Abstract
Red palm weevil (RPW) causes severe damage to date palm trees, leading to the death of trees if not detected and treated in time. A major obstacle in RPW control is the difficulty in identifying an early stage infestation In the present study, we measured the efficacy of some non-invasive optical devices including cameras (digital camera and thermal camera), TreeRadarUnit™ (TRU) (Radar 2000, Radar 900), resistograph, magnetic DNA biosensor, and Near-infrared Spectroscopy (NIRS) to detect RPW infestation in date palm trees under field conditions at Riyadh, Saudi Arabia. Date palm trees used in these experiments were selected based on visual observations. After inspection of date palm trees with different devices to detect RPW infestation, each tree was taken down and dissected in detail to validate the accuracy of each device. Results indicated that the visual RPW detection approach presented the highest accuracy (87%) followed by Radar 2000 (77%), Radar 900 (73%), resistograph (73%), thermal camera (61%), and digital camera (52%).
Moreover, different stages of RPW placed in plastic cups were fastened onto the healthy date palm trunks to judge RPW presence, the magnetic DNA biosensor correctly detected RPW eggs 75% of the time, followed by detection of larvae (64%) and the control (empty cup) (54%). In another experiment where determinations were made in an open area, the efficiency of the DNA biosensor for detecting adults was 100%, followed by 83%, 63%, 60%, and 39% for pupae, larvae, eggs, and control, respectively. Absorption spectra generated through NIRS for infested, wounded, and control samples of date palm tissue showed a remarkable variation in the gradient of the corresponding peaks between 1850 nm and 1950 nm. Based on the detection efficiency of the tested devices, the resistograph and NIRS have the best potential to detect RPW infestation in date palm trees.
Keywords: Phoenix dactylifera, TreeRadarUnit™, Resistograph, Thermal camera, Digital camera, Magnetic DNA biosensor, Near-infrared Spectroscopy
1. Introduction
Saudi Arabia is a leading date palm (Phoenix dactylifera L.) growing country having over 23 million date palm trees; it is ranked as the 4th major date producer worldwide, with 754,761 metric tons/ annum produced (FAO, 2017). Unfortunately, the date palms are under the constant threat of red palm weevil (RPW) Rhynchophorus ferrugineus (Coleoptera, Curculionidae) infestation, which invaded Saudi Arabia in 1987 (Abraham et al., 2001). RPW is a date palm borer that feeds on the internal tissues of the date palm trunk, ultimately causing the death of the infested tree if the infestation is not detected and treated in time (Gutiérrez et al., 2010). The female weevil lays around 180–339 eggs during its entire lifespan of 3–6 months. The eggs hatch after 2–3-days of incubation, and neonate larvae start feeding on date palm tissues and reach pupal stage through 10-larval instars in 35–40 days. The adult weevils emerge after 15–20 days and the entire lifecycle is completed in 5–9 months (Al-Ayedh, 2008).
RPW is a very serious pest of date palm originated from South East Asia. It rapidly extended its geographical as well as host range within the last few decades. Despite tremendous efforts taken to stop its further proliferation the pest is spreading globally. Weak quarantine enforcement and difficult early detection are major obstacles in its successful management.
Saudi Arabia has over 80,000 date palm trees infested with RPW that are posing a considerable threat to the neighboring healthy trees (Al-Sheaby, 2010). RPW activities are well concealed in the date palm trunk, and visual detection at early stages of infestation is quite challenging. Identification of date palm trees at early stages of RPW infestation, particularly when the palm heart is still intact, can be successfully cured. Visual signs of RPW infestation appear at advanced stages when the chances of recovery are almost negligible. RPW infestations have been reported from the majority of date palm producing countries, covering the entire Middle East (Faleiro, 2006).
Although several pre-emptive and curative measures have been taken, including adoption of the cultural practices, mass pheromone trapping, biological control (entomo-pathogenic fungi and nematodes), and application of insecticides. The success of all these control programs is based on the detection of RPW infestation at early stages. Indeed, the main impediment for the successful management of RPW has been the absence of an effective early detection technique. Various approaches and devices have been developed for detection of RPW infestations, including visual inspections and use of pheromone traps (Faleiro and Kumar, 2008), acoustic sensors (Potamitis et al., 2009), and sniffer dogs (Nakash et al., 2000); but each technique suffers certain limitations impeding their success. Therefore, an effective and efficient method is needed to be explored to detect RPW infestation in date palm at its early stages to take a rapid remedial action to save trees life.
