Abstract
A total of 7386 samples of adult honey bees from different areas of Serbia (fifteen regions and 79 municipalities) were selected for light microscopy analysis for Nosema species during 1992–2017. A selection of honey bee samples from colonies positive for microsporidian spores during 2009–2011, 2015 and 2017 were then subjected to molecular diagnosis by multiplex PCR using specific primers for a region of the 16S rRNA gene of Nosema species. The prevalence of microsporidian spore-positive bee colonies ranged between 14.4% in 2013 and 65.4% in 1992. PCR results show that Nosema ceranae is not the only Nosema species to infect honey bees in Serbia. Mixed N. apis/N. ceranae infections were detected in the two honey bee samples examined by mPCR during 2017. The beekeeping management of disease prevention, such as replacement of combs and queens and hygienic handling of colonies are useful in the prevention of Nosema infection.
Keywords: Apis mellifera, Nosema ceranae, Nosema apis, Serbia
1. Introduction
Nosema ceranae (N. ceranae) and Nosema apis (N. apis), two microsporidian species, can infect adult honey bees (Forsgren and Fries, 2010). Spores N. apis are larger than spores N. ceranae and at the ends rounded, symmetrical while spores N. ceranae at the ends end sharply (Fries et al., 1996, Fries et al., 2006). Spores of N. apis are approximately 6 × 3 μm and the number of coils of the polar filament inside spores are >30 (Zander and Bottcher, 1984, Fries, 1989, Liu, 1984). According to Fries et al., (1996) spores of N. ceranae are approximately 4.4 × 2.2 μm and the number of coils of the polar filament inside spores are 18–21. Natural infections where hosts are infected by a single parasite are rare (Read and Taylor, 2001). Within the bee host, interactions between parasites can influence disease severity, and mixed infections with N. apis and N. ceranae are common (Paxton et al., 2007, Fries and Forsgren, 2008, Chen et al., 2008, Milbrath et al., 2015 Huang et al., 2007). One species can significantly influence transmission rates of the other or have no measurable effect on transmission (Pilarska et al., 2006).
Both microsporidia are widespread worldwide today and each microsporidia crossinfects the other host. (Ansari et al., 2017, Sinpoo et al., 2018). Martín-Hernández et al., 2007, Fries and Forsgren, 2008 state that N. ceranae infections appear to dominate in warmer climates compared to more temperate regions in Europe. The climate could be an important factor explaining differences in parasite species distribution and impact (Pacini et al., 2016, Ansari et al., 2017). Martín-Hernández et al. (2009) compared the increase in spore numbers in bee abdomens at different times post-infection and found N. ceranae numbers increased over a wider temperature range compared to N. apis (Milbrath et al., 2015).
Higes et al., (2006) found 11 out of 12 samples from 2005 from Spanish apiaries positive for microsporidia to contain N. ceranae, based on homology to the original 16S ssrRNA GenBank entry for N. ceranae.
Nosemosis in European honey bees was attributed to N. ceranae and N. apis (Higes et al., 2006, Gisder et al., 2010, Huang, 2011, Colin et al., 2009, Stevanovic et al., 2013). In Sweden, 83% of colonies had N. apis and mixed N. apis/N. ceranae infections were detected in 17% colonies (Fries and Forsgren, 2008). In the 70.4% of colonies in Scotland, had mixed N. apis/N. ceranae infections (Bollan et al., 2013). Also, in Argentina and Australia the presence of both types of microsporidia has been proven (Giersch et al., 2009). In Greek, Italy, the eastern Azerbaijan and Saudi Arabia, only spores of N. ceranae were detected (Bacandritsos et al., 2010, Papini et al., 2017, Razmaraii et al., 2013, Ansari et al., 2017). N. ceranae is highly pathogenic when experimentally inoculated into European honey bees (Higes et al., 2007) and is associated with reduced honey production and increased winter mortality, it has been shown in Spain (Higes et al., 2006). Serbia has a long tradition of beekeeping (Ivanović et al., 2015, Matović et al., 2018). In Serbia and neighbouring countries (Croatia, Bosnia and Herzegovina, Montenegro, the North Macedonia), N. ceranae dominates in microsporidia infections in honey bees (Stevanovic et al., 2011). Stevanovic et al. (2011) indicated that N. ceranae has been present in Serbian bees (collected between 2000 and 2005) since at least the year 2000.
Because N. ceranae has been diagnosed in Serbia (Stevanovic et al., 2011) and because of the high prevalence of Nosema infections, we have suspected its presence in mixed infections with N. apis. The aim of this study was to investigate and determine the prevalence of N. ceranae and N. apis in different locations in Serbia (encompassing almost 70% of the total territory), because is still, using microscopic examination and multiplex PCR (mPCR).
