopathy | 4 (4·3) | – | – | 1 (3·6) |
| - Cytomegalovirus end organ disease | 3 (3·2) | – | 2 (11·1) | 2 (7·1) |
| - Herpes zoster | 2 (2·2) | – | – | – |
| - Non-Hodgkin lymphoma | – | – | 1 (5·6) | – |
| - Histoplasmosis | 1 (1·1) | – | – | – |
| - Fever of unknown origin, inflammation | 12 (12·9) | 1 (25·0) | 1 (5·6) | 4 (14·3) |
| - Lymphadenopathy, not previously existing | 1 (1·1) | – | – | 1 (3·6) |
| - Skin conditions: dermatitis, eczema | 5 (5·4) | 1 (25·0) | 2 (11·1) | 2 (7·1) |
Fig. 3.
Occurrence of IRIS in patients diagnosed with various OI. The numbers in the stacked bars represent the percentage of patients diagnosed with a particular OI being diagnosed with IRIS. The numbers on top of the stacked bars represent the total number of patients diagnosed with a particular OI. Note that some patients were diagnosed with multiple OI. CMV = cytomegalovirus end organ disease, Toxo = cerebral toxoplasmosis, TB = (extra)pulmonary tuberculosis, MAC = non-tuberculous mycobacterial infections, CM = cryptococcal meningitis, PML = progressive multifocal leukoencephalopathy, PJP = pneumocystis jirovecii pneumonia, KS = Kaposi's sarcoma.
Table 4.
multivariable logistic regression analysis of possible risk factors for occurrence of IRISFRENCH and IRISFRENCH+CLINICAL. Univ. = univariate analysis, Multiv. = multivariable analysis, OR = Odds Ratio, INI = INtegrase Inhibitor, TB = tuberculosis, MAC = non-tuberculous mycobacterial infections, KS = Kaposi's sarcoma, OI = opportunistic infection.
| IRISFRENCHOR (95% CI), p-value | IRISFRENCH+CLINICALOR (95% CI), p-value | |
|---|---|---|
| Use of INI | 2·46 (1·45–4·18), 0·0009 | 1·83 (1·16–2·87), 0·009 |
| Diagnosed with Cryptococcal meningitis | 2·83 (1·05–7·66), 0·040 | |
| Diagnosed with TB | 2·43 (1·26–4·69), 0·008 | |
| Diagnosed with MAC | 2·96 (1·29–6·80), 0·011 | 2·89 (1·37–6·09), 0·005 |
| Diagnosed with KS | 2·05 (1·15–3·67), 0·016 | |
| CD4+ T-cell count prior to start cART (per 10 cells/mm3 increment) | 0·96 (0·91–1·02), 0·17 | 0·96 (0·93–1·00), 0·062 |
| Plasma HIV RNA prior to start cART (/log10 c/mL) | 0·97 (0·68–1·38), 0·85 | 1·10 (0·83–1·46), 0·51 |
| Use of corticosteroids prior to IRIS diagnosis | 0·67 (0·40–1·14), 0·14 | 1·02 (0·67–1·54), 0·94 |
