Abstract
This study underpins the therapeutic potential of SEL001, a bioactive product isolated from Lactobacillus sakei probio65, in terms of its anti-inflammatory properties and its effect on gut-microbiota in a TNBS-induced ulcerative colitis mouse model. Ulcerative colitis was developed in mice by intra rectal administration of trinitrobenzene sulfonic acid. Bioactive product SEL001 (50 mg/kg b.w.) was administered orally. Myeloperoxidase activity was measured using 3,3′, 5,5′-tetramethylbenzidine. The entire colon was sampled for post-mortem clinical assessment. Colonic injury was assessed through histological and histomorphometric examinations. The 454 pyrosequencing and QIIME pipeline were used for gut microbiota analysis and statistical analysis were conducted using R. mRNA extraction from colon tissue and RT-PCR approaches were employed to determine the changes in the level of specific biomarker genes associated with UC. The results depict that SEL001 significantly lowered pro-inflammatory cytokines, including CD4, TNF-α, and interleukin-6. Examination of clinical and histopathological traits revealed that SEL001 was effective and potent in reducing the inflammatory signatures of UC to a similar extent as did by the standard drug mesalamine (5-ASA). Pyro-sequencing 16S data revealed that the reduction in the major member of phylum Firmicutes, which has been previously associated with a higher risk of UC. The SEL001, an anti-inflammatory bioactive product originated from a probiotic strain L. sakei probio65 could be an alternative therapeutic agent for treatment of UC.
Keywords: Lactobacillus sakei probio65, Ulcerative colitis, Anti-inflammatory effect, Histopathology, Gut-microbiota
1. Introduction
Inflammatory bowel disease (IBD) is a designation for the idiopathic chronic inflammatory gastrointestinal disorder, remains one of the major challenges for gastroenterologists and World Gastroenterology Organization (WGO). The term IBD covers Crohn’s disease (CD) and ulcerative colitis (UC), and are known to affect approximately 10–20 per 100,000 people per year (Mallon et al., 2007), has a high recurrence rate and eventually lead to high morbidity. Ulcerative colitis affects the large bowel and the associated clinical manifestations include chronic hemorrhagic diarrhea, abdominal cramps and pain, gut perforation, and complete bowel obstruction, which significantly affect the quality of life of the ailing person. The precise etiology of the disease is not well recognized; however, host genetic factors, immune disequilibrium, environmental factors, and host-microbial composition are believed to be responsible for the development and progression of the disease (Loftus, 2004, Hatoum and Binion, 2005, Xia et al., 2019, Kengo et al., 2019). Current medical interventions for managing IBD are not satisfactory and does not lead to complete remission of symptoms. Further, UC patients frequently experience relapse and conventional treatments are not sufficient to keep remission for longer durations. Failure of standard treatments for UC leads to colon removed in about 33–50% of cases. The front-line therapy for UC consists of a large derivative chain of aminosalicylates, the components actively known for their anti-inflammatory properties (Rousseaux et al., 2005, Sandborn, 2007). Mesalamine is a new generation formulation of 5-ASA and its clinical trials have displayed significant effectiveness for the treatment of mild to moderate form of UC (Sutherland and MacDonald, 2007a, Sutherland and MacDonald, 2007b). Mesalamine along with 5-ASA lines of drugs has adverse clinical side effects, sub-grouped into dose-related intolerance effects and dose-independent idiosyncratic reactions. Clinical side effects of these drugs can lead to symptoms like regurgitation, nausea, headache, and abdominal pain, which are results of dose-related intolerance (Ham & Moss, 2012). Whereas, idiosyncratic reactions include male infertility, anemia, hepatic and pulmonary dysfunction, hypersensitivity rash, worsening colitis, renal toxicity, interstitial lung disease, cardiopulmonary hypersensitivity etc (Ransford and Langman, 2002, Ferrusquía et al., 2015). The increased burden of the UC patients is further worsened by the limited spectrum UC curable by 5-ASA lines of drugs and their alarming side effects have placed an inevitable demand for the development of broad-spectrum, effective, and safe alternatives for the treatment of UC.
The term “probiotic” refers to the administration of live microorganisms when taken in sufficient amount (>107 CFU/mL) have a beneficial effect on the host health (Plaza-Diaz et al., 2017). Also, cellular components of these probiotic microorganisms confer health benefits (Matsumoto et al., 2005, Giahi et al., 2012). The probiotics are taken in different forms and are believed to maintain the healthy gut function (Matsumoto et al., 2005, Tojo et al., 2014). Additionally, probiotics are suggested as therapeutic mediators for gastrointestinal disorders and many pathologies, including inflammatory diseases (Plaza-Díaz et al., 2017, Tojo et al., 2014). The extract of probiotic Lactobacillus sakei proBio-65 has shown promising effects in treating imiquimod induced psoriasis like skin inflammation (Rather et al., 2018). Suggesting efficacy of extract from probiotic strains, and this could be explored for the treatment of other diseases.
As a consequence of intestinal micro-flora dysbiosis, the epithelium mounts an uncoordinated and constitutively ongoing immune response, which has been linked with initiation, and the establishment of IBD. An increasing body of evidence advocates that restoration of healthy intestinal microbiota through the administration of probiotics could be beneficial for short as well as long-term management of UC. Preciously, we have reported that L. sakei probio65 and its extract SEL001 exhibits number of novel pharmacological effects against atopy and psoriasis (Park et al., 2008, Kim and Pyo, 2012, Kim et al., 2013, Park et al., 2014, Kim et al., 2015, Rather et al., 2018).
In this research work, we aimed to evaluate the therapeutic potential of SEL001 for its anti-inflammatory efficacy in a mouse model and its effect on gut-microbiota composition.
2. Materials and methods
2.1. Chemicals and reagents
For extraction of SEL001, commercial grade ethanol was acquired from Duksan pure chemical Co. Ltd, (Gyeonggi-do, Korea). TNBS, mesalamine, and 3,3′, 5,5′-tetramethylbenzidine were acquired from Sigma-Aldrich (St. Louis, MO, USA). Trizol™ reagent was acquired from Life Technologies (Carlsbad, USA).
2.2. Experimental animals
Colitis studies were performed in 6-wk old ICR mice (n = 30) procured from Samtaco Bio Co. (Osan, Korea). Mice were maintained in a regulated facility of 22 ± 2 °C temperature, 55 ± 10% relative humidity and were exposed to a 12-h cycle of light and dark. Further, two groups of mice; control and treatment were marked, and each group consisted six mice. All the mice were fed on a standard rodent diet and had regular access to water. The Animal Ethical Committee of the Yeungnam University (Gyeongsan, Korea) approved the approval for research (YNU-ANETCOMM-2015-00120).
