Abstract
Based on multiple studies in animal models, mesenchymal stem cell (MSC)‐based therapy appears to be an innovative intervention approach with tremendous potential for the management of kidney disease. However, the clinical therapeutic effects of MSCs in either acute kidney injury (AKI) or chronic kidney disease (CKD) are still under debate. Hurdles originate from the harsh microenvironment in vivo that decreases the cell survival rate, paracrine activity and migratory capacity of MSCs after transplantation, which are believed to be the main reasons for their limited effects in clinical applications. Melatonin is traditionally regarded as a circadian rhythm‐regulated neurohormone but in recent years has been found to exhibit antioxidant and anti‐inflammatory properties. Because inflammation, oxidative stress, thermal injury, and hypoxia are abnormally activated in kidney disease, application of melatonin preconditioning to optimize the MSC response to the hostile in vivo microenvironment before transplantation is of great importance. In this review, we discuss current knowledge concerning the beneficial effects of melatonin preconditioning in MSC‐based therapy for kidney disease. By summarizing the available information and discussing the underlying mechanisms, we aim to improve the therapeutic effects of MSC‐based therapy for kidney disease and accelerate translation to clinical application.
Keywords: acute kidney injury, chronic kidney disease, melatonin preconditioning, mesenchymal stem cells
1. INTRODUCTION
Kidney disease is still a global public health problem in modern society and can be classified as acute kidney injury (AKI) or chronic kidney disease (CKD).1 AKI is characterized by an abrupt decline in glomerular filtration, while CKD is defined as a gradual loss of kidney function over 3 months.2, 3 The morbidity rate of kidney disease has increased rapidly in the last decade, and it is estimated that approximately 20% of patients will experience AKI during their hospitalization worldwide, while CKD affects approximately 13.6% of people in the United States.4, 5 AKI and CKD share an interactive pathophysiological process.6 Patients with AKI have an 8.8‐fold increased risk for developing CKD.7 Conversely, multiple studies have confirmed a significantly higher morbidity rate of AKI in patients with prior CKD.8, 9 For treatment, beyond the primary cause management, the therapeutic choice for AKI is still confined to supportive care or dialysis, which helps little in regenerating the injured kidneys.10 In CKD, although the application of renin‐angiotensin system inhibitors (RASIs) has shown benefits in delaying renal failure, treatments are unable to induce regression of glomerulosclerosis.11 The formation of fibrosis tissues in glomeruli and interstitial/tubules eventually leads to end‐stage renal disease (ESRD) in both of these diseases. In addition to renal involvement, renal function disorder will undoubtedly affect extrarenal organs given that the kidneys are important in maintaining body homoeostasis. Pathophysiological alterations following loss of renal function, such as dysregulation of extracellular volume and electrolytes and abnormal hormone secretion, can subsequently affect multiple extrarenal organs and induce a series of severe complications.12, 13 It has been reported that even mild renal injury was relevant to the development of cardiovascular system complications.14 The respiratory system, central nervous system, endocrine system and haematologic system, among others, may also be aggravated whether the abnormality is extensive or prolonged.15 The high incidence and poor prognosis of kidney disease are associated with significant economic costs, accounting for more than $10 billion for the treatment of AKI and over $80 billion to care for CKD patients annually.16, 17 Exploring new interventions to delay the progression of AKI and CKD is an urgent need.
In the last 10 years, we have witnessed an explosion in MSC‐based therapy for the management of AKI and CKD in multiple preclinical models. MSCs are fibroblast‐like multipotent cells that possess robust self‐renewal, regeneration, proliferation and multilineage differentiation ability.18, 19 Although the specific mechanisms underlying AKI and CKD are still not very clear, abnormalities in cell apoptosis or necrosis,20 inflammation,21, 22 immunoregulation,23 microvascular function,24, 25 oxidative stress injury26 and the expansion of fibroblasts/myofibroblasts11 are thought to play important roles during disease development. Numerous candidate agents targeting these abnormalities, such as Nec‐1,27 bindarit,28 OPN‐305,29 ephrinB2,30 SS‐31,31 bardoxolone methyl32 and pirfenidone,33 have shown promising therapeutic activity in some animal and clinical kidney disease models. While pharmacologic management is often confined to a single aspect of the highly complex pathophysiological process in AKI or CKD, MSCs are able to promote kidney repair through multiple mechanisms.34 A principal mechanism of these numerous MSC benefits resides in their paracrine/endocrine capacity. In general, it is considered that MSCs have the advantage of secreting a series of cytokines and growth factors that induce anti‐apoptotic,35 antioxidative,36 anti‐inflammatory,37 anti‐fibrotic,38 angiogenic39 and immunomodulatory40 activities (Figure 1). The development of MSC‐based regenerative medicine may bring hope to the billions of patients who suffer from kidney disease worldwide.
Figure 1.
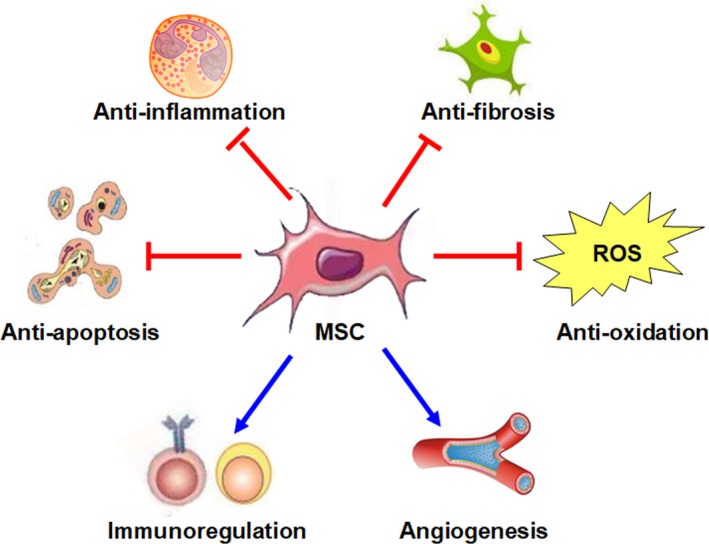
The principal mechanism by which MSCs exert their potential beneficial effects in kidney disease. The beneficial effects of MSC‐based therapy in kidney disease are reliant on the anti‐apoptotic, antioxidative, anti‐inflammatory, anti‐fibrotic, angiogenic and immunomodulatory properties of MSCs
In this review, we will describe the current knowledge concerning MSC‐based therapy in kidney disease. We begin with the promising outcome of MSC‐based therapy in animal models, and the obstacles met in attempting to translate these outcomes into clinical application. Then, the underlying reasons are analysed, and different preconditioning strategies are presented. Finally, we will discuss the beneficial effects of melatonin preconditioning on protecting MSC function after transplantation and the underlying mechanism. By summarizing the current research, we hope to provide an integral and updated view of melatonin preconditioning in MSC‐based therapy for kidney disease.