| No OI treatment/no AIDS prior to start cART | Ref | Ref |
| >2 weeks between start OI treatment and start cART | 1·80 (0·88–3·66), 0·11 | 1·11 (0·66–1·87), 0·71 |
| ≤2 weeks between start OI treatment and start cART | 1·91 (0·99–3·67), 0·053 | 0·87 (0·52–1·45), 0·60 |
| Use of efavirenz | 0·62 (0·37–1·01), 0·057 |
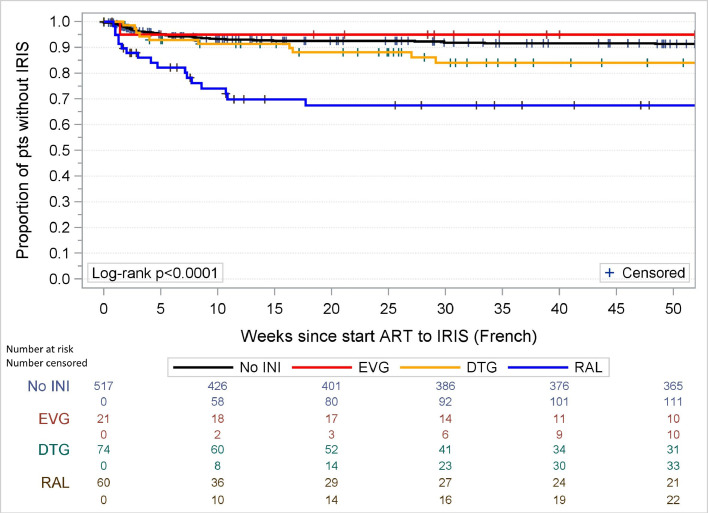
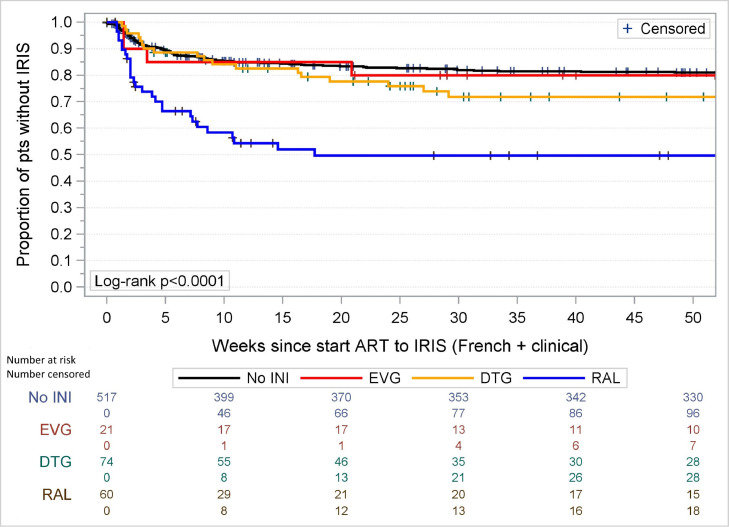
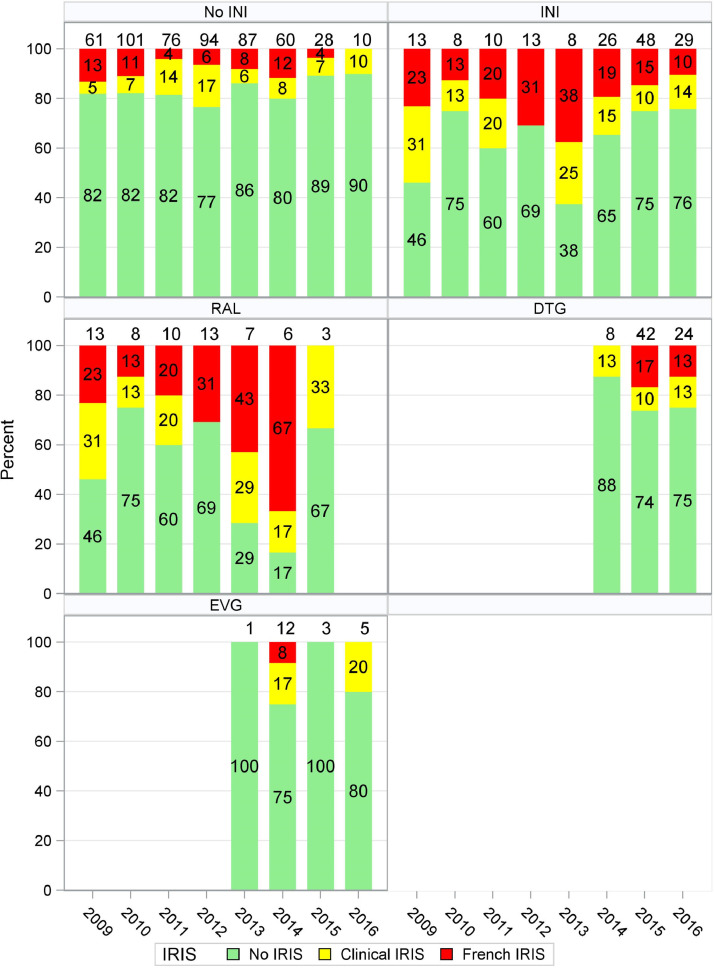
When we investigated the three different INI separately, only RAL remained significantly associated with IRISFRENCH (OR 4·04 (95%CI 1·99–8·19) as well as IRISFRENCH+CLINICAL (OR 3·07, 95%CI 1·66–5·69) while no significant associations were found between the use of DTG and IRISFRENCH or IRISFRENCH+CLINICAL (OR 1·78 (95%CI 0·84–3·79) and 1·33 (95%CI 0·72–2·45)). The number of patients starting EVG was too small (N = 21) to draw meaningful conclusions in multivariable analyses. As there were no differences in risk of developing IRIS in patients using PI or NNRTI, we did not further subdivide the non-INI group in the analyses. As the observed increased incidence of IRIS with RAL but not DTG was unexpected we repeated the final logistic regression models for the IRISFRENCH and IRISFRENCH+CLINICAL endpoints using inverse probability of treatment weighting and obtained similar results, in which the association of RAL with IRIS was not attenuated (data not shown). Kaplan–Meier analysis showed that the timing of the IRISFRENCH and IRISFRENCH+CLINICAL events was comparable for all groups (see Fig. 4, Fig. 5) with the majority of events occurring within 25 weeks after initiating cART. The increased risk of IRIS in patients using RAL was seen with both IRISFRENCH and IRISCLINICAL, but was most pronounced with IRISFRENCH (see Table 3). Table 3 also shows the distribution of specific OI and clinical conditions underlying the IRIS diagnoses (IRISFRENCH and IRISCLINICAL combined) in patients using INI- and non-INI-based cART. The increased risk of IRIS in patients using RAL compared to those using non-INI-based cART, was seen in all OI commonly associated with IRIS which were diagnosed in more than 5 patients using RAL (see Supplementary Figure S2). The risk of developing