2.3. Preparation of SEL001
The preparation and chemical composition of SEL001 was carried out by the method described in our previous study (Rather et al., 2018). Briefly, Lactobacillus sakei Probio65 was cultured for 48 h in De Man, Rogosa and Sharpe (MRS) medium. Then the culture was assorted with commercial grade ethanol (1:2 ratio). Further, the combination was incubated at room temperature for 6 h with constant shaking at 150 rpm followed by centrifugation for 10 min centrifuged at 8000g. Finally, supernatant was carefully collected, filtered (whatman No. 1) and freeze dried. A detailed chemical composition profile of ethanolic extract of a probiotic strain L. sakei probio65 (SEL001) has been summarized in Table 2. The results are described in our previous publication (Rather et al., 2018).
Table 2.
The GC–MS chemical composition profile of ethanolic extract (SEL001) derived from a probiotic strain L. sakei probio65.
| SN | Compound | Retention time (min) | Relative peak area (%) |
|---|---|---|---|
| Organic acids | |||
| 1 | D-lactic acid | 4.84 | 33.86 |
| 2 | L-lactic acid | 8.52 | 6.03 |
| 3 | Propanoic acid | 9.79 | 16.65 |
| 4 | Oleic acid | 64.46 | 0.16 |
| Amino acids | |||
| 5 | 1,2-Bis (trimethylsiloxy) ethane | 6.86 | 0.30 |
| 6 | Valine | 54.72 | 1.12 |
| 7 | L-proline | 59.00 | 0.38 |
| Phenols | |||
| 8 | Taxicatigenin | 55.73 | 0.33 |
| 9 | Hexahydro-3-phenylmethyl | 71.20 | 0.78 |
| 10 | 4-(Methylsulfanylphenyl) carbamic acid | 57.97 | 1.32 |
| Imidazole derivatives | |||
| 11 | 8-Allyl-2-amino-6-methylimidazo-1,3,5-triazin | 16.50 | 0.95 |
| 12 | Imidazo diazapinone | 65.55 | 0.35 |
| Sugar alcohols | |||
| 13 | Threitol | 15.70 | 0.24 |
| 14 | Glycerol | 21.48 | 16.6 |
| 15 | myo-inositol | 63.15 | 1.87 |
| Others | |||
| 16 | 1,3-Butandiol | 8.87 | 1.35 |
| 17 | Pentasiloxane | 15.13 | 0.42 |
| 18 | 3-Methyl octahydropyrrolo pyrazine | 50.16 | 0.54 |
| 19 | Clemastine | 51.57 | 0.88 |
| 20 | Pyrrolo[1,2–1] pyrazine-1,4-dione | 52.56 | 1.26 |
| 21 | 3,6-Dioxa-2,7-disilaoctane | 7.33 | 1.31 |
| 22 | Diethyldithiophosphinic acid | 58.96 | 0.63 |
| 23 | 2,5-Piperazinedione | 64.63 | 0.36 |
| 24 | 1,3-Bis (4-chlorobenzyl)-5,6-dihydrobenzo-quinazoline | 66.33 | 0.22 |
| 25 | Dimethoxyphenyl tetrahydropyran | 66.95 | 0.55 |
| 26 | 3-benzyl-1,4-diaza-2,5-dioxobicyclononane | 69.60 | 0.33 |
| 27 | Oleamide | 71.02 | 0.25 |
| 28 | Erucylamide | 87.07 | 0.39 |
2.4. Colitis induction in experimental mice and treatments
Colitis induction procedure followed in this study was similar as described by the Morris et al. (1989). In brief, mice were allowed to fast for 24 h before induction of colitis and subsequent therapeutic treatments. Mice were exposed to light anesthesia-using diethyl ether and then exposed to the intra-rectal administration of 0.6 mg of TNBS in 0.1 ml of 30% ethanol using a trocar needle for colitis induction. A polyethylene catheter was introduced intra-rectally 4–5 cm into the distal part of the colon, which was followed by intraluminal application of a calculated dose of TNBS for colitis induction. Each mouse was hold in head-down positing for sixty seconds to ascertain proper distribution of TNBS within the entire colon and cecum and avoid leakage of the solution. Following a similar procedure, mice (n = 12) were similarly randomized in two control groups consists of 6 mice in each group; the first control group (n = 6) without TNBS induction, designated as healthy, received sterile water intra-rectally on day 0, and second control group (n = 6) received 0.1 ml of 30% ethanol (EtOH).
Twenty-four hours post-TNBS application, mice were randomized to three oral-treatment protocols; vehicle-saline (untreated group n = 6), 5-ASA (n = 6) and freeze-dried ethanolic extract (SEL001; 50 mg/kg; n = 6) for 7 days. The healthy control group (n = 6) received water at consequent interims that paralleled to treatment protocols. Mice were routinely inspected for their behavioral output and body mass. Mice were sacrificed with an overdose of diethyl ether at the end of day 7.
2.5. Myeloperoxidase (MPO) activity assay
Myeloperoxidase (MPO), a marker for neutrophil infiltration of tissues represent a measure of severity caused by colonic inflammation. The peroxidase activity was checked using 3,3′, 5,5′-tetramethylbenzidine (TMB) reagent as a colorimetric substrate (Fitzpatrick et al., 2000). Briefly, a 3 cm colonic segment was homogenized, followed by centrifugation at 10,000 rpm for 10–15 mins. The pellet obtained was retained and used for the measurement of MPO activity. The MPO activity was expressed as units per 3 cm of the colon.
2.6. Clinical and histological evaluation of colitis
Clinical assessment of TNBS induced colitis included a routine monitoring of changes in body weight and diarrhea for each mouse. The entire colon was sampled for post-mortem clinical assessment of parameters such as variation in colon length, and colon weight across for all treatment groups.
The colonic injury was further assessed through histological and histomorphometric examinations. Crossly trimmed colonic tissue sections, 6–7 cm proximal to the rectum are excised, and then fixed in 10% neutral buffered formalin phosphate for 24 h. In order to address the extent of inflammation, the paraffin-embedded tissue sections (3–4 μm) were stained with hematoxylin and eosin (H&E), and toluidine blue staining for microscopic quantification of mast cell population (Park et al., 2014, Park et al., 2012, Regmi et al., 2014). The degree of fibrosis was examined by staining the transverse colonic sections with Masson’s trichome method (Kim et al., 2013). Further, histological profiles of stained colonic tissue samples were examined under a light microscopy (Axio Oser Z1, Germany; magnification 6200), and then scored by pathologists, unaware to the experimental groups. The microscopic features were evaluated on a scale established by Park et al., 2012, Regmi et al., 2014, modified from Stucchi et al., 2000, Peran et al., 2017. For performing morphometric analysis of regions occupied by ulcerative lesions (%), number of infiltrated mast and inflammatory cells (cells/mm2 of fields), number of microvessels formed (vessels/mm2 of fields), and percentage of collagen tissue deposited (Masson’s trichome stain; %/mm2 of fields), an automatic image analyzer was utilized.