Thermal cameras have been used for years to assess plant stress in agriculture and to find concealed activities within tree trunks (Prince et al., 2015, Gutiérrez et al., 2018, Hoffmann et al., 2013). RPW larvae cut off the vascular system while feeding on the date palm trunk tissues and produce water stress that can be identified with thermal imaging. Digital cameras generate digital images of the plants and help in visual analysis to detect any variation between healthy and infested plant images (Miranda et al., 2014, Vanegas et al., 2018).
Tree radar units (TreeRadarUnit™) use a ground-penetrating radar technique to sense the decaying of the internal tissues of the tree trunk (Xiao et al., 2018, Butnor et al., 2009). This innovative technology is commonly used in arboriculture to observe the internal status of woody plants. Resistographs are reliable devices used to detect decay and cavities in woody trees (Butnor et al., 2009, Halabe et al., 2009, Li et al., 2018). It comprises a micro drill that is introduced horizontally into the trunk and the variation in resistance in the wood is depicted into a graph. The instrument used in the present study was “IML RESI PD500”, USA.
Development of biosensors is an emerging field, and they involve the use of bio-entities including RNA, DNA, and proteins. Biosensors receive biological signals and transform them into a detectable electrical signal. Biosensors are widely used in food industries (Murugaboopathi et al., 2013), environmental studies (Justino et al., 2017), bio-terrorism prevention (Walper et al., 2018), and disease diagnostics (Fracchiolla et al., 2013).
Near-infrared Spectroscopy (NIRS) has been substantially documented because of its promising results in agriculture (Williams and Norris, 1987, Kemsley et al., 2008) food (Johnsen, 1997, Büning-Pfaue et al., 1998, Kaffka, 2008, Pallav et al., 2009, Hoyer, 1997), petroleum (Falla et al., 2006, Syunyaev et al., 2009), and pharmaceutical industries (Amigo et al., 2008, Blanco et al., 2008, Xiang et al., 2009, Sando and Dubois, 2010). Also, NIRS approach has been widely used for non-invasive analysis and monitoring of biological systems. Several studies have been conducted to determine physiological changes in living organisms using NIRS (Tsenkova et al., 1996, Ciurczak, 2001).
Several studies have also been conducted involving NIRS and insects (Dowell et al., 1998, Dowell et al., 1999, Ridgway et al., 1999). The particular chemical configuration of an object having a specific level of excited molecules absorbs light in the NIR region and vibrates at a distinct frequency level (Murray, 1987). The pattern of NIR spectra is mainly influenced by the concentration and type of chemical bonds (CH, NH and OH) of the analyzed material (Foley et al., 1998).
The present study was conducted to evaluate the performance of some devices including cameras (digital camera and thermal camera), TreeRadarUnit™ (TRU) (Radar 2000, Radar 900), resistograph, magnetic DNA biosensor, and NIRS in detecting RPW infestation with the actual infestation (determined by dissection of the date palm trees) used as a standard.
2. Materials and methods
The current study was undertaken to measure efficacy of the different optical devices including cameras (digital camera and thermal camera), radar units (Radar 2000, Radar 900), resistograph, magnetic DNA biosensor, and NIRS to detect RPW infestation in date palm trees under field conditions in Riyadh, Saudi Arabia.
Initially, 80 date palm trees were identified as RPW infested trees through visual observations at random, and tagged. Only 31 date palm trees were selected to examine with cameras and other devices.
2.1. Digital camera
Images taken with the digital camera were analyzed using computer aided drafting (CAD) software to differentiate between RPW-infested and healthy palm trees.
2.2. Thermal cameras
Images taken with the thermal camera were analyzed to compare the internal temperature of the date palm trees present under similar weather conditions. A palm tree was considered infested if its internal temperature was higher than that of the other neighboring trees.
2.3. TreeRadarUnit™ (TRU)
TRUs (TRU 2000 MHz and TRU 900 MHz) were fixed at three different heights (20, 100, and 150 cm) from the ground level depending upon the height of the date palm trunk. To avoid any discrepancy in the data, the observations were first taken by fixing the units on the rough surface (of the date palm trunk), which was then cleaned (shaved) enough for better fit of the TRU on the trunk.
2.4. Resistograph
Date palm trees were also examined with the resistograph, a probe that was horizontally inserted into the date palm trunks to monitor the relative resistance of trunk tissues. The data were generated in terms of graph on the machine. Resistograph generated instant results that were recorded as under:
| Healthy | = (–) |
| Low infestation | = (+1) |
| Medium infestation | = (+2) |
| Heavy infestation | = (+3) |
After inspection of date palm trees with different devices, each tree was taken down, and subsequently, the trunk was cut longitudinally into 1 m sections, i.e., logs. Each log was examined from the base and to the top to record any sign of RPW infestation. Additionally, for comprehensive internal inspection, each log was first cut into two equal halves and then into four quarters. Each quarter was observed thoroughly i.e., peripheral side, inner side, top, and base, to record any sign of RPW infestation (galleries) and to record the live and dead RPW individuals to validate the accuracy of the device for detection of RPW infestation. The procedure of cutting the trees has been presented in Fig. 2 and log coding is represented in Table 1.
