Abstract
The oleo gum resin of Boswellia sacra Fleuck. (Burseraceae) is widely consumed for treatment of several diseases and disorders. To determine the effect of repeated administration of this resin on liver and kidney functions, three different doses of standardized methanolic extract were administered orally to rats for 28 days. Apart from histological studies and determination of biomarkers of hepatotoxicity and nephrotoxicity, other parameters of sub-chronic toxicity such as behavioral change, food consumption and change in body weight were assessed. The extract contained about 36.91% of total boswellic acids; of which 11-keto beta boswellic acid, acetyl-11-keto beta boswellic acid, boswellic acids (α and β) and acetyl boswellic acid (α and β) were found to be 5.81%, 1.91%, 21.92% and 7.27% respectively. Oral administration of the extract for 28 consecutive days did not show any sign of behavioral toxicity and did not affect food consumption or weight gain significantly. Determination of biomarkers of hepatic and nephrotoxicity revealed that extract was safe at the tested doses as it did not produce any significant change in the studied biomarkers except producing a dose dependent increase in serum total protein levels. The histological examination supported biochemical findings. To conclude, methanolic extract of Boswellia sacra doen not produce any significant toxicity to liver and kidney up to doses of 100 mg/kg body weight. The results contradict earlier reports that members of boswellia species produce organ toxicity in rats.
Keywords: Frankincense, Boswellic acid, Biomarkers, Sub-chronic toxicity, HPLC
1. Introduction
Adverse reaction and toxicity due to use of herbal drugs is increasing throughout the world as a result of increase in their use as food supplements and for therapeutic purposes. Several herbs or their products have been reported to affect important organs such as liver, kidney, brain and testes.
Boswellia sacra Flueck. (Burseraceae) oleo gum resin commonly known as frankincense is a widely used house hold medicine, especially in the Arab countries. Apart from this, it is used as perfume, in aromatherapy, and to perform religious rites. The boswellia oleo gum resin known in Arabic as ‘‘Luban’’ is also chewed for its essence in many Middle East and Arab-African countries. In Oman, frankincense is burnt and the essence from smoke is used as perfume (Al-Harrasi et al., 2018).
Frankincense resin is widely used for treatment of bronchial and urinary infections. In some Asian countries, it is used as a rejuvenating medicine and to cure menstrual pain, gum, mouth, and throat complaints. It is also known to heal wounds, sores, ulcers, carbuncles, hemorrhoids, and inflammation. Frankincense oil possesses carminative, digestive and diuretic properties (Khan, 2012). The water extract of this gum resin called ‘Moh-Lubban’ is traditionally used for relieving cough and stomach problems (Al-Harrasi et al., 2018). Recently, boswellia species are reported to possess anticancer properties by inhibiting topoisomerase I and II-alpha and stimulating programmed cell death (Mazzio et al., 2017, Al-Yasiry and Kiczorowska, 2016). Boswellia serrata is also used for management of knee osteoarthritis in humans (Bolognesi et al., 2016). Boswellia is reported to have beneficial effect on the rearing efficiency in chickens (Kiczorowska et al., 2016a, Kiczorowska et al., 2016b)
Our lab had reported earlier that water extract of Boswellia sacra oleo gum resin increases ulcer formation and possesses proulcerogenic effect in rats (Asad and Alhomoud, 2016). There are few reports on the organ toxicity of some boswellia species though many of them have reported that boswellia species is safe. For instance, a 90-day safety assessment study of Boswellia serrata reported that a dose of 1000 mg/kg of boswellia oleo gum resin is toxic and the no observed adverse effect level (NOAEL) is 500 mg/kg in rats (Singh et al., 2012). Similarly, alcoholic extract of Boswellia ovalifoliata was reported to have NOAEL at 500 mg/kg in rats (Devi et al., 2012). However, several studies reported that Boswellia serrata is safe up to the dose of 1000 mg/kg in rats (Singh et al., 2012, Lalithakumari et al., 2006). None of the above mentioned studies used standardized extract containing known amount of the active constituents; boswellia acids. In the Middle Eastern countries, the most widely used frankincense is Boswellia sacra. There is no study on the effect of this herbal drug or its extract/constituents on the organ toxicity. Hence, a repeated dose 28-day oral toxicity study of standardized extract of Boswellia sacra oleo gum resin containing known amount of boswellic acids was done in rats to determine its safety.