2. Materials and methods
2.1. Sample collection
A total of 7386 samples from fifteen regions (79 municipalities) in Serbia were examined for Nosema infection between 1992 and 2017. Selected apiaries (a total of 250) were situated at locations distributed in both flatland and mountainous zones, i.e. from different climatic conditions (Fig. 1). Beside dead honey bees collected during winter loss, adult live bees and feces were sampled (in June and/or October). The first sampling was conducted in March 1992, and the last in June 2017. Each sample honey bees consisted of 60 adult bees (minimum). The live bees were collected from the hive entrance after closing it for 30 min (Meana et al., 2010, Botías et al., 2012). During 2009–2017, after light microscopy, collected samples were frozen at −20 °C and stored until molecular analysis Figure 1.
Fig. 1.
Map of bee samples collected in regions in Serbia.
2.2. Spore detection
The abdomens of 60 live young or corpses of honey bees were macerated in 5 ml of distilled water (Sigma-Aldrich, Germany). Microscopic analyses of homogenates collected fecal samples were also performed. Pellets were analyzed by light microscopy (x400 magnification) to verify the presence of spores, according to OIE Manual (1992–2017). This methodology was employed for determination of the presence of Nosema spores in all the samples used in this study during 1992–2017. Part of the macerated bee suspensions collected during 2009–2017 were stored at −20 °C and subsequently subjected to mPCR analysis (the samples from 1992 to 2009 were not suitable for analysis, because technical possibilities.
2.3. DNA extraction
The commercial DNA extraction kit (QIAamp DNA Kit, USA) was used to obtain DNA from macerated honeybee abdomens using a procedure described by manufacturer. Extracted DNA was stored at −20 °C until mPCR.
2.4. PCR methodology
The primers used are shown in Table 1.
Table 1.
Primers selected for detection of N. ceranae and N. apis (OIE Manual 2008, Chapter 2.2.4).
| Primer | Sequence | PCR product size (bp) | Specificity |
|---|---|---|---|
| 218MITOC-FOR | 5′-CGGCGACGATGTGATATGAAAATATTAA-3′ | 218–219 | N. ceranae |
| 218MITOC-REV | 5′-CCCGGTCATTCTCAAACAAAAAACCG-3′ | 218–219 | N. ceranae |
| 321APIS-FOR | 5′-GGGGGCATGTCTTTGACGTACTATGTA-3′ | 321 | N. apis |
| 321APIS-REV | 5′-GGGGGGCGTTTAAAATGTGAAACAACTATG-3′ | 321 | N. apis |
The thermocycler program consisted of 94 °C for 15 s followed by 35 cycles of 15 s at 94 °C, 30 s at 61.8 °C and 45 s at 72 °C and a final extension step at 72 °C for 7 min. Positive and negative controls were included in PCR runs. Amplicons were separated on 2% agarose gel in Mini-Sub Cell GT (Bio-Rad, USA) and products were visualized using Gel Doc XR system (Bio-Rad, USA). The positive controls used were from AFSSA, Sophia-Antipolis, Anses (France). The genomes are Clone plasmidique K3, Nosema apis APIS-FOR/APIS-REV (321 bp) ds pGEM-T-easy (Isolate AFSSA, September 15, 2009) and Clone plasmidique L2, Nosema ceranae MITOC-FOR/MITOC-REV (218 bp) ds pGEM-T-easy (Isolate Higes, August 26, 2009) from Reference Laboratory OIE AFSSA. The PCR products were purified with a QIA quick PCR purification Kit (QIAGEN, Germany).
3. Results and discussion
A total 7386 samples were submitted for light microscopic examination (Table 2). These samples were primarily from individual beekeepers from widely dispersed geographical locations within the 15 regions studied in Serbia. The results of this 25-year survey of Nosema species incidence (Table 2) revealed the continual high frequency of Nosema-positive bee colonies within each investigated year, ranging from 14.4% (2013) to 65.4% (1992). Lower, but still considerable percentages of bee colonies infected with Nosema species were detected in 2014 (18.8%) and 2015 (26.4%) (see Figure 2).
Table 2.
