The Kidney Disease
Improving Global Outcomes (KDIGO) Clinical Practice Guideline on Chronic Kidney Disease–Mineral and Bone Disorder (CKD–MBD) 2009 provided recommendations on the detection, evaluation, and treatment of CKD-MBD in patients CKD who are and are not undergoing dialysis. Because of the accumulation of evidence since this initial publication, the CKD-MBD Guideline underwent a selective update in 2017. In April 2018, KDIGO convened a CKD-MBD Guideline Implementation Summit in Japan with the key objective to discuss various barriers to the uptake and implementation of the CKD-MBD Guideline in 8 Asian countries/regions. These countries/regions were comparable according to their high-to-middle economic ranking assigned by the World Bank. The discussion took into account the availability of CKD-MBD medication therapies and government health policies that may influence reimbursement and practice patterns in the region. Most importantly, Summit participants developed a framework of multifaceted strategies aimed at overcoming barriers to guideline implementation. The Summit attendees suggested a shared decision-making approach between clinicians and patients in CKD-MBD management, as well as individualized care based on the treatment risk-benefit ratio. The Summit participants also discussed how KDIGO, as a guideline development organization, may work in partnership with local and national nephrology societies to provide education and facilitate implementation of the guideline by clinicians. The conclusions drawn from this Summit in Asia may serve as an important guide for other regions to follow.
Keywords: bone mineral density, calcium, CKD, dialysis, hyperparathyroidism, hyperphosphatemia, KDIGO CKD-MBD Guideline
CKD-MBD is one of the key complications in patients with CKD. Disturbances in various biochemical parameters, as well as CKD-MBD–related complications, are highly prevalent and cause significant morbidity and mortality in this population.1,2 KDIGO developed the first clinical practice guideline (CPG) on the diagnosis, evaluation, prevention, and treatment of CKD-MBD in 2009.3 In October 2013, KDIGO held a Controversies Conference entitled “CKD-MBD: Back to the Future” in Madrid, Spain. During the conference, recommendation statements from the 2009 CPG were selected for revisiting based on a newly accumulated body of evidence. A KDIGO Guideline Work Group was later convened, and a subsequent CKD-MBD CPG Update was published in July 2017.4 KDIGO decided to explore how different parts of the world, and specifically Asia, have implemented KDIGO’s CPG on the diagnosis, evaluation, prevention, and treatment of CKD-MBD through a Guideline Implementation Summit.
Recognizing that a country’s government health and reimbursement policies, economic position, and regulatory issues all impact local practice patterns and potential implementation of the KDIGO CPG in CKD-MBD management, KDIGO organized a CKD-MBD Guideline Implementation Summit in April 2018 in Japan with several key meeting objectives. The first aim was to understand the availability and reimbursement of drugs recommended by the KDIGO CKD-MBD CPG in 8 Eastern and Southern Asian countries/regions with a comparable high-to-middle economic ranking by the World Bank. The second goal was to understand variations in government health policies that may influence CKD-MBD practice patterns in the region. The third objective was to understand the current implementation status of the CKD-MBD CPG and address specific barriers to its implementation in the region. Lastly, the Summit aimed to develop a framework of practical, multifaceted strategies to overcome recognized barriers and improve KDIGO CKD-MBD CPG implementation and quality of care for patients with CKD-MBD in the region. With these aims in mind, the Summit attendees discussed both the need for individualized implementation strategies and suggestions for what KDIGO could do to further facilitate CPG implementation in the region.
The Summit was attended by nephrologists from China, Hong Kong, Japan, Malaysia, Singapore, South Korea, Taiwan, and Thailand. The conference participants specifically identified 7 recommendation statements from the 2017 CKD-MBD CPG Update as the basis for focused discussions. The selection of these recommendations took into consideration the strength of the recommendation statement, the quality of the evidence supporting the recommendation, current practice patterns, and the need for better implementation. To highlight the similarities and important differences among the countries in the region, the Summit participants discussed specific barriers and challenges to implementing these 7 recommendations.
Incidence and Prevalence of End-Stage Kidney Disease in Asia
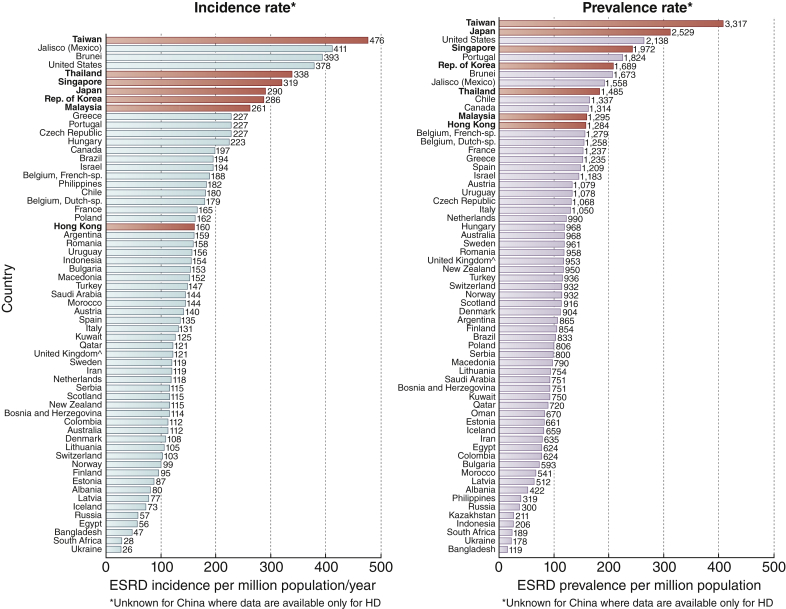
Data from the United States Renal Data System 2017 showed that 7 of the 8 Asian countries/regions represented at the Summit, with the exception of China, are among the top 25 in the world with respect to incidence and prevalence of end-stage kidney disease (ESKD)5 (Figure 1). Of the represented countries/regions, Taiwan leads this list with an annual ESKD incidence rate of 476 per million population (pmp), followed by Thailand (338 pmp), Singapore (319 pmp), Japan (290 pmp), South Korea (286 pmp), Malaysia (261 pmp), and Hong Kong (160 pmp). Similarly, Taiwan leads in the annual ESKD prevalence rate (3317 pmp), followed by Japan (2529 pmp), Singapore (1972 pmp), South Korea (1689 pmp), Thailand (1482 pmp), Malaysia (1295 pmp), and Hong Kong (1284 pmp). Thailand, Singapore, Malaysia, and South Korea are among the top 10 countries with the highest percentage increase in their ESKD incidence rate from 2002–2003 to 2014–2015.5 The recent annual data report from the China Kidney Disease Network estimated that the age-adjusted incidence rate for dialysis was 122 pmp with an estimated prevalence of hemodialysis and peritoneal dialysis of 402 pmp and 40 pmp, respectively.6
Figure 1.
International comparisons of incidence and prevalence of end-stage renal disease (ESRD). Red bars indicate countries/regions from which participants attended the implementation summit.
