Abstract
Recent references have showed crucial roles of several miRNAs in neural stem cell differentiation and proliferation. However, the expression and role of miR‐485‐3p remains unknown. In our reference, we indicated that miR‐485‐3p expression was down‐regulated during NSCs differentiation to neural and astrocytes cell. In addition, the TRIP6 expression was up‐regulated during NSCs differentiation to neural and astrocytes cell. We carried out the dual‐luciferase reporter and found that overexpression of miR‐485‐3p decreased the luciferase activity of pmirGLO‐TRIP6‐wt but not the pmirGLO‐TRIP6‐mut. Ectopic expression of miR‐485‐3p decreased the expression of TRIP6 in NSC. Ectopic miR‐485‐3p expression suppressed the cell growth of NSCs and inhibited nestin expression of NSCs. Moreover, elevated expression of miR‐485‐3p decreased the ki‐67 and cyclin D1 expression in NSCs. Furthermore, we indicated that miR‐485‐3p reduced proliferation and induced differentiation of NSCs via targeting TRIP6 expression. These data suggested that a crucial role of miR‐485‐3p in self‐proliferation and differentiation of NSCs. Thus, altering miR‐485‐3p and TRIP6 modulation may be one promising therapy for treating with neurodegenerative and neurogenesis diseases.
Keywords: Alzheimer's disease, miR‐485‐3p, neural stem cells, TRIP6
1. INTRODUCTION
Neural stem cells (NSCs) in mammalian brain possess two necessary properties of the stem cells, multipotency and self‐renewal, and it has ability to differentiate to new neuron that can function into extant neural circuits.1, 2, 3, 4, 5 The transplantation of NSCs supplies a potential therapeutic way for several neurological disorders including Parkinson's disease, Alzheimer's disease, Huntington's disease and spinal cord injuries.6, 7, 8, 9, 10, 11 Despite the great accomplishment has been achieved, there are still some challenges to resolve before clinical use of NSCs was adopted.12, 13, 14, 15 Therefore, it is crucial to exploit the molecular signal pathway and molecular mechanism modulating NSCs differentiation and proliferation.
MicroRNAs (miRNAs) are a class of noncoding, short RNAs molecules that negatively modulate gene expression through binding a perfectly complementary or a partially complementary sequence in 3′‐untranslated region (UTR) region of their target gene to influence mRNA stability and/or translation.16, 17, 18, 19 Altered specific miRNAs expression has been shown in diverse human tumours such as breast cancer, lung cancer, prostate cancer, osteosarcoma and hepatocellular carcinoma.20, 21, 22, 23, 24, 25 Increasing studies also demonstrated that miRNAs involved in the process of diverse cell biological processes including cell differentiation, growth, migration, metastasis and invasion.26, 27 More recently, growing evidence suggested that miRNAs play important roles in the differentiation and proliferation of NSCs.
In this study, we indicated that miR‐485‐3p expression was down‐regulated during NSCs differentiation to neural and astrocytes cell. Ectopic miR‐485‐3p expression suppressed the cell growth of NSCs and inhibited nestin expression of NSCs.
2. MATERIALS AND METHODS
2.1. Cell culture and transfection
NSCs were isolated and cultured using previous standard way.28, 29 These cells were isolated from embryos of rat and kept in growth medium supplement with bFGF, EGF and N2. This reference was agreed with our hospital's ethical board and complied with Helsinki Declaration. miR‐485‐3p, miR‐485‐3p control (scramble), pcDNA‐control and pcDNA‐TRIP6 were bought from GenePharma and then transfected to NSCs by Lipofectamine with the final concentration of 10 nmol/L.