2. SUCCESSFUL ATTEMPTS AT MSC‐BASED THERAPY IN ANIMAL MODELS AND HURDLES MET IN CLINICAL SETTINGS
The therapeutic effects of MSC‐based therapy in kidney disease have been confirmed in multiple animal models. The first study that indicated the renotropic property and tubular regenerative potential of MSCs was conducted in a mouse model of cisplatin‐induced AKI.41 This phenomenon was further verified in many subsequent studies that utilized many other AKI models, such as ischemia/reperfusion (I/R), sepsis and glycerol models.42 In the field of CKD, a meta‐analysis that included 71 articles also demonstrated that cell‐based therapy was valid for slowing the progression of CKD in preclinical settings.43 Based on these excellent results, some clinical trials were explored.
Currently, there are 18 completed or ongoing clinical trials associated with the application of MSCs in kidney disease according to ClinicalTrials.gov (Table 1). The first clinical trial in which the safety and efficacy of MSC therapy for AKI was evaluated was completed in 2013 (NCT00733876). MSCs were prophylactically transplanted into patients who were at high risk of developing AKI following cardiac surgery. Neither AKI nor any other adverse events occurred in the treatment group, while 20% of patients in the case‐controlled group developed AKI.44 Six autosomal dominant polycystic kidney disease (ADPKD) patients also presented tolerability to MSC therapy (NCT02166489). Moreover, a slowdown in the decline in the estimated glomerular filtration rate (eGFR) was also observed after MSC transplantation, suggesting the efficacy of this therapy. However, this trial was limited due to the lack of a control group.45 Saad et al transplanted MSCs via intra‐arterial injection into 14 patients with atherosclerotic renovascular disease (RVD). Patients in the intervention group showed better cortical perfusion and renal blood flow than those in the control group, demonstrating the therapeutic effects of the treatment (NCT01840540).46 Systemic lupus erythematosus (SLE) is another major cause of CKD, despite powerful immunosuppression regimens applied for its clinical management. To explore the safety and efficacy of MSC transplantation in refractory SLE patients, 13 patients underwent MSC therapy. At the end of the study, all patients presented tolerance to the regimen, together with decreased systemic lupus erythematosus disease activity index (SLEDAI) scores and proteinuria (NCT00698191).47 Similarly, a sequential multicenter clinical trial designed by the same team further confirmed that in patients with active and refractory SLE, MSC transplantation can significantly ameliorate disease activity, reduce proteinuria and improve renal function (NCT01741857).48
Table 1.
Clinical trials of MSCs application in kidney disease
| Condition | Aim of study | Enrolment | Phase | Status | Outcomes | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|
| AKI | To demonstrate the safety of allogeneic MSCs in patients who are at high risk of developing AKI following cardiac surgery | 16 | Phase I | Completed | Safe and efficient | NCT00733876 |
| To evaluate the safety and efficacy of allogeneic MSCs for the treatment of AKI after cardiac surgery | 156 | Phase II | Terminated | Safe but not efficient | NCT01602328 | |
| To determine the safety and tolerability of extracorporeal MSCs in subjects with AKI receiving continuous renal replacement therapy | 24 | Phase I | Recruiting | NCT03015623 | ||
| CKD | To provide confirmation of the safety of MSCs in patients with chronic renal failure due to ADPKD | 6 | Phase I | Completed | Safe and efficient | NCT02166489 |
| To develop a novel treatment via intra‐arterial MSC injection in atherosclerotic RVD patients | 28 | Phase I | Completed | Safe and efficient | NCT01840540 | |
| To explore the safety and efficacy of MSC transplantation in refractory SLE | 13 | Phase I & II | Completed | Safe and efficient | NCT00698191 | |
| To assess the safety and efficacy of MSCs in patients with active and refractory SLE | 40 | Phase I & II | Completed | Safe and efficient | NCT01741857 | |
| To investigate the efficacy of MSCs for treatment of lupus nephritis | 18 | Phase II | Terminated | Safe but not efficient | NCT01539902 | |
| To assess the safety, tolerability and therapeutic effects of mesenchymal precursor cells in patients with moderate to severe DN | 30 | Phase I & II | Completed | Safe but not efficient | NCT01843387 | |
| To investigate the safety, feasibility, tolerability and efficacy of MSCs in subjects with progressive DN | 48 | Phase I & II | Recruiting | NCT02585622 | ||
| To determine whether intra‐renal delivery of MSCs would further enhance changes in single kidney blood flow and restoration of kidney function in human subjects with advanced RVD | 42 | Phase I | Not yet recruiting | NCT02266394 | ||
| To test the safety of intra‐parenchymal Wharton jelly‐derived MSC injection in the treatment of DN | 20 | Phase I & II | Not yet recruiting | NCT03288571 | ||
| To assess the safety, tolerability and efficacy of intra‐arterially delivered MSCs in patients with progressive DN | 30 | Phase I | Not yet recruiting | NCT03840343 | ||
| To investigate the treatment effects of MSCs in chronic renal failure patients | 100 | Not Applicable | Recruiting | NCT03321942 | ||
| To evaluate the safety and efficacy of MSCs for treatment of adults with active proliferative LN | 36 | Phase II | Not yet recruiting | NCT03673748 | ||
| To investigate the efficacy and safety of MSC transplantation in the treatment of LN compared with mycophenolate mofetil | 230 | Phase II | Not yet recruiting | NCT03580291 | ||
| To demonstrate the safety and efficacy of MSCs in patients with SLE and LN | 30 | Not Applicable | Not yet recruiting | NCT03458156 | ||
| To assess the safety and tolerability of MSCs (CS20AT04) in subjects with LN | 6 | Phase I | Not yet recruiting | NCT03174587 |
Abbreviations: ADPKD, autosomal dominant polycystic kidney disease; AKI, acute kidney injury; CKD, chronic kidney disease; DN, diabetic nephropathy; DN, diabetic nephropathy; LN, lupus nephritis; MSCs, mesenchymal stem cells; RVD, renal vascular disease; SLE, systemic lupus erythematosus.