Reprinted with permission from the United States Renal Data System.5 ^United Kingdom: England, Wales, Northern Ireland (Scotland data reported separately).
Given such high incidence and prevalence of ESKD in Eastern and Southern Asia, a significant burden of CKD-MBD and related complications also can be expected. Thus there is a clinical need to drive better implementation of the KDIGO CKD-MBD CPG and to optimize CKD-MBD care to reduce mortality and morbidity and improve patient outcomes.
Race also may influence treatment effects and treatment targets. For example, the target parathyroid hormone (PTH) level was lower for Japanese CKD G5D patients (60–240 pg/ml) than other Asian populations that generally adopted KDIGO-recommended 2- to 9-fold upper laboratory reference as the PTH target. A recent analysis from the Dialysis Outcomes and Practice Patterns Study showed that Japanese patients undergoing dialysis maintained a more steady PTH level over a 9-month period in contrast to the European, Australian, New Zealand, and North American counterparts, which were more likely to show an increase in PTH level.7
Availability and Reimbursement Policies of Medications Used to Treat CKD-MBD In Asia
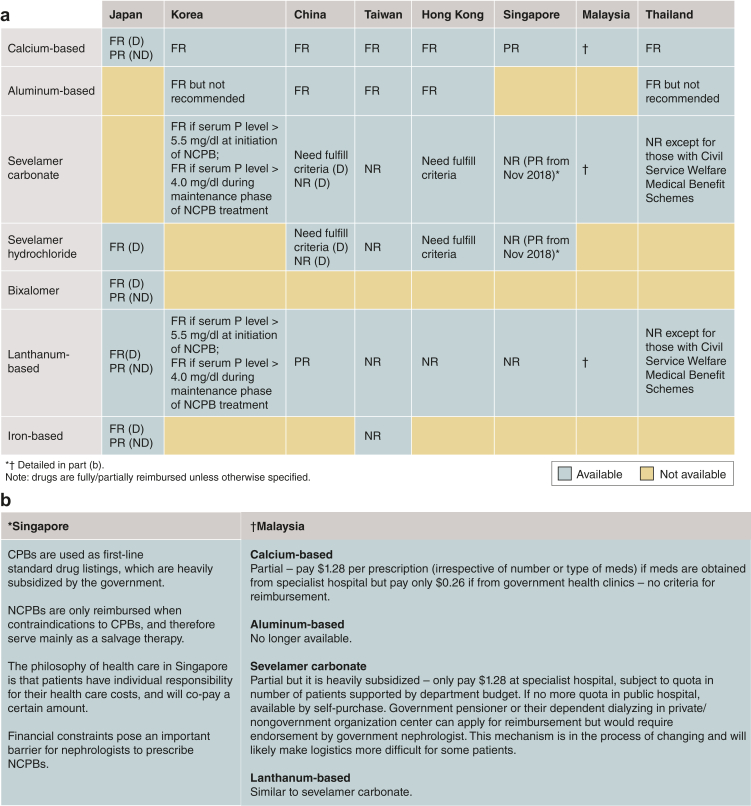
The 8 Asian countries/regions represented at the Summit have notable variations in the availability of CKD-MBD–related drugs and their reimbursement policies (Figure 2a and b). Calcium-based phosphate binders (CPBs) are most commonly used and reimbursed in all the 8 countries/regions. Sevelamer carbonate/hydrochloride, although available, requires specific criteria for reimbursement in some countries/regions such as China, Hong Kong, Korea, Malaysia, and Singapore (Figure 2a and b). Lanthanum carbonate also requires specific criteria for reimbursement in Korea and Malaysia (Figure 2a and b). In Taiwan, neither sevelamer- nor lanthanum-based phosphate binders are reimbursed. Aluminum-based phosphate binders are still prescribed in China, Taiwan, Hong Kong, and Thailand. Other newer classes of phosphate binders, such as bixalomer and iron-based phosphate binders, are available in very few countries.
Figure 2.
(a) Availability and (b) reimbursement policy of various phosphate binders in the 8 Asian countries/regions. CPB, calcium-based phosphate binder; D, dialysis; FR, full reimbursement; NCPB, non–calcium-based phosphate binder; ND, nondialysis; NR, not reimbursed; P, phosphate; PR, partial reimbursement.
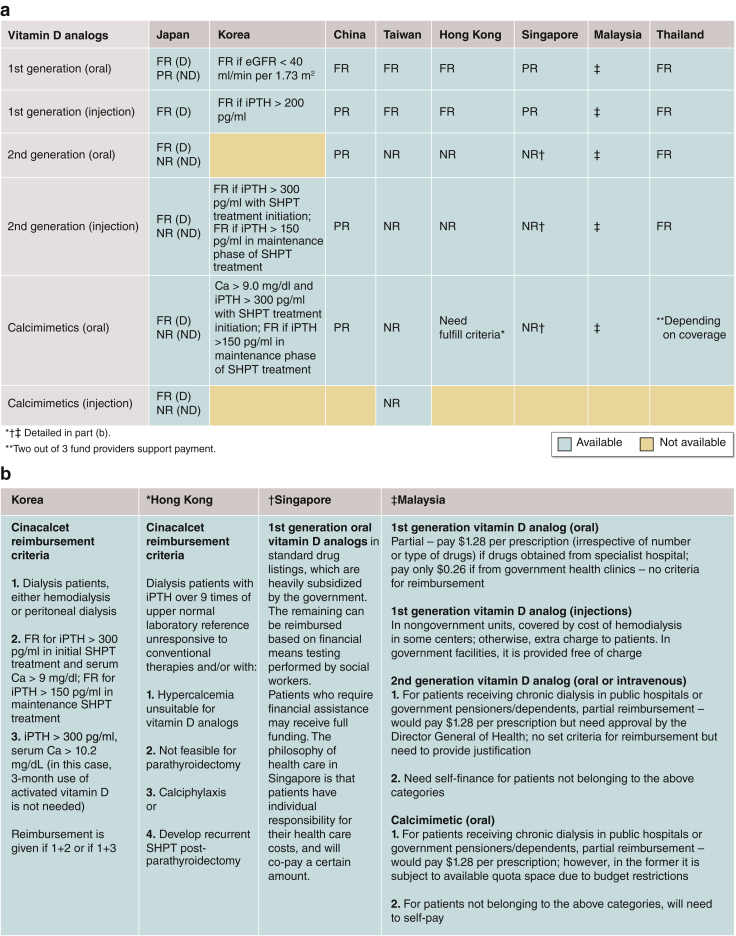
Figure 3a and b summarize the availability and reimbursement policies of various vitamin D analogs and calcimimetics in the region. Second-generation oral vitamin D analogs are reimbursed in all countries except Taiwan and Hong Kong. Oral calcimimetic agents are available in all countries/regions; however, reimbursement of calcimimetics requires the fulfilment of specific indications in many countries/regions. Intravenous calcimimetics are available only in Japan and Taiwan, yet neither oral nor intravenous calcimimetics are reimbursed in Taiwan.