2.7. DNA extraction, PCR, and sequencing
For microbiome analysis, the DNA extraction from the fecal samples was carried out with the help of a commercial kit, FastDNA® spin kit for soil (Qiagen Inc., Valencia, CA, USA). DNA quality was checked using 0.8% agarose. Further, the concentration of DNA (ng/μl) was checked by NanoDrop. Further, the primer was designed to target V1-V3 regions of prokaryotic 16S rRNA gene. The pre-denaturation was carried out at 95 °C for 5 min; then 30 cycles of denature for 30 s was done at 95 °C. The annealing was done at 72 °C for approximately 30 sec, followed by 5 min elongation at 72 °C. After confirmation of PCR product, the sequencing was performed on 454 pyrosequencing platform (Roche, USA) at ChunLab (South Korea).
2.8. Bioinformatic analysis
Quantitative Insights into Microbial Ecology (QIIME) pipeline (v.1.8.0; Morris et al., 1989) was used for the analysis of sequencing data. The statistical analysis was done with R. The reads that did not match with the specific sample barcode were discarded. In addition read shorter or longer than 50 pm and 480 bp, respectively, were also discarded. Further, OTUs with 97% similarly were formed by UCLUST (Edgar, 2010). PyNAST was used to align both representative and reference sequences (Caporaso et al., 2010). Further, taxonomic assignments and relationships between microbial communities were quantified.
2.9. RNA extraction and PCR analysis
The RNA extraction from the colon tissue was isolated using Trizol™ reagent (Life Technologies, Carlsbad, USA) following manufacturer’s instructions. The extracted RNA was stored in diethylpyrocarbonate-treated (DEPC-treated) H2O at –80 °C for further use. Further, the isolated RNA was reverse transcribed into cDNA using RevertAid kit (MBI, Thermo-Scientific, USA) following the manufacturer’s protocol. A 20 μl reverse transcription reaction mixture consisted of 1.0 μg of RNA, then primed with oligo (dT) 20 primers. The cDNA was used for quantification and expression of various genes associated with ulcerative colitis such as CD4, interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α). Furthermore, the quantitative real-time -PCR was performed using the Power SYBR Green PCR Master Mix (Applied Biosystems, Seattle, USA) on an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was taken as an internal control. Primers for this study were designed with Primer-BLAST Software using the sequence information listed at the National Center for Biotechnology Information (NCBI). The primer sequence sets used are provided in Table 1.
Table 1.
The table shows a list of primer indicating species, gene names, product size, Tm (temperature), and primer sequences (F: forward, R: reverse).
| Species | Gene | Product size (bp) | Tm (℃) | Sequence (F) | Sequence (R) |
|---|---|---|---|---|---|
| Mouse | GAPDH | 113 | 60.11 | 5′-gagagtgtttcctcgtcccg-3′ | 5′-caatctccactttgccactgc-3′ |
| CD4 | 105 | 59.89 | 5′-ccagacagtgttcctggctt-3′ | 5′- tgcctggcgctgttgg-3′ | |
| TNF-α | 185 | 59.53 | 5′-ggcctccctctcatcagttc-3′ | 5′-cacttggtggtttgctacgac-3′ | |
| IL-6 | 130 | 59 | 5′-agtccggagaggagacttca-3′ | 5′-gccattgcacaactcttttctca-3′ |
2.10. Statistical analysis
All the data are expressed as ± standard error of the mean. Further, unpaired Student’s t-test or non-parametric Mann–Whitney test was used to determine the statistical significance test differences in treatment groups. p values of <0.05 were considered statistically significant.
3. Results
3.1. Effect of SEL001 on clinical disease activity
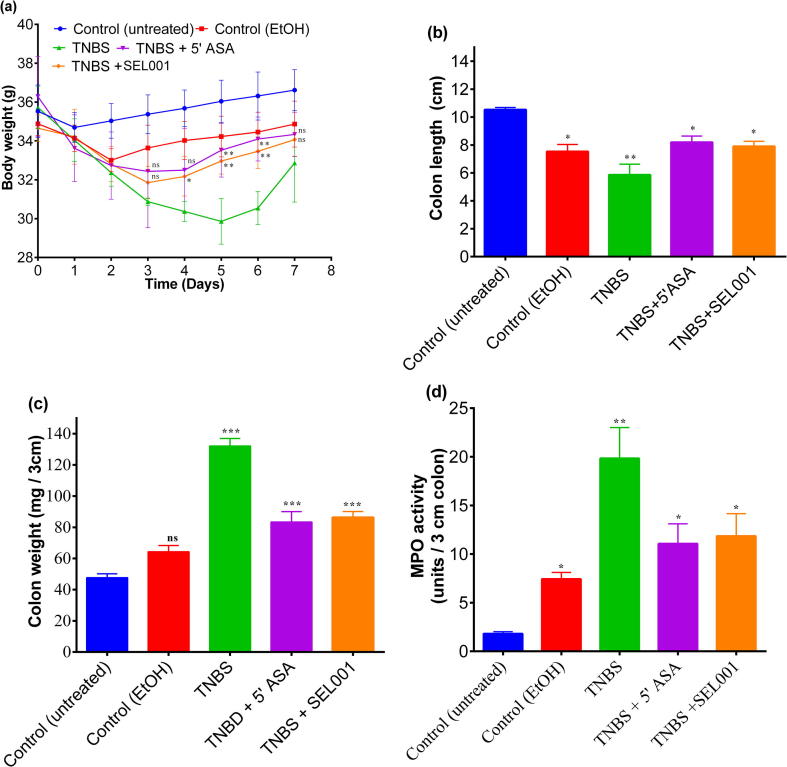
The mice in all the treatment groups exhibited uniform and steep weight loss within first 24 h after receiving TNBS as compared with the untreated control mice. However, an initial weight loss of 5.0% was also observed in animals treated with 30% ethanol compared with untreated control mice. This initial weight loss in ethanol control group was due to a non-specific perturbation of mucosal wall by ethanol. This initial weight loss was diluted immediately after day 2, after a steady and gradual weight gain trend was noted in ethanol control group. Mice treated with TNBS continued to loose weight till 5th day post treatment. The weight loss in mice treated with TNBS was 15.0% in body mass when compared with untreated control mice. However, mice treated with standard drug 5-ASA and SEL001 displayed a divergent pattern of significant gradual weight gain as early as day 3 when compared with TNBS treated mice. The body weight data (Fig. 1a) suggested that SEL001 and 5-ASA were equally effective (P ≥ 0.05) in ameliorating the colonic inflammatory reaction to TNBS, thereby allowing the mice to recommence normal eating pattern and weight (Fig. 1a). Nevertheless, after 5 days a steady increase in weight gain was observed in all groups.
Fig. 1.
Effect of oral administration of freeze-dried ethanol extract (SEL001) isolated from a probiotic strain L. sakei probio65 on body weight (a); colon length (b); colon weight (c); and MPO activity (d) in TNBS-induced colitis experimental mice.