In addition to the above‐mentioned exciting results, contradictory results have been reported in other studies. A phase II, randomized, multicenter trial was terminated due to the uncertain therapeutic effects in patients with postcardiac surgical AKI (NCT01602328).49 Packham et al aimed to assess the safety, tolerability and therapeutic effects of mesenchymal precursor cells in patients with moderate to severe diabetic nephropathy, which is a major cause of CKD. After follow‐up for 12 weeks, the measured parameters, including serum creatinine, creatinine clearance, albumin‐creatinine ratio, protein‐creatinine ratio, HbA1c and blood pressure, were comparable in both groups (NCT01843387).50 In the aspect of lupus nephritis (LN), the two studies mentioned above were both observational studies, which might provide insufficiently strong evidence. In a randomized double‐blind, placebo‐controlled trial that was published in 2017, MSC therapy did not show better therapeutic effects than the placebo (NCT01539902).51 These contradictory results confuse physician when making clinical decisions. However, there are still approximately ten registered or ongoing trials in this field. We are looking forward to the results of these studies, which may lead to a clear conclusion about the clinical effects of MSC‐based therapy for kidney disease.
Why is there still a huge gap between experimental evidence and clinical applications? One major reason for the limited clinical effects of MSCs in kidney disease is the reduced cell function after transplantation.52 As mentioned above, the beneficial effects of MSCs are related to their paracrine/endocrine activity and largely rely on the number of MSCs at the site and the concentration of secreted cytokines/growth factors around the damaged tissues.53 However, different from the appropriate culture conditions ex vivo, MSCs face an undesirable microenvironment after transplantation that may induce early cell death and loss of function. In one study, more than 80%–90% of grafted cells died within 72 hours.54 Data from Freyman et al also showed that only approximately 3% of administered MSCs remained alive at 14 days after injection.55 Moreover, decreased angiogenesis capacity, defects in migration activity and accelerated cellular senescence were also observed in MSCs after exposure to uremic toxins during AKI or CKD.56, 57 For the secretory activity, Idziak et al and Klinkhammer et al noticed that after exposure to uremic toxins, the secretomes of MSCs were largely altered. Up‐regulated secretion of IL‐8 and PDGF and decreased expression of VEGF converted MSCs into a more inflammatory, more fibrotic and less regenerative phenotype, which had a profound consequence on its anti‐inflammatory, anti‐fibrotic, angiogenic and immunomodulatory functions.58, 59 Finding a proper preconditioning strategy to optimize MSCs and enhance their survival, paracrine effects and migratory ability before transplantation is of great importance.60
3. MELATONIN PRECONDITIONING AND MSC‐BASED THERAPY FOR KIDNEY DISEASE
Multiple preconditioning strategies have been developed in recent years. Generally, these strategies can be sorted into four categories: incubation with cytokines or chemical compounds, hypoxia preconditioning, application of supporting materials and genetic modification. Different preconditioning strategies have unique advantages and drawbacks (Figure 2). Hypoxia preconditioning is simple and fast but faces the issues of standardization and optimization.61 Genetic modification is a more accurate approach compared with other preconditioning strategies but presents a risk of vector toxicity and tumorigenicity.62 The advantages of application with supporting materials are greater biocompatibility and targeting; however, the current price of these newly developed materials is still high, which may restrict their application in the clinic.63 In addition to the above‐mentioned methods, incubation of MSCs with cytokines or chemical compounds is another preconditioning strategy that has long been explored. The simple operation and safety guarantee of this technology make it a promising preconditioning strategy for clinical application, especially when the cells are incubated with certain physiological hormones.52 However, the key challenge of this technique is to find a proper substrate.
Figure 2.