Fig. 2.
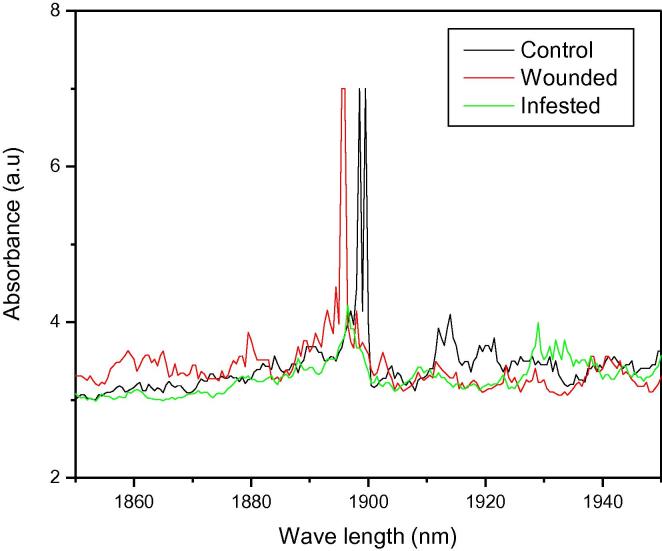
Response of the leaf spectral absorbance to control, wounded, and infested date palm leaves in NIR range (1850 nm to 1950 nm).
Table 1.
Comparative efficacy of different optical devices for red palm weevil infestation detection in date palm trees at Riyadh, Saudi Arabia.
| Method | n | Accuracy (%) |
|---|---|---|
| Actual | 31 | 100 ± 0a |
| Visual | 31 | 87 ± 6ab |
| Thermal Camera | 31 | 61 ± 9b |
| Digital Camera | 30 | 52 ± 9b |
Means followed by the same letter in same column were not significantly different at α: 0.05. n = number of date palm trees observed
2.5. Detection with magnetic DNA biosensor
2.5.1. Experiment 1
A handy antenna-based magnetic DNA biosensor was tested for RPW detection in a date palm orchard. The experiment was carried out using RPW eggs, larvae, and control (moist tissue paper only).
Stages of RPW were wrapped in moist tissue paper and placed in a plastic cup with perforated lid for aeration. In control, only tissue papers were placed in plastic cups. After screening date palm trees for RPW infestation, the cups with RPW stages and control were attached with healthy date palm trees using adhesive tape. Each tree with a plastic cup was monitored for RPW detection using the magnetic DNA biosensor. Judgment was recorded for RPW presence to calculate the accuracy percentage.
2.5.2. Experiment 2
2.5.2.1. Sample preparations
One hundred and sixty samples were prepared comprising of seven groups: eggs, larvae, pupae, adults, control (empty), fresh diet without exposure to RPW, and old diet exposed to RPW. Each group contained 15 replicates. Samples were prepared by wrapping the samples in moist tissue paper and placed in plastic cups with perforated lids for aeration.
2.5.2.2. Testing area set up
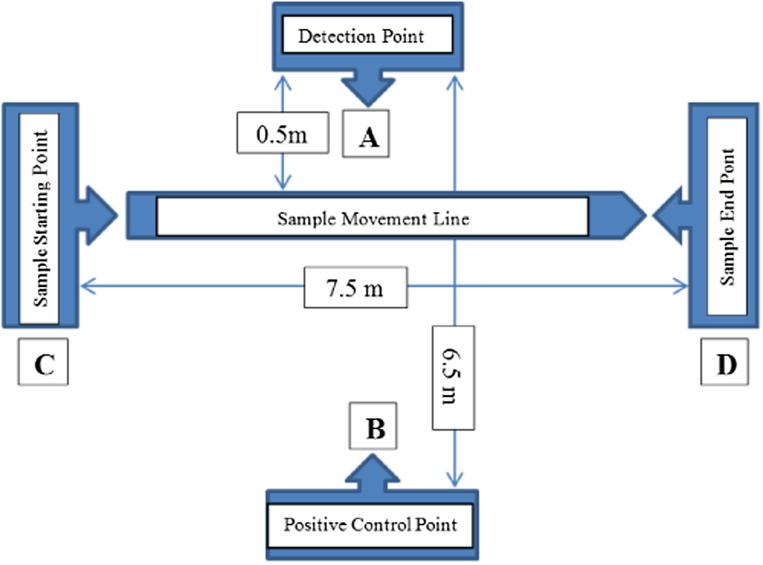
The testing area was mapped as given in the picture below. It consisted of five points: (A) Detection point, (B) Positive control point (where the RPW were placed inside the plastic cups), (C) Sample movement starting point, (D) Sample movement endpoint.