IRIS over calendar time in patients using INI- and non-INI-based regimens is shown in Fig. 6. The risk of developing IRISFRENCH and IRISCLINICAL was highest in all individual calendar years that RAL was used. From 2014 onwards DTG became the most common treatment, with the number of late presenting patients using non-INI and other INI-based regimen decreasing sharply. In 2016 no patients initiated RAL-based cART anymore. From 2015 on, the risk of IRISFRENCH and IRISCLINICAL in patients using DTG sharply increased. In the period 2015–2016 all but one IRISFRENCH events occurred in patients using DTG. When limiting the multivariable logistic regression analysis of IRISFRENCH+CLINICAL to the period 2015–2016, use of DTG was borderline significantly associated with an increased risk (OR 4·08, 95%CI 0·99–16·82, p = 0·052) compared to non-INI-based cART. Patients initiating DTG-based cART before 2015 had higher pre-cART CD4 counts and CD4/8-ratios (median [IQR] 170 [56–170] cells per µL and 0·15 [0·11–0·19], respectively) compared to those initiating DTG-based cART after 2015 (39 [20–110] cells per µL, p = 0·053 and 0·010 [0·06–0·12], p = 0·029). Furthermore, the number of opportunistic infections commonly associated with IRIS was lower in those initiating DTG-based cART before 2015 than in those initiating DTG-based cART after 2015: mean (SD) 0·63 (0·74) versus 1·08 (0·85) opportunistic infections, p = 0·13, respectively. There were no differences in pre-cART plasma HIV-1 RNA levels and BMI between the two groups, but statistical power was very limited.
Fig. 4.
Kaplan–Meier analysis of occurrence of IRISFRENCH in users of integrase inhibitor-containing cART versus non-integrase inhibitor-containing cART. INI = integrase inhibitor, EVG = elvitegravir, DTG = dolutegravir, RAL = raltegravir.
Fig. 5.
Kaplan–Meier analysis of occurrence of IRISFRENCH+CLINICAL in users of integrase inhibitor-containing cART versus non-integrase inhibitor-containing cART. INI = integrase inhibitor, EVG = elvitegravir, DTG = dolutegravir, RAL = raltegravir.
Fig. 6.
Risk of IRIS over calendar time is patients using INI- and non-INI-based cART. INI = integrase inhibitor, DTG = dolutegravir, RAL = raltegravir, EVG = elvitegravir.
