Abstract
Background
Adverse drug reactions (ADRs) are an important cause of morbidity and mortality. Reports on differences in reporting patterns between women and men exist nationally. The goal of the present study was to assess the global evidence on spontaneous post-marketing ADR reporting differences between reports for women and men.
Methods
We analysed data collected within VigiBase, the WHO global database of individual case safety reports, between 1967-2 January 2018. VigiBase contains more than 18 million reports from the 131 member countries of the WHO Programme for International Drug Monitoring.
Findings
Of the reports with information on sex, 9,056,566 (60.1%) concerned female and 6,012,804 (39.9%) male children and adults. More female ADR reports were submitted in all regions of the world and by all types of reporters. A higher proportion of female reports was seen in all age groups from the age group 12-17 years and older. The largest difference was observed in the age group of 18–44 years and could not be explained by hormonal contraceptive use. The proportion of serious and fatal reports was higher for male reports.
Interpretation
Global post marketing surveillance data on spontaneous reports indicate that women, from puberty and onwards and especially in their reproductive years, report more ADRs than men. However, there is a higher proportion of serious and fatal ADRs among male reports. Our results suggest important underlying sex-related differences in ADRs. These findings highlight the importance of considering sex throughout the entire life-cycle of drug development and surveillance and understanding the underlying reasons for reporting ADRs.
Keywords: Pharmacovigilance, Sex differences, Sex distribution, Drug safety, Adverse drug reactions, Gender medicine, Adverse events
Research in context
Evidence before this study
Several studies have shown sex differences in national data on reported adverse drug reactions (ADRs), which consistently show more female than male reports of ADRs. Global reporting patterns however have not been reported before.
Added value of this study
This study show that more ADR reports are submitted for women from all regions of the world and by all types of reporters.
There are more female ADR reports in all age groups from puberty and onwards. The largest difference was observed in the age group 18–44 years and could not be explained by hormonal contraceptive use.
The proportion of serious and fatal reports are higher among male reports than female reports using global spontaneous ADR reporting data.
This study underscores the importance of sex and gender in medicine.
Implications of all the available evidence
The differences in reporting patterns between women and men implicate that further studies are needed to identify the underlying reasons for female dominance, especially around reproductive age. Women need to be enrolled into clinical trials to a similar degree as men and data in clinical trials needs to be stratified and analysed by sex to better understand sex and gender differences in ADRs.
1. Introduction
In general, women are prescribed more drugs than men [1], [2], [3], [4] more often access healthcare services, [5], [6], [7], [8] experience more adverse drug reactions (ADRs) [9,10] and are more often hospitalised due to ADRs than men [11], [12], [13].
Women are thought to be more at risk for ADRs not only because they use more drugs but also due to differences in pharmacokinetic and pharmacodynamic effects of drugs and the fact that they use higher doses in relation to their body weight [14].
A thorough review of 300 new drug applications between 1995 and 2000 to the United States Food and Drug Administration (FDA) showed that out of 163 applications that included an analysis by sex, 11 demonstrated a difference of 40% or more in pharmacokinetics between women and men [15]. Apart from pharmacokinetic and pharmacodynamic differences between women and men, drug-induced cardiac arrhythmias, including torsade de pointes, indicate that factors other than differences in body composition and pharmacokinetics may be involved in the occurrence of ADRs that differ between the sexes [16]. Healthcare professionals may also report ADRs for women more often than for men, even when adjusted for dispensed drugs and age-related differences. This was seen in a Swedish study investigating the reporting patterns of healthcare professionals. Regarding seriousness of the reports, men were reported to experience more serious ADRs as compared to women [17].
ADRs are an important cause of morbidity and mortality, [18] even though many ADRs are potentially avoidable if medicines are used by the right patient at the right dose. In a prospective study of over 18,000 patients admitted to two large hospitals in England during a period of six months, it was concluded that most admissions related to ADRs were either definitely or possibly avoidable [13]. ADRs are also an important reason for non-adherence to drugs. Not only because they increase the risk of hospitalisation but also because patients may discontinue taking the drug due to ADRs and thereby lose the potential benefit [19]. In a study investigating the reasons for non-adherence to drug regimens, women reported ADRs as the reason for non-adherence more commonly than men [20]. The discontinuation of drugs is problematic as a patient's non-adherence represents an important health problem: it is associated with reduced treatment benefits and significant financial burden.
In order to be able to prevent future ADRs there is a need for better understanding of the reporting patterns of ADRs as well as reported sex differences. The safety of drugs is evaluated in clinical trials and through post-marketing surveillance of ADRs. The latter is important because clinical trials in general include a limited number of patients, are of short duration, and include individuals who are younger and healthier than those who will use the drug therapy in the population. As such, many ADRs may not be well recognised in clinical trials and become apparent during post marketing surveillance using population-level data. VigiBase, the WHO global database of individual case safety reports, is the largest database of spontaneous ADR reports in the world. It contains over 18 million reports, submitted by member countries of the WHO Programme for International Drug Monitoring (WHO PIDM), dating back to 1967 [21].
In national settings, sex differences in ADR reporting have been noted [10,17,22,23]. To our knowledge, no dedicated investigation of global ADR reporting data on spontaneous reports has been performed. This study aims to complement previous national findings by assessing sex differences in post-marketing ADR reporting globally. Specifically, we aim to investigate differences in the female-to-male reporting distribution by geographical origin, patient age, type of reporter, type of ADR, and drug groups as well as the proportion of serious and fatal reports for female and male reports, respectively.
2. Data and methods
This study is performed in VigiBase, which at the time of the study contained more than 18 million reports from 131 WHO PIDM member countries, including reports from the US FDA Adverse Event Reporting System (FAERs) and reports from various European countries through EudraVigilance. These data provide the opportunity to explore differences in ADR reporting for female and male individuals of all ages at a global level. Data were collected from the end of 1967 up to 2 January 2018 and include both serious and non-serious reports. We excluded reports where patient sex was not provided. Further, suspected duplicate reports were excluded [24].
