Abstract
A differentiation of human induced pluripotent stem cells (hiPSCs) into definitive endoderm linage is required for a preparation of metabolic organ derived cells. The differentiation consumed high-priced cytokines and small molecules, which have hampered the manufacturability of differentiated cells. Although the cytokines and small molecules are remained or cells produce the autocrine factors, daily culture medium change should be proceeded to remove toxic metabolites generated from cells. In this study, we developed a simple dialysis culture system to refine the medium during definitive endodermal differentiation. We demonstrated that dialysis culture prevented cell damage to remove lactate. The hiPSCs cultured with dialysis also differentiated similarly as usual differentiation without dialysis even if they were not supplied Activin A for latter culture days in the differentiation. With this dialysis culture system, hiPSCs were differentiated into endodermal lineage with medium refinement and recycling and autocrine factors as well as cytokines, which may lead to reduce differentiation cost.
Keywords: Dialysis culture, Human induced pluripotent stem cell, Suspension culture, Definitive endoderm
Abbreviations: hiPSCs, human induced pluripotent stem cells; VTN-N, vitronectin human protein; E8, essential 8 medium; PSA, penicillin–streptomycin–amphotericin B suspension; KSR, knockout serum replacement; DE, definitive endoderm; NEAA, non-essential amino acids
Highlights
-
•
We developed a simple plate based dialysis culture system for suspension culture.
-
•
The continuous glucose supply and lactate removal using dialysis culture.
-
•
The differentiation promoting cytokines and autocrine factors were retained.
1. Introduction
Human induced pluripotent stem cells (hiPSC) are expected as a promising cell source for drug screening and regenerative medicine because of their ability of proliferation and pluripotency. Especially towards manufactural application, efficient preparation of plenty amount of cells and keeping production cost down are required. The required transplanted cell number of pancreastic β cells which is expected to be a replacement of donor derived pancreatic islet is more than tens of million cells [1]. A suspension culture is profitable compare to the adhesion culture which depends on the cultured surface area [2], [3]. However, the increase of cultured cell number causes increment of toxic metabolite including lactate an ammonium [4]. A energy metabolic pathway related to lactate production of hiPSCs is activated during differentiation [5], [6], which is expected actively production of lactate from differentiated cells. Considering the removal toxic metabolite, daily medium change is generally performed.
Definitive endodermal differentiation is generally performed to prepare metabolic organ derived cells; hepatocyte [7], [8], pancreatic β cells [9], kidney podocytes [10], and intestine epithelial cells [11] are generally prepared with definitive endodermal differentiation from hiPSCs using Activin A. The existence of autocrine factors produced by hiPSCs has been reported. Nodal is one of the autocrine factor and perform similarly as Activin A in their pathway [12], [13]. The recycling of autocrine factors is promising to reduce the usage of costly cytokines for the differentiation. However, daily medium change looses those autocrine factors with removal of waste including toxic metabolites produced by cells.
Dialysis culture is a potential solution to refine the culture medium with retaining of cytokines and autocrine factors. Previous study focused on mass production and their reactors were generally large scale using hollow fiber or perfusion culture [14], [15]. The application dialysis culture to hiPSCs culture was reported for expansion of hiPSCs [15]. However, the definitive endoderm differentiation using dialysis culture has not been studied yet.
In this study, we developed a plate-based dialysis culture system to differentiate hiPSCs into definitive endoderm. We also demonstrated that the differentiation without Activin A addition at the latter culture days of differentiation to evaluate the existence of autocrine factor influences toward the differentiation. Our dialysis culture suggested that there were continuous glucose supply, lactate removal, and autocrine factors retaining. However, further study to remove the higher molecule toxic metabolite than 3 kDa should be performed to improve the culture condition. This study is expected to contribute the manufactural application to prepare the plenty number of cells with keeping the usage of costly cytokines down.
2. Materials and methods
2.1. Preparation of dialysis culture plate
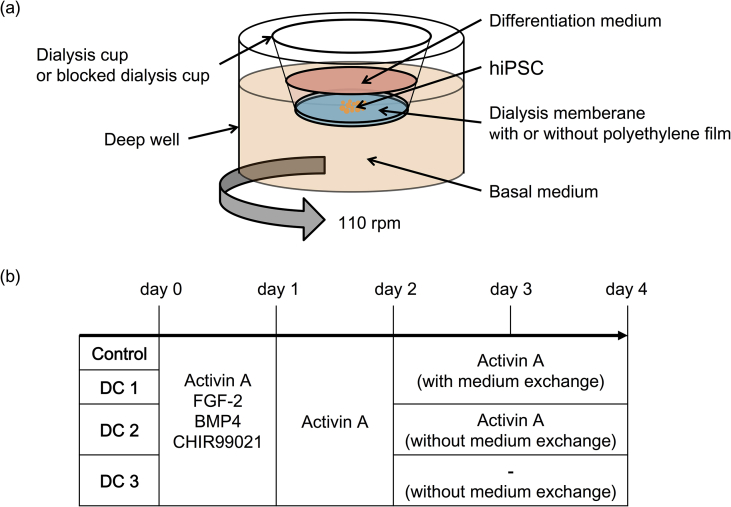
A dialysis culture plate was prepared with a deep well plate (Corning, USA) and a culture insert (Corning, USA), which had a dialysis membrane (Spectrum, USA). The polycarbonate membrane of the culture insert was removed and replaced with a dialysis membrane, which had a molecular weight cutoff (MWCO) of 3 kDa and a surface area of 0.53 cm2 (herein after, this is called “dialysis cup”). The bottom of the dialysis membrane was covered with a polyethylene film (Diversified Biotech, USA) to avoid mass transfer as the comparison to dialysis culture (hereinafter, this is called “blocked dialysis cup”) (Fig. 1a).