2.2. qRT‐PCR
RNA from NSCs was gained using TRIzol kit (Invitrogen) by standard way. qRT‐PCR was used to analyse miR‐485‐3p and mRNA expression on Applied Biosystems machine (Applied Biosystems) utilizing TaqMan mix and primer for 45 cycles. miR‐485‐3p expression was related to U6, and GAPDH was done as control for mRNA. The primers were shown: Nestin, 5′‑GATCTAAACAGGAAGGAAATCCAG G‑3′; and 5′‑TCTAGTGTCTCATGGCTCTGGTTTT‑3′; Tuj1, 5′‑CGCCATGTTCAGACGCAAG‑3′ and 5′‑CTCGGACACCAGGTCGTTCA‑3′; Ki‐67, 5′‑CAGTACTCGGAATGCAGCAA‑3′ and 5′‑CAGTCTTCAGGGGCTCTGTC‑3′; GAPDH, 5′‑ATTCCATGGCACCGTCAAGGCTGA‑3′ and 5′‑TTC TCCATGGTGGTGAAGACGCCA‑3′.
2.3. Cell viability
Cell growth of NSCs was detected with MTT (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl‐tetrazolium bromide) assay. The OD (absorbance) at the 490 nm was recorded by microplate reader. The cell viability at 0, 1, 2 and 3 days was analysed.
2.4. Dual‐luciferase assay
Full‐length 3′UTR of TRIP6 gene and one fragment consisting of putative miR‐485‐3p binding site was amplified from genomic DNA and then specific cloned into pGl3‐promoter plasmid (Promega). A mutant plasmid in seed area of miR‐485‐3p binding site was also established. Cell was treated with one mixture of Renilla, miR‐485‐3p mimic, miR‐NC, pLuc‐3′‐UTR and mut or WT pGl3‐TRIP6 plasmid using Lipofectamine. Luciferase activity was detected with Promega Dual‐Luciferase kit.
2.5. Western blot analysis
Western blot assay was done using the standard way. Protein was isolated with SDS‐PAGE (12%) and diverted to PVDF membrane (Millipore, USA). After blocking with milk (5%) for 2 hours, membrane was stained in primary antibodies (anti‐TRIP6 and anti‐GAPDH, 1:1,000, Abcam) at 4°C overnight. After washing in TBST, membrane was incubated in second antibody. Blot was observed with ECL detection reagent.
2.6. Immunohistochemistry
Cell was fixed by paraformaldehyde (4%) in PBS for about 10 min and blocked with TritonX‐100 (0.1%), FBS (1%) and serum. Then, cell was stained in primary antibodies (anti‐nestin and anti‐Tuj1, 1:2,000, Abcam) at 4°C overnight. After washed three times in PBS, cell was incubated with second antibody for 1 hour at 37°C. Cell was observed by Leica camera (Leica Germany).
2.7. Statistical analysis
Result was present as the mean ± standard deviation and was calculated via SPSS 17.0 software. Student's t test was utilized to determine the difference between these two groups. A P value < .05 was regarded to be significant.
3. RESULTS
3.1. NSCs have self‐proliferation and differentiation capacity
NSCs were isolated from mouse forebrain and they can form neurosphere (Figure 1A). After withdraw of bFGF, these cells differentiated to astrocytes and neurons (Figure 1B). Moreover, NSCs were expressed the nestin, which is the NSC maker (Figure 1C).
Figure 1.
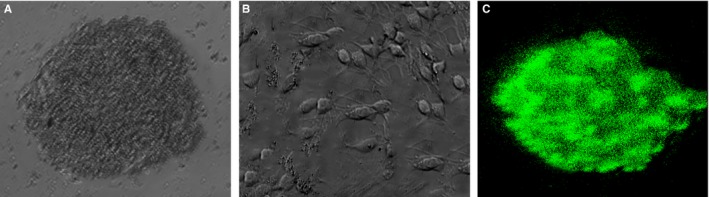
NSCs have self‐proliferation and differentiation capacity. A, NSCs were isolated from mouse forebrain and they can form neurosphere. B, These cells differentiated to astrocytes and neurons. C, NSCs were expressed the nestin, which is the NSC maker
3.2. miR‐485‐3p is down‐regulated a during cell differentiation of NSC
miR‐485‐3p expression level was measured by qRT‐PCR assay during differentiation of NSCs. It was shown that miR‐485‐3p expression was down‐regulated during NSCs differentiation to neural cell (Figure 2A). We also found that expression of miR‐485‐3p was reduced during NSCs differentiation to astrocytes cell (Figure 2B).