The distance between detection point (A) and Positive control point (B) was 6.5 m; the distance from sample starting point (C) to endpoint (D) was 7.5 m; whereas, the distance from detector point to the sample movement line (C-D line) was 0.5 m. The groups of samples prepared for testing were placed out of this area.
2.5.2.3. Red palm weevil detection procedures
a. Detector calibration
The detector (A) represented by the antennae movement (left–right movement) should direct towards point B consistently, indicating the detector was in excellent working condition. This step was repeated for every sample testing.
b. Initial checking:
Before passing sample for detection the bearer of sample was screened by passing on sample movement line without sample.
Testing procedure
The sample bearer was asked to move from C point to D point crossing the detector point (A) and adjust if needed. The antennae detector should face toward the positive control point (B) before the sample bearer crosses the detector. After the sample bearer crosses the detector point (A), there were two possibilities: first, the antennae detector did not move and stays facing toward the positive point (B) indicating the sample was negative; second, the direction of the antennae detector moves toward the sample bearer, deviating from the positive point (B), indicating the sample was positive. The working principle of the detector was to detect the closest positive sample.
Observations of the tested samples were recorded and analyzed using the analysis of variance (ANOVA) PROC GLM procedure of SAS (SAS Institute, 2009), and means were separated using the Least Significant Difference (LSD) (P = 0.05).
2.6. Near Infrared Spectroscopy (NIRS)
NIRS was applied to measure the absorbance spectra for RPW infested, wounded, and control fresh date palm leaves samples using the JASCO Spectrophotometer Model JASCO-V670.
Date palm plants belonging to Khudry cultivar were purchased from Al Rahji laboratory for tissue culture, Riyadh. Plants were distributed into three groups (G1, G2, and G3) each containing three plants where each plant was considered as a replicate. G1 plants were infested with larval stage of RPW, G2 plants were mechanically wounded, whereas G3 plants were considered as control. G1 plants were infested by introducing 5 RPW larvae (2nd instar) by drilling holes in the lower part of the date palm trunk using a 6-mm size bit. Wounds were created in G2 date palm plants with the same drill machine. Artificial wound treatment was undertaken to differentiate between RPW and artificial wound effects as the RPW larvae were also to be introduced into the date palm plants after making holes with drill machine as mentioned above. All experimental plants were covered with fine steel mesh. Plants with each treatment were kept in separate places having uniform conditions to avoid any stress-related communication among the plants. Leaf samples were taken from the control, wounded, and infested date palm plants three days after treatment and stored immediately at −80 °C for further studies. To take NIR absorbance spectra, 1 × 1 cm2 sample disks were cut from these leaves and cleaned with fine tissue in order to remove dust or other unwanted impurities. Then these samples were fixed in the sampling port to take absorption spectra between 760 nm and 2100 nm range.
3. Results and discussions
3.1. Comparative efficacy of different optical devices
In the first experiment 31date palm trees were examined visually as well as with digital and thermal cameras, and results revealed that the RPW detection accuracy with both cameras was significantly lower than the actual observation. On the other hand, visual detection was closer to actual RPW infestation with no statistical difference (Table 1). Visual detection presented the highest accuracy (87%), followed by thermal (61%) and digital camera (52%), but remained statistically at par with the others.
TRUs (Radar 2000, Radar 900) and resistograph were also used to examine the date palm trees at 20, 100, and 150 cm above ground level. The RPW detection accuracy (percentage) of these devices was confirmed through dissection of the trees examined (Fig. 1, Fig. 2) and was considered as actual infestation. Results obtained with different devices were statistically compared with actual infestation. Results indicated no statistical difference for RPW detection accuracy percentage among Radar 2000, Radar 900, and Resistograph at 20 cm height (Table 2). Radar 2000 exhibited significantly lower accuracy percentage as compared to actual infestation but Radar 900 and Resistograph were found at par with actual infestation. The non-significant difference is probably because of high variation in the data and standard error. The highest RPW infestation detection accuracy (78%) was achieved with Radar 900, followed by resistograph (76%) and Radar 2000 (73%).
Fig. 1.