Figure 3.
(a) Availability and (b) reimbursement policy of various vitamin D analogs and calcimimetics in the 8 Asian countries/regions. Ca, serum calcium; D, dialysis; eGFR, estimated glomerular filtration rate; FR, full reimbursement; iPTH, intact parathyroid hormone; ND, nondialysis; NR, not reimbursed; PR, partial reimbursement; SHPT, secondary hyperparathyroidism.
Although calcimimetics are available, parathyroidectomy is still considered the first-line treatment for severe secondary hyperparathyroidism refractory to locally available medical therapies in all countries/regions except Japan. Different PTH thresholds were used as an indication for parathyroidectomy across all 8 countries/regions (Supplementary Table S1A), with or without considering other local indications for parathyroidectomy (Supplementary Table S1B). Supplementary Table S2 provides the different PTH levels treatment targets adopted in the region.
Government Health and Reimbursement Policies
Thailand, South Korea, and Japan have their own local government policies for the reimbursement of sevelamer-based or lanthanum-based phosphate binders, second-generation activated vitamin D, and cinacalcet. Singapore, Hong Kong, and China have reimbursement policies for these drugs that are not linked to any guideline recommendations. Taiwan and Malaysia are mixed in their reimbursement policies.
Current Uptake of Kdigo Ckd-Mbd Guideline
Most countries follow the KDIGO CKD-MBD CPG, but some countries continue to refer to the Kidney Disease Outcomes Quality Initiative (KDOQI) 2003 guideline. Japan has developed its own national Japanese Society for Dialysis Therapy CPG for CKD-MBD, and the Malaysian Society of Nephrology, in collaboration with the Ministry of Health, recently has developed a specific national CKD-MBD and parathyroidectomy CPG and standard of practice.
KDIGO CKD-MBD 2017 Guideline Recommendations Identified For Better Implementation
The Summit participants assessed 7 KDIGO CKD-MBD CPG statements for better implementation in the region (Table 1).4
Table 1.
KDIGO CKD-MBD CPG recommendation statements identified for better implementation in the region
| Guideline no. | Recommendation statement |
|---|---|
| Monitoring of CKD-MBD | |
| 3.1.1 | We recommend monitoring serum levels of calcium, phosphate, PTH, and alkaline phosphatase activity beginning in CKD G3a (1C) |
| Calcium and phosphorus control | |
| 4.1.3 | In adult patients with CKD G3a–G5D, we suggest avoiding hypercalcemia (2C) |
| 4.1.6 | In adult patients with CKD G3a–G5D receiving phosphate-lowering treatment, we suggest restricting the dose of calcium-based phosphate binders (2B) |
| 4.1.7 | In patients with CKD G3a–G5D, we recommend avoiding the long-term use of aluminum-containing phosphate binders and, in patients with CKD G5D, avoiding dialysate aluminum contamination to prevent aluminum intoxication (1C) |
| PTH control | |
| 4.2.4 | In patients with CKD G5D requiring PTH-lowering therapy, we suggest calcimimetics, calcitriol, or vitamin D analogs, or a combination of calcimimetics with calcitriol or vitamin D analogs (2B) |
| Osteoporosis management | |
| 4.3.1 | In patients with CKD G1–G2 with osteoporosis and/or high risk of fracture, as identified by World Health Organization criteria, we recommend management as for the general population (1A) |
| 4.3.2 | In patients with CKD G3a–G3b with PTH in the normal range and osteoporosis and/or high risk of fracture, as identified by World Health Organization criteria, we suggest treatment as for the general population (2B) |
CKD-MBD, chronic kidney disease–mineral bone disorder; CPG, clinical practice guidelines; KDIGO, Kidney Disease: Improving Global Outcomes; PTH, parathyroid hormone.
KDIGO CKD-MBD Guideline Recommendation 3.1.1
Monitoring Biochemical Parameters of CKD-MBD in the Region
The Summit participants recognized wide variations in the frequency of monitoring of CKD-MBD biochemical parameters in the region. Taiwan’s national structured pre-ESKD education program supported by the government requires referral of patients with CKD to nephrologists starting at CKD G3a when routine, regular monitoring of CKD-MBD parameters is initiated. The government of Taiwan reimburses all biochemical tests for CKD-MBD including calcium, phosphorus, alkaline phosphatase performed every 3 months, and PTH testing every 6 to 12 months starting from CKD G4. The government provides incentives to physicians who refer cases early to nephrologists for structured CKD care.
In Singapore, PTH testing is variably performed in patients with CKD G3a–G5. Family physicians or non-nephrologists would rarely order PTH testing in CKD G3a–G5 patients and timing of referral to nephrologists therefore is variable. As such, many patients with CKD G3a–G3b may not have their case managed by nephrologists. Recently, Singapore set up the National CKD Program by which all patients with CKD G3b are referred to nephrologists. Raising the awareness among primary care physicians or other clinicians of early referral of patients with CKD to nephrologists starting at CKD G3b may help promote earlier PTH testing and standardize monitoring frequency in persons with CKD.
On the other hand, some hospitals in Hong Kong set restrictions on the frequency of PTH testing in persons with CKD because of resource limitations. For example, testing cannot be done more than once per year in nondialysis CKD G3a–G5 patients. In China, it is common to have late presentations and referrals of patients with CKD to nephrologists; thus many patients may have missed PTH, calcium, and phosphorus monitoring in the nondialysis CKD stages. Rural areas of China may also lack the required nephrology expertise and biochemical tests for CKD-MBD monitoring. In Malaysia there is a gap in the availability of PTH tests in CKD G3a–G4, but not in CKD G5. In Thailand, the government does not fully reimburse all PTH testing in CKD G3a–G5 patients. Other countries, including South Korea and Japan, do not restrict biochemical testing of CKD-MBD in patients with CKD G3a–G5 as long as it is reasonably compatible with the diagnosis for health insurance; however, there is no standardized monitoring frequency for these parameters.
Barriers to the Implementation of KDIGO 2017 CKD-MBD Guideline Recommendation 3.1.1 in Asia
Across the 8 countries/regions participating in the Summit, some barriers at the governmental, hospital, physician, and patient levels may make it difficult to implement Recommendation 3.1.1. Except for Taiwan, most Asian countries do not have structured CKD care programs; therefore, there is generally late referral of patients with CKD to nephrologists and low awareness of the need to monitor PTH levels regularly. In China, a large discrepancy in health policy and reimbursement coverage for CKD, bundled payment, and cost containment exists between urban and rural areas. Some basic biochemical tests such as PTH and alkaline phosphatase may not be available in rural areas. Patients may move between different nephrology centers for follow-up and lack continuity in CKD care. Some public hospitals in Hong Kong set restrictions on PTH testing frequency in persons with CKD because of resource limitation.