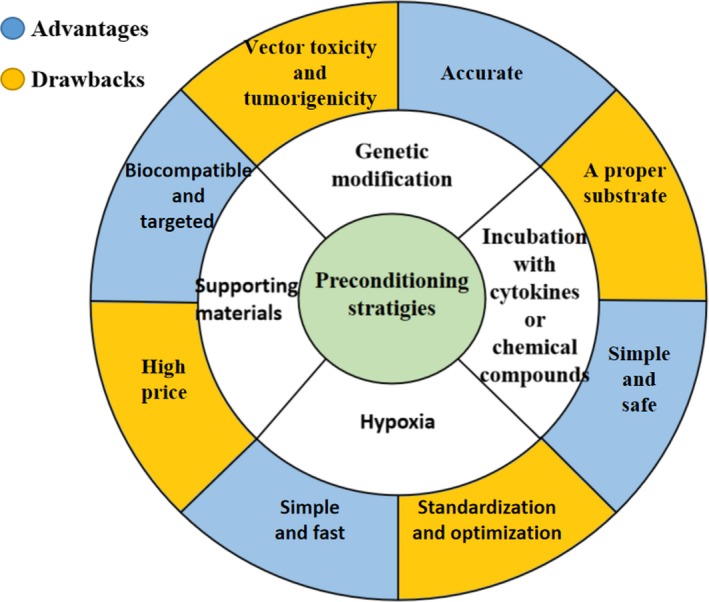
Advantages and drawbacks of different preconditioning strategies. Incubation with cytokines or chemical compounds, hypoxia preconditioning, application of supporting materials and genetic modification are currently the four main preconditioning strategies. These four preconditioning strategies have unique advantages and drawbacks. Currently, it is difficult to say one strategy is greater than another
Melatonin is a neurohormone that is primarily secreted by the pineal gland. The physiological role of melatonin is a key regulatory molecule in circadian rhythms.64 A low blood level of melatonin during the daytime and an increased level at night‐time guarantees a sleep‐wake cycle in mammals.65 In addition to its traditional role, in recent years, melatonin has been found to take part in many other pathophysiologic processes. By possessing antioxidant and anti‐inflammatory properties,66, 67 melatonin was found to be a potent free radical scavenger and present protective effects in multiple dysfunctional organs, including the kidneys.68, 69 Immune function during the course of prostate cancer therapy has also been demonstrated.70 Based on the fact that inflammation, oxidative stress, thermal injury and hypoxia are four main factors that cause the dysfunction of injected MSCs under disease conditions, preconditioning with melatonin may become a wonderful strategy.71 In addition, melatonin is currently applied as a dietary complement and shows little risk of genetic mutation, tumorigenicity or other major side effects.72, 73
Incubation of MSCs with melatonin prior to transplantation has been confirmed to be able to induce an enhanced therapeutic outcome in multiple animal models, including myocardial infarction, cerebral ischemia and limb ischemia models.74, 75, 76 In a broad sense, it was considered that melatonin itself could efficiently serve as an antioxidant and protect MSCs from oxidation injury by biologically eliminating free radicals.66 In addition to receptor‐independent pathways, the melatonin receptors MT1 and MT2 have also been found to be highly expressed on the surface of MSCs, indicating that melatonin may regulate the fate of MSCs in a receptor‐dependent manner.77, 78 Moreover, enhanced PrPC‐dependent mitochondrial function,79 Erk1/2 overexpression80 and up‐regulated phosphorylation of AMPK pathway proteins were observed after melatonin preconditioning.81 Melatonin preconditioning can rely on various mechanisms to protect injected MSCs against premature senescence or early apoptosis after transplantation and definitely exaggerate their therapeutic effects in diseased tissues (Figure 3). However, whether these benefits still exist in kidney disease is unclear. In the following section, we will discuss this question (Table 2).
Figure 3.
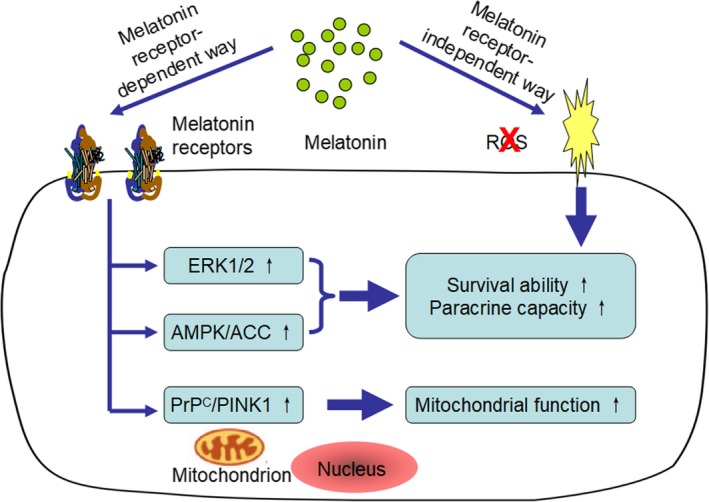
The mechanism underlying the protective effects of melatonin on MSCs. Melatonin preconditioning exerts beneficial effects on MSCs through receptor‐dependent and receptor‐independent pathways. By eliminating ROS and up‐regulating the ERK1/2, AMPK/ACC, and PrPC/PINK1 signaling pathways, this method can facilitate cell survival and promote paracrine activity and mitochondrial function
Table 2.
Studies demonstrating the beneficial effects of melatonin preconditioning in MSC‐based therapy for kidney disease
| Condition | References | Year | Animal | Model | Stem cells source | Renal outcomes | MSCs outcomes |
|---|---|---|---|---|---|---|---|
| AKI | Chen83 | 2014 | Rats | Sepsis‐AKI | A‐AMSCs | ↓Inflammatory; ↑Antioxidants; ↓ROS; ↓Apoptosis; ↓Fibrosis; ↓Kidney injury score; ↑Renal function | Not mentioned |
| Zhao84 | 2015 | HK‐2 cells | Cisplatin‐AKI | AMSCs | ↑Proliferation; ↑Migration; ↑Prosurvival and anti‐apoptotic ability | ↑Proliferation; ↑Prosurvival, anti‐apoptotic and antioxidative ability; | |
| Mias53 | 2008 | Rats | I/R‐AKI | BMMSCs | ↑Angiogenesis; ↑Proliferation; ↓Apoptosis; ↑Renal function | ↓Apoptosis; ↑Survival rate; ↑Paracrine ability (b‐FGF, HGF); | |
| CKD | Saberi71 | 2019 | Rats | UUO | BMMSCs | ↓TGF‐β and TNF‐α; ↓α‐SMA; ↑E‐cadherin; ↓Fibrotic areas | ↑Survival rate; ↑Migratory activity |
| Rashed87 | 2018 | Rats | DN | MSCs | ↓TGF‐β; ↑SOD‐1; ↑Beclin‐1; ↑Renal function | ↑Proliferation |
Abbreviations: A‐AMSCs, apoptotic adipose‐derived MSCs; AKI, acute kidney injury; AMSCs, adipose‐derived MSCs; b‐FGF, basic fibroblast growth factor; BMMSCs, bone marrow‐derived mesenchymal stem cells; Cisplatin‐AKI, cisplatin‐induced AKI; CKD, chronic kidney disease; DN, diabetic nephropathy; HGF, hepatocyte growth factor; I/R, ischemia/reperfusion; I/R‐AKI, I/R induced AKI; MSCs, mesenchymal stem cells; ROS, reactive oxygen species; Sepsis‐AKI, sepsis induced AKI; SOD‐1, superoxide dismutase‐1; TGF‐β, tumour growth factor β; TNF‐α, tumour necrosis factor‐ α; UUO, unilateral ureteral obstruction; α‐SMA, α‐smooth muscle actin.