Number and percentage of bee samples and colonies in Serbia positive for Nosema spp. spores after light microscopic examination (1992–2017).
| Year | Number of examined bee samples | Number of municipalities where the infection was detected | Number of infected apiaries | Number of positive bee samples | % of Nosema-positive bee colonies |
|---|---|---|---|---|---|
| 1992 | 130 | 38 | 68 | 85 | 65.4 |
| 1993 | 107 | 34 | 34 | 34 | 31.8 |
| 1994 | 160 | 40 | 80 | 90 | 56.3 |
| 1995 | 127 | 7 | 34 | 38 | 29.9 |
| 1996 | 183 | 16 | 98 | 90 | 49.2 |
| 1997 | 140 | 21 | 57 | 62 | 44.3 |
| 1998 | 171 | 38 | 44 | 60 | 35.1 |
| 1999 | 217 | 24 | 76 | 93 | 42.9 |
| 2000 | 132 | 15 | 51 | 86 | 65.2 |
| 2001 | 176 | 18 | 45 | 60 | 34.1 |
| 2002 | 330 | 34 | 132 | 93 | 28.2 |
| 2003 | 337 | 8 | 42 | 155 | 46.0 |
| 2004 | 253 | 25 | 56 | 113 | 44.7 |
| 2005 | 384 | 16 | 58 | 157 | 40.9 |
| 2006 | 415 | 34 | 48 | 225 | 54.2 |
| 2007 | 719 | 25 | 78 | 230 | 32.0 |
| 2008 | 657 | 27 | 60 | 208 | 31.7 |
| 2009 | 245 | 21 | 41 | 148 | 60.4 |
| 2010 | 457 | 27 | 99 | 263 | 57.5 |
| 2011 | 504 | 21 | 66 | 242 | 48.0 |
| 2012 | 522 | 14 | 70 | 176 | 33.7 |
| 2013 | 355 | 12 | 17 | 51 | 14.4 |
| 2014 | 207 | 8 | 23 | 39 | 18.8 |
| 2015 | 148 | 8 | 29 | 39 | 26.4 |
| 2016 | 162 | 13 | 27 | 64 | 39.5 |
| 2017 | 148 | 10 | 11 | 45 | 30.4 |
| Total | 7386 | 2946 |
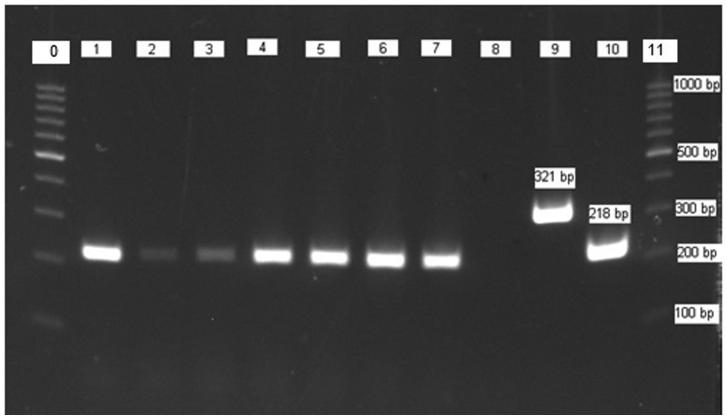
Fig. 2.
Typical PCR products of N. apis and N. ceranae: Lane 0 and 11, molecular weight markers (DNA Ladder 10 × 100 bp); lanes 1–7, PCR products of positive samples; lane 8 negative control; lane 9 positive control for N. apis; lane 10 positive control for N. ceranae.
Based on the data reported by Republic Hydrometeorological Service of Serbia in the last 25 years (1992–2017), the average temperature in Serbia increased by 0.05 °C, annually (total 1.25 °C for the period). During the same period, the highest measured temperature was 44.9 °C, in 2007, the lowest was −34.8 °C, in 2000. Annually number of days with a temperature below 0 °C ranged from 59 (2014) to 111 (1994). Such temperature fluctuations may have influenced the incidence of nosmosis, which is maintained or even increased in Serbia. Previous studies have shown that the incidence of N. apis and N. ceranae significantly different in years, is possible also as climate change in Serbia (e.g. 1994 and 2000) (Stevanovic et al., 2013). After light microscopy, a total of 138 samples from different localities and years (2009–2011, 2015, 2017) were selected and investigated by mPCR for the presence of N. apis and N. ceranae. PCR amplicons from representative bee samples, plus negative and matching positive controls are presented in Table 3. N. ceranae was detected more frequently than N. apis in the monitored bee colonies during the years examined. Also, mixed N. apis/N. ceranae infections were detected using mPCR during 2017 but not in previous years.
Table 3.
PCR results in relation to Nosema spp. genome presence in adult bee samples.
| Year | Number of bee samples examined by PCR | N. apis | N. ceranae |
|---|---|---|---|
| 2009 | 107 | 0 | 107 |
| 2010 | 21 | 0 | 21 |
| 2011 | 7 | 0 | 7 |
| 2015 | 1 | 0 | 1 |
| 2017 | 2 | 2 | 2 |
| Total | 138 | 2 | 138 |
N. ceranae-infected bees were found in honey bee samples collected from 2009 to 2017, in 15 examined regions and in all climatic areas (mountain and lowland zones) of Serbia that were included for study by mPCR. Also, the results showed that both examined bee samples that were positive for N. apis were also positive for N. ceranae during 2017.