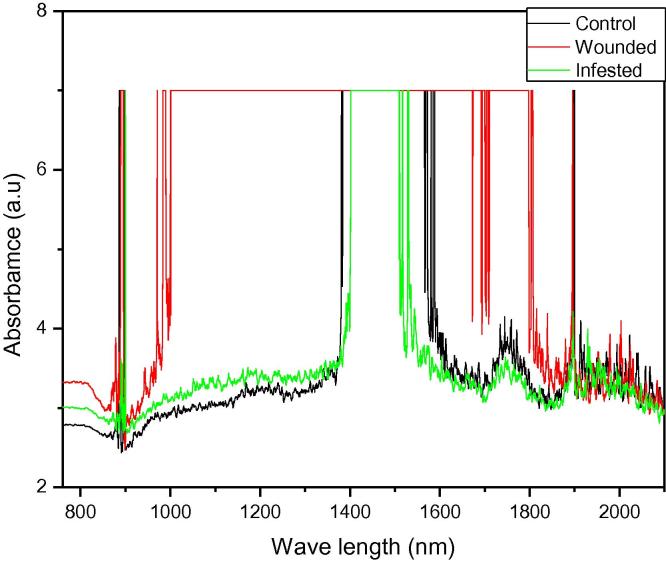
Response of the leaf spectral absorbance to control, wounded, and infested date palm leaves in NIR range.
Table 2.
Comparative efficacy of different optical devices for red palm weevil infestation detection at different heights of date palm trunk above ground level at Riyadh, Saudi Arabia.
| Devices | Red palm weevil infestation detection accuracy assessment at different heights of date palm trunk |
|||||
|---|---|---|---|---|---|---|
| n | 20 cm | n | 100 cm | n | 150 cm | |
| Actual | 31 | 100 ± 0.0a | 31 | 100 ± 0.0a | 31 | 100 ± 0.0a |
| Radar 2000 | 41 | 73 ± 7b | 33 | 72 ± 8a | 23 | 91 ± 6ab |
| Radar 900 | 9 | 78 ± 15ab | 3 | 67 ± 33a | 3 | 67 ± 33b |
| Resistograph | 25 | 76 ± 9ab | 21 | 62 ± 11a | 13 | 85 ± 10ab |
Means followed by the same letter in same column were not significantly different at α: 0.05. n = number of date palm trees observed.
Similarly, results indicated no statistical difference for RPW detection accuracy percentage among Actual, Radar 2000, Radar 900, and Resistograph at 100 cm height (Table 2). The highest accuracy was observed where trees were examined with Radar 2000 (72%) followed by Radar 900 (67%) and Resistograph (62%).
Next, no statistical difference for RPW detection accuracy was observed between actual, Radar 2000, and resistograph except Radar 900, which presented significantly lower accuracy as compared with actual accuracy but at par with Radar 2000 and resistograph at 150 cm height (Table 3). However, highest accuracy was observed where trees were examined with Radar 2000 (91%), followed by resistograph (85%), and Radar 900 (67%).
Table 3.
Overall, comparative efficacy of different optical devices for red palm weevil infestation detection at Riyadh, Saudi Arabia.
| Devices | Red palm weevil infestation detection accuracy assessment |
|
|---|---|---|
| n | Mean ± SE | |
| Actual | 31 | 100 ± 0 a |
| Visual | 31 | 87 ± 6 ab |
| Radar 2000 | 97 | 77 ± 4b |
| Radar 900 | 15 | 73 ± 12b |
| Resistograph | 59 | 73 ± 6b |
Means followed by the same letter in same column were not significantly different at α: 0.05. n = number of date palm trees observed.
Comparing the detection accuracy among three heights (20, 100 and 150 cm), greater accuracy was observed at 150 cm with Radar 2000 (91%), followed by resistograph (85%).
Overall, comparative efficacy of visual, Radar 2000, Radar 900, and resistograph RPW detection indicated that visual detection showed highest accuracy percentage (87%), followed by Radar 2000 (77%), Radar 900 (73%), and resistograph (73%).
3.2. DNA magnetic sensor
In the first experiment, where RPW stages placed in plastic cups provided with moist tissue paper were tied onto the trunk of healthy date palm trees to determine RPW presence, results indicated that highest correct reading was recorded for egg stage (75%), followed by larva (64%), and lowest in control (54%). Results did not show any significant difference between eggs, larvae and control judgment accuracy. But the accuracy level was found to be higher in RPW stages as compared to control (Table 4).
Table 4.
Evaluation of magnetic DNA biosensor for Red palm weevil detection.
| Life stage | Average correct judgment percentage |
|
|---|---|---|
| n | Mean ± SE | |
| Eggs | 8 | 75 ± 16a |
| Larvae | 14 | 64 ± 13a |
| Control | 26 | 54 ± 10a |
Means followed by the same letter in same column were not significantly different at α: 0.05. n = number of date palm trees observed.
In the second experiment, where judgement was made in an open area, the efficiency of the DNA biosensor for detecting adults was 100%, followed by 83%, 63%, 60%, and 39% for pupae, larvae, eggs, and control (empty cup). No significant difference was observed in detection of adults, larvae, pupae, and eggs, but that of the control was significantly lower than detection of adults, but statistically at par with pupae, larvae, and egg stages (Table 5).