2. Materials and methods
2.1. Materials
Methanol used in extraction process was obtained from Loba Chemie (Mumbai, India). Boswellic acids were purchased from Sigma Aldreich (USA) and kits for estimation of biomarkers were purchased from local suppliers and they were from different manufacturers. The kits for estimation of alanine aminotransferase, aspirate aminotransferase, alkaline phosphatase, bilirubin (total and direct), serum creatinine, total cholesterol, total proteins and albumin were purchased from Crescent Diagnostics (Jeddah, Saudi Arabia). All the above estimations were carried out using spectrophotometer (Biochrom Libra S22, UK) The sodium and potassium were estimated by using kits procured from Human Diagnostics (Germany) and the estimations were done using a flame photometer (BWB Technologies, England). The HPLC grade solvents were from Vicam (England).
2.2. Preparation and standardization of the extract
The oleo gum resin of Boswellia sacra was purchased from local market. It was identified by Prof A M Sadaby (College of Applied Medical Sciences, Shaqra University). A voucher specimen was deposited in the college for future reference.
The oleo gum resin was powdered and subjected to methanol extraction in a Soxhlet extractor followed by drying in a rotavapor. The yield of the extract was 12.76%w/w. The extract was subjected to quantification of boswellic acid by high performance liquids chromatography (HPLC) method that developed in our institute.
2.3. Quantification of boswellic acids
The sample was prepared by weighing accurately 500 mg of extract into 100 ml volumetric flask. This was dissolved in 50 ml of methanol and made up to 100 ml with methanol and filtered. The standard was prepared by weighing accurately 10 mg of 11-keto-β-boswellic acid, 5 mg of acetyl-11-keto-β-boswellic acid and 10 mg each of boswellic acid (α + β) and acetyl-boswellic acid (α + β) in separate 50 ml volumetric flask. These were dissolved in 20 ml of methanol and made up to 50 ml with methanol.
A shimadzu liquid chromatography system equipped with C18-ODS; 5μ size, 100 × 3.0 mm (Luna) was employed for analyses. The mobile phase was prepared by mixing 100 ml of solvent A and 900 ml of solvent B. The solvent A consisted of 950 ml water and 50 ml acetonitrile and the pH was adjusted to 2.8 with ortho-phosphoric acid. Solvent B was 100% methanol. The injection volume was 5 µl (for 100 × 3 mm column) and flow rate was 0.4 ml/min. The detection was carried out 247 nm for 11-keto-β-boswellic acid and acetyl-11-keto-β-boswellic acid and at 210 nm for boswellic acid (α + β) and acetyl-boswellic acid (α + β). The chromatographic analytes were identified by comparing retention times with those of standard. The percentage of total triterpene acid content from the peak areas was calculated using the formula
2.4. Animals and grouping
2.4.1. Animals
Adult male Sprague-Dawley rats weighing between 173 g and 196 g were used. The animals were housed in a well-maintained animal house with temperature and humidity control. The animals were fed rat chow (Lipton India, Mumbai, India), containing all essential nutrients and trace elements (Table 1) and water ad libitum. All rats were bred in the institutional animal house. The protocol of the experiment was approved by the scientific committee of the university that also financed this study (1/1/2005).
Table 1.
Composition of the rat diet.
| Contents | Percentage |
|---|---|
| Moisture | 8.55% |
| Crude protein | 18.18% |
| Crude fat | 3.20% |
| Crude fiber | 5.00% |
| Calcium | 1.26% |
| Phosphorous | 0.54% |
| Total ash | 5.45% |
| Carbohydrates | 63.00% |
| Energy | 3080 kcal/kg |
The animals were divided into five groups consisting of six animals each. The first group served as control and received vehicle (Sodium carboxymethyl cellulose 1% w/v) at a dose of 1 ml/kg. The next three groups received Boswellia extract suspended in sodium carboxymethyl cellulose (1% w/v) at doses of 250 mg/kg, 500 mg/kg and 1000 mg/kg orally for 28 days. The last group of animals received Boswellia extract suspended in sodium carboxymethyl cellulose (1% w/v) at a dose of 1000 mg/kg and served as recovery group (OECD, 2019). The treatment was given once daily for 28 days.
2.5. Effect on behavior, food consumption and weight
The effect of extract on general behavior such as activity, grooming and convulsion as indicator of central nervous system activity was determined (OECD, 2019). The daily intake of food and water in each group of animals was determined. Weight of each animal was determined weekly and total weight gain was calculated.
2.6. Effect on serum biochemical parameters and histology
At the end of the treatment period, blood was withdrawn from all animals and serum was used for estimation of biochemical parameter using commercially available kits. The serum was separated from blood by keeping the blood for 30–45 min to clot followed by centrifugation at 1000 g for 15 min. The liver function was assessed by estimating aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and bilirubin (both direct and total) levels. To determine the effect of kidney; serum creatinine levels, sodium and potassium levels in serum were estimated. The animals were sacrificed to remove the liver and kidney. Weight of these organs after blotting dry using a filter paper was determined. The organs were then used for histological studies. The organs were studied for degenerative, infiltrative and circulatory changes.