When we compared the use of corticosteroids as therapy for IRISFRENCH+CLINICAL, an increased use of corticosteroids for IRIS was more likely to be observed in the INI group compared to the non-INI group (OR 1·56, 95% CI 0·95–2·58). The hospital (re)admission rates after cART-initiation were comparable in both groups, being 60/155 (39%) in the INI versus 181/517 (35%) in the non-INI group (OR 1·17, 95% CI 0·81–1·70). Similarly, the mortality rate within 12 months was comparable at 11·6% (18/155) and 8·7% (45/517) in the INI and non-INI groups respectively (OR 1·38, 95% CI 0·77–2·46).
4. Discussion
The primary goal of this study was to examine whether initiating INI-containing cART in HIV-1 late presenters is a risk factor for IRIS. Initiation of RAL-containing cART was identified as independent risk factor for IRIS in multivariable logistic regression models, but channeling bias can at least partially explain this finding. It should be taken into consideration that the use of INI-containing cART was preferred by physicians in patients with specific OIs, a factor which we were not able to fully correct for in the models. The observed association of IRIS with the use of DTG was not statistically significant during the entire observation period, but we did observe an increased risk for IRIS (p = 0.052) in patients starting DTG-based cART in the years after RAL had been abandoned and had been replaced by DTG-based cART in HIV late-presenters after clinicians became more confident in using this new drug by 2015. Additionally, our analysis confirmed that a pre-cART diagnosis of Mycobacterium avium complex or other infections including tuberculosis, cryptococcal meningitis, and KS increased the risk for IRIS. Our models did not show any evidence that our observation was confounded by differences in patient, OI-related, and HIV-related characteristics at the start of cART. Although we incorporated all available measures of disease severity and presence of specific OI in our multivariable models and performed inverse probability of treatment weighted models in an attempt to adjust for channeling bias, it remains very well possible that channeling bias and unmeasured confounders caused the observed associations. Indeed, our study was non-randomized and observational. As such, RAL may have been used preferentially in the sickest patients and/or in patients in whom drug-drug interactions with concomitant medication had to be avoided. The possibility of channeling bias being involved is made even more likely by our observation that when DTG became more popular (2015–2016) and no new late-presenting patients were started on RAL anymore, the risk of IRIS in patients using DTG sharply increased, suggesting that since 2015 the patients most at risk for IRIS are now preferentially channeled to DTG-based cART. Patients initiating DTG-based cART after 2015 were indeed more severely ill with lower pre-cART CD4 counts and CD4/8-ratios and a higher number of opportunistic infections. Furthermore, the absolute risk of IRIS in late-presenting patients starting cART has not decreased since the use of RAL has completely stopped and DTG has become the most often used treatment option in late-presenting patients in the Netherlands. However, late presenting patients, who started on DTG, developed IRIS in substantially lower proportions compared to the risk observed in patients starting RAL-based cART only a few years earlier. This absolute risk difference of IRIS in patients starting RAL- and DTG-based cART may be explained by the fact that RAL-based cART was a regimen that was only prescribed by clinicians when drug-drug interactions needed to be avoided in late-presenting patients with OIs, while DTG-based cART is generally preferentially recommended by the Dutch HIV treatment guidelines, resulting in a much larger group of late-presenting patients being started on DTG-based cART instead of just the sickest late-presenting patients with OIs and potential drug–drug-interactions. And finally, EVG-based cART always contains the pharmacologic booster cobicistat and is hence not a popular option in late presenting patients with OIs and co-medications with drug-drug interactions with cobicistat, possibly explaining why IRIS is not often seen in patients using EVG.
Two recent studies have previously described an association between INI and IRIS. First, a French multicenter observational study investigated the incidence of IRIS requiring hospitalization in patients initiating cART with or without an INI. In this study, baseline characteristics were more favorable with higher nadir CD4+ T-lymphocyte counts. More importantly, the study design and IRIS definition differed from our study as only IRIS-cases requiring hospitalization were included. Second, a Greek small single center observational study described an association of INI and IRIS as well. However, the number of patients on any of the three INI was limited to 80. Also, patients with any CD4 cell count at the time of cART initiation were included. Still, despite these limitations, the authors described a significant association of INI use and IRIS [17,18]. Therefore, compared with these two studies, the strengths of our study are its larger sample size, the availability of a substantial amount of clinical data systematically registered in the ATHENA cohort, combined with additional on-site data extraction using a detailed IRIS CRF. This allowed us to check for IRIS according to two predefined IRIS definitions, one of which also included the clinical diagnosis of IRIS by the treating physician.