VigiBase contains individual case safety reports (ICSRs), i.e. reports of adverse events occurring in relation to the use of drugs, which have been submitted from the diverse range of countries that are members of the WHO PIDM. The reports shared in VigiBase generally describe a suspicion that has arisen from an observation of an unexpected or unwanted event in relation to the use of a drug. In most instances it cannot be proven that a specific drug (rather than, for example, underlying illness or concomitant drugs) is the cause of an event. In addition, the likelihood that the drug caused the event may vary across countries and between reporters. In this paper we use the term ‘adverse drug reaction’ or ‘ADR’ for any adverse event or reaction reported to VigiBase.
Each report represents one person, but the same person might have several reports shared within the database. One report can contain several different ADRs and several suspected, interacting, or concomitant drugs. For any report to be valid, there are four minimum requirements: an identifiable patient, an identifiable reporter, at least one ADR described, and at least one suspected drug identified.
Reports are submitted to national member countries both from healthcare practitioners and, in some countries, directly from patients. Reports can also be forwarded from the manufacturing pharmaceutical company.
The reports shared within the WHO Programme are deidentified, and research based on this data is not subject to ethics approval or informed consent.
2.1. Report variables
While patient sex is the primary variable of interest, our analyses make use of the following additional report variables: country of origin, geographical region of origin, type of reporter, patient age group, MedDRA System Organ Class (SOC), Anatomical Therapeutic Chemical (ATC) classification system group, seriousness, and fatality. Country of origin, geographical region of origin, type of reporter, patient age group, SOC, and ATC group are variables used to calculate stratified proportions of female reports, while seriousness and fatality are outcome variables when comparing female and male reports, as described in the following sub-sections. Reports with information missing on a particular variable were excluded for any analysis including that variable.
The country of origin is the country that submitted the report to VigiBase, which is not necessarily the home country of the patient concerned. There are 131 different countries in this data set. Each report has a single country of origin (information missing on 0.1% of the reports).
The geographical region of origin is the UN geographical region to which the country of origin belongs. There are six different regions represented: Africa, Asia, Europe, Latin America and The Caribbean, North America (both subdivisions of Americas), and Oceania (information missing on 0.1% of the reports).
The type of reporter is either patient, physician, pharmacist, or other health professional. A report may have several people contributing and processing its content, and hence a single report may have multiple reporter types. Since some reporter types used in older reporting formats have been deprecated (specifically ‘Lawyer’ and ‘Other’), this variable may be missing (information missing on 25.5% of the reports).
The patient age group is the patient age at ADR onset, put in one of eight age groups ranging between 0-27 days and 75 years and older. While there is only one age group per report, information about age might be missing (information missing on 22.3% of the reports).
The MedDRA SOCs are the 27 groups of ADR terms at the top of the MedDRA hierarchy (MedDRA version 20.1) [25]. A report belongs to a given SOC if any of its reported ADR terms belongs to that SOC. As each report contains at least one ADR term, this variable is not missing on any of the reports.
The ATC groups are all groups at the second level of the ATC classification system, such as A01 [26]. A report belongs to a given ATC group if any of its reported drugs (whether characterised as suspected, interacting, or concomitant) is included in that ATC group. As each report contains at least one suspected drug, this variable is not missing on any of the reports.
Seriousness is a derived binary reporting variable. A report is considered serious if either the general seriousness field or any of the six ICH-E2B seriousness sub-categories was indicated [27]. Because seriousness can only be reported in the E2B report format, this variable is missing for reports using older report formats (information missing on 31.1% of the reports).
Fatality is also a derived binary reporting variable, which is present on a report if there is any coded information that suggests the patient died. This information could be either an ADR term (e.g. ‘Death’), a reported lethal outcome from any of the coded ADRs, or (for E2B reports) presence of ‘Death’ as a seriousness criterion (information not missing on any reports).
Any sub-group smaller than 500 reports was excluded from the results for that specific variable. This number was chosen to include only large enough groups of reports for a relevant presentation of the sex distributions. This cut-off only caused exclusion of individual countries of origin and the single ATC group ‘Surgical dressings’, since all sub-groups for all other investigated variables were substantially larger than 500 reports. Individual case reports can have multiple reporters (see previous sub-section); a single report can thus be counted in multiple reporter type categories. The same applies to MedDRA SOCs and ATC groups.
2.2. Statistical analysis
2.2.1. Prevalence of female reports
The proportion of female reports was computed for the entire dataset, and for individual sub-groups based on the following variables: country of origin, geographical region of origin, type of reporter, patient age, MedDRA SOC, and ATC group. For patient age, a separate sensitivity analysis was performed in the age group 18–44 years, excluding all reports containing any drug from the ATC-group G (”Genito urinary system and sex hormones”). This was done to eliminate the influence of contraceptive drugs which are frequently used by otherwise healthy women in this age group. For MedDRA SOCs and ATC groups, only the ten groups with the largest proportion of female reports as well as the ten groups with the lowest proportion of female reports (and thus the highest proportion of male reports) were considered.
2.2.2. Comparison between female and male reports
In addition to analysing the prevalence of female reports within different sub-groups, reporting characteristics among female reports were quantitatively compared to those among male reports. For this purpose, a method called vigiPoint was utilised [28]. vigiPoint compares the reporting frequencies of a large number of features, e.g. reported drugs, patient outcome, or country of origin, between a report set of interest and a suitable comparator report set. Here, all female reports were compared to all male reports on seriousness and fatality. The comparison entails both the crude reporting frequencies of the features in the two groups, as well as highlighting of key characteristics, here called vigiPoint key features. These key features are derived from a statistical comparison using log odds ratios, while both accounting for multiple testing and requiring a sufficiently large effect size. This means further that a vigiPoint key feature (e.g. ‘fatality’) is both statistically significantly different between female and male reports, and there is a sufficiently large relative difference (upwards or downwards) between female and male reports to be of practical relevance (at least about 40% on the odds ratio scale).
Because sex differences are likely to be age-dependent to some extent, vigiPoint analyses for seriousness and fatality were also performed separately for each age group. This means that seriousness and fatality reporting frequencies were compared between female and male reports within each of the considered age groups, as well as in total for all reports.
3. Results
3.1. Prevalence of female reports
The total number of global ADR reports included in this analysis is 15,069,370. There are 9,056,566 (60,1%) female reports and 6,012,804 (39.9%) male reports.