In the last few years, infection of honey bees by Nosema spp. has been reported in numerous European countries, including Spain, France, Greece, Italy, Germany, Switzerland, Denmark, Finland and in our neighborhood (Hungary, Slovenia, Bosnia and Herzegovina) (Fries et al., 2006, Klee et al., 2007, Topolska and Kasprzak, 2007, Paxton et al., 2007, Tapaszti et al., 2009, Higes et al., 2010, Papini et al., 2017), as well as in other continents (Guerrero-Molina et al., 2016, Ansari et al., 2017). The exact date of entry of N. ceranae into European countries has not been reported (Higes et al., 2010). Our results show that N. ceranae was in Serbia in 2009, in agreement with earlier findings by Stevanovic et al. (2011), who discovered the parasite in Serbia, in 2000. By implication, N. ceranae has probably been in Europe for at least the past two decades. It seems clear that N. ceranae is an pathogen of A. mellifera, having become distributed across the world, possibly within the last decade of the last century (Paxton et al., 2007, Klee et al., 2007, Guerrero-Molina et al., 2016). The detection of only N. ceranae in 2015 and in prior years in this study is in agreement with previously reports on Nosema in honey bees from Serbia by Stevanovic et al., 2011, Stevanovic et al., 2013. In 2017, N. apis was detected in our study using a PCR method commonly used for molecular identification of Nosema species (Martín-Hernández et al., 2007, Martín-Hernandez et al., 2009, Higes et al., 2010, Hedtke et al., 2011). This is the first time they have N. apis and N. cerana detected in bee samples at the same time/co-infection, in the Republic of Serbia. This is the second time that N. apis has been molecular detected in Serbian bees. The present study showed that Nosema infections in Serbian honey bees can be caused by N. ceranae, either alone or in association with N. apis. In most of the examined beehives from the honey bee societies of infected European countries were also primarily N. ceranae (Higes et al., 2006, Higes et al., 2006, Stevanovic et al., 2011, Stevanovic et al., 2013, Odnosum, 2017, Papini et al., 2017).
The detection of microsporidian infections in honey bees may not be related to its increased prevalence in the insects but rather to the development of the new, highly sensitive and specific molecular technique, multiplex PCR. Using this molecular technique, the percent of positive samples is higher than is obtained using microscopic examination. Given that the spores of N. apis and N. cerana microscopy is not easily distinguishable, the use of molecular technique for species identification has played an important role in the study studies (Klee et al., 2007, Papini et al., 2017).
The world trade of honey bee products (honey, propolis and royal jelly) and beekeeping materials could also play an important role in the expansion of infective spores of N. ceranae from apiary to apiary over different geographical regions (Klee et al., 2007, Ansari et al., 2017). Commerce of queens and trade of worker bees can be a source of infection in some regions (Giersch et al., 2009). Education of beekeepers and good beekeeping practice are necessary in apiculture (Pacini et al., 2016). All these means of transmitting infective N. ceranae spores should be taken into account to explain the presence of N. ceranae in honey bees in remote geographical sites theoretically isolated from any source of infective spores (Colin et al., 2009, Ansari et al., 2017). The queen is susceptible to most of the diseases that attack her offspring, and N. ceranae can be transmitted horizontally from infected worker honey bees to queens by feeding (Higes et al., 2009). The behavioural changes described in confined honey bees could modify this mode of transmission since infected bees are less inclined to share their available sucrose solution with other bees (Naug and Gibbs, 2009). One of the reservoirs of N. ceranae is pollen stored in honeycombs. The presence of infective N. ceranae spores in pollen must be due to self-contamination during the process of pollen collection. The effect of pollen fermentation (to bee bread) on Nosema spore viability has yet to be evaluated. Honey and royal jelly have been reported as sources of N. ceranae spores (Giersch et al., 2009, Cox-Foxter et al., 2007). N. ceranae-infected colonies have a long incubation period that usually appears to be asymptomatic, and which is usually passed over by apiarists. This includes a longer breeding period during cold months and diminished honey production. Finally, colonies become weakened and depleted of adult bees, and they collapse in a period of 1.5 to 2 years (Higes et al., 2010). Higes et al. (2009) found a close connection between N. ceranae infection and colony loss in professional apiaries in Spain. These findings were not present in Serbia, according to research by Stevanovic et al. (2013) (Brodschneider et al., 2018). In other European countries, the presence of N. ceranae has not been related to colony death, including the reported massive colony loss in North Europe (Paris et al., 2018, Papini et al., 2017). The largest losses of bee colonies in Serbia were in 2014 and 2016, in the years without clinical symptoms of the disease and laboratory slightly confirmed cases of nosemosis (Brodschneider et al., 2018). Based on these researches, the greatest winter losses and disappearances of bee colonies are not always related to the presence of N. ceranae (Ansari et al., 2017, Brodschneider et al., 2018, Paris et al., 2018).