The extent of damage caused by acute TNBS-induced colitis was also reflected by shortening of colon length. During 7-day course of treatment, the TNBS-treated mice exhibited approximately 50% reduction in colon length when compared to the control mice (Fig. 1b). In contrast, TNBS-treated mice showed a drastic increase of colon weight approximately by 3-fold when compared to the control mice (Fig. 1c). Both 5-ASA and SEL001 treatments resulted in a lesser (P < 0.05) reduction of colon length, and a milder colon weight gain when compared to the control mice (Fig. 1b, c). This data on disease activity parameters suggest that SEL001 was as effective as 5-ASA in ameliorating the colonic inflammatory response to TNBS.
3.2. Effects of SEL001 on MPO activity
TNBS-induced colitis in mice resulted in significantly elevated MPO activity in the colon when measured 7 days after the induction in comparison to the healthy controls. Six days of monotherapy with either 5-ASA or SEL001 resulted in approximately a 50% reduction in MPO activity and these reduced values were similar to ethanol control group mice (Fig. 1d).
3.3. Effects of SEL001 on colonic mucosal injury and inflammation
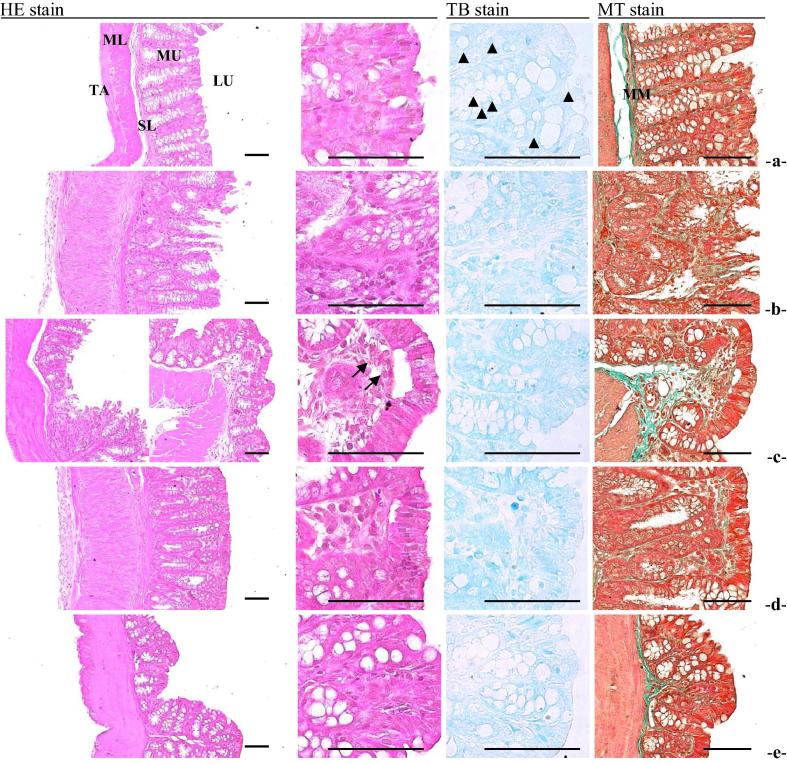
The histological examination of TNBS induced colitis tissues depicted an aggregate microscopic injury score of 14.02 ± 2.45 at 7 days post induction. Totalized microscopic colonic damage scores observed in TNBS-treated colonic tissue were significantly higher when compared with untreated control and ethanol control (Table 3). However, these TNBS-induced microscopic colonic damage scores were reduced (P > 0.05) upon treatment with 5-ASA or SEL001 (mean measures of colonic mucosal injury: 5.40 ± 2.30 and 6.80 ± 2.39, respectively). Moreover, the histomorphometric analysis further corroborated with previous microscopic injury score examination, where TNBS treatment resulted in deterioration of mucosal tissue and displayed a score of 25.68 ± 2.23. However, the histopathological signatures associated with TNBS-induced colitis were ameliorated by 5-ASA and SEL001 monotherapies (Fig. 2).
Table 3.
Histomorphometrical values on the TNBS-induced colitis mice.
| Criteria | Control (untreated) | Control (EtOH) | TNBS | TNBS + 5′-ASA | TNBS + SEL001 |
|---|---|---|---|---|---|
| Ulcerative lesions (%)* | 2.36 ± 2.47 | 7.05 ± 1.70ad | 15.34 ± 4.75ace | 5.87 ± 1.22bf | 9.07 ± 2.36acf |
| Mucosa | |||||
| Thickness (μm) | 249.42 ± 13.15 | 208.79 ± 9.89ac | 100.50 ± 12.03ace | 246.45 ± 36.29ef | 151.36 ± 17.78acef |
| Inflammatory cells (/mm2) | 54.20 ± 14.86 | 227.40 ± 26.15hj | 460.40 ± 62.92hjl | 231.20 ± 28.26hjn | 343.00 ± 43.06hjlo |
| Microvessels (/mm2) | 10.80 ± 2.05 | 17.20 ± 4.87ik | 56.80 ± 12.79hjl | 24.40 ± 3.78hjmn | 29.80 ± 5.40hjmn |
| Mast cells (/mm2) | 27.60 ± 3.58 | 18.40 ± 2.97ac | 4.80 ± 0.84ace | 12.80 ± 1.64acef | 9.00 ± 1.58aceg |
| Collagen fibers (%) | 6.77 ± 3.12 | 14.38 ± 2.04ac | 25.68 ± 2.23ace | 16.51 ± 2.25acf | 19.29 ± 2.37acef |
Values are expressed as Mean ± S.D. of five histological fields.
Ulcerative lesions = [(Length of any desquamated lesions in crossly trimmed colon mucosa/Total cross length of colon mucosa (mm)] × 100.
Fig. 2.
Effect of oral administration of freeze-dried ethanolic extract (SEL001) isolated from a probiotic strain L. sakei probio65 on representative histopathological images of colon tissues (intact or TNBS-induced) in experimental mice. (a) Control (untreated), (b), Control (EtOH), (c) TNBS treated colon, (d). TNBS and 5′ASA treated colon, (e). TNBS and SEL001 treated colon. [LU = Lumen; MU = Mucosa layer; MM = Muscularis mucosa; SL = Submucosa layer; ML = Muscle layer; TA = Tunica adventitia]. Stains: HE = Hematoxylin-eosin; MT = Masson’s trichrome; TB = Toluidine blue. Scale bars = 80 μm.