Table 5.
Evaluation of magnetic DNA biosensor for red palm weevil detection.
| Objects | n | Mean ± SE |
|---|---|---|
| Adult | 5 | 100 ± 0a |
| Pupa | 5 | 80 ± 20 ab |
| Larva | 24 | 63 ± 10 ab |
| Egg | 20 | 60 ± 11 ab |
| Control (empty cups) | 69 | 39 ± 6b |
Means followed by the same letter in same column were not significantly different at α: 0.05. n = number of date palm trees observed.
Results indicated a high efficiency of the magnetic DNA biosensor for adult detection followed by other stages of RPW, but very poor performance with the control, which is a question mark either for the efficiency of the machine or user expertise. Therefore, further improvements may be required either in the machine or user expertise or both.
3.3. Near Infrared Spectroscopy (NIRS)
The absorption spectra for different treatments, including infested, wounded, and control samples generated by NIRS are shown in Fig. 1, Fig. 2. A remarkable variation in intensity of the corresponding peaks of these spectra was observed. The spectra revealed change in the elemental concentration of the constituents of the samples. More prominent lines of the spectra between 1850 nm and 1950 nm have been shown in Fig. 2. We have also observed a shift in the corresponding peaks. Results indicated that NIRS has great potential to be exploited for the detection of RPW infestation in date palm trees. Moreover, such investigation with absorption spectroscopy will not only detect the RPW infestation, but it will also suggest the remedy to the infection by identifying the elemental concentration before and after infection.
Devices tested for RPW detection in date palm, including digital and thermal cameras, TreeRadarUnit™ (TRU) (Radar 2000, Radar 900), resistograph, magnetic DNA biosensor, and NIRS have been used for decades to detect anomalies in plants caused by biotic or abiotic stresses (Halabe et al., 2009, Li et al., 2018, Fracchiolla et al., 2013, Pallav et al., 2009). In the present study, the accuracy of digital and thermal cameras was around 50 to 60%, whereas visual detection was 87%. Such a low accuracy might be because of the fact that in date palm, RPW infestation occurs generally at the basal portion of the trunk while both cameras rely on changes appearing in the crown. The accuracy of TRU 2000 and the resistograph was quite promising; they successfully detected RPW infestation at 1.5 m height 91% and 85% of the time, respectively. RPW infestation activities occur mostly at this position in date palm. Overall comparison of TRU and resistograph indicated that Radar 2000 (77%) was more accurate than Radar 900 (73%) and resistograph (73%). In all cases, the accuracy of these devices was found to be quite lower than that of actual (validated by dissecting the date palm trees) and visual detection approaches.
The magnetic DNA biosensor was used to detect adult, pupa, larva, and egg stages of RPW in comparison with control (empty box). The highest accuracy was observed with adults (100%), followed by pupa (80%), larva (63%), eggs (60%), and control (39%).
NIRS absorption spectra for infested, wounded, and control date palm samples generated a remarkable variation in gradient of the corresponding peaks. Results indicated that NIRS is a promising technique for the early detection of RPW infestation.
4. Conclusions
Non-invasive optical devices including cameras (digital camera and thermal camera), TreeRadarUnit™ (radar 2000, radar 900), resistograph, magnetic DNA biosensor, and NIRS used to detect RPW infestation in date palm trees under field conditions indicated that resistograph and NIRS have great potential to detect RPW infestation in date palm trees.
Acknowledgement:
The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support and for funding the present work through Research group (No: RGP-1438-009).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Khawaja Ghulam Rasool, Email: gkhawaja@ksu.edu.sa.
Mureed Husain, Email: mbukhsh@ksu.edu.sa.
Shehzad Salman, Email: shehzadsalman@gmail.com.
Muhammad Tufail, Email: mtufail.ksu@gmail.com.
Sukirno Sukirno, Email: sukirnobiougm@ugm.ac.id.
Khalid Mehmood, Email: sirum_mk@yahoo.com.
Wazirzada Aslam Farooq, Email: awazirzada@ksu.edu.sa.
Abdulrahman S. Aldawood, Email: aldawood@ksu.edu.sa.
References
- Abraham V.A., Faleiro J.R., Al-Shuaibi M.A., Al-Abdan S. Status of pheromone trap captured female red palm weevils from date gradens in Saudi Arabia. J. Trop. Agri. 2001;39:197–199. [Google Scholar]
- Al-Ayedh H. Evaluation of date palm cultivars for rearing the red date palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) Fla. Entomol. 2008;91:353–359. [Google Scholar]
- Al-Sheaby, F., 2010. SABIC launches Red Palm Weevil workshop as part of its commitment to global corporate social responsibility. <http://www.sabic.com/corporate/en/newsandmediarelations/news/20100331-2.aspx>.