Our study has several limitations. This was an unblinded study for the investigators, but we used several methods to avoid possible association bias by making the adjudication of IRIS as objective as possible with the IRIS definitions described by French et al. Strict criteria were used to diagnose paradoxical IRIS in a patient with KS (see methods in the online supplement) to avoid a subjective interpretation. The study's observational design made us rely on the diagnostics that the treating physician had used for a clinical IRIS diagnosis and on its documentation in the patient files. The fact that the treating physician was aware of the type of prescribed cART to the patient may be considered a limitation as well. Indeed, in theory an increasing awareness of a possible association between INI and IRIS over the years may have caused clinicians to avoid INI in particular in those patients starting cART in the more recent INI era with DTG available and specifically in patients considered to be at very high risk for IRIS (e.g. patients with cryptococcal meningitis or a proven or suspected mycobacterial infection). However, no such trend could be identified when we evaluated the type of cART given to patients with a mycobacterial infection or cryptococcal meningitis between 2009 and 2017. Actually, the opposite was true; from 2009 to 2017, the use of INI progressively increased from 28% in patients with mycobacterial and 0% in patients with cryptococcal infections in the years 2009–2011 to 60% and 100% respectively in the years 2015–2017. The lack of a plausible mechanism behind the observed higher incidence of IRIS in patients on RAL than on DTG is another limitation of our study.
A causal interpretation of the association between use of RAL and to a lesser extent DTG, and the increased risk of IRIS is difficult to provide in a retrospective observational study like ours, but instead should ideally be based on prospective studies in which a large number of HIV-1 late presenters are included, started on cART as soon as possible and randomized to an INI or a non-INI containing cART regimen. This should be possible now that the rollout of DTG containing cART in resource-limited settings has started. The ongoing ADVANCE trial (NCT03122262) will and the completed REALITY trial already has provided valuable data in this regard. The randomized REALITY trial used a factorial design to study the addition of RAL to NNRTI-based cART as initial therapy and found no association between the use of RAL and risk of IRIS. With a majority (89·7%) on efavirenz the results of this study are consistent with our interpretation of the observed relationship between the use of RAL (and DTG) and IRIS in our study potentially being caused by channeling bias [14]. While the REALITY and ADVANCE trials recruited patients in sub-Saharan Africa, a third randomized study that is about to start in Europe will provide important insights into the matter as well. This study has IRIS as one of its predefined endpoints and will be the first large study to compare a PI-based with an INI-based cART regimen in a large number of patients newly diagnosed with AIDS (NCT03696160, LAPTOP).
In conclusion, INI-containing cART was independently associated with IRIS, an observation mainly driven by patients on RAL in the period up to 2014 and in more recent years by patients on DTG. This increased incidence of IRIS was not associated with mortality nor risk of hospital (re-) admissions and should therefore not be a reason to avoid INI-containing cART in late presenters.
Declaration of Competing Interest
IW reports personal fees from Gilead Sciences, non-financial support from ViiV, outside the submitted work. FW reports personal fees from Gilead and ViiV outside the submitted work, CR reports grants and personal fees from Merck, Gilead, ViiV outside the submitted work. KB reports grants and personal fees from ViiV, grants and personal fees from Gilead, personal fees from MSD, personal fees from Janssen, outside the submitted work. WB reports grants from Janssen Pharmaceuticals, other from GSK, non-financial support from Janssen Pharmaceuticals, outside the submitted work. PR reports grants from Gilead, grants from ViiV, grants from Janssen Pharmaceutica, grants from Merck, other from Gilead, other from Janssen Pharmacutica, other from Merck, other from ViiV, other from Teva Pharmaceutical Industries outside the submitted work. BR reports grants from Gilead, other from VIIV, other from Gilead, outside the submitted work. EL, SL, MK, AP, VB, GB, AH, and ME have nothing to disclose.