3.4. Effect on SEL001 on the gut bacterial composition
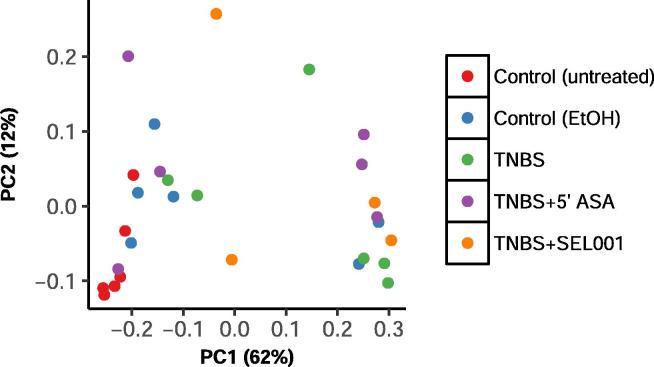
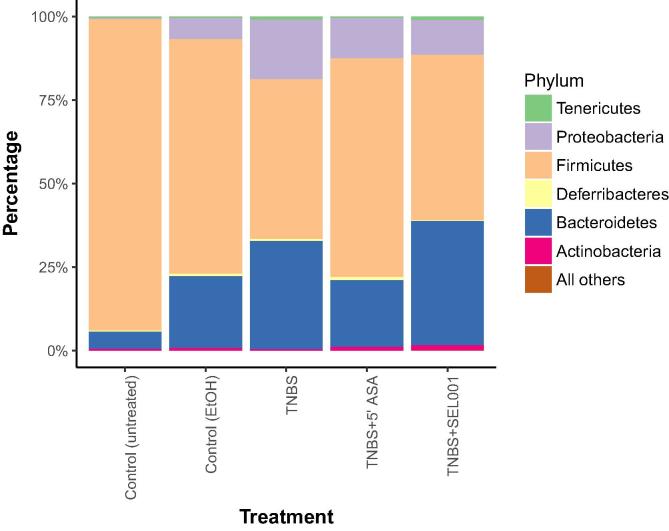
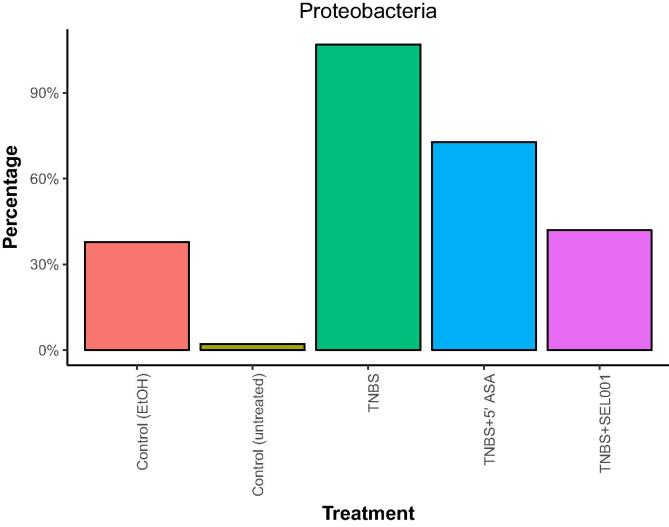
Weighted UniFrac distance was calculated as per relative abundance of operational taxonomic units (OTUs) in prokaryotic communities. In control and treated groups, substantial differences were seen in relative abundance of specific populations (Fig. 3). The taxonomic assignment of the OTUs recognized 6 major phyla across all groups. Firmicutes constituted >95% in the control group. However, in the treated groups, the lineage of Firmicutes decreased with a concomitant increase in Bacteroidetes and Proteobacteria (Fig. 4). The relative abundance of Proteobacteria was higher in the TNBS + ASA group as compared to TNBS + SEL001 group (Fig. 5).
Fig. 3.
Effect of oral administration of freeze-dried ethanolic extract (SEL001) isolated from a probiotic strain L. sakei probio65 on weighed Unifrac-Principal Coordinate Analysis (PCoA) in experimental mice.
Fig. 4.
Effect of oral administration of freeze-dried ethanolic extract (SEL001) isolated from a probiotic strain L. sakei probio65 on phylum level composition of experimental mice.
Fig. 5.
Effect of oral administration of freeze-dried ethanolic extract (SEL001) isolated from a probiotic strain L. sakei probio65 on phylum level composition of Proteobacteria in experimental mice.
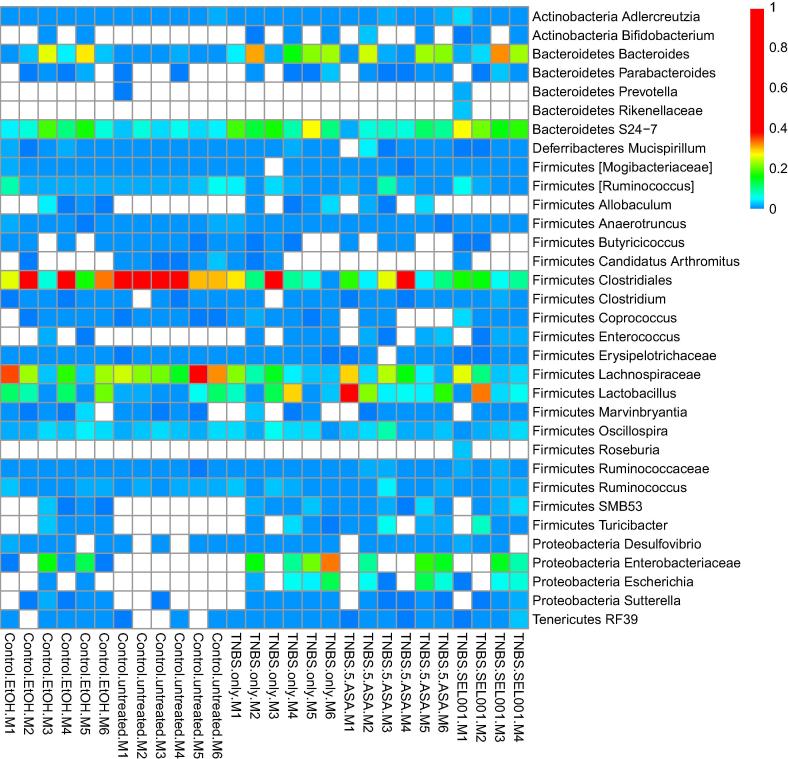
At the genus level, 2 genera from Actinobacteria, 3 genera from Proteobacteria, 5 genera from Bacteroidetes, and 18 genera from Firmicutes was identified. Among Bacteroidetes, Bacteroides and members of Bacteroidales family S24-7 were predominant in treated groups. Conversely, members of Clostriadales (Firmicutes) were predominant (<0.4%) in control group compared to treated group. Genus Enterococcus and members of the family Enterobacteriaceae (Proteobacteria) were either not detected or in a very low abundance in control group as compared to treatment groups (Fig. 6).
Fig. 6.
Effect of oral administration of freeze-dried ethanolic extract (SEL001) isolated from a probiotic strain L. sakei probio65 on genus-level heat-map in experimental mice.