- Amigo J.M., Cruz J., Bautista M., Maspoch S., Coello J., Blanco M. Study of pharmaceutical samples by NIR chemical-image and multivariate analysis. Trends Analyt. Chem. 2008;27:696–713. [Google Scholar]
- Blanco M., Cruz J., Bautista M. Development of a univariate calibration model for pharmaceutical analysis based on NIR spectra. Anal. Bioanal. Chem. 2008;392:1367–1372. doi: 10.1007/s00216-008-2426-9. [DOI] [PubMed] [Google Scholar]
- Büning-Pfaue H., Hartmann R., Harder J., Kehraus S., Urban C. NIR-spectrometric analysis of food. Methodical development and achievable performance values. Fresenius J. Anal. Chem. 1998;360:832–835. [Google Scholar]
- Butnor J.R., Pruyn M.L., Shaw D.C., Harmon M.E., Mucciardi A.N., Ryan M.G. Detecting defects in conifers with ground penetrating radar: applications and challenges. For. Pathol. 2009;39:309–322. [Google Scholar]
- Ciurczak E.W. Biomedical applications of near-infrared spectroscopy. In: Burns D.A., Ciurczak E.W., editors. Handbook of Near-infrared Analysis. second ed. Marcel dekker Inc; New York: 2001. pp. 633–646. [Google Scholar]
- Dowell F., Ram M., Seitz L. Predicting scab, vomitoxin, and ergosterol in single wheat kernels using near-infrared spectroscopy. Cereal Chem. 1999;76:573–576. [Google Scholar]
- Dowell F.E., Throne J.E., Baker J.E. Automated nondestructive detection of internal insect infestation of wheat kernels by using near-infrared reflectance spectroscopy. J. Econ. Entomol. 1998;91:899–904. [Google Scholar]
- Faleiro J.R. A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sci. 2006;26:135–154. [Google Scholar]
- Faleiro J.R., Kumar J. A rapid decision sampling plan for implementing area–wide management of the red palm weevil, Rhynchophorus ferrugineus, in coconut plantations of India. J. Insect Sci. 2008;8:1–9. doi: 10.1673/031.008.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falla F.S., Larini C., Le Roux G.A.C., Quina F.H., Moro L.F.L., Nascimento C.A.O.D. Characterization of crude petroleum by NIR. J. Pet. Sci. Eng. 2006;51:127–137. [Google Scholar]
- FAO, 2017. FAOSTAT database collection. Food and Agriculture Organization of the United Nation, Rome. http://www.fao.org (accessed on 21 February 2017).
- Foley W.J., McIlwee A., Lawler I., Aragones L., Woolnough A.P., Berding N. Ecological applications of near infrared reflectance spectroscopy–a tool for rapid, cost-effective prediction of the composition of plant and animal tissues and aspects of animal performance. Oecologia. 1998;116:293–305. doi: 10.1007/s004420050591. [DOI] [PubMed] [Google Scholar]
- Fracchiolla N., Artuso S., Cortelezzi A. Biosensors in clinical practice: focus on oncohematology. Sensors. 2013;13:6423–6447. doi: 10.3390/s130506423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez A., Ruiz V., Moltó E., Tapia G., del Mar Téllez M. Development of a bioacoustic sensor for the early detection of Red Palm Weevil (Rhynchophorus ferrugineus Olivier) Crop Prot. 2010;29:671–676. [Google Scholar]
- Gutiérrez S., Diago M.P., Fernández-Novales J., Tardaguila J. Vineyard water status assessment using on-the-go thermal imaging and machine learning. PloS One. 2018;13:e0192037. doi: 10.1371/journal.pone.0192037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halabe U.B., Agrawal S., Gopalakrishnan B. Nondestructive evaluation of wooden logs using ground penetrating radar. Nondestruct. Test. Eva. 2009;24:329–346. [Google Scholar]
- Hoffmann N., Schröder T., Schlüter F., Meinlschmidt P. Potential of infrared thermography to detect insect stages and defects in young trees. J. Kulturpflanzen. 2013;65:337–346. [Google Scholar]
- Hoyer H. NIR on-line analysis in the food industry. Process Control Qual. 1997;9:143–152. [Google Scholar]
- Johnsen, E., 1997. How to use on-line NIR in the feed and food industries Process Control and Quality. 9, 205–206.