In Singapore a physician’s workload is generally very high, and they often need reminders to order biochemical tests of CKD-MBD. Furthermore, CKD G3b patients may not be managed by nephrologists. In some countries such as Malaysia, where a bundled payment exists, physicians in the private sector have to absorb the costs of biochemical tests in patients’ consultation fees and may skip ordering of these tests to save costs.
Patients’ noncompliance poses another important barrier to adherence of monitoring. In Singapore, some patients who are followed up in private dialysis centers may refuse biochemical tests of CKD-MBD because of high cost, as they need to pay out of pocket. PTH tests are not fully reimbursed in Singapore, China, and Thailand (Table 2).
Table 2.
Barriers in implementation of KDIGO CPG on monitoring biochemical parameters of CKD-MBD
| Japan | Korea | Taiwan | Singapore | China | Hong Kong | Thailand | Malaysia | |
|---|---|---|---|---|---|---|---|---|
| Government/policy level | Nil | Nil; in Korea, reimbursement on monitoring covers CKD from G3a | Not a barrier as there is structured CKD program with standardized monitoring frequency with incentive available for referral of CKD G3b to nephrologist | No policy requirements for monitoring of biochemical parameters of CKD-MBD | Diverse policies in different parts of the country—urban vs. rural | Lack of structured CKD program | Late referral of patients with CKD to nephrologists | Lack of government policy on referral of CKD3a and 3b patients, hence patients with CKD are cared for mostly by primary care physicians |
| Hospital level | Nil | Nil | Nil | Heavy workload in nephrology unit, and reminder needed on timing of monitoring | Many institutions in rural areas do not have biochemical tests available | Cost of PTH testing high and restrictions on PTH testing in hospitals | Nil | Lag time in results reporting as tests are processed in batches; results may not be acted upon until much later |
| Physician level | Low awareness of the need to monitor PTH | Low awareness of the need to institute frequent monitoring | CKD guidelines require referral of CKD G3b to nephrologists | CKD G3a is mostly managed by primary care physicians and PTH test may not be performed | Low awareness of the need for regular monitoring; goals of management is cost containment | A large proportion of predialysis patients may be managed in the private sector; lack of education of general and primary care physicians | CKD G3a–G4 are mostly managed by family or general physicians, thus PTH and alkaline phosphatase testing not likely being done | Non-nephrologists may not be aware of the need for regular monitoring |
| Patient level | Nil | Nil | Nil | Patients may refuse tests in private settings because they require out-of-pocket costs | Out-of-pocket costs; noncompliance of follow-up, which will affect monitoring frequency | Nil | Out-of-pocket costs | Nil |
| Reimbursement issues | Nil | Nil | Nil | Tests not fully reimbursed, but may be offset by certain schemes | Reimbursement may not be available in some hospitals | Nil | PTH/alkaline phosphatase testing may not be reimbursed, depending on the reimbursement policy | Reimbursement may not be available for some institutions |
CKD-MBD, chronic kidney disease–mineral bone disorder; CPG, clinical practice guidelines; KDIGO, Kidney Disease: Improving Global Outcomes; PTH, parathyroid hormone.
Consensus on Monitoring Frequency for Various Biochemical Parameters of CKD-MBD in the Region
To improve implementation of the KDIGO CKD-MBD CPG, the Summit reached a consensus on the minimum monitoring frequency of various biochemical parameters of CKD-MBD in persons with CKD (Table 3). The participants believed that more frequent, structured monitoring of these biochemical parameters is justified as the estimated glomerular filtration rate decreases below 30 ml/min per 1.73 m2.
Table 3.
Consensus on suggested monitoring frequency of biochemical parameters of CKD-MBD according to CKD GFR categories in the region
| CKD GFR category | Calcium /phosphate |
Intact PTH | 25-hydroxyvitamin D | Alkaline phosphatase |
|---|---|---|---|---|
| G3a | Yearly | Yearly | Nil | Yearly |
| G3b | Yearly | Yearly | Nil | Yearly |
| G4 | 6 mo | Yearly | Nil | 6 mo |
| G5 | 3 mo | 6 mo | Nil | 3 mo |
| G5D | 3 mo | 6 mo | Nil | 3 mo |
CKD-MBD, chronic kidney disease–mineral bone disorder; GFR, glomerular filtration rate; PTH, parathyroid hormone.
How Can We Improve Adherence to CKD-MBD Monitoring Recommendation 3.1.1 in Asia?
Potential ways to improve adherence to Recommendation 3.1.1 include (i) establishing structured patient referral systems so that patients with CKD are referred early to nephrologists; (ii) setting up structured CKD care programs with protocol-guided CKD management; and (iii) incorporating regular monitoring of CKD-MBD biochemical tests starting at CKD G3b. Taiwan’s national, multidisciplinary CKD care program may serve as a good reference model for other Asian countries in the region. Continuous education of health professionals and patients on the need for regular monitoring of biochemical parameters for CKD-MBD starting at CKD G3b is essential to raise awareness of the importance of CKD-MBD monitoring in the overall care of patients with kidney disease.
KDIGO CKD-MBD Guideline Recommendations 4.1.3, 4.1.6, and 4.1.7
Barriers to the Implementation of KDIGO 2017 CKD-MBD Guideline Recommendations 4.1.3, 4.1.6, and 4.1.7 in Asia
Except in Japan, Malaysia, and Singapore, aluminum-based phosphate binders are currently still in use. All 8 countries/regions use aluminum-free dialysis fluid. It was believed that aluminum exposure is not a major issue for patients with CKD in the 8 Asian countries/regions.
Calcium exposure and hypercalcemia appear to be a highly prevalent problem in the region. Epidemiologic studies demonstrated an important association between serum calcium level and mortality in patients undergoing dialysis: the higher the serum calcium level, the greater the mortality risk.8, 9, 10 A calcium balance study showed that taking a daily dose of 1500 mg calcium carbonate for only 3 weeks caused significant positive calcium balance in CKD G3a–G4 subjects.11 Another study by Spiegel and Brady12 corroborated similar findings showing significantly more positive calcium balance in CKD G3b and G4 subjects receiving a 2000-mg calcium diet (500 mg from their diet and 1500 mg from calcium carbonate) than those CKD patients taking an 800-mg calcium diet or healthy individuals taking a 2000-mg calcium diet at least over 9 days of study.12 However, these calcium balance studies are not without limitations in that they could not estimate the rate of calcium deposition into extraskeletal tissues. The study time frame of 9 days to 3 weeks is also too short for the bone to achieve a steady state of calcium balance.13 An early study suggested that the mean duration to achieve calcium balance was 90 days.14 Thus, one needs to caution against overinterpreting these findings, and a more balanced view of these studies is called for. The KDIGO 2017 guideline update suggested restricting the dose of CPBs in persons with CKD G3a–G5D, and the recommendation statement was given a grading of 2B.4 It is noteworthy that there are significant regional variations in dietary calcium intake, with countries including China, India, and Indonesia having the world’s lowest average calcium intakes—often less than 400 mg a day.15 These regional differences in dietary calcium intake will need to be taken into account when prescribing the dose of CPBs.