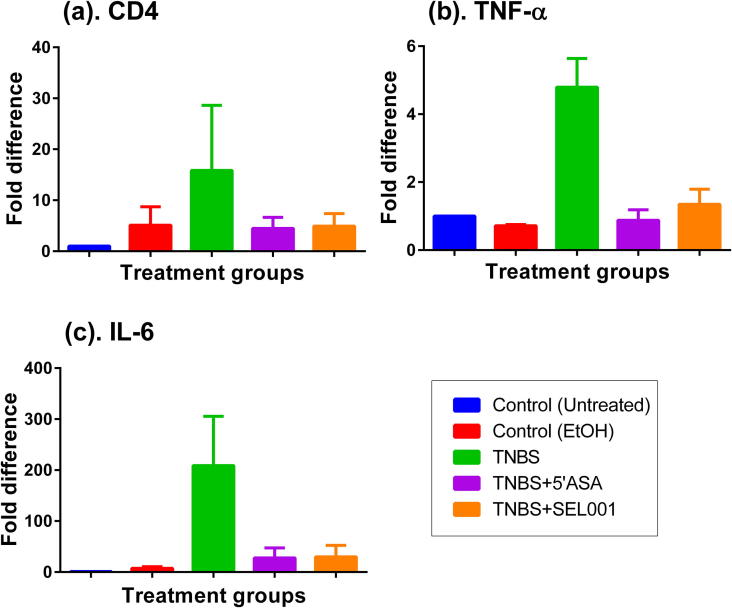
3.5. mRNA expression levels
The mRNA expression profile of pro-inflammatory cytokines such as CD4, IL-6 and TNF-α were significantly (P value) increased when compared with those of untreated controls, and ethanol-treated mice (Fig. 7). This increase in mRNA expression level of pro-inflammatory cytokines can be correlated with extensive cellular infiltration.
Fig. 7.
Effect of oral administration of freeze-dried ethanolic extract (SEL001) isolated from a probiotic strain L. sakei probio65 on mRNA expression levels in experimental mice.
4. Discussion
Ulcerative colitis is a persistent and long-lasting inflammatory condition that is known to affect the lining of the large bowel, predominantly the rectum (Awaad et al., 2013). Patients suffering from UC often develop inflammation in the colon and form minute open sores or ulcers that extravagate puss, and mucous. The combinatorial effect of inflammation and lesion formation culminates into severe abdominal discomfort accompanied by frequent emptying of the colon, and diarrhea. The basic pathophysiological principle behind ulcerative colitis is the impairment of normal immune system regulation towards an antigenic trigger, which leads to prolonged activation of mucosal immune response resulting in inflammation (Awaad et al., 2013).
Ulcerative colitis presents itself with a range of severities (e.g., mild, moderate, severe) that mainly influences and limits treatment options. Mesalamine (5-ASA) is a new generation aminosalicylate drug, and it has been recognized for its effectiveness against the mild-to-moderate form of UC. However, the application of 5-ASA suffers from adverse side-effects and also this drug has limited effectiveness for treating the most severe form of UC disorder (Karagozian and Burakoff, 2007).
The current study demonstrated that oral administration of SEL001, which contained various bioactive components, significantly attenuated the severity of disease in a TNBS-induced colitis mouse model. These compounds or the extracts of various origins (e.g. lactic acid from Lactobacillus, nipa vinegar, Gastrodia elata root extract) exhibit anti-inflammatory effects both in vitro and in vivo (Lee et al., 2006a, Lee et al., 2006b, Hearps et al., 2017, Beh et al., 2017). Also, it has been confirmed that amino acids have significant therapeutic potential to control IBD (Liu et al., 2017). In addition, the SEL001 contains myo-inositol, which has shown to possess anti-inflammatory effects in animal mouse model (Claxson et al., 1990); this depicts that the anti-inflammatory effect of SEL001 could be due to the presence of myo-inositol. Nevertheless, synergistic properties of other bioactive components existing in the SEL001 could not be ruled out.
The freeze dried extract SEL001 was also found to be effective in attenuating the miss-regulated inflammatory responses as measured by histopathological markers. The SEL001 treatment group also showed much better healing effect in disease activity measures when compared with the impact of the synthetic drug (5-ASA). Morphological signatures such as early rescue, and steady increase in body weight, a decrease in the rate of stool passage/diarrhea, inhibition of shortening of colon length, and recovery of the colon weight showed the healing potential of SEL001 for treating TNBS-induced colitis. In a similar study Yang et al. (2016) showed efficacy of ethanol extract of Portulaca oleracea L., for treating symptoms on dextran sulphate sodium induced UC in C57BL/6 mice model. Application of ethanol extract of Portulaca oleracea L. resulted in significant improvement in body weight gain and a reduction in the disease activity index scores.
Based on the course of TNBS-application, acute and chronic forms of experimental intestinal fibrosis could be induced in rodents, which mimic conditions of UC in patients. The gut inflammation in patients with UC or in TNBS-induced colitis mice is associated with the active phase inflammatory reaction and relocation of lymphocytes to the gastrointestinal tract mucosa. Chronic inflammation of colon is marked by migration of lymphocytes, and plasma cells, whereas acute inflammation results in granulocyte infiltration, local aggregation of neutrophils, eosinophilic and chemotactic mediators (Krawisz et al., 1984). Neutrophil granulocytes carry multiple enzymes e.g., myeloperoxidase (MPO), which upon release from the granules confers local immunity and helps to combat bacterial infections. The MPO activity derived from the solubilized inflamed tissue is used as a direct measure of neutrophils count (Krawisz et al., 1984). In this study, reduced MPO activity was observed for the SEL001 treatment group, which reflects the diminished neutrophils counts present in the tissue and shows the therapeutic-anti-inflammatory effect of SEL001.
In order to induce UC mice were administered with TNBS dissolved in ethanol. The ethanol caused initial breakdown of the epithelium and TNBS resulted in transmural inflammation of the colon (Neurath et al., 2000). The histopathology and histomorphometric assessment revealed the colitis severity and recuperation effect of SEL001 in TNBS-induced colitis mice. The histopathological inspection of TNBS-induced colitis mouse revealed features such as establishment of ulceration, mucous cell depletion, inflammatory cell infiltration, neovascularization, collagen deposition, mucosal mast cell depletion, and edematous changes, severely enhanced (Fig. 5). The marked reduction in aforementioned histopathological signs and partial or complete restoration of the normal state exhibited the therapeutic prospect of SEL001. The mucosal lining of the healthy gastrointestinal tract is composed of delicate collagen fibrils that allow the movement of mucosa over the muscularis propria (Rieder et al., 2007). It has been reported that inflammation in UC leads to the accumulation of abnormal fibrotic collagens through the abnormal deposition of extracellular matrix (Rieder et al., 2007). This phenomenon disturbs the normal relationship between mucosa, and underlying muscularis propria, which translates into a condition called as obstructive symptomatology (Rieder et al., 2007). Histopathology examination revealed that the treatment of TNBS-inflamed mice with SEL001 significantly reduced the deposition of abnormal collagen fibers, further supporting its therapeutic potential. Similar results were reported by Yang et al. (2016), where ethanol extract from P. oleracea exhibited protective effect on dextran sulphate sodium (DSS)-induced ulcerative colitis in mouse model (Yang et al., 2016). In addition, ethanolic extract derived from a medicinal mashroom Hericum erinaceus also showed anti-inflammatory effects on DSS-induced UC. These results suggest the therapeutic potential of ethanolic extract obtained from lactic acid bacteria and medicinal herbs.