In other studies, infection by N. ceranae does not seem to have any effect on colony loss (Guerrero-Molina et al., 2016, Ansari et al., 2017, Papini et al., 2017). Thus, many different views on the consequences of N. ceranae infection in European colonies have been reported. Huang (2011) found that infection with this parasite can affect the physiology, behavior and survival of bees. N. ceranae infection certainly disrupts the integrity of the central midgut epithelial cells and changes the energy needs of bees (Martín-Hernández et al., 2011, Mayack and Naug, 2010, Paris et al., 2018). The infection can also significantly affect the immune response of the bees and modify the production of pheromones, leading to a disorder of bee nutrition (Antúnez et al., 2009, Paris et al., 2018, Sinpoo et al., 2018). This can also be explained by other stress factors (pesticide poisoning, parasites, poor nutrition, flaws in bee technology, heavy metal contamination, etc.) (Alaux et al., 2010, Ansari et al., 2017, Vidau et al., 2011, Cox-Foxter et al., 2007; Genersch et al., 2010, Gisder et al., 2010, Ruschioni et al., 2013, Odnosum, 2017, OIE, 2013, Paris et al., 2018, Papini et al., 2017, Sinpoo et al., 2018).
It has been reported that N. ceranae is more prevalent in warmer climates than is N. apis (Fries, 2010). It appears that N. ceranae is better adapted to complete its endogenous cycle with a higher biotic index in a greater temperature range (Martín-Hernández et al., 2007). This difference in temperature sensitivity between parasite species has epidemiological implications and could mean decreased transmission opportunities for N. ceranae. Also, it is generally accepted that the earth’s temperature is progressively increasing, with great effect on parasites’ life cycles (Brooks and Hoberg, 2007). Changes in climate could affect the distribution and seasonality of nosemosis in honey bees (De la Rocque et al., 2008). Thus, more research will be needed to establish the epidemiological field characteristics of nosemosis in different European countries (Van der Zee et al., 2009). In conclusion, beekeeping management of disease prevention, such as replacement of combs and queens and hygienic handling of colonies are useful in the prevention of Nosema species infection (Pacini et al., 2016, OIE, 2013).
Acknowledgments
Acknowledgements
The authors would like to express their gratitude to the EU Reference Laboratory for Honey Bees’ Health – ANSES – the Sophia-Antipolis Laboratory, French Agency for Food, Environmental and Occupational Health and Safety, France. Part of this study was conducted within Projects TR 31088 funded by the Serbian Ministry of Education, Science and Technological Development of the Republic of Serbia. The authors want to thank the Kraljevo Veterinary Specialized Institute, Kraljevo, Serbia.
Disclosure statement
No potential conflict of interest was reported by the authors.
Compliance with ethics requirements
The manuscript does not contain clinical studies or patient data.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alaux C., Brunet J.L., Dussaubat C., Mondet F., Tchamitchan S., Cousin M., Brillard J., Baldy A., Belzunces L.P., Le Conte Y. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera) Environ. Microbiol. 2010;12(3):774–782. doi: 10.1111/j.1462-2920.2009.02123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Nuru A., Khan K.A., Alattal Y. Geographical distribution and molecular detection of Nosema ceranae from indigenous honey bees of Saudi Arabia. Saudi J. Biol. Sci. 2017;24(5):983–991. doi: 10.1016/j.sjbs.2017.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antúnez K., Martín-Hernández R., Prieto L., Meana A., Zunino P., Higes M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia) Environ. Microbiol. 2009;11(9):2284–2290. doi: 10.1111/j.1462-2920.2009.01953.x. [DOI] [PubMed] [Google Scholar]
- Bacandritsos N., Granato A., Budge G., Papanastasiou I., Roinioti E., Caldon M., Falcaro C., Gallina A., Mutinelli F. Sudden deaths and colony population decline in Greek honey bee colonies. J. Invertebr. Pathol. 2010;105(3):335–340. doi: 10.1016/j.jip.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Bollan K.A., Hothersall J.D., Moffat C., Durkacz J., Saranzewa N., Wright G.A., Connolly C.N. The microsporidian parasites Nosema ceranae and Nosema apis are widespread in honeybee (Apis mellifera) colonies across Scotland. Parasitol. Res. 2013;112(2):751–759. doi: 10.1007/s00436-012-3195-0. [DOI] [PubMed] [Google Scholar]
- Botías C., Martín-Hernández R., Garrido-Bailón E., González-Porto A., Martínez-Salvador A., De La Rúa P., Higes M. The growing prevalence of Nosema ceranae in honey bees in Spain, an emerging problem for the last decade. Res. Vet. Sci. 2012;93(1):150–155. doi: 10.1016/j.rvsc.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Brodschneider R., Gray A., Adjlane N., Ballis A. Multi-country loss rates of honey bee colonies during winter 2016/2017 from the COLOSS survey. J. Apicult. Res. 2018;57(3):452–457. [Google Scholar]
- Brooks D., Hoberg E.P. How will global climate change affect parasites? Trends Parasitol. 2007;23(12):571–574. doi: 10.1016/j.pt.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Chen Y., Evans J.D., Smith I.B., Pettis J.S. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 2008;97(2):186–188. doi: 10.1016/j.jip.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Colin, M.E., Tournaire, M., Gauthier, L., 2009. On the epidemiology of Nosema ceranae in France. In: Abstracts of 41st Congress Apimondia, 2009, Montpellier, France, pp. 144.