Figure 2.
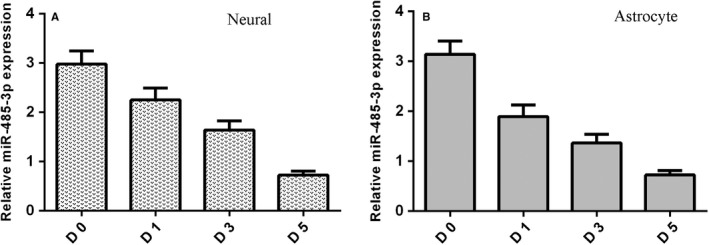
miR‐485‐3p is down‐regulated a during cell differentiation of NSC. A, miR‐485‐3p expression level was measured by qRT‐PCR assay. B, The expression of miR‐485‐3p was reduced during NSCs differentiation to astrocytes cell
3.3. TRIP6 is overexpressed during NSC differentiation
TRIP6 expression level was determined by qRT‐PCR assay during differentiation of NSCs. It was shown that TRIP6 expression was up‐regulated during NSCs differentiation to neural cell (Figure 3A). We also found that expression of TRIP6 was overexpressed during NSCs differentiation to astrocytes cell (Figure 3B).
Figure 3.
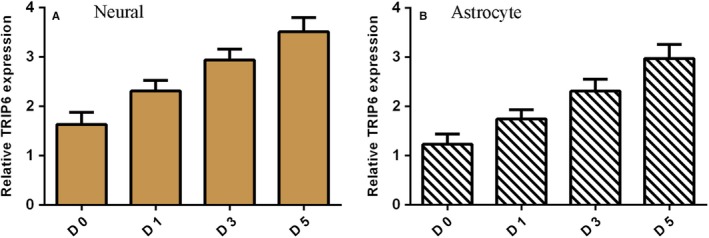
TRIP6 is overexpressed during NSC differentiation. A, TRIP6 expression level was determined by qRT‐PCR assay. B, The expression of TRIP6 was overexpressed during NSCs differentiation to astrocytes cell
3.4. miR‐485‐3p targets TRIP6 expression in NSC
To find potential target gene of miR‐485‐3p, we exploited TargetScan software. It was shown that miR‐485‐3p has target sites in 3′‐UTR of TRIP6 (Figure 4A). qRT‐PCR assay demonstrated that miR‐485‐3p was up‐regulated in the NSCs after transfected with miR‐485‐3p mimic (Figure 4B). Ectopic expression of miR‐485‐3p decreased the expression of TRIP6 in NSC (Figure 4C). Dual‐luciferase reporter analysis was carried out to confirm that overexpression of miR‐485‐3p decreased the luciferase activity of pmirGLO‐TRIP6‐wt but not the pmirGLO‐TRIP6‐mut (Figure 4D).
Figure 4.

miR‐485‐3p targets TRIP6 expression in NSC. A, It was shown that miR‐485‐3p has target sites in 3′‐UTR of TRIP6. B, The expression of miR‐485‐3p was detected by qRT‐PCR assay. C, Ectopic expression of miR‐485‐3p decreased the expression of TRIP6 in NSC. D, Dual‐luciferase reporter analysis was carried out to confirm that overexpression of miR‐485‐3p decreased the luciferase activity of pmirGLO‐TRIP6‐wt but not the pmirGLO‐TRIP6‐mut. *P < .05
3.5. miR‐485‐3p reduced proliferation and induced differentiation of NSCs
Ectopic expression of miR‐485‐3p suppressed the cell growth of NSCs by using MTT assay (Figure 5A). Elevated expression of miR‐485‐3p decreased the expression of nestin, which is one maker of NSCs (Figure 5B). As determined by qRT‐PCR, ectopic expression of miR‐485‐3p suppressed the expression of ki‐67 (Figure 5C). Moreover, we proved that miR‐485‐3p overexpression inhibited the cyclin D1 expression (Figure 5D). Furthermore, overexpression of miR‐485‐3p induced the Tuj1 expression, which is a maker of neuronal (Figure 5E). As measured by Tuj1 immunofluorescence analysis, data showed that elevated expression of miR‐485‐3p increased the Tuj1 expression (Figure 5F).