3.1.1. Distribution by region and country of origin
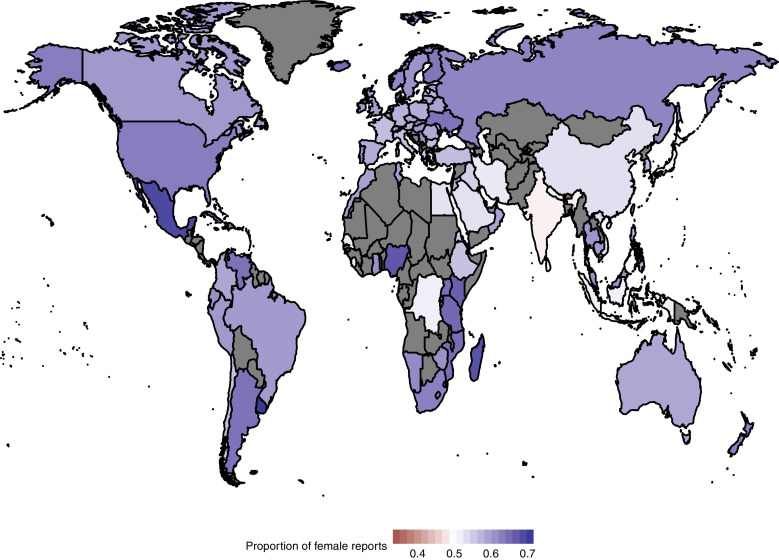
Fig. 1 shows that most of the countries have a similar proportion of female reports as the whole of VigiBase. Looking closer at the countries with at least 500 reports in VigiBase, Uruguay has the highest proportion of female reports at 71% of about 3300 reports in total. In contrast, Macedonia has only 34% female reports of approximately 2000 in total. In total, 96% of the countries have a higher proportion of female reports as compared to males. Overall, only 4 of 96 countries (4%) have a higher proportion of male reports. These are, apart from Macedonia, Andorra (45% female reports out of about 800 reports in total), India (49% of about 281,000 reports), and Sri Lanka (just below 50% of about 2100 reports).
Fig. 1.
Proportion of female reports per country for all countries with at least 500 report submitted to the WHO Programme for International Drug Monitoring. Countries that have less than 500 reports or are not yet members of the program are plotted in grey. The results presented in this figure are available in tabular format in Appendix 1.
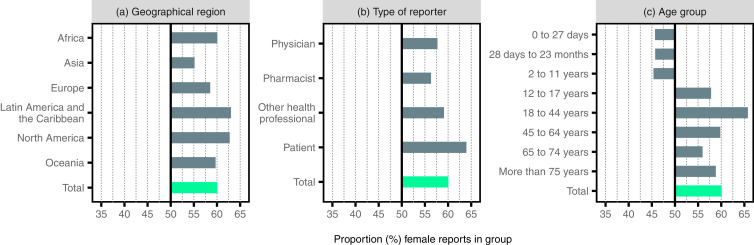
As seen in Fig. 2a, Asia as a region has the lowest proportion of female reports, with 55% female reports, as compared to the other regions where the proportion of female reports varies between 59% and 63%.
Fig. 2.
Proportion of female reports in VigiBase categorized by (a) geographical region, (b) age group, and (c) type of reporter. Note that reporter types available only in older reporting formats (specifically ‘lawyer’ and ‘other’) have been excluded. The results presented in this figure are available in tabular format in Appendix 1.
3.1.2. Distribution by reporter type
Fig. 2b displays female and male proportion of reports categorised by reporter type. Pharmacists represent the reporter type with the smallest difference between the proportion of female and male reports (54% female reports, 46% male reports) whilst patients are the group of reporters where the difference in distribution of reports between the sexes is greatest (64% female reports, 36% male reports).
3.1.3. Distribution by patient age
Fig. 2c displays patient age groups: For children in age groups 2-11 years or younger, there are more reports for boys than for girls. However, there is a shift around puberty and for the age group 12–17 years and in all adult age groups, there are more female reports. The difference between female and male reports is most pronounced in the group of 18–44 years, which corresponds roughly to the fertile female age. Within this age group, 66% of all reports concern women. Excluding all reports from the ATC-group G (”Genito urinary system and sex hormones”), the proportion of female reports decreases to 61%.
3.1.4. Distribution by MedDRA system organ class
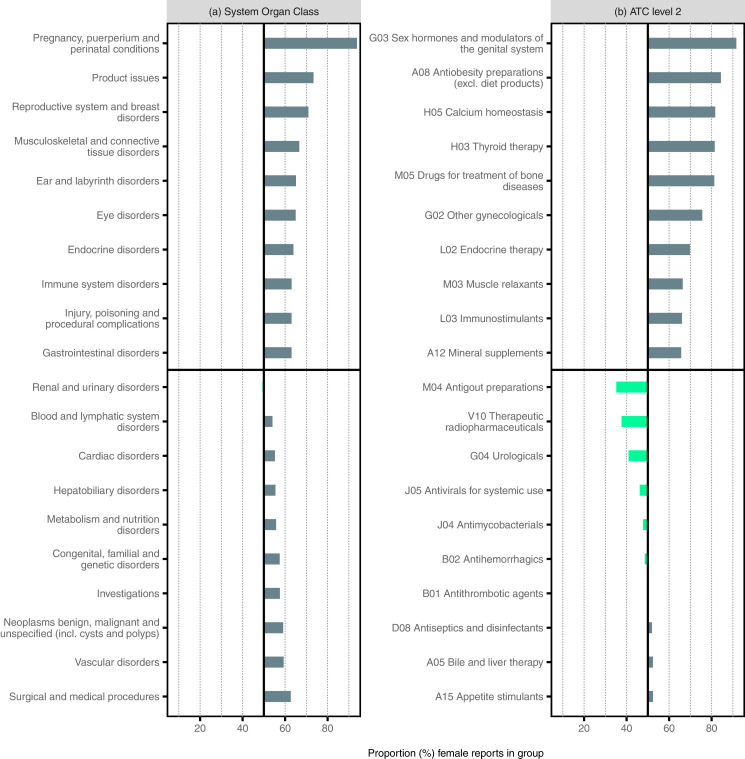
Fig. 3a shows the proportion of female reports for the ten SOCs with the highest and lowest proportion of female reports, respectively. The only SOC with a male dominance of reports is the SOC ‘Renal and urinary disorders’ with slightly less than half the reports being female. More than 70% female reports were noted in the SOCs Pregnancy, puerperium and perinatal conditions’ (94%), ‘Product issues’ (73%), and ‘Reproductive system and breast disorders’ (71%).