- Cox-Foxter D.L., Conlan S., Holmes E.C., Palacios G., Evans J.D., Moran N.A., Quan P.L., Brise T., Horning M., Geiser D.M., Martinson V., VanEngelsdorp D., Kalkstein A.L., Drysdale A., Hui J., Zhai J., Cui L., Hutchinson S.K., Simons J.F., Egholm M., Pettis J.S., Lipkin W.I. A metagenomic survey of microbes in honey bee colony collapse disorder. Science. 2007;318(5848):283–287. doi: 10.1126/science.1146498. [DOI] [PubMed] [Google Scholar]
- De la Rocque S., Rioux J.A., Slingenbergh J. Climate change: effects on animal disease systems and implications for surveillance and control. Int. Office Epizootics. 2008;27(2):339–354. [PubMed] [Google Scholar]
- Forsgren E., Fries I. Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet. Parasitol. 2010;170(3–4):212–217. doi: 10.1016/j.vetpar.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Fries I. Observation on the development and transmission of Nosema apis Z. in the ventriculus of the honey bee. J. Api. Res. 1989;28(2):07–117. [Google Scholar]
- Fries I., Feng F., da Silva A., Slemenda S.B., Pieniazek N.J. Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae) Eur. J. Protistol. 1996;32(3):356–365. [Google Scholar]
- Fries I. Nosema ceranae in European honey bees (Apis mellifera) J. Invertebr. Pathol. 2010;103:S73–S79. doi: 10.1016/j.jip.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Fries, I., Forsgren, E., 2008. Undersökning av spridningen av Nosema ceranae i Sverige. In: Investigation of the distribution of Nosema ceranae in Sweden. Bitidningen 107, januari/februari, pp. 26–27. (in Swedish).
- Fries I., Martín-Hernández R., Meana A., GarcíaPalencia P., Higes M. Natural infections of Nosema ceranae in European honey bees. J. Apic. Res. 2006;45(4):230–233. [Google Scholar]
- Genersch E., von der Ohe W., Kaatz H., Schroeder A., Otten C. The German bee monitoringproject: a long term study to understand periodicallyhigh winter losses of honey bee colonies. Apidologie. 2010;41(3):332–352. [Google Scholar]
- Giersch T., Berg T., Galea F., Hornitzky M. Nosema ceranae infects honey bees (Apis mellifera) and contaminates honey in Australia. Apidologie. 2009;40(2):117–123. [Google Scholar]
- Gisder S., Hedtke K., Möckel N., Frielitz M.-C., Linde A., Genersch E. Five-year cohort study of Nosema spp. in Germany: does climate shape virulence and assertiveness of Nosema ceranae? Appl. Environ. Microb. 2010;76(9):3032–3038. doi: 10.1128/AEM.03097-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Molina C., Correa-Benítez A., Hamiduzzaman M.M., Guzman-Novoa E. Nosema ceranae is an old resident of honey bee (Apis mellifera) colonies in Mexico, causing infection levels of one million spores per bee or higher during summer and fall. J. Invertebr. Pathol. 2016;141:38–40. doi: 10.1016/j.jip.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Hedtke K., Jensen P.M., Jensen A.B., Genersch E. Evidence for emerging parasites and pathogens influencing outbreaks of stress-related diseases like chalkbrood. J. Invertebr. Pathol. 2011;108(3):167–173. doi: 10.1016/j.jip.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Higes M., García-Palencia P., Martín-Hernández R., Meana A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia) J. Invertebr. Pathol. 2007;94(3):211–217. doi: 10.1016/j.jip.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Higes M., Martin R., Meana A. Nosema ceranae. A new microsporidian parasite in honey bees in Europe. J. Invertebr. Pathol. 2006;92(2):93–95. doi: 10.1016/j.jip.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Higes M., Martín-Hernández R., Meana A. Nosema ceranae in Europe: an emergent type C nosemosis. Apidologie. 2010;41(3):375–392. [Google Scholar]