2.7. Statistical analysis
All data are mean ± SEM. Statistical significance was determined using one-way ANOVA followed by Tukey’ test using Graphpad Software Inc., (Graphpad Instat, San Diego, USA). P < 0.05 was considered to indicate significant difference.
3. Results
3.1. Quantification of boswellic acids
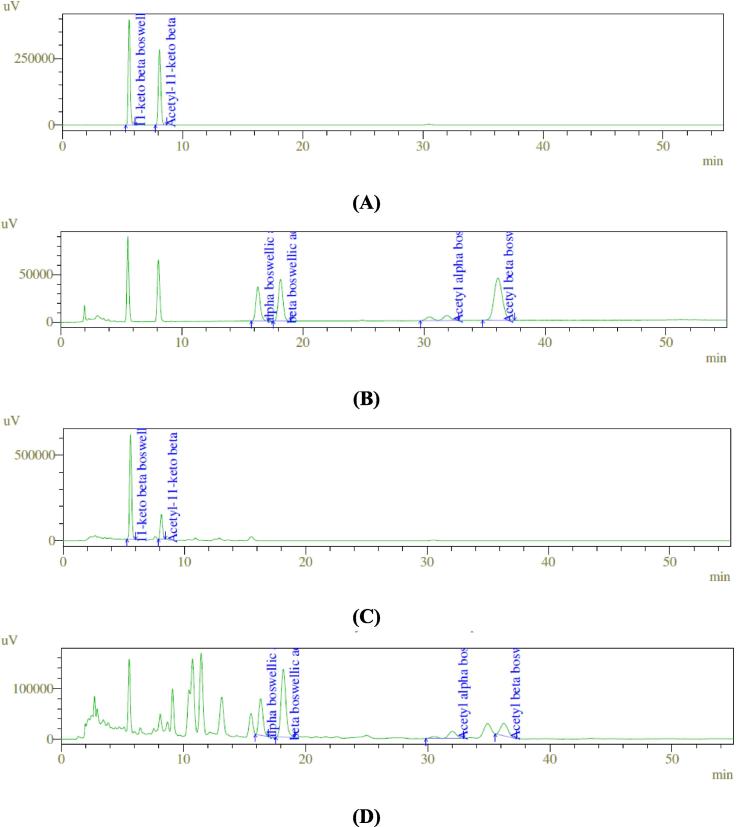
Analysis of the Bowellia sacra oleo gum resin extract revealed that it contains high amount of total boswellic acids (36.91% w/w). Out of the total boswellic acids, the maximum amount was that of boswellic acid (α + β), which was around 21.92% w/w of the extract while acetyl-11-keto-β-boswellic acid was present in the least amount (Table 2). The chromatogram is shown in Fig. 1.
Table 2.
Quantification of boswellic acids.
| Constituent | Amount (%w/w) |
|---|---|
| Total boswellic acid | 36.91 |
| 11-keto-β-boswellic acid | 5.81 |
| Acetyl-11-keto-β-boswellic acid | 1.91 |
| Boswellic acid (α + β) | 21.92 |
| Acetyl-boswellic acid (α + β) | 7.27 |
Fig. 1.
Chromatograms showing 11-keto beta boswellic acid and acetyl-11-keto beta boswellic acid in standard mix (A), alpha boswellic acid, alpha boswellic acid, beta boswellic acid, acetyl alpha boswellic acid and acetyl beta boswellic acid in standard mix (B), 11-keto beta boswellic acid and acetyl-11-keto beta boswellic acid in extract (C), alpha boswellic acid, alpha boswellic acid, beta boswellic acid, acetyl alpha boswellic acid and acetyl beta boswellic acid in extract (D).
3.2. Effect on behavior, food consumption and weight
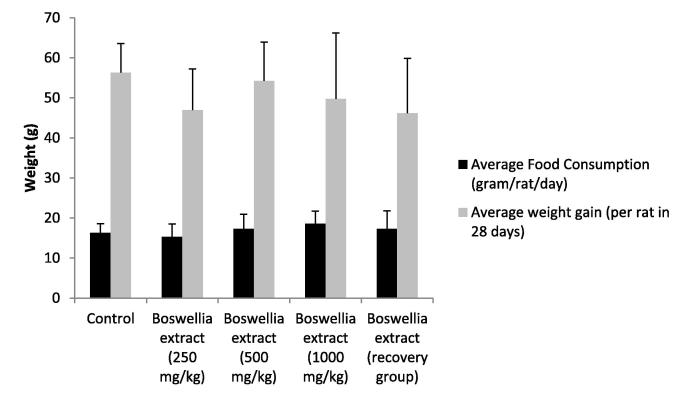
The extract at three different doses tested did not show any notable change in the behavior of the treated animals in comparison to control animals. The food consumption and weight changes in the treated animals were not significantly different from control animals (Fig. 2).