Histomorphometrical analysis revealed the decrease of mucosal mast cell population in the colon tissue after induction of UC in the experimental mice, which is the indicative of inflammation-induced loss and/or degranulation of mast cells (Sutton et al., 2008). Treatment with SEL001 partially restored mast cell population in mucosa by inhibiting the inflammation-dependent mast cell degranulation. This evidence depicts the possible anti-inflammatory role exhibited by SEL001, which could be mediated through the regulation of mast cell degranulation and associated inflammatory processes.
Pro-inflammatory cytokines such as IL-6 and TNF-α are believed to determine the degree of inflammation in UC patients. It has been reported as the expression levels of IL-6 and TNF-α increases as the severity of UC increases, suggesting a positive correlation between cytokine expression and severity of UC (Akazawa et al., 2002, Indaram et al., 2002, Tian et al., 2002). Treatment of TNBS-induced UC in experimental mice with 5 ASA or SEL001 greatly reduced the levels of pro-inflammatory cytokine production. Similarly, Lee et al., 2006a, Lee et al., 2006b reported anti-inflammatory effects of Uro-Vaxom® a bacterial extract derived from 18 Escherichia coli strains containing immunostimulating components and observed that the levels of the cytokines (IL-6, IL-10, and TNF-α) in the bladder tissues were significantly changed in the experimental mice upon treatment of bacterial extract (Lee et al., 2006a, Lee et al., 2006b). Mice treated with Vaxom® exhibited milder inflammation, suggesting a possible role of bioactive components present in the bacterial extract (Lee et al., 2006a, Lee et al., 2006b).
The altered microbiota can directly alter and affect host health. Many probiotics are used as traditional ulcer medication, similar to the gold-standard treatment medication mesalamine probiotics has been reported to exhibit similar curative effects on the patients (Kruis et al., 2004). Similar to the earlier studies, there is substantial increase in the proportion of Proteobacteria at the expense of reduction in the proportion of phyla Firmicutes and Bacteroidetes in the colitis induced mice (Manichanh et al., 2006, Kang et al., 2010). Fecteau et al. (2016) showed similar reduction in Firmicutes while studying dysbiosis in cattle with Jhone’s disease parallel to Crohn’s disease in humans. However, there is no direct report on effect of probiotics on gut microbiota of IBD/ UC to our knowledge. Increase in abundance of Firmicutes and low expression on inflammatory cytokines in SEL001 treated group indicates the positive effect of probiotic in balancing the gut microbiota and immune cells.
5. Conclusion
In summary, in this study, SEL001 as a novel extract of probiotic-origin containing significant immunostimulating components showed promising anti-inflammatory activity in a murine model. In contrast to the detrimental side effects of the synthetic drug such as 5-ASA, the products of biological/probiotic origin such as SEL001 conferring protective anti-inflammatory effect could be considered as an attractive and safer alternative for the treatment of UC.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgements
This work was supported by the 2015 Yeungnam University Research Grant.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Irfan A. Rather, Email: ammm@kau.edu.sa.
Sanjay Kumar, Email: sanjayvrm@gmail.com.
Yong-Ha Park, Email: peter@ynu.ac.kr.
References
- Akazawa A. Increased expression of tumor necrosis factor-alpha messenger RNA in the intestinal mucosa of inflammatory bowel disease, particularly in patients with disease in the inactive phase. J. Gastroenterol. 2002;37:345–353. doi: 10.1007/s005350200048. [DOI] [PubMed] [Google Scholar]
- Awaad S., El-Meligy R.M., Soliman G.A. Natural products in treatment of ulcerative colitis and peptic ulcer. J. Saudi Chem. Soc. 2013;17:101–124. [Google Scholar]
- Beh B.K. Anti-obesity and anti-inflammatory effects of synthetic acetic acid vinegar and Nipa vinegar on high-fat diet-induced obese mice. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-06235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J.G. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxson A. The anti-inflammatory effects of D-myo-inositol-1.2.6-trisphosphate (PP56) on animal models of inflammation. Agents Actions. 1990;29:68–70. doi: 10.1007/BF01964724. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Fecteau M.E. Dysbiosis of the fecal microbiota in cattle infected with Mycobacterium avium subsp. Paratuberculosis. PlosOne. 2016;11 doi: 10.1371/journal.pone.0160353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrusquía J., Pérez-Martínez I., Gómez de la Torre R., Fernández-Almira M.L., de Francisco R., Rodrigo L., Riestra S. Gastroenterology case report of mesalazine-induced cardiopulmonary hypersensitivity. World J. Gastroenterol. 2015;21(13):4069–4077. doi: 10.3748/wjg.v21.i13.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick L.R., Wang J., Le M. In vitro and in vivo effects of gliotoxin, a fungal metabolite: efficacy against dextran sodium sulfate-induced colitis in rats. Digest Disease Sci. 2000;45:2327–2336. doi: 10.1023/a:1005630723111. [DOI] [PubMed] [Google Scholar]
- Giahi L., Aumueller E., Elmadfa I., Haslberger A.G. Regulation of TLR4, p38 MAPkinase, IκB and miRNAs by inactivated strains of lactobacilli in human dendritic cells. Beneficial Microbes. 2012;3:91–98. doi: 10.3920/BM2011.0052. [DOI] [PubMed] [Google Scholar]
- Ham M., Moss A.C. Mesalamine in the treatment and maintenance of remission of ulcerative colitis. Expert Rev. Clin. Pharmacol. 2012;5:113–123. doi: 10.1586/ecp.12.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatoum O.A., Binion D.G. Contribution to pathogenesis and clinical pathology. The vascular and inflammatory bowel disease: contribution to pathogenesis and clinical pathology. Inflammatory Bowel Disease. 2005;11:304–313. doi: 10.1097/01.mib.0000160772.78951.61. [DOI] [PubMed] [Google Scholar]
- Hearps A.C. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017 doi: 10.1038/mi.2017.27. [DOI] [PubMed] [Google Scholar]
- Indaram A.V., Visvalingam V., Locke M., Bank S. Mucosal cytokine production in radiation-induced proctosigmoiditis compared with inflammatory bowel disease. Am. J. Gastroenterol. 2002;95:1221–1225. doi: 10.1111/j.1572-0241.2000.02013.x. [DOI] [PubMed] [Google Scholar]
- Kang S. Dysbiosis of fecal microbiota in Crohn’s disease patients as revealed by a custom phylogenetic microarray. Inflamm. Bowel Dis. 2010;16:2034–2042. doi: 10.1002/ibd.21319. [DOI] [PubMed] [Google Scholar]
- Karagozian R., Burakoff R. The role of mesalamine in the treatment of ulcerative colitis. Therapies Clin. Risk Manage. 2007;3:893–903. [PMC free article] [PubMed] [Google Scholar]