3.1. Application of MSCs with melatonin preconditioning to treat AKI
Sepsis‐induced AKI (sepsis‐AKI) is a major subtype of AKI with high morbidity and mortality.82 To assess whether MSCs treated with melatonin can exert additional benefits in attenuating sepsis‐AKI, Chen et al conducted a series of experiments that aimed to compare a combination of melatonin and apoptotic adipose‐derived MSCs (A‐ADMSCs) with A‐ADMSCs alone in a rat model of sepsis. After cecal‐ligation and puncture (CLP), the combined treatment presented better therapeutic effects in the aspects of anti‐inflammation, antioxidization, anti‐apoptosis, and anti‐fibrosis activity and the circulating level of creatinine. Haematoxylin and eosin (HE) staining also confirmed less kidney injury in the combined treatment group.83 To gain further sight into the mechanisms underlying the protective effects of melatonin on MSCs, Zhao et al evaluated the action of melatonin on adipose‐derived MSCs (AMSCs) ex vivo. Melatonin pretreatment significantly enhanced the proliferation of AMSCs according to MTT assays. Moreover, higher expression of P‐Erk1/2, P‐Akt, superoxide dismutase‐1 (SOD‐1) and haeme oxygenase‐1 (HO‐1) was observed, indicating better prosurvival, antiapoptotic and antioxidative capacity of AMSCs after exposure to melatonin. Furthermore, their study also demonstrated the superiority of conditioned medium from melatonin‐pretreated AMSCs in enhancing the proliferative, migratory, prosurvival and antiapoptotic abilities of human HK‐2 cells exposed to cisplatin.84 To assess whether these protective mechanisms are still effective in vivo, Mias et al transplanted bone marrow‐derived mesenchymal stem cells (BMMSCs) preconditioned with melatonin into ischemia/reperfusion‐induced AKI (I/R‐AKI) rats. More surviving grafted MSCs, overstimulation of angiogenesis and proliferation in renal cells and more rapid renal function recovery were observed in the preconditioning group, indicating a potential role of melatonin in protecting transplanted MSCs in the in vivo microenvironment. In vitro analysis demonstrated a higher level of catalase and SOD‐1 expression and overexpression of basic fibroblast growth factor (bFGF) and hepatocyte growth factor (HGF) in the preconditioning group. However, all these beneficial effects were reversed by the melatonin receptor antagonist luzindole, suggesting that melatonin is able to enhance the intrinsic prosurvival and paracrine abilities of MSCs through its receptors.53
3.2. Application of MSCs with melatonin preconditioning to treat CKD
To date, only two studies have described the beneficial effects of melatonin on MSC‐based therapy in the field of CKD. Saberi et al pretreated MSCs with melatonin and then transplanted them into unilateral ureteral obstruction (UUO) rats. They found that pretreated MSCs could, on the one hand, decrease the expression of transforming growth factor‐β (TGF‐β), tumour necrosis factor‐α (TNF‐α) and α‐smooth muscle actin (α‐SMA) and, on the other hand, increase the expression of E‐cadherin compared with the control group, which ultimately induced fewer fibrotic areas in renal tissues. In addition, more engrafted and surviving pretreated MSCs were observed in the injured kidneys, suggesting a better survival and migratory activity of MSCs after melatonin incubation.71
Diabetic nephropathy (DN) is a major cause of CKD and shows increasing morbidity worldwide.85 MSC‐based therapy could become a promising therapeutic strategy for DN.86 However, local oxidative stress and the inflammatory state might largely impact the migratory and survival capacities of MSCs in diabetic tissues.57 Rashed et al preincubated MSCs with melatonin and found that melatonin pretreatment significantly enhanced the proliferation of MSCs in vitro. Then, they injected melatonin‐preincubated MSCs into rats with DN. Compared with the control group, the rats that received preincubated MSC therapy presented better kidney function and greater amelioration of the underlying pathogenic factors. This study verified the superiority of ex vivo melatonin treatment, which acts as a preconditioning agent to enhance the efficiency of MSCs therapy in DN.87
4. CONCLUSION AND FUTURE PERSPECTIVES
Given the increasing prevalence of AKI and CKD worldwide, continued breakthroughs in the field of MSC‐based therapy have a large potential impact and social benefits. Numerous clinical trials have confirmed the tolerability and safety of MSC treatment in kidney disease. However, some concerns regarding their unproven therapeutic effects in clinical applications remain due to their reduced cell function after transplantation. In this review, we summarized the currently available studies in which melatonin was used as a preconditioning substrate to enhance the therapeutic effects of MSCs in kidney disease. These studies definitively demonstrate the superiority of this strategy both in vivo and ex vivo.
In terms of mechanism, the benefits of melatonin include but are not limited to its antioxidative and anti‐inflammation effects. Current evidence indicates that melatonin preconditioning can also significantly enhance the proliferative, prosurvival, paracrine secretion and migratory abilities of MSCs after transplantation. One major advantage of this strategy over other preconditioning methods is the safety issue; melatonin can be applied as a dietary complement. However, at the moment, there are no available articles comparing the ability of melatonin with that of other preconditioning methods to enhance the capacity of MSCs. Whether melatonin preconditioning is superior to other preconditioning strategies should be explored in future studies.