Fig.3.
Proportion of top disproportionally reported female and male reports for System Organ Classes (SOCs) and Anatomical Therapeutic Chemical (ATC)-groups. The ten SOCs with the highest and the lowest proportion of female reports respectively (a) and the ten ATC-groups (ATC level 2) with the highest and lowest proportion of female reports respectively (b) are visualised. The results presented in this figure are available in tabular format in Appendix 1.
3.1.5. Distribution by ATC group
Fig. 3b shows the proportion of female reports for the ten ATC groups (at the second level) with the highest and lowest proportion of female reports, respectively. Five different ATC codes had more than 80% female reports: ‘Sex hormones and modulators of the genital system’ (92%), ‘Anti-obesity preparations (excluding diet products)’ (84%), ‘Calcium homeostasis’ (82%), ‘Thyroid therapy’ (81%), and ‘Drugs for treatment of bone diseases’ (81%), The ATC-groups with fewer female than male reports were ‘Antihemorrhagics’ (49%), ‘Antimycobacterials’ (48%), Antivirals for systemic use’ (46%), ‘Urologicals’ (41%), ‘Therapeutic Radiopharmaceuticals’ (38%), and ‘Antigout preparations’ (35%).
3.2. Comparison between female and male reports
Fig. 4a and b display the proportion of serious and fatal reports, respectively, separately for female and male reports. Results are displayed both in total (including unknown ages) and separately for each considered age group. Age was reported in about 11,700,000 (78%) reports.
Fig. 4.
Proportion serious and fatal reports for female and male reports in VigiBase, in total and by age group. VP indicates that the variable is a vigiPoint key feature when comparing female to male reports, which means that there is a large relative difference that is also statistically significant. Note that seriousness is only available for reports of the newer E2B format, and other reports are not included in this analysis. Also, note that ‘Total’ includes reports with unknown age. The results presented in this figure are available in tabular format in Appendix 1.
3.2.1. Seriousness
As seen in Fig. 4a, serious reports are less common among female reports than among male reports. For all reports taken together, the proportion of serious reports is 42% among female reports and 49% among male reports. The proportion of serious reports is also higher for male reports in each individual age group, even though the differences are minor for children and adolescents (below 18 years). The largest difference between female and male reports is seen for the age group of 65–74-year olds where 47% of female reports are serious, compared to 57% among male reports. In this age group, seriousness is also a highlighted vigiPoint key feature in the comparison between female and male reports. This means that the relative difference between female and male reports in seriousness is large (at least about 40% on the odds ratio scale) and statistically significant.
3.2.2. Fatality
The results for fatality (see Fig. 4b) follow the same pattern as those for seriousness, only with more distinct differences between the sexes. For children and adolescents (ages below 18 years), there are no or only minor differences between female and male reports. However, for adults there are clearly lower proportions of fatal reports among female reports as compared to male reports. All reports taken together, 4.2% of female reports are fatal compared to 6.7% for male reports. Fatality is a vigiPoint key feature in the comparison between female and male reports for all reports and for all adult age groups except 75 years and older.
In addition to the results presented here for our two variables of primary interest, seriousness and fatality, the full vigiPoint results including all available variables have been made publicly accessible from an external repository [41]. This allows for further exploration of the characteristics of female and male reports in VigiBase by anyone interested.
4. Discussion
We find that female ADR reports outnumber male ADR reports across the globe, in all adult ages and by all available reporter types. Male reports however, to a larger extent, more often contain serious and fatal ADRs than female reports.
Our results are in line with several previous studies which have shown that female ADR reports are more common than male ones [10,17]. In global data, cultural differences could be expected to appear. However, our results show that female reports outnumber male reports in 96% of the countries that have at least 500 reports shared within the WHO PIDM. Authors Saikia et al. point out in their recent study that in India, one of the four countries with a male dominance of reports in our study, there is a differences in access to healthcare for men and women, which could contribute to our observation of excess male reports in this particular country [29].
For the geographical regions, the figure varied between 55-63% female reports between the different regions with Asia having the lowest proportion of female reports. A previous VigiBase study characterizing ADR reporting with respect to national income level, classified in accordance with the World Bank definition (low, lower-middle, upper-middle and high), found no significant difference for the distribution of sex in the ADR reports across different income groups [30].
Notably, the data show that the excess of female reports starts within the age group of 12–17 years and is consistent from that age group and above. The finding that ADRs are more commonly reported for boys than girls has previously been noted [31,32]. This was in one study hypothesised to be partly explained by a statistically slightly higher use of medications among boys than girls [32] and thus could potentially be due to sex differences in prevalence of childhood diseases. The largest difference between female and male reports is seen in the age group 18–44-years, which corresponds roughly to the female reproductive age. In this age group, contraceptive drugs are commonly used all over the world. A plausible explanation would be that these drugs contribute to the excess in female ADR reports. However, when removing the reports which included hormonal contraceptive drugs, we observed that the female predominance of reports in this particular age group decreased only from 66% to 61%. Therefore, within our global data, this only explains a relatively small fraction of female ADR reporting. It has also been suggested that older age in combination with more co-morbidities resulting in polypharmacy and drug-drug interactions is a major factor contributing to the excess ADR reports in particularly women [33]. In VigiBase there are actually slightly lower proportions of female reports in the older age groups of 65–74 years and 75 years and older, respectively, as compared to the age groups of 18–44 years and of 44–64 years.
The higher reporting rate for female reports could be explained by a higher use of drugs in the female population compared to the male population. This was found in a study investigating the reporting patterns for three regional centres in three different countries in southern Europe. It was particularly noted for psychotropic drugs, of which antidepressants were the most reported [34]. Also in our data we find a different proportion of reports between the sexes for different ATC groups of drugs. The groups with the largest proportion of female and male reports are expected as drugs belonging to the ATC group ‘Sex hormones and modulators of the genital system’ are more commonly used in women and ‘Antigout preparations’ more commonly used in men. However, the proportion of reports in other ATC groups might have less obvious explanations, as for the male dominance of reports in the ATC groups ‘Antimycobacterials’ (48% female reports) and ‘Antihemorrhagics’ (49%).