Acknowledgments
We thank all participating hospitals for their willingness to make data collection possible. We thank L de Groot – Berndsen for her assistance with data retrieval.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2019.11.003.
Appendix. Supplementary materials
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentsGL.pdf. [Accessed 12 May 2017].
- 2.European AIDS Clinical Society. European guidelines for treatment of HIV-positive adults. Available at: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. [Accessed 12 May 2017].
- 3.Rockstroh J.K., DeJesus E., Lennox J.L. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from Startmrk. J Acquir Immune Defic Syndr. 2013;63:77–85. doi: 10.1097/QAI.0b013e31828ace69. [DOI] [PubMed] [Google Scholar]
- 4.Sax P.E., DeJesus E., Mills A. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379:2439–2448. doi: 10.1016/S0140-6736(12)60917-9. [DOI] [PubMed] [Google Scholar]
- 5.Clotet B., Feinberg J., van Lunzen J. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383:2222–2231. doi: 10.1016/S0140-6736(14)60084-2. [DOI] [PubMed] [Google Scholar]
- 6.Andrade B.B., Singh A., Narendran G. Mycobacterial antigen driven activation of CD14++CD16- monocytes is a predictor of tuberculosis-associated immune reconstitution inflammatory syndrome. PLoS Path. 2014;10 doi: 10.1371/journal.ppat.1004433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan H.Y., Yong Y.K., Shankar E.M. Abbarant inflammasome activation characterized tuberculosis-associated immune reconstitution inflammatory syndrome. J Immunol. 2016;196:4052–4063. doi: 10.4049/jimmunol.1502203. [DOI] [PubMed] [Google Scholar]
- 8.Hsu D.C., Breglio K.F., Pei L. Emergence of polyfunctional cytotoxic CD4+ T cells in mycobacterium avium immune reconstitution inflammatory syndrome in human immunodeficiency virus-infected patients. Clin Infect Dis. 2018;67:437–446. doi: 10.1093/cid/ciy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manabe Y.C., Campbell J.D., Sydnor E., Moore R.D. Immune reconstitution inflammatory syndrome: risk factors and treatment implications. J Acquir Immune Defic Syndr. 2007;46:456–462. doi: 10.1097/qai.0b013e3181594c8c. [DOI] [PubMed] [Google Scholar]
- 10.Lawn S.D., Myer L., Bekker L.G., Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 11.Haddow L.J., Moosa M.Y., Mosam A., Moodley P., Parboosing R., Easterbrook P.J. Incidence, clinical spectrum, risk factors and impact of HIV-associated immune reconstitution inflammatory syndrome in South Africa. PLoS ONE. 2012 doi: 10.1371/journal.pone.0040623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tadokera R., Meintjes G., Skolimowska K.H. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J. 2011;37:1248–1259. doi: 10.1183/09031936.00091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahnke Y.D., Greenwald J.H., DerSimonian R. Selective expansion of polyfunctional pathogen-specific CD4(+) T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood. 2012;119:3105–3112. doi: 10.1182/blood-2011-09-380840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kityo C., Szubert A.J., Siika A. Raltegravir-intensified initial antiretroviral therapy in advanced HIV disease in Africa: a randomised controlled trial. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Sighem AI, Boender TS, Wit FWNM, Smit C, Matser A, Reiss P. Monitoring Report 2016. Human Immunodeficiency Virus (HIV) Infection in the Netherlands. Amsterdam: Stichting HIV Monitoring, 2016. Available at: www.hiv-monitoring.nl. [Accessed 12 May 2017].
- 16.French M.A., Price P., Stone S.F. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 17.Dutertre M., Cuzin L., Demonchy E. Initiation of antiretroviral therapy containing integrase inhibitors increases the risk of IRIS requiring hospitalization. J Acquir Immune Defic Syndr. 2017 doi: 10.1097/QAI.0000000000001397. [DOI] [PubMed] [Google Scholar]
- 18.Psichogiou M., Basoulis D., Tsikala-Vafea M., Vlachos S., Kapelios C.J., Daikos G.L. Integrase strand transfer inhibitors and the emergence of immune reconstitution inflammatory syndrome (IRIS) Curr HIV Res. 2017;15:405–410. doi: 10.2174/1570162X15666171122155708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.