In all 8 countries/regions, non–calcium-based phosphate binders (NCPBs) such as sevelamer-based phosphate binders are not reimbursed in patients with CKD who are not undergoing dialysis. In Japan, NCPBs are fully reimbursed in patients undergoing dialysis, but CPBs are still prescribed and often used in combination with NCPBs so as to minimize the dose of NCPBs. According to the Japan CKD-MBD Guideline, the daily prescribed dose of calcium carbonate is limited to a maximum of 3 g, which corresponds to 1.2 g of elemental calcium. In South Korea, sevelamer-based phosphate binders are reimbursed as a first-line drug for phosphate control in patients undergoing dialysis when serum phosphate levels exceed 5.5 mg/dl. In Taiwan and Singapore, NCPBs are not reimbursed in patients undergoing dialysis, although in Taiwan patients undergoing dialysis with disability cards may receive subsidies with an NCPB prescription. In Malaysia, reimbursement of NCPBs is restricted by individual hospital quotas in the public sector or only in certain groups of patients.
In Singapore, financial constraints pose an important barrier for nephrologists to prescribe NCPBs, although subsidies are available to patients who require financial assistance. NCPBs often are used as second-line drug treatment for hyperphosphatemia when there are contraindications to the use of CPBs. In China, payment policy is bundled and the prescription of NCPBs is highly restricted because of cost issues. NCPBs are not reimbursed in most parts of Mainland China and therefore incur out-of-pocket costs by patients. Thus their prescription remains highly restrictive. In Thailand, only government officials, civil servants, and their family members are entitled to reimbursement of NCPBs. In Hong Kong, the reimbursement policy only allows NCPB prescriptions as a second-line drug under the fulfillment of certain criteria, including poor calcium phosphorus control despite maximal dose of CPBs, aluminium toxicity, or hypercalcemia with first-line CPBs. Furthermore, there are variations in practice patterns among different public hospitals because some may choose to restrict prescription of NCPBs further due to budget constraints.
Apart from financial issues and reimbursement policies of NCPBs, there are other significant barriers to implementation of Recommendations 4.1.3 and 4.1.6 in China, Hong Kong, Malaysia, and Thailand. These include physicians’ low awareness of the importance of phosphorus control, the need to avoid hypercalcemia, and the need to restrict the dose of CPB. Nephrologists may not place phosphorus control as a high priority in the management of patients with CKD. Patient factors also may be potential barriers in CPG implementation because large pill burden could contribute to poor adherence to phosphate binders (Table 4).
Table 4.
Barriers in implementing KDIGO CKD-MBD guidelines 4.1.3, 4.1.6, and 4.1.7
| Japan | Korea | Taiwan | Singapore | China | Hong Kong | Thailand | Malaysia | |
|---|---|---|---|---|---|---|---|---|
| Government/policy level | Nil | Reimbursement policy for NCPBs and cinacalcet has been broadened since 2018 | Nil | Only CPBs are in the national list of subsidized drugs | Bundled payment policy may restrict the use of NCPBs because of cost | CRC sets limitations on the use of NCPBs | Only CPBs are in the national list of essential drugs | Nil |
| Hospital level | Nil | Nil | Nil | Some hospitals may preferentially stock only one kind of NCPBs | Variability in practice in rural and urban areas | Variability in practice among hospitals; hospital is free to restrict use of NCPB based on budget beyond restrictions by CRC | Some hospitals are allowed to stock only one kind of NCPBs | Nil |
| Physician level | Nil | Nil | Nil | Physicians not recognizing the importance to avoid hypercalcemia and need to restrict CPBs | Physicians not recognizing the importance to avoid hypercalcemia and need to restrict CPBs | Physicians not recognizing the importance to avoid hypercalcemia and need to restrict CPBs; physicians may not place phosphorus control as a high priority | Physicians not recognizing the importance to avoid hypercalcemia and need to restrict CPBs | Physicians not recognizing the importance to avoid hypercalcemia and need to restrict CPBs |
| Patient level | Low adherence of phosphate binders | Nil | Dietary calcium intake is low in Taiwanese patients with CKD, so use of calcium-based binders is not a contraindication | Drug adherence problem due to high pill burden; financial resistance to start NCPB because they need to self-finance the drug | In early CKD, hypocalcemia and vitamin D deficiency allows liberal use of CPBs | Failure to recognize the importance of phosphorus control and how dietary control may facilitate phosphorus control; phosphorus control is also suboptimal with twice weekly hemodialysis | Failure to recognize the importance of phosphorus control and how dietary intake may facilitate phosphorus control | High dietary phosphorus intake and low adherence to phosphate binders |
| Drug availability | CPBs are often used in combination with NCPBs so as to reduce dose of NCPBs to save cost | Sevelamer and lanthanum used as the first-line agents for dialysis patients since 2018; aluminum is not prescribed any more | Nil | CPBs are heavily subsidized in standard drug list, promoting their use; NCPB is used as a second-line drug therapy | CPBs are heavily used; NCPB is used as a second-line drug therapy in limited institutions and only partially reimbursed | Nil | CPBs are heavily subsidized in the national drug list, promoting their use; NCPB is used as a second-line drug therapy | Nil |
| Reimbursement issues | Cost of NCPBs fully reimbursed in dialysis patients but not in CKD | NCPBs are reimbursed in patients undergoing dialysis but not in patients with CKD | NCPBs are not reimbursed in patients undergoing dialysis; disability cards for dialysis patients may allow subsidies | NCPBs are not reimbursed, although assistance schemes may be available to some patients | Bundled payment as above | NCPBs are reimbursed as a second-line therapies under specific criteria in patients undergoing dialysis; NCPBs are not reimbursed in patients with CKD | NCPBs are not reimbursed under most health care schemes; they are only available to persons with Civil Service Welfare Medical Benefits | NCPBs are reimbursed under specific criteria in patients undergoing dialysis and not reimbursed in CKD |
CKD-MBD, chronic kidney disease–mineral bone disorder; CPBs, calcium-based phosphate binders; CRC, Central Renal Committee; KDIGO, Kidney Disease: Improving Global Outcomes; NCPBs, non–calcium-based phosphate binders.
KDIGO CKD-MBD Guideline Recommendation 4.2.4
Barriers to the Implementation of KDIGO 2017 CKD-MBD Guideline Recommendation 4.2.4 in Asia
Cinacalcet use is restricted across the region, except in Japan, because of high costs. Gastrointestinal side effects of cinacalcet may sometimes affect its prescription and compliance. As such, vitamin D analogs are more widely used in the region.
In Japan, cinacalcet is reimbursed until PTH levels reach the Japanese CKD-MBD guideline target range. Both etelcalcetide and evocalcet are available and reimbursed in patients undergoing dialysis. In patients not undergoing dialysis, cinacalcet can be used only in patients with parathyroid cancer or noncontrollable primary hyperparathyroidism after surgery.