Interestingly, we noticed that male reports more often than female reports were classified as serious. This is in agreement with what has been found in a national study of Swedish ADR reports [17]. The same pattern is highlighted as a vigiPoint key feature for fatal reports which are more common among the male reports than the female reports. The reasons behind the larger proportion serious and fatal reports among the male reports in VigiBase are not clear. One reason might be that women are more prone to access healthcare services and thus ADRs might potentially get addressed earlier. However, more research within this field is needed.
The safety of drugs is to a large part evaluated using post-marketing surveillance data because many ADRs occur rarely in clinical trial populations. Even if ADRs occur in clinical trials, they are scarcely reported in a sex-specific manner [35,36], making it difficult to predict post-marketing risks in women and men. Additionally, women have long been underrepresented in clinical trials, historically due to concerns to involve women in reproductive ages due to risks for pregnant women and the foetus. Although laws now exist mandating women to be included in phase III clinical trials in adequate numbers to allow for valid analyses of data, this is not always the case [36,37]. ADR databases such as VigiBase are reliant on the experiences of ADRs being shared with others, but it has been shown that healthcare professionals do not report all ADRs they observe to the responsible authority. The median under-reporting rate across 37 studies included in a systematic review of under-reporting of ADRs was shown to be as high as 94% [38]. The underreporting might have an influence on the sex distribution of the reports in VigiBase as it is possible that for one sex experienced ADRs are reported to a lesser extent than for the other, which would influence our figures. Left out information about seriousness of the reports could also affect the proportion of serious reports for each sex. Therefore, proper reporting of ADRs by sex in clinical trials is warranted, even if the individual numbers of ADRs per trials are low, and all outcomes should be reported, as this would allow for meta-analyses to be done in the future.
In most instances the causality between a specific drug (rather than, for example, underlying illness or concomitant drugs) and an ADR cannot be proven solely from the reports in our data set. In addition, the likelihood that the drug caused the event may vary across countries and between reporters, for example due to differences in regulations or methods to assess causality. However, there is no reason to believe that these issues should have any substantial impact on our results.
In later years, several countries have implemented a spontaneous reporting system that also includes self-reporting by patients. The addition of patient reporting has been shown to increase the value of these systems, as patients send in different ADR reports compared to healthcare professionals [39], [40], [42]. For example, it has been found that patients often provide detailed clinical stories that describe their experiences and the impact of ADRs on their quality of life [40,42]. Recent studies using VigiBase data also show that patient reports can contribute to identifying possible safety issues [40,43]. From the reports in VigiBase it is notable that the proportion of female reports is slightly larger for patients compared to other groups of reporters. This may reflect a higher incidence of ADRs in women but may also reflect a higher willingness to report among women as compared to men. If patient reporting continues to increase and be advocated, and women continue to report more than men, this could influence the proportion of female reports to be even larger in the future.
5. Conclusion
In conclusion, this global analysis of ADR reporting shows that female reports outnumber male reports across the world. The large number of reports and the consistency of this result across different sub-groups of the data adds to the strength of this finding. For children, there are more reports for boys than for girls, but from puberty and onwards there are more female reports, with the largest difference seen around a woman's reproductive age. This finding could not be explained by oral contraceptive use. However, the proportion of serious and fatal ADRs is higher for males. Our results clearly suggest, but do not prove, true and important underlying sex-related differences in ADRs. It is therefore imperative to improve the consideration of sex throughout the entire life-cycle of drug development and surveillance. For example, a balanced inclusion of women and men, as well as sex-stratified analyses of all experienced ADRs, in clinical trials of all phases is warranted. Further, more research is needed to better understand the underlying reasons for the differences in ADR reporting observed in this study.
CRediT authorship contribution statement
Sarah Watson: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Ola Caster: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Paula A Rochon: Conceptualization, Formal analysis, Writing - review & editing. Hester den Ruijter: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors Sarah Watson, Hester den Ruijter, Ola Caster and Paula Rochon declare no conflicts of interest.
Acknowledgements
The authors wish to thank the MATERA Alliance for supporting this global study.
The authors are indebted to the national centres which make up the WHO Programme for International Drug Monitoring and provide reports to VigiBase. However, the opinions and conclusions of this study are not necessarily those of the various centres nor of the WHO.
This study was for one of the authors, Hester van den Ruijter, funded by the Dutch Heart Foundation (2013T084, Queen of Hearts Program) and by ZonMw grant (849100003, Reviews en Kennissyntheses Gender en Gezondheid). The funders had no role in the choice of study design, in data collection, data analysis, interpretation or writing of the report. All authors approved the final Article as submitted and agree to be accountable for all aspects of the work.
Appendix 1
Proportion of female reports per country for all countries with at least 500 report shared to VigiBase, as presented in Fig. 1.