Fig. 2.
Effect on food consumption and weight gain. All values are mean ± SEM, n = 6, there was no significant difference.
3.3. Effect on serum biochemical parameters and histology
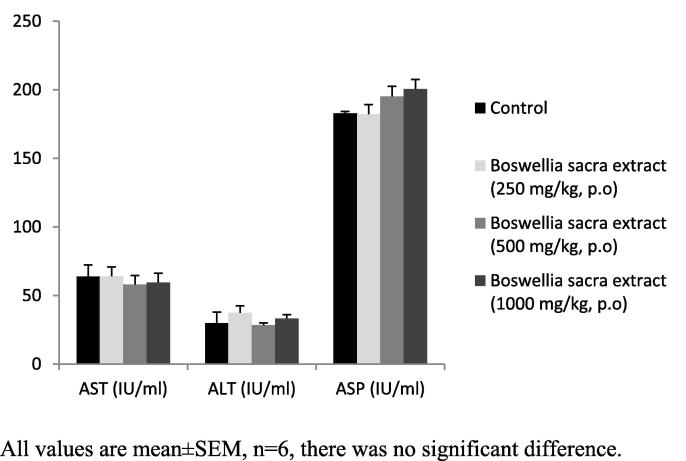
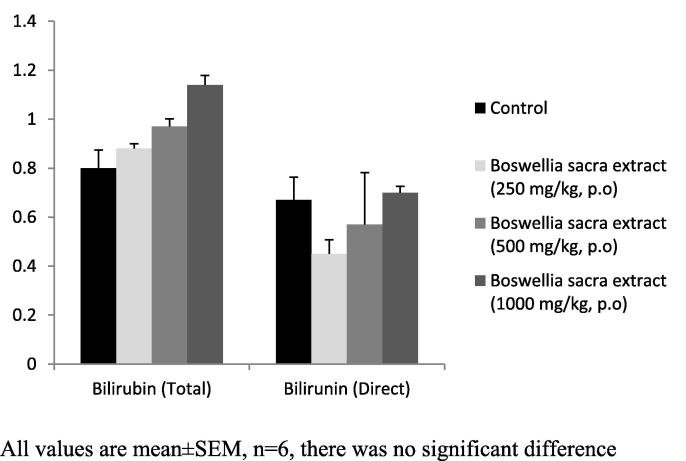
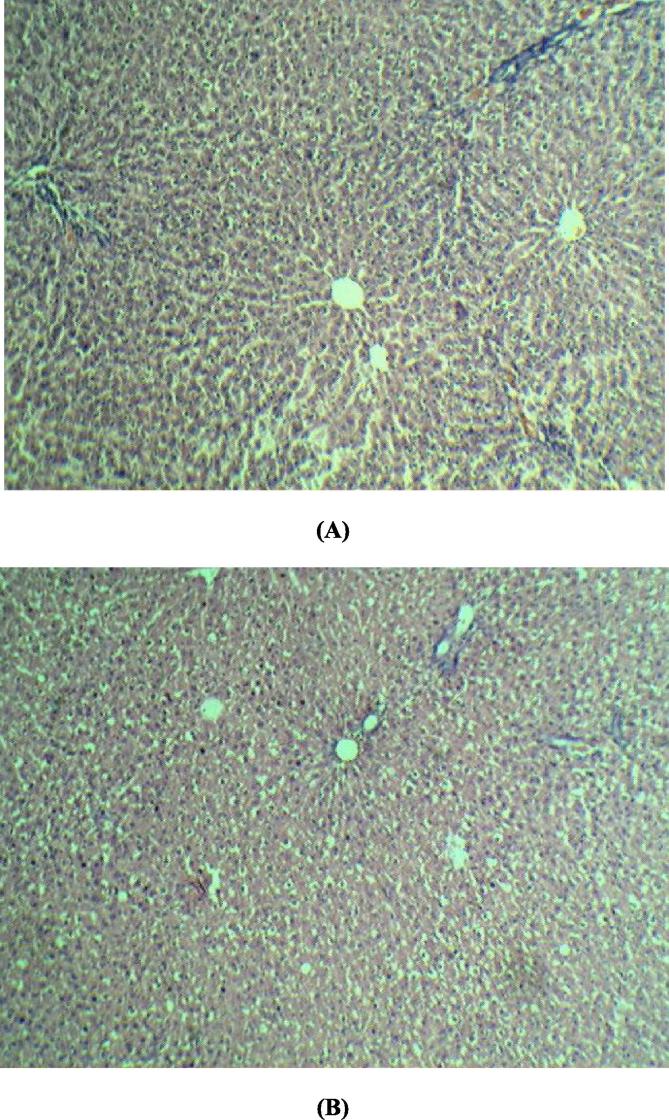
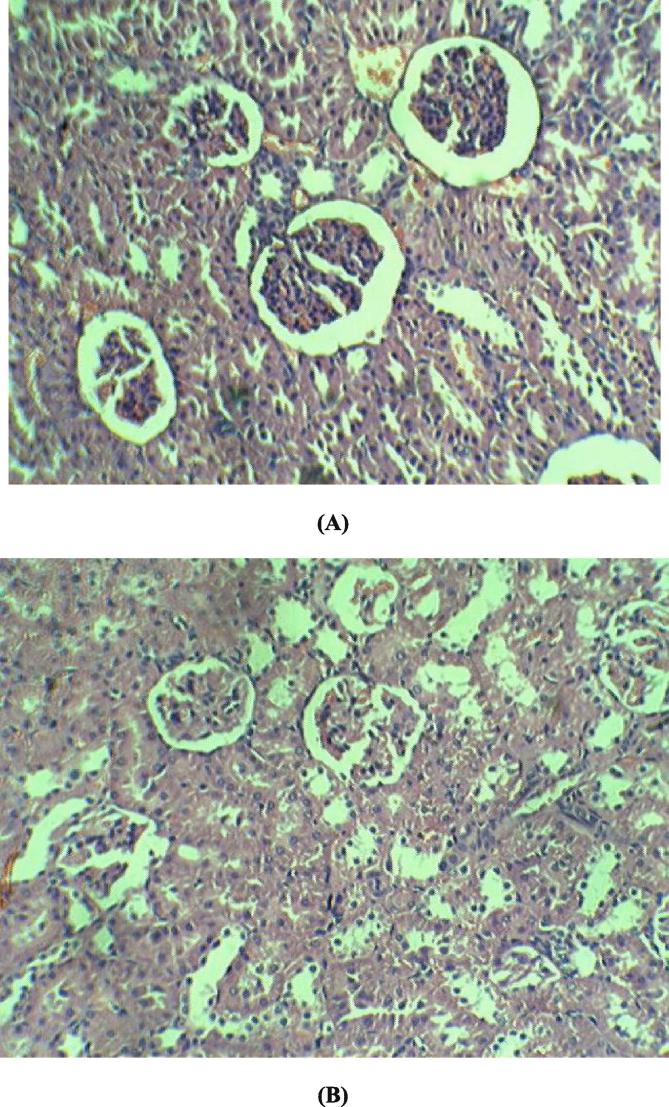
Determination of biomarkers for liver and kidney injury showed that Boswellia sacra extract did not significantly affect liver or kidney functions (Table 3; Fig. 3, Fig. 4). Histological examination of the tissues revealed that there was no degenerative, circulatory and infiltrative changes (Fig. 5). In the kidney, uniformly spread nephrons with mild to moderate degenerative distal and proximal tubes were noticed (Fig. 6).
Table 3.
Effect on biomarkers of kidney function.