In South Korea, cinacalcet has been reimbursed as a first-line agent for treatment of secondary hyperparathyroidism since 2018. In Singapore, cinacalcet is available but not reimbursed. Cost remains a significant barrier for its prescription. In Hong Kong, cinacalcet is reimbursed as a second-line drug in patients with ESKD when parathyroidectomy is either not feasible or contraindicated or in those who experience recurrent secondary hyperparathyroidism after surgery. Because Recommendation 4.2.4 does not prioritize the use of cinacalcet over vitamin D analogs, the Summit attendees did not perceive barriers to implementing this specific guideline recommendation.
KDIGO CKD-MBD Guideline Recommendations 4.3.1 and 4.3.2
Barriers to the Implementation of KDIGO 2017 CKD-MBD Guideline Recommendations 4.3.1 and 4.3.2 in Asia
These 2 recommendation statements are especially relevant for patients with CKD who have a high risk of fracture, such as those receiving glucocorticoid treatment. The implementation of these recommendations requires a concerted effort that entails working with endocrinologists or orthopedic surgeons who usually manage osteoporosis. Major barriers to implementing these 2 recommendation statements may relate to the lack of awareness among nephrologists of the importance of osteoporosis treatment in persons with CKD because nephrologists mostly focus on CKD management. Furthermore, a dual energy x-ray absorptiometry (DEXA) scan is not reimbursed for patients with CKD in this region, except in Japan and in the dialysis population in South Korea. Thus there are financial barriers to ordering DEXA scans in CKD G1–G3b patients who are at high risk of the development of osteoporosis. Patients with CKD G1 and G2 also are followed up mostly by primary care physicians who may lack awareness of this complication in persons with CKD. Implementation of these recommendation statements thus requires a concerted, multidisciplinary effort among primary care physicians, nephrologists, endocrinologists, and orthopedic surgeons. Furthermore, there is insufficient knowledge about the efficacy of anti-osteoporosis drugs in persons with advanced CKD (i.e., subjects with CKD G4–G5), and existing noninvasive investigations have limitations in differentiating osteoporosis from adynamic bone disease. Lastly, many patients are unwilling to undergo an invasive bone biopsy.
Would the KDIGO 2017 CPG Change Your Practice Pattern Regarding Performing DEXA Scans in Your Patients With CKD?
The Summit recognized that fracture is an important complication in patients with CKD and causes significant morbidity. The Dialysis Outcomes and Practice Patterns Study reported a significantly higher incidence of fractures for patients receiving hemodialysis than in the general population, with a 3.7-fold increase in the unadjusted relative risk of death.16 Five prospective cohort studies demonstrated that DEXA-derived bone mineral density predicted fractures across CKD G3a–G5D.17, 18, 19, 20, 21 However, DEXA scans currently are not reimbursed in the 8 Asian countries/regions for patients with CKD, except in Japan, and there is limited evidence to inform the best practice for osteoporosis treatment in this population. Uncertainties also exist about the safety and efficacy of anti-osteoporotic drugs such as bisphosphonates and denosumab in patients with advanced CKD.22, 23, 24 Thus the Summit did not insist strongly on performing and reimbursing a DEXA scan for patients with CKD G3a–G5D. However, the Summit participants unanimously suggested on performing a DEXA scan and treating osteoporosis in patients with CKD G1–G2 as in the general population with osteoporosis and/or high risk of fracture. More evidence will be required to drive better implementation of this recommendation statement in the region.
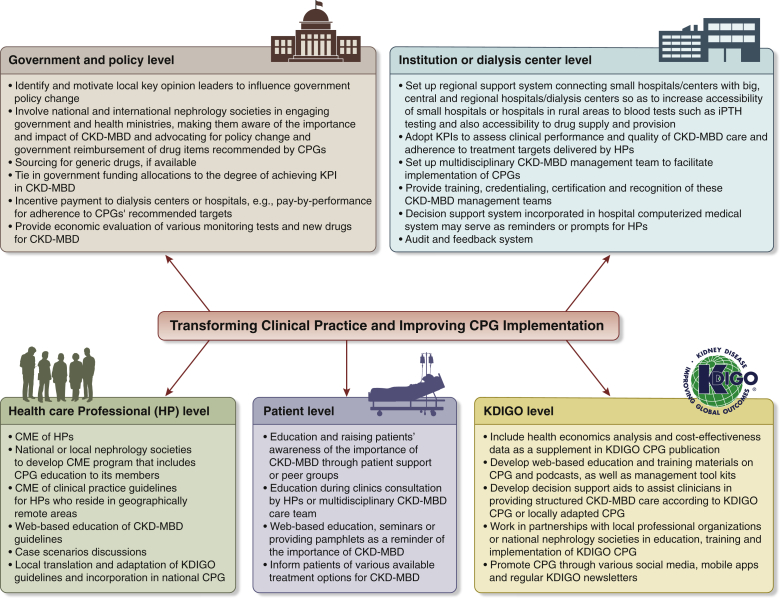
Strategies to Improve Implementation of KDIGO CKD-MBD CPG
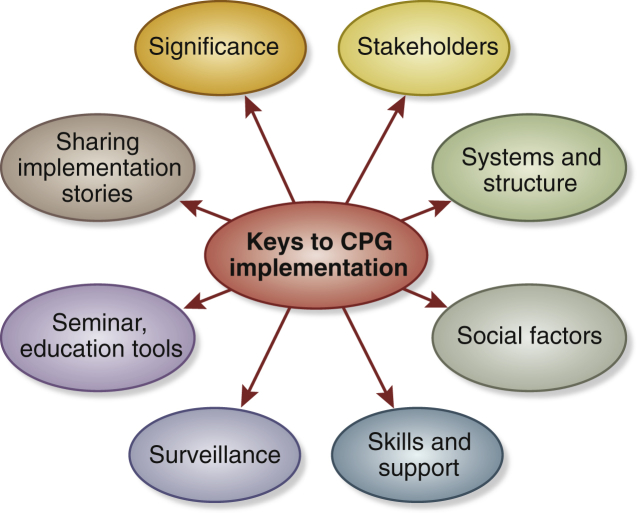
Figure 4 depicts the 8 key elements that facilitate CPG implementation. The Summit participants discussed potential strategies to improve implementation of the KDIGO CKD-MBD CPG. Figure 5 outlines a framework of multifaceted strategies that aim to improve CPG implementation.
Figure 4.
Eight key steps prior to clinical practice guideline (CPG) implementation.
Figure 5.
A framework of multi-level implementation strategies to overcome barriers for chronic kidney disease-mineral bone disease (CKD-MBD) Guideline implementation in the region. CME, continuing medical education; CPG, clinical practice guideline; HP, health professionals; iPTH, intact parathyroid hormone; KDIGO, Kidney Disease: Improving Global Outcomes; KPI, key performance indicator.