Figure 5.
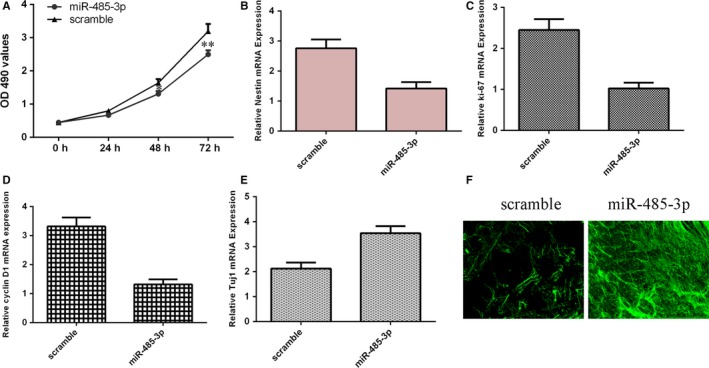
miR‐485‐3p reduced proliferation and induced differentiation of NSCs. A, Ectopic expression of miR‐485‐3p suppressed the cell growth of NSCs. B, Elevated expression of miR‐485‐3p decreased the expression of nestin. C, Ectopic expression of miR‐485‐3p suppressed the expression of ki‐67. D, miR‐485‐3p overexpression inhibited the cyclin D1 expression. E, Overexpression of miR‐485‐3p induced the Tuj1 expression, which is a maker of neuronal. F, As measured by Tuj1 immunofluorescence analysis, data showed that elevated expression of miR‐485‐3p increased the Tuj1 expression. *P < .05 and **P < .01
3.6. miR‐485‐3p reduced proliferation and induced differentiation of NSCs via targeting TRIP6 expression
To further consider contribution of TRIP6 to cell biological effect of miR‐485‐3p on differentiation and proliferation of NSCs, we induced TRIP6 expression in the NSCs and co‐transfected with miR‐485‐3p mimic. Ectopic expression of TRIP6 increased miR‐485‐3p‐overexpressing NSCs proliferation with using MTT assay (Figure 6A). Elevated expression of TRIP6 promoted the expression of nestin in miR‐485‐3p‐overexpressing NSCs (Figure 6B). Elevated expression of TRIP6 enhanced the expression of ki‐67 (Figure 6C) and cyclin D1 (Figure 6D) in the miR‐485‐3p‐overexpressing NSCs. Restoration expression of TRIP6 over‐turned the function effect of miR‐485‐3p on NSCs differentiation (Figure 6E). As measured by Tuj1 immunofluorescence analysis, results indicated that restoration expression of TRIP6 decreased the Tuj1 expression in the miR‐485‐3p‐overexpressing NSCs (Figure 6F).
Figure 6.
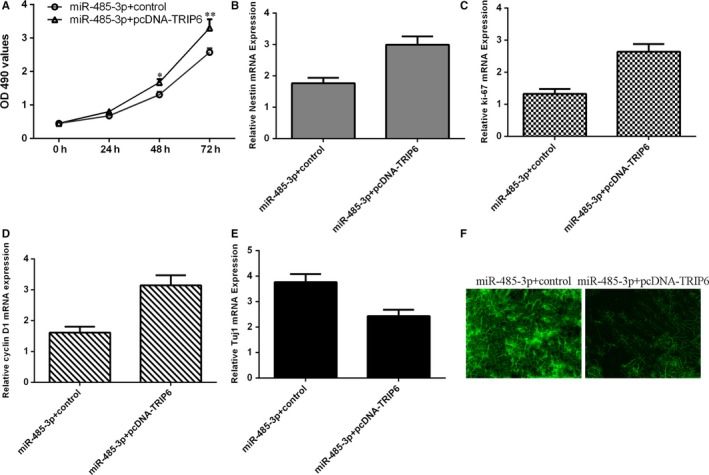
miR‐485‐3p reduced proliferation and induced differentiation of NSCs via targeting TRIP6 expression. A, Ectopic expression of TRIP6 increased miR‐485‐3p‐overexpressing NSCs proliferation with using MTT assay. B, The expression of nestin was determined by using qRT‐PCR assay. C, The expression of ki‐67 was measured by using qRT‐PCR assay. D, The expression of cyclin D1 was measured by using qRT‐PCR assay. E, Restoration expression of TRIP6 over‐turned the function effect of miR‐485‐3p on NSCs differentiation. F, As measured by Tuj1 immunofluorescence analysis, results indicated that restoration expression of TRIP6 decreased the Tuj1 expression in the miR‐485‐3p‐overexpressing NSCs. * P < .05 and ** P < .01
4. DISCUSSION
In the present research, we found that miR‐485‐3p expression was down‐regulated during NSCs differentiation to neural and astrocytes cell. In addition, the TRIP6 expression was up‐regulated during NSCs differentiation to neural and astrocytes cell. We carried out the dual‐luciferase reporter and found that overexpression of miR‐485‐3p decreased the luciferase activity of pmirGLO‐TRIP6‐wt but not the pmirGLO‐TRIP6‐mut. Ectopic expression of miR‐485‐3p decreased the expression of TRIP6 in NSC. Ectopic miR‐485‐3p expression suppressed the cell growth of NSCs and inhibited nestin expression of NSCs. Moreover, elevated expression of miR‐485‐3p decreased the ki‐67 and cyclin D1 expression in NSCs. Furthermore, we indicated that miR‐485‐3p reduced proliferation and induced differentiation of NSCs via targeting TRIP6 expression. These data suggested that a crucial role of miR‐485‐3p in self‐proliferation and differentiation of NSCs. Thus, altering miR‐485‐3p and TRIP6 modulation may be one promising therapy for treating with neurodegenerative and neurogenesis diseases.