| Country | Proportion female reports | Total number of reports in country |
|---|---|---|
| Uruguay | 70.7% | 3301 |
| Mexico | 69.0% | 77,190 |
| Madagascar | 67.7% | 1335 |
| Nigeria | 67.2% | 10,306 |
| Kenya | 66.4% | 9594 |
| Costa Rica | 65.7% | 717 |
| Tanzania | 65.2% | 1670 |
| Denmark | 65.1% | 91,423 |
| Cuba | 64.8% | 42,068 |
| Iceland | 64.8% | 1921 |
| Mozambique | 64.8% | 1010 |
| Uganda | 64.6% | 2432 |
| Venezuela | 64.2% | 13,046 |
| Argentina | 64.1% | 18,164 |
| Ukraine | 64.0% | 1676 |
| Finland | 63.8% | 42,404 |
| Cape Verde | 63.0% | 501 |
| USA | 63.0% | 6,961,190 |
| New Zealand | 62.7% | 99,796 |
| South Africa | 62.7% | 39,881 |
| Slovakia | 62.5% | 14,378 |
| United Arab Emirates | 62.4% | 837 |
| Russia | 62.2% | 3273 |
| Norway | 62.0% | 58,351 |
| El Salvador | 61.7% | 715 |
| Namibia | 61.7% | 1930 |
| Netherlands | 61.4% | 196,142 |
| Ghana | 61.1% | 3051 |
| Ecuador | 61.1% | 1700 |
| Montenegro | 61.0% | 939 |
| Zimbabwe | 60.8% | 2788 |
| Malaysia | 60.8% | 73,324 |
| Sweden | 60.7% | 155,321 |
| Thailand | 60.6% | 344,139 |
| Hungary | 60.5% | 11,708 |
| Serbia | 60.4% | 7243 |
| Estonia | 60.3% | 1500 |
| Portugal | 60.3% | 36,783 |
| Canada | 60.3% | 540,681 |
| Peru | 60.2% | 55,715 |
| Cambodia | 60.1% | 919 |
| Brazil | 60.0% | 6154 |
| Switzerland | 59.7% | 89,267 |
| Tunisia | 59.6% | 7669 |
| UK | 59.6% | 746,548 |
| Morocco | 59.4% | 20,252 |
| Croatia | 59.3% | 32,180 |
| Ireland | 59.3% | 56,293 |
| Belarus | 59.2% | 929 |
| Oman | 59.2% | 14,329 |
| Singapore | 59.0% | 165,173 |
| Colombia | 58.8% | 83,570 |
| Australia | 58.8% | 327,503 |
| Lithuania | 58.6% | 2313 |
| Romania | 58.5% | 20,494 |
| Czech Republic | 58.4% | 38,580 |
| Malta | 58.3% | 861 |
| Spain | 58.2% | 289,139 |
| Poland | 58.1% | 13,472 |
| Austria | 58.0% | 45,240 |
| Germany | 57.7% | 563,141 |
| Jordan | 57.3% | 534 |
| Latvia | 57.3% | 1498 |
| Philippines | 57.2% | 20,158 |
| Belgium | 57.2% | 51,528 |
| South Korea | 57.0% | 993,765 |
| Bulgaria | 56.6% | 8465 |
| Turkey | 56.5% | 30,783 |
| France | 56.4% | 627,065 |
| Italy | 56.1% | 391,027 |
| Chile | 56.1% | 11,292 |
| Ethiopia | 56.1% | 1065 |
| Slovenia | 55.7% | 5057 |
| Greece | 55.7% | 29,503 |
| Cyprus | 55.6% | 1036 |
| Eritrea | 55.5% | 5223 |
| Moldova | 54.7% | 1352 |
| Israel | 54.6% | 11,683 |
| Iraq | 54.6% | 3994 |
| Vietnam | 53.2% | 10,707 |
| Armenia | 53.2% | 2501 |
| China | 53.1% | 732,344 |
| Saudi Arabia | 52.9% | 4874 |
| Egypt | 52.9% | 13,980 |
| Sierra Leone | 52.2% | 1328 |
| Senegal | 52.1% | 706 |
| Iran | 52.1% | 18,436 |
| Democratic Republic of the Congo | 51.6% | 13,180 |
| Indonesia | 51.3% | 7123 |
| Nepal | 51.3% | 675 |
| Japan | 50.4% | 300,849 |
| Sri Lanka | 49.6% | 2121 |
| India | 48.7% | 281,246 |
| Andorra | 44.7% | 759 |
| Macedonia | 34.2% | 1987 |
Proportion of female reports per geographical region for reports shared to VigiBase, as presented in Fig. 2a.
| Geographical region | Proportion female reports | Total number of reports in region |
|---|---|---|
| Africa | 60.1% | 140,059 |
| Asia | 55.2% | 3,022,513 |
| Europe | 58.5% | 3,648,727 |
| Latin America and the Caribbean | 63.0% | 312,279 |
| North America | 62.8% | 7,501,871 |
| Oceania | 59.7% | 427,310 |
| Total | 60.1% | 15,056,524 |
Proportion of female reports for different reporter types of reports shared to VigiBase, as presented in Fig. 2b.
| Type of reporter | Proportion female reports | Total number of reports in group |
|---|---|---|
| Physician | 57.7% | 5,212,044 |
| Pharmacist | 56.3% | 979,580 |
| Other health professional | 59.1% | 1,648,294 |
| Patient | 64.0% | 4,252,156 |
| Total | 60.1% | 14,007,807 |
Proportion of female reports per age group for reports shared to VigiBase, as presented in Fig. 2c.
| Age group | Proportion female reports | Total number of reports in group |
|---|---|---|
| 0–27 days | 45.7% | 28,873 |
| 28 days to 23 months | 45.7% | 392,645 |
| 2–11 years | 45.4% | 555,106 |
| 12–17 years | 57.8% | 364,188 |
| 18–44 years | 65.8% | 3,154,641 |
| 45–64 years | 59.8% | 3,872,048 |
| 65–74 years | 56.0% | 1,843,428 |
| 75 years and older | 58.8% | 1,498,918 |
| Total | 60.1% | 15,069,370 |
Proportion of female reports per MedDRA System Organ Class for reports shared to VigiBase, as presented in Fig. 3(a).
| MedDRA System Organ Class | Proportion female reports | Total number of reports |
|---|---|---|
| Pregnancy, puerperium and perinatal conditions | 93.8% | 101,011 |
| Product issues | 73.4% | 296,291 |
| Reproductive system and breast disorders | 71.0% | 369,864 |
| Musculoskeletal and connective tissue disorders | 66.7% | 1,178,485 |
| Ear and labyrinth disorders | 65.1% | 172,194 |
| Eye disorders | 65.0% | 547,524 |
| Endocrine disorders | 63.9% | 64,779 |
| Immune system disorders | 63.0% | 438,229 |
| Injury, poisoning and procedural complications | 63.0% | 1,399,621 |
| Gastrointestinal disorders | 63.0% | 2,776,517 |
| Renal and urinary disorders | 49.3% | 447,485 |
| Blood and lymphatic system disorders | 54.1% | 619,235 |
| Cardiac disorders | 55.2% | 835,851 |
| Hepatobiliary disorders | 55.4% | 295,839 |
| Metabolism and nutrition disorders | 55.8% | 639,331 |
| Congenital, familial and genetic disorders | 57.5% | 48,349 |
| Investigations | 57.5% | 1,390,244 |
| Neoplasms benign, malignant and unspecified (incl. cysts and polyps) | 59.1% | 317,088 |
| Vascular disorders | 59.3% | 858,231 |
| Surgical and medical procedures | 62.7% | 211,682 |
Proportion of female reports per ATC-code (level two) for reports shared to VigiBase, as presented in Fig. 3(b).