- Kengo S., Jun I., Daisuke S., Namiko H., Tomokazu S., Itsuko F., Takeshi A., Akihiko K., Ro O. Construction of a model culture system of human colonic microbiota to detect decreased Lachnospoiracease abundance and Butyrogensiss in the feces of ulcerative colitis patients. Biotechnol. J. 2019;5 doi: 10.1002/biot.201800555. [DOI] [PubMed] [Google Scholar]
- Kim H. A double-blind, placebo controlled-trial of a probiotic strain Lactobacillus sakei probio-65 for the prevention of canine atopic dermatitis. J. Microbiol. Biotechnol. 2015;25:1966–1969. doi: 10.4014/jmb.1506.06065. [DOI] [PubMed] [Google Scholar]
- Kim J.Y., Pyo S. Oral administration of dead Lactobacillus sakei inhibits atopic dermatitis-like skin lesion in NC/Nga mice. FASEB J. 2012;26 [Google Scholar]
- Kim J.Y. Atopic dermatitis-mitigating effects of new Lactobacillus strain, Lactobacillus sakei probio 65 isolated from Kimchi. J. Appl. Microbiol. 2013;115:517–526. doi: 10.1111/jam.12229. [DOI] [PubMed] [Google Scholar]
- Krawisz J.E., Sharon P., Stenson W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- Kruis W. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y. Anti-inflammatory action of phenolic compounds from Gastrodia elata root. Arch. Pharmacal Res. 2006;29:849–858. doi: 10.1007/BF02973905. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Kim S.W., Cho Y.H., Yoon M.S. Anti-inflammatory effect of an Escherichia coli extract in a mouse model of lipopolysaccharide-induced cystitis. World J. Urol. 2006;24:33–38. doi: 10.1007/s00345-005-0046-y. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang X., Andy Hu C.A. Therapeutic potential f amino acids in inflammatory bowel disease. Nutrient. 2017;9 doi: 10.3390/nu9090920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus E.V. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- Mallon P., McKay D., Kirk S., Gardiner K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2007;17 doi: 10.1002/14651858.CD005573.pub2. [DOI] [PubMed] [Google Scholar]
- Manichanh C. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin. Exp. Immunol. 2005;140:417–426. doi: 10.1111/j.1365-2249.2005.02790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.P. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Neurath M., Fuss I., Strober W. TNBS-colitis. Int. Rev. Immunol. 2000;19:51–62. doi: 10.3109/08830180009048389. [DOI] [PubMed] [Google Scholar]
- Park C.W. New functional probiotic Lactobacillus sakei probio 65 alleviates atopic symptoms in the mouse. J. Med. Food. 2008;11:405–412. doi: 10.1089/jmf.2007.0144. [DOI] [PubMed] [Google Scholar]
- Park S.B. Effect of emollients containing vegetable-derived lactobacillus in the treatment of atopic dermatitis symptoms: split-body clinical trial. Ann. Dermatol. 2014;26:150–155. doi: 10.5021/ad.2014.26.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Ku S.K., Lee E.S., Kim J.A. 1,3-Diphenylpropenone ameliorates TNBS-induced rat colitis through suppression of NF-κB activation and IL-8 induction. Chem. Biol. Interactions. 2012;196:39–49. doi: 10.1016/j.cbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Peran L., Camuesco D., Comalada M., Nieto A., Concha A., Diaz-Ropero M.P. Preventative effects of a probiotic, Lactobacillus salivarius ssp. salivarius, in the TNBS model of rat colitis. World J. Gastroenterol. 2017;11:5185–5192. doi: 10.3748/wjg.v11.i33.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Díaz J., Ruiz-Ojeda F.J., Vilchez-Padial L.M., Gil A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients. 2017;9 doi: 10.3390/nu9060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransford R.A.J., Langman M.J.S. Sulphasalazine and mesalazine: serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the committee on safety of medicines. Gut. 2002;51:536–539. doi: 10.1136/gut.51.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather I.A. Probiotics Lactobacillus sakei proBio-65 extract ameliorates the severity of imiquimod induced psoriasis-like skin inflammation in a mouse model. Front. Microbiol. 2018;9:1021. doi: 10.3389/fmicb.2018.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regmi S.C., Park S.Y., Ku S.K., Kim J.A. Serotonin regulates innate immune responses of colon epithelial cells through Nox2-derived reactive oxygen species. Free Radical Biol. Med. 2014;69:377–389. doi: 10.1016/j.freeradbiomed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Rieder F., Brenmoehl J., Lee S., Scholmerich J., Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56:130–139. doi: 10.1136/gut.2006.090456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseaux C. Intestinal anti-inflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator–activated receptor-γ. J. Exp. Med. 2005;18:1205–1215. doi: 10.1084/jem.20041948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborn W.J. Step-up versus top-down therapy in the treatment of ulcerative colitis. Gastroenterol. Hepatol. 2007;3:16–17. [PMC free article] [PubMed] [Google Scholar]
- Stucchi A.F., Shofer S., Leeman S., Materne O., Beer E., McClung J. NK-1 antagonist reduces colonic inflammation and oxidative stress in dextran sulfate-induced colitis in rats. Am. J. Physiol.-Gastrointestinal Liver Physiol. 2000;279:1298–1306. doi: 10.1152/ajpgi.2000.279.6.G1298. [DOI] [PubMed] [Google Scholar]
- Sutherland L., MacDonald J.K. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2007;3:1–35. [Google Scholar]
- Sutherland L., MacDonald J.K. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2007;3:1–26. doi: 10.1002/14651858.CD000544.pub2. [DOI] [PubMed] [Google Scholar]
- Sutton T.L. Anti-Inflammatory mechanisms of enteric Heligmosomoides polygyrus infection against trinitrobenzene sulfonic acid-induced colitis in a murine model. Infect. Immun. 2008;76:4772–4782. doi: 10.1128/IAI.00744-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Huang Y.X., Wen Q.S., Wang Q.L. Therapeutic effect and mechanism of electro-acupuncture on rats with ulcerative colitis. World Chin. J. Digestol. 2002;10:916–921. [Google Scholar]
- Tojo R. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014;20:15163–15176. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S.L., Ying S.J., Lin Q.R. Association of ulcerative colitis with FOXP3 Gene polymorphisms and its colonic expression in Chinese patients. Gastroenterol. Res. Practice. 2019;40521686:10. doi: 10.1155/2019/4052168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. Protective effects of ethanol extract from Portulaca oleracea L. on dextran sulphate sodium-induced mice ulcerative colitis involving anti-inflammatory and antioxidant. Am. J. Transgenic Res. 2016;8:2138–2148. [PMC free article] [PubMed] [Google Scholar]