Increasing evidence indicated that miR‐485 has involved in the progression of varied diseases such as oesophageal cancer, glioma, osteosarcoma, hepatocellular carcinoma, osteoarthritis.30, 31, 32, 33 For instance, Chen and workmates found that miR‐485‐5p expression was negatively related with differentiation degree of bone marrow mesenchymal stem cells (BMSCs).34 Ectopic miR‐485‐5p expression suppressed cartilage surface‐related genes and toluidine blue, while promoted tumour necrosis factor and interleukin partly regulating SOX9 expression. It has been shown that miR‐485‐5p overexpression decreased breast tumour development and promoted chemosensitivity partly via modulating survivin expression.35 Previous study indicated that miR‐485‐5p expression was down‐regulated in the serum of NSCLC cells and patients. Epigallocatechin‐3‐gallate (EGCG) inhibited cancer stem cells characteristics through regulating RXRα/miR‐485‐5p axis.36 Du et al37 demonstrated that overexpression miR‐485‐3p suppressed osteosarcoma cell colony formation, growth, sphere formation and migration and inhibited CtBP1 expression. However, the role of miR‐485‐3p in NSC differentiation and proliferation remains unknown. In this reference, we showed that miR‐485‐3p expression was down‐regulated during NSCs differentiation to neural and astrocytes cell. Ectopic miR‐485‐3p expression suppressed the cell growth of NSCs and inhibited nestin expression of NSCs. Moreover, elevated expression of miR‐485‐3p decreased the ki‐67 and cyclin D1 expression in NSCs.
TRIP6 is one member of zyxin family of the LIM proteins and is one focal adhesion element with the capacity to the shuttle between cell nucleus and surface.38, 39 TRIP6 was played roles in modulation of signal transduction and actin dynamics during cell migration and adhesion.40, 41 Increasing studies showed that TRIP6 was expressed in neurons of hippocampal and regulated biological function of neurological.42 Previous reference indicated that TRIP6 was sufficient and essential for proliferation and self‐renewal of NSCs, but suppressed NSCs differentiation.43 Another reference suggested that miR‐138‐5p modulated differentiation and proliferation of NSCs via inhibiting TRIP6 expression.44 In our reference, we exploited TargetScan software to find potential target gene of miR‐485‐3p and found that miR‐485‐3p has target sites in 3′‐UTR of TRIP6. Ectopic expression of miR‐485‐3p decreased the expression of TRIP6 in NSC. Dual‐luciferase reporter analysis was carried out to confirm that overexpression of miR‐485‐3p decreased the luciferase activity of pmirGLO‐TRIP6‐wt but not the pmirGLO‐TRIP6‐mut. Furthermore, we found that miR‐485‐3p reduced proliferation and induced differentiation of NSCs via targeting TRIP6 expression.