| ATC level 2 | Female | Total number |
|---|---|---|
| G03 Sex hormones and modulators of the genital system | 91.6% | 621,046 |
| A08 Antiobesity preparations (excl. diet products) | 84.4% | 84,423 |
| H05 Calcium homeostasis | 81.7% | 145,936 |
| H03 Thyroid therapy | 81.5% | 394,606 |
| M05 Drugs for treatment of bone diseases | 81.3% | 273,779 |
| G02 Other gynecologicals | 75.6% | 697,444 |
| L02 Endocrine therapy | 69.8% | 246,985 |
| M03 Muscle relaxants | 66.4% | 289,198 |
| L03 Immunostimulants | 66.1% | 414,916 |
| A12 Mineral supplements | 65.7% | 475,153 |
| M04 Antigout preparations | 35.2% | 141,519 |
| V10 Therapeutic radiopharmaceuticals | 37.6% | 8600 |
| G04 Urologicals | 40.9% | 456,927 |
| J05 Antivirals for systemic use | 46.2% | 439,626 |
| J04 Antimycobacterials | 47.7% | 118,945 |
| B02 Antihemorrhagics | 48.6% | 77,143 |
| B01 Antithrombotic agents | 50.3% | 1,271,349 |
| D08 Antiseptics and disinfectants | 51.9% | 44,991 |
| A05 Bile and liver therapy | 52.4% | 67,371 |
| A15 Appetite stimulants | 52.4% | 13,217 |
References
- 1.Anthony M., Lee K.Y., Bertram C.T. Gender and age differences in medications dispensed from a national chain drugstore. J Womens Health. 2008;17(5):735–743. doi: 10.1089/jwh.2007.0731. [DOI] [PubMed] [Google Scholar]
- 2.Correa-de-Araujo RM G.E., Banthin J.S., Trinh Y. Gender differences in drug use and expenditures in a privately insured population of older adults. J Womens Health. 2005;14(1):73–81. doi: 10.1089/jwh.2005.14.73. [DOI] [PubMed] [Google Scholar]
- 3.Stock S.A., Stollenwerk B., Redaelli M., Civello D., Lauterbach K.W. Sex differences in treatment patterns of six chronic diseases: an analysis from the German statutory health insurance. J Womens Health. 2008;17(3):343–354. doi: 10.1089/jwh.2007.0422. [DOI] [PubMed] [Google Scholar]
- 4.Thorell K., Skoog J., Zielinski A., Borgquist L., Halling A. Licit prescription drug use in a Swedish population according to age, gender and socioeconomic status after adjusting for level of multi-morbidity. BMC Public Health. 2012;12:575. doi: 10.1186/1471-2458-12-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redondo-Sendino A., Guallar-Castillon P., Banegas J.R., Rodriguez-Artalejo F. Gender differences in the utilization of health-care services among the older adult population of Spain. BMC Public Health. 2006;6:155. doi: 10.1186/1471-2458-6-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schappert S.M., Burt C.W. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001–02. Vital Health Stat. 2006;13(159):1–66. [PubMed] [Google Scholar]
- 7.Osika Friberg I., Krantz G., Maatta S., Jarbrink K. Sex differences in health care consumption in Sweden: a register-based cross-sectional study. Scand J Public Health. 2016;44(3):264–273. doi: 10.1177/1403494815618843. [DOI] [PubMed] [Google Scholar]
- 8.Ladwig K.H., Marten-Mittag B., Formanek B., Dammann G. Gender differences of symptom reporting and medical health care utilization in the German population. Eur J Epidemiol. 2000;16(6):511–518. doi: 10.1023/a:1007629920752. [DOI] [PubMed] [Google Scholar]
- 9.Zopf Y., Rabe C., Neubert A. Women encounter ADRs more often than do men. Eur J Clin Pharmacol. 2008;64(10):999–1004. doi: 10.1007/s00228-008-0494-6. [DOI] [PubMed] [Google Scholar]
- 10.Martin R.M., Biswas P.N., Freemantle S.N., Pearce G.L., Mann R.D. Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: analysis of 48 cohort studies. Br J Clin Pharmacol. 1998;46(5):505–511. doi: 10.1046/j.1365-2125.1998.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodenburg E.M., Stricker B.H., Visser L.E. Sex-related differences in hospital admissions attributed to adverse drug reactions in the Netherlands. Br J Clin Pharmacol. 2011;71(1):95–104. doi: 10.1111/j.1365-2125.2010.03811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onder G., Pedone C., Landi F. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA) J Am Geriatr Soc. 2002;50(12):1962–1968. doi: 10.1046/j.1532-5415.2002.50607.x. [DOI] [PubMed] [Google Scholar]
- 13.Pirmohamed M., James S., Meakin S. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soldin O.P., Mattison D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143–157. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson G.D. Sex and racial differences in pharmacological response: where is the evidence? Pharmacogenetics, pharmacokinetics, and pharmacodynamics. J Womens Health. 2005;14(1):19–29. doi: 10.1089/jwh.2005.14.19. [DOI] [PubMed] [Google Scholar]
- 16.Anderson G.D. Gender differences in pharmacological response. Int Rev Neurobiol. 2008;83:1–10. doi: 10.1016/S0074-7742(08)00001-9. [DOI] [PubMed] [Google Scholar]
- 17.Holm L., Ekman E., Jorsater Blomgren K. Influence of age, sex and seriousness on reporting of adverse drug reactions in Sweden. Pharmacoepidemiol Drug Saf. 2017;26(3):335–343. doi: 10.1002/pds.4155. [DOI] [PubMed] [Google Scholar]
- 18.Formica D., Sultana J., Cutroneo P.M. The economic burden of preventable adverse drug reactions: a systematic review of observational studies. Expert Opin Drug Saf. 2018;17(7):681–695. doi: 10.1080/14740338.2018.1491547. [DOI] [PubMed] [Google Scholar]
- 19.Leporini C., De Sarro G., Russo E. Adherence to therapy and adverse drug reactions: is there a link? Expert Opin Drug Saf. 2014;13(Suppl 1):S41–S55. doi: 10.1517/14740338.2014.947260. [DOI] [PubMed] [Google Scholar]
- 20.Thunander Sundbom L., Bingefors K. Women and men report different behaviours in, and reasons for medication non-adherence: a nationwide Swedish survey. Pharm Pract. 2012;10(4):207–221. doi: 10.4321/s1886-36552012000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inf J. 2008;42(5):409–419. [Google Scholar]