Government and Policy Level
Two approaches may help overcome barriers at the government or health policy level, depending on the health care financing infrastructure of the country. The first approach is to identify and motivate key local physicians, particularly leaders of local nephrology societies or key opinion leaders who could help to advocate and influence health policy change of provincial or local governments. The second approach is to involve national and international nephrology organizations in engaging government and health ministries, making them aware of the importance and impact of CKD-MBD so they may further advocate for policy change and government reimbursement of drugs recommended by the KDIGO CPG, such as NCPBs.
Cost poses a key barrier for CPG implementation in the region. This encompasses not only the costs of various biochemical tests of CKD-MBD but also the costs of newer drug treatment such as NCPBs, second-generation vitamin D analogs, and calcimimetics. The group felt that a cost-effectiveness analysis of regular biochemical testing and the use of newer, yet more costly drugs for CKD-MBD management could shed light on their use in CKD-MBD management in the region and could help facilitate discussions with local health policy makers or health ministers for their potential reimbursement. An alternative strategy to overcome cost or financial barriers for both the government and patients may include sourcing for generic versions of more costly drugs such as NCPBs and calcimimetics, if available.
Key performance indicators (KPIs) could be used to assess clinical performance and quality of CKD-MBD care in individual health care institutions, as well as adherence to treatment targets recommended by the KDIGO CPG. The achievement of various KPIs may correlate with government funding allocations to health care institutions or incentive payments such as pay-by-performance or pay-by-adherence to targets recommended by KDIGO. This may serve as a useful strategy to motivate healthcare institutions or dialysis centers and add incentives to drive for better CKD-MBD care and better adherence to the global CKD-MBD CPG.
Institution Level
The availability of biochemical tests of CKD-MBD, such as PTH, is vital in ensuring adherence to CKD-MBD monitoring and achieving treatment targets recommended by KDIGO. However, some tests may not be readily available in rural areas or small peripheral hospitals. Therefore, a regional support system should be established to connect small hospitals with large, central, or regional hospitals whereby patient blood samples could be transported for testing. For the availability and supply of NCPBs and calcimimetics, a similar regional support and transport system could be set up, connecting small peripheral hospitals with large central and regional hospitals for drug supply and provision.
Health Professional Level
Continuous education of health professionals is essential in facilitating CPG implementation and changing practice patterns. National or local nephrology societies may assist KDIGO guideline implementation by educating health professionals including nephrologists, non-nephrology physicians, and allied health professionals through developing continuing medical education programs. Continuing medical education of the KDIGO CPG also could be delivered to health professionals who reside in geographically remote areas. Web-based education of the CKD-MBD CPG with patient-case discussions are an effective means of training health professionals and facilitating continuing medical education. In addition, because some countries such as Japan and Malaysia have developed their own national CPG for CKD-MBD care, the KDIGO CPG may be translated or adapted and incorporated into the national CPG by local professional societies or guideline groups.
A CKD-MBD management task force that involves a multidisciplinary team of nephrologists, nurses, dieticians, social workers, and pharmacists to coordinate the monitoring and treatment of CKD-MBD may be useful for the case management of patients with CKD and may facilitate better implementation of the KDIGO CPG in individual health care institutions and dialysis centers. In addition, training, credentialing, and certification of this CKD-MBD management task force may provide recognition and more motivation to health professionals, such that the implementation of the KDIGO CKD-MBD CPG would become a routine and standard part of the clinical practice. Decision support systems incorporated in hospital electronic medical record systems may serve as prompts and useful reminders for health professionals in their daily clinical practice.
Assessing the Effectiveness of KDIGO CKD-MBD CPG Implementation
Adopting KPIs may be an important strategy for facilitating KDIGO CPG’s implementation and monitoring the degree of adherence at health institutions or dialysis centers in the region. Some suggested examples of KPI in CKD-MBD management may include the following:
-
(i)
percentage of patients with CKD who have frequent testing of serum calcium, phosphorus, PTH and alkaline phosphatase presented according to severity of CKD;
-
(ii)
percentage of patients with hypercalcemia who are undergoing dialysis (with a threshold to be defined using the local laboratory reference values) in dialysis centers;
-
(iii)
percentage of patients undergoing dialysis who are prescribed CPBs and NCPBs in dialysis centers;
-
(iv)
percentage of patients by dose of CPBs prescribed (e.g., patients with daily elemental calcium dose prescribed exceeding 1.5 g); and
-
(v)
percentage of patients prescribed aluminium-based phosphate binders.
Because currently there is a lack of strong evidence supporting a specific treatment target for each biochemical parameter of CKD-MBD and reimbursement policies also differ across the region, threshold levels for various KPIs may be locally adapted to suit local situations and needs for an individual country. Installing an audit and feedback system will be useful in monitoring CPG implementation.
What KDIGO Can Do to Assist CKD-MBD CPG Implementation
Because cost and reimbursement policies are among the key barriers to implementing the CKD-MBD CPG, KDIGO could consider incorporating health economics analysis and relevant data as a supplement to publication of a CPG. General formulas for calculating the incremental cost-effectiveness ratio could be developed and calculations could be personalized based on the actual costs incurred in individual regions or countries. These data are of direct relevance and interest to health policy makers and are important considerations that may help bridge the gap between guideline recommendations and implementation.
KDIGO also may consider developing web-based education and training materials on CPGs, such as podcasts, regionally relevant management toolkits, and decision support aids to assist clinicians in providing structured CKD-MBD care. KDIGO may work in partnership with local professional organizations or national nephrology societies on education, training, and implementation of a KDIGO CPG. KDIGO also may consider promoting their CPG through various social media, mobile apps, and regular newsletters.
Conclusions
Although the 8 Eastern and Southern Asian countries/regions represented in this Summit have a similar high-to-middle income ranking, clinical practices in CKD-MBD and related drugs reimbursement policies vary among them. This Summit identified specific barriers in implementing CKD-MBD Guidelines in this region, among which financial barriers and reimbursement policies of NCPBs appear to be the most significant. The Summit also presented a framework of strategies that target various facilitators, namely local government, health, and reimbursement policy; institution and dialysis centers; health professionals; patients; and KDIGO itself, with an aim to improve both the implementation of the CKD-MBD CPG and the translation of knowledge in CKD-MBD care in the region. There is a need to recognize possible racial differences in treatment effects; however, given the paucity of published data on Asian patients, more studies are required from this region if local data are to contribute to future KDIGO CPG development. It is hoped that this first Asia Summit on KDIGO CKD-MBD Guideline Implementation will help facilitate knowledge translation on a local level and serve as an important example for other regions of the world to follow.