We believe that summarizing the related articles and clarifying the underlying mechanism will shed light on the protective role of melatonin preconditioning in MSC‐based therapy for kidney disease. However, robust clinical evidence concerning this strategy in clinical settings is still lacking. We look forward to a promising future of MSC‐based therapy for kidney disease and call for more clinical trials in this field.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR'S CONTRIBUTION
LF Zhao and JH Chen contributed to the conception of the manuscript. LF Zhao and CX Hu were responsible for the literature review. LF Zhao, P Zhang and H Jiang drafted and revised the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 81700553, No. 81770752). The authors would like to thank the laboratory members for their contributions and gratefully acknowledge the funding support from the sources indicated.
Zhao L, Hu C, Zhang P, Jiang H, Chen J. Melatonin preconditioning is an effective strategy for mesenchymal stem cell‐based therapy for kidney disease. J Cell Mol Med. 2020;24:25–33. 10.1111/jcmm.14769
Zhao and Hu contributed equally to this work.
REFERENCES
- 1. Fraser S, Roderick PJ. Kidney disease in the Global Burden of Disease Study 2017. Nat Rev Nephrol. 2019;15:193‐194. [DOI] [PubMed] [Google Scholar]
- 2. Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813‐818. [DOI] [PubMed] [Google Scholar]
- 3. Jha V, Garcia‐Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260‐272. [DOI] [PubMed] [Google Scholar]
- 4. Saran R, Li Y, Robinson B, et al. Renal Data System 2015 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2016;67:Svii, S1‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levey AS, Acute JMT, Injury K. Acute kidney injury. Ann Intern Med. 2017;167:ITC66‐66ITC80. [DOI] [PubMed] [Google Scholar]
- 6. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta‐analysis. Kidney Int. 2012;81:442‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. James MT, Grams ME, Woodward M, et al. A meta‐analysis of the association of estimated GFR, albuminuria, diabetes mellitus, and hypertension with acute kidney injury. Am J Kidney Dis. 2015;66:602‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu V‐C, Huang T‐M, Lai C‐F, et al. Acute‐on‐chronic kidney injury at hospital discharge is associated with long‐term dialysis and mortality. Kidney Int. 2011;80:1222‐1230. [DOI] [PubMed] [Google Scholar]
- 10. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20:67‐75. [DOI] [PubMed] [Google Scholar]
- 11. Ruggenenti P, Cravedi P, Remuzzi G. Mechanisms and treatment of CKD. J Am Soc Nephrol. 2012;23:1917‐1928. [DOI] [PubMed] [Google Scholar]
- 12. Lameire NH, Bagga A, Cruz D, et al. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170‐179. [DOI] [PubMed] [Google Scholar]
- 13. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238‐1252. [DOI] [PubMed] [Google Scholar]
- 14. Odutayo A, Wong CX, Farkouh M, et al. AKI and long‐term risk for cardiovascular events and mortality. J Am Soc Nephrol. 2017;28:377‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eckardt K‐U, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158‐169. [DOI] [PubMed] [Google Scholar]
- 16. Honeycutt AA, Segel JE, Zhuo X, Hoerger TJ, Imai K, Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24:1478‐1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rewa O, Bagshaw SM. Acute kidney injury‐epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10:193‐207. [DOI] [PubMed] [Google Scholar]
- 18. Choi JR, Yong KW, Choi JY. Effects of mechanical loading on human mesenchymal stem cells for cartilage tissue engineering. J Cell Physiol. 2018;233:1913‐1928. [DOI] [PubMed] [Google Scholar]
- 19. Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739‐2749. [DOI] [PubMed] [Google Scholar]
- 20. Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J Am Soc Nephrol. 2014;25:2689‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rabb H, Griffin MD, McKay DB, et al. Inflammation in AKI: Current understanding, key questions, and knowledge gaps. J Am Soc Nephrol. 2016;27:371‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anders HJ. Of inflammasomes and alarmins: IL‐1β and IL‐1α in kidney disease. J Am Soc Nephrol. 2016;27:2564‐2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang M‐Z, Yao B, Yang S, et al. CSF‐1 signaling mediates recovery from acute kidney injury. J Clin Invest. 2012;122:4519‐4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Houben A, Martens R, Stehouwer C. Assessing microvascular function in humans from a chronic disease perspective. J Am Soc Nephrol. 2017;28:3461‐3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Humphreys BD, Cantaluppi V, Portilla D, et al. Targeting endogenous repair pathways after AKI. J Am Soc Nephrol. 2016;27:990‐998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szeto HH. Pharmacologic approaches to improve mitochondrial function in AKI and CKD. J Am Soc Nephrol. 2017;28:2856‐2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Linkermann A, Bräsen JH, Himmerkus N, et al. Rip1 (receptor‐interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81:751‐761. [DOI] [PubMed] [Google Scholar]
- 28. Zoja C, Corna D, Benedetti G, et al. Bindarit retards renal disease and prolongs survival in murine lupus autoimmune disease. Kidney Int. 1998;53:726‐734. [DOI] [PubMed] [Google Scholar]