- 22.Montastruc J.L., Lapeyre-Mestre M., Bagheri H., Fooladi A. Gender differences in adverse drug reactions: analysis of spontaneous reports to a Regional Pharmacovigilance Centre in France. Fundam Clin Pharmacol. 2002;16(5):343–346. doi: 10.1046/j.1472-8206.2002.00100.x. [DOI] [PubMed] [Google Scholar]
- 23.Aagaard L., Weber C.B., Hansen E.H. Adverse drug reactions in the paediatric population in Denmark: a retrospective analysis of reports made to the Danish Medicines Agency from 1998 to 2007. Drug Saf. 2010;33(4):327–339. doi: 10.2165/11319100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Norén G.N., Orre R., Bate A., Edwards I.R. Duplicate detection in adverse drug reaction surveillance. Data Min Knowl Discov. 2007;14(3):305–328. [Google Scholar]
- 25.2019. MedDRA introductory guide version 22.1.https://www.meddra.org/sites/default/files/guidance/file/000354_intguide_22.1.pdf Accessed 3 September 2019. [Google Scholar]
- 26.WHO Collaborating Centre for Drug Statistics . 2018. ATC structure and priniciples.https://www.whocc.no/atc/structure_and_principles/ 2018-02-15. Accessed 6 September 2019. [Google Scholar]
- 27.ICH Expert Working Group . 2001. ICH harmonised tripartite guideline.http://estri.ich.org/e2br22/E2B_R2_Guideline.pdf Accessed 10 January 2019. [Google Scholar]
- 28.Juhlin K., Star K., Noren G.N. A method for data-driven exploration to pinpoint key features in medical data and facilitate expert review. Pharmacoepidemiol Drug Saf. 2017;26(10):1256–1265. doi: 10.1002/pds.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saikia N., Moradhvaj, Bora J.K. Gender difference in health-care expenditure: evidence from India human development survey. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0158332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aagaard L., Strandell J., Melskens L., Petersen P.S., Holme Hansen E. Global patterns of adverse drug reactions over a decade: analyses of spontaneous reports to Vigibase. Drug Saf. 2012;35(12):1171–1182. doi: 10.1007/BF03262002. [DOI] [PubMed] [Google Scholar]
- 31.Star K., Noren G.N., Nordin K., Edwards I.R. Suspected adverse drug reactions reported for children worldwide: an exploratory study using Vigibase. Drug Saf. 2011;34(5):415–428. doi: 10.2165/11587540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Ferrajolo C., Sultana J., Ientile V. Gender differences in outpatient pediatric drug utilization: a cohort study from Southern Italy. Front Pharmacol. 2019;10:11. doi: 10.3389/fphar.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvi F., Marchetti A., D'Angelo F., Boemi M., Lattanzio F., Cherubini A. Adverse drug events as a cause of hospitalization in older adults. Drug Saf. 2012;35(Suppl 1):29–45. doi: 10.1007/BF03319101. [DOI] [PubMed] [Google Scholar]
- 34.D'Incau P., Lapeyre-Mestre M., Carvajal A. No differences between men and women in adverse drug reactions related to psychotropic drugs: a survey from France, Italy and Spain. Fundam Clin Pharmacol. 2014;28(3):342–348. doi: 10.1111/fcp.12032. [DOI] [PubMed] [Google Scholar]
- 35.Bots SH G., .F, Eikendal A.L., Tannenbaum C., Rochon P.A., Regitz-Zagrosek V., Miller V.M., Day D., Asselbergs F.W., den Ruijter H.M. Adverse drug reactions to guideline-recommended heart failure drugs in women: a systematic review of the literature. JACC Heart Fail. 2019;7(3):258–266. doi: 10.1016/j.jchf.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Kim E.S., Carrigan T.P., Menon V. Enrollment of women in National Heart, Lung, and Blood Institute-funded cardiovascular randomized controlled trials fails to meet current federal mandates for inclusion. J Am Coll Cardiol. 2008;52(8):672–673. doi: 10.1016/j.jacc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Vitale C., Fini M., Spoletini I., Lainscak M., Seferovic P., Rosano G.M. Under-representation of elderly and women in clinical trials. Int J Cardiol. 2017;232:216–221. doi: 10.1016/j.ijcard.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 38.Hazell L., Shakir S.A. Under-reporting of adverse drug reactions : a systematic review. Drug Saf. 2006;29(5):385–396. doi: 10.2165/00002018-200629050-00003. [DOI] [PubMed] [Google Scholar]
- 39.McLernon D.J., Bond C.M., Hannaford P.C. Adverse drug reaction reporting in the UK: a retrospective observational comparison of yellow card reports submitted by patients and healthcare professionals. Drug Saf. 2010;33(9):775–788. doi: 10.2165/11536510-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Watson S., Chandler R.E., Taavola H. Safety concerns reported by patients identified in a collaborative signal detection workshop using Vigibase: results and reflections from Lareb and Uppsala monitoring centre. Drug Saf. 2018;41(2):203–212. doi: 10.1007/s40264-017-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson S., Caster O. A vigiPoint characterisation of female versus male reports in VigiBase, the WHO global database of individual case safety reports. Dryad, Dataset. 2019 [Google Scholar]
- 42.Rolfes L., van Hunsel F., Wilkes S., van Grootheest K., van Puijenbroek E. Adverse drug reaction reports of patients and healthcare professionals-differences in reported information. Pharmacoepidemiol Drug Saf. 2015;24(2):152–158. doi: 10.1002/pds.3687. [DOI] [PubMed] [Google Scholar]
- 43.Rolfes L., van Hunsel F., Caster O., Taavola H., Taxis K., van Puijenbroek E. Does patient reporting lead to earlier detection of drug safety signals? A retrospective comparison of time to reporting between patients and healthcare professionals in a global database. Br J Clin Pharmacol. 2018;84(7):1514–1524. doi: 10.1111/bcp.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]