- Higes M., Martín-Hernández R., Garrido-Bailón E., Botías C., Meana A. First detection of Nosema ceranae (Microsporidia) in African Honey bees (Apis mellifera intermissa) J. Apic. Res. 2009;48:217–219. [Google Scholar]
- Huang W.F., Jiang J.H., Chen Y.W., Wang C.H. A Nosema ceranae isolate from the honeybee Apis mellifera. Apidologie. 2007;38(1):30–37. [Google Scholar]
- Huang Z. Effects of Nosema on honey bee behavior and physiology. Am. Bee J. 2011;151:9. [Google Scholar]
- Ivanović J., Baltić M.Ž., Jelić D., Janjić J., Bošković M., Marković R., Dokmanović- & Starčević M. Research of production volume and market turnover of honey from 2004 to 2014. Veterinarski Glasnik. 2015;69(5/6):467–478. [Google Scholar]
- Klee J., Besana A.M., Genersch E., Gisder S., Nanetti A., Tam D.Q., Hatjina F. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee. Apis mellifera. J. Invertebr. Pathol. 2007;96(1):1–10. doi: 10.1016/j.jip.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Liu T.P. Ultrastructure of the midgut of the worker honey bee Apis mellifera heavily infected with Nosema apis. J. invertebr. Pathol. 1984;44(3):282–291. [Google Scholar]
- Martín-Hernández R., Botías C., Barrios L., Martínez-Salvador A., Meana A., Mayack C., Higes M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera) Parasitol. Res. 2011;109(3):605–612. doi: 10.1007/s00436-011-2292-9. [DOI] [PubMed] [Google Scholar]
- Martín-Hernandez R., Meana A., Garcia-Palencia P., Marin P., Botias C., Garrido-Bailon E., Barrios L., Higes M. Effect of temperature on the biotic potential of honeybee microsporidia. Appl. Environ. Microbiol. 2009;75(8):2554–2557. doi: 10.1128/AEM.02908-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Hernández R., Meana A., Prieto L., Salvador A.M., Garrido-Bailón E., Higes M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007;73(20):6331–6338. doi: 10.1128/AEM.00270-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matović K., Ćirić J., Kaljević V., Nedić N., Jevtić G., Vasković N., Baltić Ž.M. Physico-chemical parameters and microbiological status of honey produced in an urban environment in Serbia. Environ. Sci. Pollut. Res. Int. 2018;25(14):14148–14157. doi: 10.1007/s11356-018-1659-1. [DOI] [PubMed] [Google Scholar]
- Mayack C., Naug D. Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. 2010;56(11):1572–1575. doi: 10.1016/j.jinsphys.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Meana A., Martín-Hernández R., Higes M. The reliability of spore counts to diagnose Nosema ceranae infections in honey bees. J. Apic. Res. 2010;49(2):212–214. [Google Scholar]
- Milbrath M.O., van Tran T., Huang W.F., Solter L.F., Tarpy D.R., Lawrence F., Huang Z.Y. Comparative virulence and competition between Nosema apis and Nosema ceranae in honey bees (Apis mellifera) J. Invertebr. Path. 2015;125:9–15. doi: 10.1016/j.jip.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Naug D., Gibbs A. Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie. 2009;40(6):595–599. [Google Scholar]
- Odnosum H.V. Distribution of the Nosema ceranae (Microspora, Nosematidae) in the Apiaries in Ukraine. Vestnik zoologii. 2017;51(2):161–166. [Google Scholar]
- Office International des Épizooties (OIE). 2013. Listed diseases, infections and infestations. URL http://www.oie.int/en/animal-health-in-the-world/oielisteddiseases-2013.
- OIE Manual of Standards for Diagnostic Tests and Vaccines 1992 – OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2017.