| Treatment | Serum creatinine (mg/dl) | Na (mmol/L) | K (mmol/L) | Total proteins (g/dl) | Albumin (g/dl) | Total cholesterol (mg/dl) |
|---|---|---|---|---|---|---|
| Control | 0.65 ± 0.028 | 140.75 ± 0.487 | 5.15 ± 0.478 | 6.32 ± 0.118 | 2.90 ± 0.204 | 63.50 ± 8.655 |
| Boswellia sacra extract (250 mg/kg, p.o) | 0.62 ± 0.025 | 142.00 ± 0.577 | 4.85 ± 0.307 | 7.70 ± 0.103* | 2.85 ± 0.095 | 63.25 ± 6.981 |
| Boswellia sacra extract (500 mg/kg, p.o) | 0.67 ± 0.063 | 140.50 ± 1.708 | 5.32 ± 0.155 | 7.42 ± 0.253** | 2.82 ± 0.137 | 57.75 ± 7.169 |
| Boswellia sacra extract (1000 mg/kg, p.o) | 0.70 ± 0.041 | 142.50 ± 1.555 | 5.37 ± 0.085 | 8.03 ± 0.146*** | 2.40 ± 0.248 | 64.75 ± 4.535 |
All values are mean ± SEM, n = 6, *P < 0.05, **P < 0.01, ***P < 0.001 compared to control.
Fig. 3.
Effect on serum levels of liver enzymes. All values are mean ± SEM, n = 6, there was no significant difference.