- 29. Reilly M, Miller RM, Thomson MH, et al. Randomized, double‐blind, placebo‐controlled, dose‐escalating phase I, healthy subjects study of intravenous OPN‐305, a humanized anti‐TLR2 antibody. Clin Pharmacol Ther. 2013;94:593‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kida Y, Ieronimakis N, Schrimpf C, Reyes M, Duffield JS. EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury. J Am Soc Nephrol. 2013;24:559‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Szeto HH, Liu S, Soong YI, et al. Mitochondria‐targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22:1041‐1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327‐336. [DOI] [PubMed] [Google Scholar]
- 33. Cho ME, Smith DC, Branton MH, Penzak SR, Kopp JB. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2007;2:906‐913. [DOI] [PubMed] [Google Scholar]
- 34. Liu KD, Brakeman PR. Renal repair and recovery. Crit Care Med. 2008;36:S187‐S192. [DOI] [PubMed] [Google Scholar]
- 35. Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation‐independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31‐F42. [DOI] [PubMed] [Google Scholar]
- 36. Liu H, McTaggart SJ, Johnson DW, Gobe GC. Original article anti‐oxidant pathways are stimulated by mesenchymal stromal cells in renal repair after ischemic injury. Cytotherapy. 2012;14:162‐172. [DOI] [PubMed] [Google Scholar]
- 37. Chen Y‐T, Sun C‐K, Lin Y‐C, et al. Adipose‐derived mesenchymal stem cell protects kidneys against ischemia‐reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alfarano C, Roubeix C, Chaaya R, et al. Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia‐reperfusion in cyclosporine‐immunosuppressed rats. Cell Transplant. 2012;21:2009‐2019. [DOI] [PubMed] [Google Scholar]
- 39. Jia X, Pan J, Li X, et al. Bone marrow mesenchymal stromal cells ameliorate angiogenesis and renal damage via promoting PI3k‐Akt signaling pathway activation in vivo. Cytotherapy. 2016;18:838‐845. [DOI] [PubMed] [Google Scholar]
- 40. Casiraghi F, Perico N, Cortinovis M, Remuzzi G. Mesenchymal stromal cells in renal transplantation: opportunities and challenges. Nat Rev Nephrol. 2016;12:241‐253. [DOI] [PubMed] [Google Scholar]
- 41. Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794‐1804. [DOI] [PubMed] [Google Scholar]
- 42. Tögel FE, Westenfelder C. Mesenchymal stem cells: a new therapeutic tool for AKI. Nat Rev Nephrol. 2010;6:179‐183. [DOI] [PubMed] [Google Scholar]
- 43. Papazova DA, Oosterhuis NR, Gremmels H, van Koppen A, Joles JA, Verhaar MC. Cell‐based therapies for experimental chronic kidney disease: a systematic review and meta‐analysis. Dis Model Mech. 2015;8:281‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tögel FE, Westenfelder C. Kidney protection and regeneration following acute injury: progress through stem cell therapy. Am J Kidney Dis. 2012;60:1012‐1022. [DOI] [PubMed] [Google Scholar]
- 45. Makhlough A, Shekarchian S, Moghadasali R, et al. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther. 2017;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saad A, Dietz AB, Herrmann SMS, et al. Autologous mesenchymal stem cells increase cortical perfusion in renovascular disease. J Am Soc Nephrol. 2017;28:2777‐2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liang J, Zhang H, Hua B, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis. 2010;69:1423‐1429. [DOI] [PubMed] [Google Scholar]
- 48. Wang D, Li J, Zhang YU, et al. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014;16:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Swaminathan M, Stafford‐Smith M, Chertow GM, et al. Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J Am Soc Nephrol. 2018;29:260‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Packham DK, Fraser IR, Kerr PG, Segal KR. Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: a randomized, placebo‐controlled, dose escalation study. EBioMedicine. 2016;12:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deng DQ, Zhang P, Guo Y, Lim TO. A randomised double‐blind, placebo‐controlled trial of allogeneic umbilical cord‐derived mesenchymal stem cell for lupus nephritis. Ann Rheum Dis. 2017;76:1436‐1439. [DOI] [PubMed] [Google Scholar]
- 52. Silva LHA, Antunes MA, Dos Santos CC, Weiss DJ, Cruz FF, Rocco PRM. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res Ther. 2018;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mias C, Trouche E, Seguelas M‐H, et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells. 2008;26:1749‐1757. [DOI] [PubMed] [Google Scholar]
- 54. Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti‐death strategies. J Mol Cell Cardiol. 2001;33:907‐921. [DOI] [PubMed] [Google Scholar]
- 55. Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114‐1122. [DOI] [PubMed] [Google Scholar]
- 56. Noh H, Yu MR, Kim HJ, et al. Uremia induces functional incompetence of bone marrow‐derived stromal cells. Nephrol Dial Transplant. 2012;27:218‐225. [DOI] [PubMed] [Google Scholar]
- 57. Fadini GP, Ciciliot S, Albiero M. Concise review: perspectives and clinical implications of bone marrow and circulating stem cell defects in diabetes. Stem Cells. 2017;35:106‐116. [DOI] [PubMed] [Google Scholar]
- 58. Klinkhammer BM, Kramann R, Mallau M, et al. Mesenchymal stem cells from rats with chronic kidney disease exhibit premature senescence and loss of regenerative potential. PLoS ONE. 2014;9:e92115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Idziak M, Pędzisz P, Burdzińska A, Gala K, Pączek L. Uremic toxins impair human bone marrow‐derived mesenchymal stem cells functionality in vitro. Exp Toxicol Pathol. 2014;66:187‐194. [DOI] [PubMed] [Google Scholar]