In summary, this reference revealed that miR‐485‐3p expression was down‐regulated during NSCs differentiation and miR‐485‐3p reduced proliferation and induced differentiation of NSCs via targeting TRIP6 expression. These data suggested that a crucial role of miR‐485‐3p in self‐proliferation and differentiation of NSCs. Thus, altering miR‐485‐3p and TRIP6 modulation may be one promising therapy for treating with neurodegenerative and neurogenesis diseases.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interest.
AUTHOR CONTRIBUTION
Juxian Gu, Rusheng Shao, Meng Li, Qiuyue Yan, Hongwei Hu designed and conducted the experiments and analysed data. Juxian Gu wrote and revised the manuscript.
Gu J, Shao R, Li M, Yan Q, Hu H. MiR‐485‐3p modulates neural stem cell differentiation and proliferation via regulating TRIP6 expression. J Cell Mol Med. 2020;24:398–404. 10.1111/jcmm.14743
REFERENCES
- 1. Shi Y, Sun G, Zhao C, Stewart R. Neural stem cell self‐renewal. Crit Rev Oncol/Hemat. 2008;65:43‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Juliandi B, Abematsu M, Nakashima K. Chromatin remodeling in neural stem cell differentiation. Curr Opin Neurobiol. 2010;20:408‐415. [DOI] [PubMed] [Google Scholar]
- 3. Goustard‐Langelier B, Koch M, Lavialle M, Heberden C. Rat neural stem cell proliferation and differentiation are durably altered by the in utero polyunsaturated fatty acid supply. J Nutr Biochem. 2013;24:380‐387. [DOI] [PubMed] [Google Scholar]
- 4. Garg N, Po A, Miele E, et al. microRNA‐17‐92 cluster is a direct Nanog target and controls neural stem cell through Trp53inp1. EMBO J. 2013;32:2819‐2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cui Y, Xiao Z, Chen T, et al. The miR‐7 identified from collagen biomaterial‐based three‐dimensional cultured cells regulates neural stem cell differentiation. Stem Cells Dev. 2014;23:393‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gioia U, Di Carlo V, Caramanica P, et al. Mir‐23a and mir‐125b regulate neural stem/progenitor cell proliferation by targeting Musashi1. RNA Biol. 2014;11:1105‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andersson T, Rahman S, Sansom SN, et al. Reversible block of mouse neural stem cell differentiation in the absence of dicer and microRNAs. PLoS ONE. 2010;5:e13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aranha MM, Santos DM, Sola S, Steer CJ, Rodrigues CM. miR‐34a regulates mouse neural stem cell differentiation. PLoS ONE. 2011;6:e21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lattanzi A, Gentner B, Corno D, et al. Dynamic activity of miR‐125b and miR‐93 during murine neural stem cell differentiation and in the subventricular zone neurogenic niche. PLoS ONE. 2013;8:e67411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao C, Sun G, Li S, et al. MicroRNA let‐7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci USA. 2010;107:1876‐1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Z, Li XY, Chan M, Wu W, Tan DX, Shen JX. Melatonin antagonizes interleukin‐18‐mediated inhibition on neural stem cell proliferation and differentiation. J Cell Mol Med. 2017;21:2163‐2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsai JW, Chen Y, Kriegstein AR, Vallee RB. LIS1 RNA interference blocks neural stem cell division, morphogenesis, and motility at multiple stages. J Cell Biol. 2005;170:935‐945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tan SL, Ohtsuka T, Gonzalez A, Kageyama R. MicroRNA9 regulates neural stem cell differentiation by controlling Hes1 expression dynamics in the developing brain. Gene Cell. 2012;17:952‐961. [DOI] [PubMed] [Google Scholar]