Fig. 4.
Effect on serum bilirubin levels. All values are mean ± SEM, n = 6, there was no significant difference.
Fig. 5.
Histology of liver. Control animal; Liver revealed no degenerative, circulatory and infiltrative changes were noticed (A). Animal treated with Boswellia sacra extract (1000 mg/kg, p.o); liver revealed no degenerative, circulatory and infiltrative changes were noticed (B).
Fig. 6.
Histology of liver. Control animal; kidney reveal uniformly spread nephrons, Mild to moderate degenrative distal and proximal tubes are noticed (A). Animal treated with Boswellia sacra extract (1000 mg/kg, p.o), kidney reveal uniformly spread nephrons, Mild to moderate degenrative distal and proximal tubes are noticed (B).
4. Discussion
The methanolic extract of Boswellia sacra oleo gum resin did not produce any significant change in serum biomarkers of liver and kidney damage though mild to moderate degenerations were observed in distal convoluted tubules and proximal convoluted tubular cells. Similarly, no significant change in behavior, food consumption or weight of the animals was observed after administration of extract. The extract contained high amount of the active constituents; boswellic acids.
The results indicate that extract is safe up to 1000 mg/kg in rats. This dose is quiet high considering the amount of extract consumed by humans. The extract is marketed for consumption at doses up to 1000 mg per day for humans (Del Grossi Moura et al., 2017), which roughly comes to 200 mg/kg for rats. The highest dose tested in the present study is almost 5 times of the maximum dose used in human. We would like to stress that there is no fixed dose of boswellia, the doses vary depending on the country and the diseases/disorders for which the product is used. However, the maximum dose used is 1.5 g per day for humans.
Boswellic acids are the active constituents present in all boswellia species (Ammon, 2016). It is used as a marker to determine the purity of extract. In the present study, the extract contained almost 37% w/w of boswellic acid. This indicates good extraction and it also shows that the quality of oleo gum resin used for extraction is of very good quality. Earlier reports show that the boswellic acids in the extract varies from 65% to 0.03% in Boswellia serrata, the most widely used boswellia species (Ameen et al., 2017, Beghelli et al., 2017). There are no reports on the amount of boswellic acids present in Boswellia sacra used in Arabian countries. Hence, the results of present study will contribute to information about the amount of boswelic acids in Boswellia sacra. There are different types of boswellic acids that includes boswellic acid (α + β), acetyl boswellic acid (α + β), acetyl-11-keto-β-boswellic acid and 11-keto-β-boswellic acid.
Organ toxicity is one of the common adverse effects of drugs. All organs are not equally affected by agents producing adverse actions. The vulnerability of an organ depends to a large extent on the blood flow it receives. Further, organs involved in metabolism and/or excretion are more susceptible for damage (Klaassen, 2013). The Organization for Economic Cooperation and Development (OECD) recommends rats as the preferred rodents for repeat dose 28-day oral toxicity study (OECD, 2019). Hence, these animals were used. Though the guidelines recommend the study to be conducted in both males and females, many investigators prefer only male rats to determine the effect of organ toxicity to eradicate the influence of changes in hormonal levels on toxicity (Yousef et al., 2019, Turkmen et al., 2019, Devi et al., 2012). Since, this is the first study on the organ toxicity of Boswellia sacra extract, we used only male rats in the study. Further studies on the toxicity profile in nulliparous and multiparous animals will be taken in future to determine the effect on organ toxicity in female rats. As per the OECD guidelines, a recovery group of six animals was used. Since, there was no toxicity was observed with the extract after treatment for 28 days, data obtained from recovery group has not been shown.
Liver is one of the organs that is prone for damage because it is highly perfused and contains high amount of metabolic enzymes. Though metabolism is known to reduce toxic effects by producing metabolites that can be easily excreted, there are a number of compounds, which are not toxic by themselves but their metobloites are reported to damage the organs. The best examples for compounds whose metabolites are toxic include carbon tetrachloride and paracetamol; both of which are known hepatotoxicants. The liver toxicity is usually studied by determining the leakage of intracellular enzymes from liver cells such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and bilirubin. The damage to liver is supported by histological examination of the liver tissue to study degenerations such as fatty liver or liver necrosis, especially around the central vein (Klaassen, 2013).