- Justino C., Duarte A., Rocha-Santos T. Recent progress in biosensors for environmental monitoring: a review. Sensors. 2017;17:2918. doi: 10.3390/s17122918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaffka K. How the NIR technology came to and spread in Europe for quality assessment and control in the food industry. Acta Alimentaria. 2008;37:141–145. [Google Scholar]
- Kemsley E.K., Tapp H.S., Binns R., Mackin R.O., Peyton A.J. Feasibility study of NIR diffuse optical tomography on agricultural produce. Postharvest Biol. Technol. 2008;48:223–230. [Google Scholar]
- Li W., Wen J., Xiao Z., Xu S. Application of ground-penetrating radar for detecting internal anomalies in tree trunks with irregular contours. Sensors. 2018;18:649. doi: 10.3390/s18020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda J.L., Gerardo B.D., Tanguilig B.T., III Pest detection and extraction using image processing techniques. Int. J. Comput. Commun. Eng. 2014;3:189. [Google Scholar]
- Murray, I., 1987. Chemical principles of near-infrared technology. Near-infrared technology in the agricultural and food industries, 17–34.
- Murugaboopathi G., Parthasarathy V., Chellaram C., Anand T.P., Vinurajkumar S. Applications of biosensors in food industry. Biosci. Biotechnol. Res. Asia. 2013;10:711–714. [Google Scholar]
- Nakash J., Osem Y., Kehat M. A suggestion to use dogs for detecting Red Palm Weevil (Rhynchophorus ferrugineus) infestation in date palms in Israel. Phytoparasitica. 2000;28:153–155. [Google Scholar]
- Pallav P., Diamond G., Hutchins D.A., Green R., Gan T. A Near-Infrared (NIR) technique for imaging food materials. J. Food Sci. 2009;74:E23–E33. doi: 10.1111/j.1750-3841.2008.01011.x. [DOI] [PubMed] [Google Scholar]
- Potamitis I., Ganchev T., Kontodimas D.C. On automatic bioacoustic detection of pests: the cases of Rhynchophorus ferrugineus and Sitophilus oryzae. J. Econ. Entomol. 2009;102:1681–1690. doi: 10.1603/029.102.0436. [DOI] [PubMed] [Google Scholar]
- Prince G., Clarkson J.P., Rajpoot N.M. Automatic detection of diseased tomato plants using thermal and stereo visible light images. PLoS ONE. 2015;10:e0123262. doi: 10.1371/journal.pone.0123262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway C., Chambers J., Cowe I.A. Detection of grain weevils inside single wheat darnels by a very near infrared two-wavelength model. J. Near Infrared Spectrosc. 1999;7:213–221. [Google Scholar]
- Sando G., Dubois J. Seeing the chemicals in pharmaceutical tablets: with NIR chemical imaging. Chim. Oggi. 2010;28:40–42. [Google Scholar]
- SAS Institute . SAS/STAT 9.2. Users guide. SAS Institute; Cary, NC: 2009. [Google Scholar]
- Syunyaev R., Balabin R., Akhatov I., Safieva J. Adsorption of petroleum asphaltenes onto reservoir rock sands studied by Near-Infrared (NIR) Spectroscopy. Energy Fuels. 2009;23:1230–1236. [Google Scholar]
- Tsenkova R., Itoh K., Natsuga M., Himoto J. Near infrared monitoring of biological objects on a dairy farm. Near Infrared Spectroscopy. 1996:565–572. [Google Scholar]
- Vanegas F., Bratanov D., Powell K., Weiss J., Gonzalez F. A novel methodology for improving plant pest surveillance in vineyards and crops using UAV-based hyperspectral and spatial data. Sensors. 2018;18:260. doi: 10.3390/s18010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walper S.A., Lasarte Aragonés G., Sapsford K.E., Brown C.W., III, Rowland C.E., Breger J.C., Medintz I.L. Detecting biothreat agents: From current diagnostics to developing sensor technologies. ACS Sensors. 2018;3:1894–2024. doi: 10.1021/acssensors.8b00420. [DOI] [PubMed] [Google Scholar]
- Williams P., Norris K. American Association of Cereal Chemists Inc.; 1987. Near-infrared Technology in the Agricultural and Food Industries. [Google Scholar]
- Xiang, D. Berry, J., Buntz, S., Gargiulo, P., Cheney, J., Joshi, Y Wabuyele, B., Wu, H., Hamed, M., Hussain, A.S., Khan, M.A., 2009. Robust calibration design in the pharmaceutical quantitative measurements with near‐infrared (NIR) spectroscopy: Avoiding the chemometric pitfalls. J. Pharm. Sci., 98, 1155–1166. [DOI] [PubMed]
- Xiao, X., Wen, J., Xiao, Z., Li, W., 2018. Detecting and measuring internal anomalies in tree trunks using radar data for layer identification. J. Sens.