- Pacini A., Mira A., Molineri A., Giacobino A., Cagnolo N.B., Aignasse A., Zago L., Izaguirre M., Merke J., Orellano E., Bertozzi E. Distribution and prevalence of Nosema apis and N. ceranae in temperate and subtropical eco-regions of Argentina. J. Inverteb. Pathol. 2016;141:34–37. doi: 10.1016/j.jip.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Papini R., Mancianti F., Canovai R., Cosci F., Rocchigiani G., Benelli G., Canale A. Prevalence of the microsporidian Nosema ceranae in honeybee (Apis mellifera) apiaries in Central Italy. Saudi J. Biol. Sci. 2017;24(5):979–982. doi: 10.1016/j.sjbs.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris L., El Alaoui H., Delbac F., Diogon M. Effects of the gut parasite Nosema ceranae on honey bee physiology and behavior. Curr. Opin. Insect Sci. 2018;26:149–154. doi: 10.1016/j.cois.2018.02.017. [DOI] [PubMed] [Google Scholar]
- Paxton R.J., Klee J., Korpela S., Fries I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie. 2007;38(6):558–565. [Google Scholar]
- Pilarska D.K., Solter L.F., Kereselidze M., Linde A., Hoch G. Microsporidian infections in Lymantria dispar larvae: Interactions and effects of multiple species infections on pathogen horizontal transmission. J. Invertebr. Pathol. 2006;93(2):105–113. doi: 10.1016/j.jip.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Razmaraii N., Sadegh-Eteghad S., Babaei H., Paykari H., Esmaeilnia K., Froghy L. Molecular identification of Nosema species in East Azerbaijan province. Iran. Archives Razi Institute. 2013;68(1):23–27. [Google Scholar]
- Read A.F., Taylor L.H. The ecology of genetically diverse infections. Science. 2001;292(5519):1099–1102. doi: 10.1126/science.1059410. [DOI] [PubMed] [Google Scholar]
- Ruschioni S., Riolo P., Minuz R.L., Stefano M., Cannella M., Porrini C., Isidoro N. Biomonitoring with honeybees of heavy metals and pesticides in nature reserves of the Marche Region (Italy) Biol. Trace Elem. Res. 2013;154(2):226–233. doi: 10.1007/s12011-013-9732-6. [DOI] [PubMed] [Google Scholar]
- Sinpoo C., Paxton R.J., Disayathanoowat T., Krongdang S., Chantawannakul P. Impact of Nosema ceranae and Nosema apis on individual worker bees of the two host species (Apis cerana and Apis mellifera) and regulation of host immune response. J. Insect. Physiol. 2018;105:1–8. doi: 10.1016/j.jinsphys.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Stevanovic J., Simeunovic P., Gajic B., Lakic N., Radovic D., Fries I., Stanimirovic Z. Characteristics of Nosema ceranae infection in Serbian honey bee colonies. Apidologie. 2013;44(5):522–536. [Google Scholar]
- Stevanovic J., Stanimirovic Z., Genersch E., Kovacevic R.S., Ljubenkovic J., Radakovic M., Aleksic N. Dominance of Nosema ceranae in honey bees in the Balkan countries in the absence of symptoms of colony collapse disorder. Apidologie. 2011;41(1):49–58. [Google Scholar]
- Tapaszti Z., Forgách P., Kövágó C., Békési L., Bakonyi T., Rusvai M. First detection and dominance of Nosema ceranae in Hungarian honeybee colonies. Acta. Vet. Hung. 2009;57(3):383–388. doi: 10.1556/AVet.57.2009.3.4. [DOI] [PubMed] [Google Scholar]
- Topolska G., Kasprzak S. First cases of Nosema ceranae infection in bees in Poland. Med. Weter. 2007;63(11):1504–1506. [Google Scholar]
- Van der Zee R., Gómez-Moracho T., Pisa L., Sagastume S., García-Palencia P., Maside, Van Engelsdorp D., Evans J.D., Saegerman C., Mullin C., Haubruge E. Colony collapse disorder: a descriptive study. PLoS One. 2009;4:e6481. doi: 10.1371/journal.pone.0006481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidau C., Diogon M., Aufauvre J., Fontbonne R., Viguès B., Brunet J.L., Texier C., Biron D.G., Blot N., El Alaoui H., Belzunces L.P. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS ONE. 2011;6(6):e21550. doi: 10.1371/journal.pone.0021550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander E., Bottcher F.K. 7th ed. Verlag Eugen Ulmer; Stuttgart: 1984. Krankheiten der Biene. [Google Scholar]
Further reading
- Chen Y.P., Evans J.D., Murphy C., Gutell R., Zuker M., Gundensen-Rindal D.A.W.N., Pettis J.S. Morphological, Molecular, and Phylogenetic Characterization of Nosema ceranae, a Microsporidian Parasite Isolated from the European Honey Bee, Apis mellifera. J. Eukaryot. Microbiol. 2009;56(2):142–147. doi: 10.1111/j.1550-7408.2008.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]