In the present study, the extract though administered at high doses did not significantly affect the levels of liver biomarkers in serum and histological examination also did not show any distinct damage to the organ. We had earlier reported that water extract of Boswellia sacra that is free of boswellic acids showed hepatoprotective effect against carbon tetrachloride induced hapatotoxicity in rats (Asad and Alhumoud, 2015). We attributed this effect to the antioxidants present in the extract that included carvacol, cineole, linalool and furanmethanol, which were shown to be present in the extract. Furthermore, the hepatoprotective effect of hexane extract of Boswellia serrata oleo-gum resin that is rich in boswellic acids against several hepatotoxicants such as carbon tetrachloride, paracetamol and thioacetamide has been reported wherein it showed good hepatoprotective effects (Jyothi et al., 2006). In the present study, methanolic extract of Boswellia sacra was used and this is different from Boswellia serrata and also from the water extract of Boswellia sacra that were reported to have hepatoprotective effects. Furthermore, the doses tested in the earlier studies were 500 mg/kg or less that were selected to extrapolate the results to the humans. In the present safety study, doses up to 1000 mg/kg were used to completely rule out any toxic effect of the Boswellia sacra oleo gum resin on the liver.
Kidney like liver is a highly perfused organ and is also an important excretory organ. Both these factors make it prone to damage. Similar to liver, biomarkers of kidney damage and histological examination of the tissue are used to determine the effect on kidney cytoarchitecture (Klaassen, 2013). The Boswellia sacra extract did not affect the kidney as indicated by its effect on serum creatinine levels and histological examination. Damage to glomerulus leads to excretion of albumin leading to decrease in serum albumin levels. Albumin is essential for proper transportation of lipids. Decrease in albumin levels will lead to an increase in serum cholesterol levels. In the present study, there was no significant effect on the serum albumin levels or cholesterol suggesting that there was no damage to the glomerulus. However, a dose dependent increase in the serum total proteins was observed. Since, the albumin levels were normal, this suggests an increase in proteins other than albumin. There may have been an increase in serum globulin levels. To determine the influence of this increase in serum total proteins, further studies on the type of globulin that may have been increased needs to be determined. Such studies are being planned. Apart from these, effect on excretion of ions also indicated that there was no significant effect on serum sodium and serum potassium levels, thus ruling out any effect on the function of the kidneys (Rouiller and Muller, 2013).
Though the study focused on the effect of repeated dose toxicity of boswellia, the results suggest that boswellia is quiet safe and supports earlier reports that boswellia species have hepatoprotective and renoprotective effects (Liu et al., 2018, Jyothi et al., 2006). Furthermore, absence of behavioral changes in the animals treated with boswellia extract suggests that the extract is free of any central nervous system effects.
To conclude, the methanolic extract of Boswellia sacra oleo gum resin did not produce any significant effect on the kidney and liver upon repeated dose administration for 28 days indicating that it is safe up to oral doses of 1000 mg/kg in rats.
Acknowledgement
The authors thankful to the Deanship of Research, Shaqra University for funding this research work (Grant No. 1/1/2005).
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Harrasi A., Hussain H., Csuk R., Khan H.Y. Elsevier; USA: 2018. Chemistry and Bioactivity of Boswellic Acids and Other Terpenoids of the Genus Boswellia. [Google Scholar]
- Al-Yasiry A.R., Kiczorowska B. Frankincense–therapeutic properties. Postepy. Hig. Med. Dosw. (Online) 2016;70:380–391. doi: 10.5604/17322693.1200553. [DOI] [PubMed] [Google Scholar]
- Ameen A.M., Elkazaz A.Y., Mohammad H.M.F., Barakat B.M. Anti-inflammatory and neuroprotective activity of boswellic acids in rotenone parkinsonian rats. Can. J. Physiol. Pharmacol. 2017;95(7):819–829. doi: 10.1139/cjpp-2016-0158. [DOI] [PubMed] [Google Scholar]
- Ammon H.P. Boswellic acids and their role in chronic inflammatory diseases. Adv. Exp. Med. Biol. 2016;928:291–327. doi: 10.1007/978-3-319-41334-1_13. [DOI] [PubMed] [Google Scholar]