- 60. Zhao L, Hu C, Zhang P, Jiang H, Chen J. Preconditioning strategies for improving the survival rate and paracrine ability of mesenchymal stem cells in acute kidney injury. J Cell Mol Med. 2019;23:720‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Das R, Jahr H, van Osch GJVM, Farrell E. The role of hypoxia in bone marrow‐derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010;16:159‐168. [DOI] [PubMed] [Google Scholar]
- 62. Hu C, Li L. Pre‐conditions for eliminating mitochondrial dysfunction and maintaining liver function after hepatic ischaemia reperfusion. J Cell Mol Med. 2017;21:1719‐1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li X, Tamama K, Xie X, Guan J. Improving Cell Engraftment in Cardiac Stem Cell Therapy. Stem Cells Int. 2016;2016:7168797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Reiter RJ, Tan DX, Fuentes‐Broto L. Melatonin: a multitasking molecule. Prog Brain Res. 2010;181:127‐151. [DOI] [PubMed] [Google Scholar]
- 65. Reiter RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79:C153‐C158. [DOI] [PubMed] [Google Scholar]
- 66. Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51:1‐16. [DOI] [PubMed] [Google Scholar]
- 67. Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González‐Gallego J. A review of the molecular aspects of melatonin's anti‐inflammatory actions: recent insights and new perspectives. J Pineal Res. 2013;54:1‐14. [DOI] [PubMed] [Google Scholar]
- 68. Sener G, Sehirli AO, Keyer‐Uysal M, Arbak S, Ersoy Y, Yegen BC. The protective effect of melatonin on renal ischemia‐reperfusion injury in the rat. J Pineal Res. 2002;32:120‐126. [DOI] [PubMed] [Google Scholar]
- 69. Reiter RJ, Tan D‐X, Poeggeler B, Menendez‐pelaez A, Chen L‐D, Saarela S. Melatonin as a free radical scavenger: implications for aging and age‐related diseases. Ann N Y Acad Sci. 1994;719:1‐12. [DOI] [PubMed] [Google Scholar]
- 70. Rodriguez‐Garcia A, Mayo JC, Hevia D, Quiros‐Gonzalez I, Navarro M, Sainz RM. Phenotypic changes caused by melatonin increased sensitivity of prostate cancer cells to cytokine‐induced apoptosis. J Pineal Res. 2013;54:33‐45. [DOI] [PubMed] [Google Scholar]
- 71. Saberi K, Pasbakhsh P, Omidi A, et al. Melatonin preconditioning of bone marrow‐derived mesenchymal stem cells promotes their engraftment and improves renal regeneration in a rat model of chronic kidney disease. J Mol Histol. 2019;50:129‐140. [DOI] [PubMed] [Google Scholar]
- 72. Bandyopadhyay D, Chattopadhyay A. Reactive oxygen species‐induced gastric ulceration: protection by melatonin. Curr Med Chem. 2006;13:1187‐1202. [DOI] [PubMed] [Google Scholar]
- 73. Welin A‐K, Svedin P, Lapatto R, et al. Melatonin reduces inflammation and cell death in white matter in the mid‐gestation fetal sheep following umbilical cord occlusion. Pediatr Res. 2007;61:153‐158. [DOI] [PubMed] [Google Scholar]
- 74. Han D, Huang W, Li X, et al. Melatonin facilitates adipose‐derived mesenchymal stem cells to repair the murine infarcted heart via the SIRT1 signaling pathway. J Pineal Res. 2016;60:178‐192. [DOI] [PubMed] [Google Scholar]
- 75. Tang Y, Cai B, Yuan F, et al. Melatonin pretreatment improves the survival and function of transplanted mesenchymal stem cells after focal cerebral ischemia. Cell Transplant. 2014;23:1279‐1291. [DOI] [PubMed] [Google Scholar]
- 76. Lee JH, Han YS, Lee SH. Potentiation of biological effects of mesenchymal stem cells in ischemic conditions by melatonin via upregulation of cellular prion protein expression. J Pineal Res. 2017;62. [DOI] [PubMed] [Google Scholar]
- 77. Slominski RM, Reiter RJ, Schlabritz‐Loutsevitch N, Ostrom RS, Slominski AT. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol. 2012;351:152‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101‐110. [DOI] [PubMed] [Google Scholar]
- 79. Han YS, Kim SM, Lee JH, et al. Melatonin protects chronic kidney disease mesenchymal stem cells against senescence via PrPC ‐dependent enhancement of the mitochondrial function. J Pineal Res. 2019;66:e12535. [DOI] [PubMed] [Google Scholar]
- 80. Wang F, Zhou H, Du Z, et al. Cytoprotective effect of melatonin against hypoxia/serum deprivation‐induced cell death of bone marrow mesenchymal stem cells in vitro. Eur J Pharmacol. 2015;748:157‐165. [DOI] [PubMed] [Google Scholar]
- 81. Yang Y, Fan C, Deng C, et al. Melatonin reverses flow shear stress‐induced injury in bone marrow mesenchymal stem cells via activation of AMP‐activated protein kinase signaling. J Pineal Res. 2016;60:228‐241. [DOI] [PubMed] [Google Scholar]
- 82. Brun‐Buisson C, Meshaka P, Pinton P, et al. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 2004;30:580‐588. [DOI] [PubMed] [Google Scholar]
- 83. Chen H‐H, Lin K‐C, Wallace CG, et al. Additional benefit of combined therapy with melatonin and apoptotic adipose‐derived mesenchymal stem cell against sepsis‐induced kidney injury. J Pineal Res. 2014;57:16‐32. [DOI] [PubMed] [Google Scholar]
- 84. Zhao J, Young YK, Fradette J, Eliopoulos N. Melatonin pretreatment of human adipose tissue‐derived mesenchymal stromal cells enhances their prosurvival and protective effects on human kidney cells. Am J Physiol Renal Physiol. 2015;308:F1474‐F1483. [DOI] [PubMed] [Google Scholar]
- 85. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047‐1053. [DOI] [PubMed] [Google Scholar]
- 86. Paulini J, Higuti E, Bastos RMC, Gomes SA, Rangel ÉB. Mesenchymal stem cells as therapeutic candidates for halting the progression of diabetic nephropathy. Stem Cells Int. 2016;2016:9521629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rashed LA, Elattar S, Eltablawy N, Ashour H, Mahmoud LM, El‐Esawy Y. Mesenchymal stem cells pretreated with melatonin ameliorate kidney functions in a rat model of diabetic nephropathy. Biochem Cell Biol. 2018;96:564‐571. [DOI] [PubMed] [Google Scholar]