- 14. Stevanato L, Sinden JD. The effects of microRNAs on human neural stem cell differentiation in two‐ and three‐dimensional cultures. Stem Cell Res Ther. 2014;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu S, Yin F, Zhang J, et al. Regulatory roles of miRNA in the human neural stem cell transformation to glioma stem cells. J Cell Biochem. 2014;115:1368‐1380. [DOI] [PubMed] [Google Scholar]
- 16. Yu X, Li Z, Chen G, Wu WK. MicroRNA‐10b induces vascular muscle cell proliferation through Akt pathway by targeting TIP30. Curr Vasc Pharmacol. 2015;13:679‐686. [DOI] [PubMed] [Google Scholar]
- 17. Yu X, Li Z, Shen J, et al. MicroRNA‐10b promotes nucleus pulposus cell proliferation through RhoC‐Akt pathway by targeting HOXD10 in intervetebral disc degeneration. PLoS ONE. 2013;8:e83080. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Li Z, Yu X, Shen J, Wu WK, Chan MT. MicroRNA expression and its clinical implications in Ewing's sarcoma. Cell Prolif. 2015;48:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu S, Zhang M, Sun F, et al. miR‐375 controls porcine pancreatic stem cell fate by targeting 3‐phosphoinositide‐dependent protein kinase‐1 (Pdk1). Cell Prolif. 2016;49:395‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun Y, Zhao J, Yin X, Yuan X, Guo J, Bi J. miR‐297 acts as an oncogene by targeting GPC5 in lung adenocarcinoma. Cell Prolif. 2016;49:636‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yin Z, Ding H, He E, Chen J, Li M. Up‐regulation of microRNA‐491‐5p suppresses cell proliferation and promotes apoptosis by targeting FOXP4 in human osteosarcoma. Cell Prolif. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. She K, Yan H, Huang J, Zhou H, He J. miR‐193b availability is antagonized by LncRNA‐SNHG7 for FAIM2‐induced tumour progression in non‐small cell lung cancer. Cell Prolif. 2017;51(1):e12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He JH, Han ZP, Liu JM, et al. Overexpression of long non‐coding RNA MEG3 inhibits proliferation of hepatocellular carcinoma Huh7 cells via negative modulation of miRNA‐664. J Cell Biochem. 2017;118:3713‐3721. [DOI] [PubMed] [Google Scholar]
- 24. Liu J, Song ZW, Feng C, et al. The long non‐coding RNA SUMO1P3 facilitates breast cancer progression by negatively regulating miR‐320a. Am J Transl Res. 2017;9:5594‐5602. [PMC free article] [PubMed] [Google Scholar]
- 25. Ma GX, Tang MQ, Wu YQ, Xu XM, Pan F, Xu RA. LncRNAs and miRNAs: potential biomarkers and therapeutic targets for prostate cancer. Am J Transl Res. 2016;8:5141‐5150. [PMC free article] [PubMed] [Google Scholar]
- 26. Li Z, Yu X, Shen J, Law PT, Chan MT, Wu WK. MicroRNA expression and its implications for diagnosis and therapy of gallbladder cancer. Oncotarget. 2015;6:13914‐13924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Z, Shen JX, Chan M, Wu W. MicroRNA‐379 suppresses osteosarcoma progression by targeting PDK1. J Cell Mol Med. 2017;21:315‐323. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Zheng JL, Yi D, Liu Y, Wang MQ, Zhu YL, Shi HZ. Long nonding RNA UCA1 regulates neural stem cell differentiation by controlling miR‐1/Hes1 expression. Am J Transl Res. 2017;9:3696‐3704. [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng J, Yi D, Shi X, Shi H. miR‐1297 regulates neural stem cell differentiation and viability through controlling Hes1 expression. Cell Prolif. 2017;50(4):e12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang FR, Xu SH, Wang BM, Wang F. MiR‐485‐5p inhibits metastasis and proliferation of osteosarcoma by targeting CX3CL1. Eur Rev Med Pharmacol Sci. 2018;22:7197‐7204. [DOI] [PubMed] [Google Scholar]