- Asad M., Alhumoud M. Hepatoprotective effect and GC-MS analysis of traditionally used Boswellia sacra oleo gum resin (frankincense) extract in rats. Afr. J. Tradit. Complement. Altern. Med. 2015;12(2):1–5. [Google Scholar]
- Asad M., Alhomoud M. Proulcerogenic effect of water extract of Boswellia sacra oleo gum resin in rats. Pharm. Biol. 2016;54(2):225–230. doi: 10.3109/13880209.2015.1028553. [DOI] [PubMed] [Google Scholar]
- Beghelli D., Isani G., Roncada P., Andreani G., Bistoni O., Bertocchi M., Lupidi G., Alunno A. Antioxidant and ex vivo immune system regulatory properties of Boswellia serrata extracts. Oxid. Med. Cell Longev. 2017;2017:7468064. doi: 10.1155/2017/7468064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi G., Belcaro G., Feragalli B., Cornelli U., Cotellese R., Hu S., Dugall M. Movardol® (N-acetylglucosamine, Boswellia serrata, ginger) supplementation in the management of knee osteoarthritis: preliminary results from a 6-month registry study. Eur. Rev. Med. Pharmacol. Sci. 2016;20(24):5198–5204. [PubMed] [Google Scholar]
- Rouiller C., Muller A. Volume 2. Elsevier Saunders; USA: 2013. (The Kidney: Morphology, Biochemistry, Physiology:). [Google Scholar]
- Del Grossi Moura M., Lopes L.C., Biavatti M.W., Kennedy S.A., de Oliveira E., Silva M.C., Silva M.T., de Cássia Bergamaschi C. Oral herbal medicines marketed in Brazil for the treatment of osteoarthritis: A systematic review and meta-analysis. Phytother. Res. 2017;31(11):1676–1685. doi: 10.1002/ptr.5910. [DOI] [PubMed] [Google Scholar]
- Devi P.R., Adilaxmamma K., Rao G.S., Srilatha Ch., Raj M.A. Safety evaluation of alcoholic extract of Boswellia ovalifoliolata stem-bark in rats. Toxicol. Int. 2012;19(2):115–120. doi: 10.4103/0971-6580.97198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyothi Y., Kamath J.V., Asad M. Effect of hexane extract of Boswellia serrata oleo-gum resin on chemically induced liver damage. Pak. J. Pharm. Sci. 2006;19(2):129–133. [PubMed] [Google Scholar]
- Khan A.J. Medicinal properties of frankincense. Int. J. Nutr. Pharmacol. Neurol. Dis. 2012;2:79. [Google Scholar]
- Kiczorowska B., Samolińska W., Al-Yasiry A.R.M., Kowalczyk-Pecka D. Effect of supplementation of mixtures for broiler chickens with Boswellia serrata on the condition of the gastrointestinal tract and rearing efficiency. Ann. Animal Sci. 2016;16:835–849. [Google Scholar]
- Kiczorowska B., Al-Yasiry A.R.M., Samolińska W., Pyzik E., Marek A. The effect of dietary supplementation of the broiler chicken diet with Boswellia serrata resin on growth performance, digestibility, and gastrointestinal characteristics, morphology, and microbiota. Livestock Sci. 2016;191:117–124. [Google Scholar]
- Klaassen C.D. McGraw-Hill Education; United States: 2013. Casarett and Doull's Toxicology: The Basic Science of Poisons. [Google Scholar]
- Lalithakumari K., Krishnaraju A.V., Sengupta K., Subbaraju G.V., Chatterjee A. Safety and toxicological evaluation of a novel, standardized 3-O-Acetyl-11-keto-beta-boswellic acid (AKBA)-enriched Boswellia serrata extract (5-Loxin(R) Toxicol. Mech. Methods. 2006;16(4):199–226. doi: 10.1080/15376520600620232. [DOI] [PubMed] [Google Scholar]
- Liu M., Liu T., Shang P., Zhang Y., Liu L., Liu T., Sun S. Acetyl-11-keto-β-boswellic acid ameliorates renal interstitial fibrosis via Klotho/TGF-β/Smad signalling pathway. J. Cell. Mol. Med. 2018;22(10):4997–5007. doi: 10.1111/jcmm.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzio E.A., Lewis C.A., Soliman K.F.A. Transcriptomic profiling of MDA-MB-231 cells exposed to Boswellia serrata and 3-O-acetyl-β-boswellic acid; ER/UPR mediated programmed cell death. Cancer Genomics Proteomics. 2017;14(6):409–425. doi: 10.21873/cgp.20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD, 2019. https://www.oecd-ilibrary.org/docserver/9789264070684-en.pdf?expires=1557044033&id=id&accname=guest&checksum=7533DC457BE95C1CBEE3EA0A180FECCF. (accessed on 5 May 2019, time 10.15 GMT)
- Singh P., Chacko K.M., Aggarwal M.L., Bhat B., Khandal R.K., Sultana S., Kuruvilla B.T. A-90 day gavage safety assessment of Boswellia serrata in rats. Toxicol. Int. 2012;19(3):273–278. doi: 10.4103/0971-6580.103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkmen R., Birdane Y.O., Demirel H.H., Kabu M., Ince S. Protective effects of resveratrol on biomarkers of oxidative stress, biochemical and histopathological changes induced by sub-chronic oral glyphosate-based herbicide in rats. Toxicol. Res. (Camb) 2019;8(2):238–245. doi: 10.1039/c8tx00287h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef M.I., Mutar T.F., Kamel M.A.E. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol. Rep. 2019;6:336–346. doi: 10.1016/j.toxrep.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]