Disclosure
AY-MW declared having received research grants and speaker honorarium from Sanofi Renal. TA declared having received consultancy fees from Astellas, Bayer Healthcare Pharmaceuticals, FUSO Pharmaceutical Industries, GlaxoSmithKline, JT Pharmaceuticals, Kyowa Hakko Kirin, Nipro Medical Corporation, Ono Pharmaceutical, Sanwa Chemical, and Torii Pharmaceutical; and speaker honoraria from Bayer HealthCare, Chugai Pharmaceutical, Kissei Pharmaceutical, Kyowa Hakko Kirin, Ono Pharmaceutical, and Torii Pharmaceutical. YT declared having received speaker honoraria from Bayer HealthCare, Boehringer Ingelheim, Kyowa Kirin Pharmaceutical, Otsuka Pharmaceutical, and Torii Pharmaceutical; and consultancy fees from Kyowa Kirin Pharmaceutical. MF declared having received speaker honoraria from Bayer HealthCare, Kissei Pharmaceutical, Kyowa Hakko Kirin, and Torii Pharmaceutical; and consultancy fees from Kyowa Hakko Kirin, Ono Pharmaceutical, and Torii Pharmaceutical. All the other authors declared no competing interests.
Acknowledgments
This Summit conference was sponsored by KDIGO and supported in part by unrestricted educational grants from Bayer HealthCare, Boehringer Ingelheim, Chugai Pharmaceutical, Fresenius Medical Care, Japan CKD-MBD Forum, Japanese Society of Nephrology (JSN), Kissei Pharmaceutical, Kyowa Kirin Pharmaceutical Development, Nikkiso, Ono Pharmaceutical, Suntop Healthcare, and Torii Pharmaceutical. We thank Dr. Sinee Disthabanchong for valuable input into this manuscript.
Other conference participants are Ali K. Abu-Alfa, Lebanon; Hideki Fujii, Japan; Naohiko Fujii, Japan; Kunitoshi Iseki, Japan; Nobuhiko Joki, Japan; Tilakavati Karupaiah, Malaysia; Naoki Kashihara, Japan; Junichiro Kazama, Japan; Fumihiko Koiwa, Japan; Hirotaka Komaba, Japan; Braden J. Manns, Canada; Masahide Mizobuchi, Japan; Yusuke Sakaguchi, Japan; Hiroko Segawa, Japan; Takashi Shigematsu, Japan; Tetsuo Shoji, Japan; Motoko Tanaka, Japan; Masatomo Taniguchi, Japan; Minako Wakasugi, Japan; and Suguru Yamamoto, Japan.
Footnotes
Table S1A. Intact parathyroid hormone threshold for parathyroidectomy in the region.
Table S1B. Other local indications for parathyroidectomy in the region.
Table S2. Target intact parathyroid hormone level adopted in the region.
Supplementary Material
References
- 1.Block G.A., Klassen P.S., Lazarus J.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 2.Rivara M.B., Ravel V., Kalantar-Zadeh K. Uncorrected and albumin-corrected calcium, phosphorus, and mortality in patients undergoing maintenance dialysis. J Am Soc Nephrol. 2015;26:1671–1681. doi: 10.1681/ASN.2014050472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int. 2009;76(suppl 113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 4.Ketteler M., Block G.A., Evenepoel P. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what's changed and why it matters. Kidney Int. 2017;92:26–36. doi: 10.1016/j.kint.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 5.United States Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2017. 2017 USRDS annual data report: epidemiology of kidney disease in the United States. Chapter 11: international comparisons.https://www.usrds.org/2017/view/v2_11.aspx Available at: [Google Scholar]
- 6.Wang F., Yang C., Long J. Executive summary for the 2015 Annual Data Report of the China Kidney Disease Network (CK-NET) Kidney Int. 2019;95:501–505. doi: 10.1016/j.kint.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto S., Karaboyas A., Komaba H. Mineral and bone disorder management in hemodialysis patients: comparing PTH control practices in Japan with Europe and North America: the Dialysis Outcomes and Practice Patterns Study (DOPPS) BMC Nephrol. 2018;19:253. doi: 10.1186/s12882-018-1056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tentori F., Blayney M.J., Albert J.M. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Martin J.L., Martinez-Camblor P., Dionisi M.P. Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant. 2015;30:1542–1551. doi: 10.1093/ndt/gfv099. [DOI] [PubMed] [Google Scholar]
- 10.Fukagawa M., Kido R., Komaba H. Abnormal mineral metabolism and mortality in hemodialysis patients with secondary hyperparathyroidism: evidence from marginal structural models used to adjust for time-dependent confounding. Am J Kidney Dis. 2014;63:979–987. doi: 10.1053/j.ajkd.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Hill K.M., Martin B.R., Wastney M.E. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int. 2013;83:959–966. doi: 10.1038/ki.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiegel D.M., Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int. 2012;81:1116–1122. doi: 10.1038/ki.2011.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evenepoel P., Wolf M. A balanced view of calcium and phosphate homeostasis in chronic kidney disease. Kidney Int. 2013;83:789–791. doi: 10.1038/ki.2013.21. [DOI] [PubMed] [Google Scholar]
- 14.Harrison M., Fraser R., Mullan B. Calcium metabolism in osteoporosis. Acute and long-term responses to increased calcium intake. Lancet. 1961;1:1015–1019. doi: 10.1016/s0140-6736(61)91828-1. [DOI] [PubMed] [Google Scholar]
- 15.Balk E.M., Adam G.P., Langberg V.N. Global dietary calcium intake among adults: a systematic review. Osteoporos Int. 2017;28:3315–3324. doi: 10.1007/s00198-017-4230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tentori F., McCullough K., Kilpatrick R.D. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2014;85:166–173. doi: 10.1038/ki.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denburg M.R., Tsampalieros A.K., de Boer I.H. Mineral metabolism and cortical volumetric bone mineral density in childhood chronic kidney disease. J Clin Endocrinol Metab. 2013;98:1930–1938. doi: 10.1210/jc.2012-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.West S.L., Lok C.E., Langsetmo L. Bone mineral density predicts fractures in chronic kidney disease. J Bone Miner Res. 2015;30:913–919. doi: 10.1002/jbmr.2406. [DOI] [PubMed] [Google Scholar]
- 19.Yenchek R.H., Ix J.H., Shlipak M.G. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7:1130–1136. doi: 10.2215/CJN.12871211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iimori S., Mori Y., Akita W. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients–a single-center cohort study. Nephrol Dial Transplant. 2012;27:345–351. doi: 10.1093/ndt/gfr317. [DOI] [PubMed] [Google Scholar]
- 21.Naylor K.L., Garg A.X., Zou G. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin J Am Soc Nephrol. 2015;10:646–653. doi: 10.2215/CJN.06040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khairallah P., Nickolas T.L. Management of osteoporosis in CKD. Clin J Am Soc Nephrol. 2018;13:962–969. doi: 10.2215/CJN.11031017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nitta K., Yajima A., Tsuchiya K. Management of osteoporosis in chronic kidney disease. Intern Med. 2017;56:3271–3276. doi: 10.2169/internalmedicine.8618-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moe S.M. Renal osteodystrophy or kidney-induced osteoporosis? Curr Osteoporos Rep. 2017;15:194–197. doi: 10.1007/s11914-017-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.