- 31. Sun XJ, Liu YL, Li M, Wang MC, Wang YT. Involvement of miR‐485‐5p in hepatocellular carcinoma progression targeting EMMPRIN. Biomed Pharmacother. 2015;72:58‐65. [DOI] [PubMed] [Google Scholar]
- 32. Han DL, Wang LL, Zhang GF, et al. MiRNA‐485‐5p, inhibits esophageal cancer cells proliferation and invasion by down‐regulating O‐linked N‐acetylglucosamine transferase. Eur Rev Med Pharmaco Sci. 2019;23:2809‐2816. [DOI] [PubMed] [Google Scholar]
- 33. Wang R, Zuo XH, Wang K, et al. MicroRNA‐485‐5p attenuates cell proliferation in glioma by directly targeting paired box 3. Am J Cancer Res. 2018;8:2507‐2517. [PMC free article] [PubMed] [Google Scholar]
- 34. Chen HO, Zhang L, Tang ZY, Gong ZM. MiR‐485‐5p promotes the development of osteoarthritis by inhibiting cartilage differentiation in BMSCs. Eur Rev Med Pharmacol Sci. 2018;22:3294‐3302. [DOI] [PubMed] [Google Scholar]
- 35. Wang M, Cai WR, Meng R, et al. miR‐485‐5p suppresses breast cancer progression and chemosensitivity by targeting survivin. Biochem Biophys Res Comm. 2018;501:48‐54. [DOI] [PubMed] [Google Scholar]
- 36. Jiang P, Xu CY, Chen LJ, et al. Epigallocatechin‐3‐gallate inhibited cancer stem cell‐like properties by targeting hsa‐mir‐485‐5p/RXR alpha in lung cancer. J Cell Biochem. 2018;119:8623‐8635. [DOI] [PubMed] [Google Scholar]
- 37. Du KL, Zhang XL, Lou ZK, et al. MicroRNA485‐3p negatively regulates the transcriptional co‐repressor CtBP1 to control the oncogenic process in osteosarcoma cells. Int J Biol Sci. 2018;14:1445‐1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gou HY, Liang QY, Zhang YQ, et al. Ttpal promotes colorectal tumorigenesis by activating Wnt/β‐Catenin signaling through Trip6. Gastroenterology. 2018;154:S32‐S. [Google Scholar]
- 39. Sheppard SA, Savinova T, Loayza D. TRIP6 and LPP, but not Zyxin, are present at a subset of telomeres in human cells. Cell Cycle. 2011;10:1726‐1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chastre E, Abdessamad M, Kruglov A, et al. TRIP6, a novel molecular partner of the MAGI‐1 scaffolding molecule, promotes invasiveness. Faseb J. 2009;23:916‐928. [DOI] [PubMed] [Google Scholar]
- 41. Miao XB, Xu XH, Wu YX, et al. Overexpression of TRIP6 promotes tumor proliferation and reverses cell adhesion‐mediated drug resistance (CAM‐DR) via regulating nuclear p27(Kip1) expression in non‐Hodgkin's lymphoma. Tumor Biol. 2016;37:1369‐1378. [DOI] [PubMed] [Google Scholar]
- 42. Li MY, Lai YJ, Yang CY, Huang KH, Tsai JC, Wang TW. TRIP6 regulates the maintenance of postnatal mouse neural stem cells. J Neurochem. 2014;130:50. [DOI] [PubMed] [Google Scholar]
- 43. Lai YJ, Li MY, Yang CY, Huang KH, Tsai JC, Wang TW. TRIP6 regulates neural stem cell maintenance in the postnatal mammalian subventricular zone. Dev Dynam. 2014;243:1130‐1142. [DOI] [PubMed] [Google Scholar]
- 44. Wang J, Li J, Yang J, et al. MicroRNA‐138‐5p regulates neural stem cell proliferation and differentiation in vitro by targeting TRIP6 expression. Mol Med Rep. 2017;16:7261‐7266. [DOI] [PMC free article] [PubMed] [Google Scholar]