Fig. 1.
The culture condition in this study. (a) Schematic images of culture design. (b) Schedule of cytekines addition for the differentiation.
2.2. Maintenance of hiPSC and formation of hiPSC aggregates
The human iPSC line TkDN4-M was provided by Stem Cell Bank at Institute of Medical Science, The University of Tokyo. Cells were maintained on a tissue-culture treated 6 well plate coated with 10 μg/cm2 vitronectin human recombinant protein (VTN-N; Thermo Fisher, USA) in Essential 8 medium (E8; Thermo Fisher Scientific, USA) containing 1% penicillin–streptomycin–amphotericin B suspension (PSA; FUJIFILM Wako Pure Chemical Corporation, Japan). The hiPSCs were seeded at 1.0 × 104 cell/cm2 with E8 medium containing 1% PSA and 10 μM Rho-associated kinase inhibitor (Y-27632) and were cultured at 37 °C in a humidified atmosphere with 5% CO2. Culture medium was changed every day with E8 medium containing 1% PSA. Cells were detached by Accutase (Innovative Cell Technologies, Inc., USA) after washing with phosphate buffered saline (PBS; FUJIFILM Wako Pure Chemical Corporation, Japan) for passaging and starting aggregate formation.
For aggregate formation, hiPSCs aggregates were seeded at 7.5 × 105 cell/mL in 4 mL of E8 medium containing 5 mg/mL bovine serum albumin (BSA; Proliand Biologicals, USA), 1% PSA, and 10 μM Y-27632 in a 6 well plate for suspension culture (Sumitomo Bakelite Co., Ltd., Japan). The plate was rotated at 90 rpm on an orbital shaker. The aggregates were formed within 24 h at 37 °C in a humidified atmosphere with 5% CO2.
2.3. Differentiation of hiPSC into definitive endoderm linage
One day before starting hiPSCs differentiation in a suspension culture, the culture medium was replaced with DMEM/Ham's F12 medium (FUJIFILM Wako Pure Chemical Corporation, Japan) containing 20% knockout serum replacement (KSR; Thermo Fisher Scientific, USA), 1% non-essential amino acids (NEAA; FUJIFILM Wako Pure Chemical Corporation, Japan) and 7.5 ng/mL recombinant human fibroblast growth factor 2 (bFGF; Reprocell Inc., Japan). Right before differentiation, hiPSCs aggregates in a 6 well plate were collected and partially dissociated using Accutase for cell counting. The hiPSCs aggregates were then resuspended into 2.5 mL at 1 × 106 cell/mL and transferred in the dialysis cup or the blocked dialysis cup.
The differentiation of iPSCs into definitive endoderm (DE) was performed as described previously with some modification [9]. The hiPSCs aggregates were cultured in a differentiation medium consisted of a basal medium with cytokines and small molecules for four days. The basal medium was also feeded in the deep well and changed every day with fresh basal medium; 3 μM CHIR-99021(FUJIFILM Wako Pure Chemical Corporation, Japan) was added first day. The basal medium was prepared with RPMI1640 (FUJIFILM Wako Pure Chemical Corporation, Japan) containing 5 mg/mL BSA, 1% sodium pyruvate, 1% NEAA, 1% PSA, and 55 μm 2-mercaptoethanol (Thermo Fisher Scientific, USA).
We compared four different culture conditions to evaluate the culture medium refinement and autocrine existence which promoted differentiation (Fig. 1b). First, the hiPSCs aggregates were cultured in the blocked dialysis cup for four days with every day a differentiation medium change using the basal medium containing 80 ng/mL recombinant human/mouse/rat Activin A (R&D Systems, USA); 3 μM CHIR-99021, 20 ng/mL human bone morphogenetic protein (BMP)-4 (Miltenyi Biotec K.K., Japan) and 50 ng/mL bFGF were added for first day (herein after, this is called “Control”). Second, the hiPSCs aggregates were cultured in the dialysis cup for four days with every day a differentiation medium change using basal medium containing 80 ng/mL recombinant human/mouse/rat Activin A; 3 μM CHIR-99021, 20 ng/mL human BMP-4 50 ng/mL bFGF were added for first day (herein after, this is called “DC1”). Third, the hiPSCs aggregates were cultured in the dialysis cup for four days with first two days a differentiation medium change using basal medium containing 80 ng/mL recombinant human/mouse/rat Activin A; 3 μM CHIR-99021, 20 ng/mL human BMP-4 50 ng/mL bFGF were added for first day. The hiPSCs aggregates were cultured for latter two days without a differentiation culture medium change but with removal of dead cells by centrifugation and added 80 ng/mL recombinant human/mouse/rat Activin A (herein after, this is called “DC2”). Fourth, the hiPSCs aggregates were cultured in the dialysis cup for four days with first two days a differentiation medium change using basal medium containing 80 ng/mL recombinant human/mouse/rat Activin A; 3 μM CHIR-99021, 20 ng/mL human BMP-4 50 ng/mL bFGF were added for first day. The hiPSCs aggregates were cultured for latter two days without a differentiation culture medium change but with removal of dead cells by centrifugation (herein after, this is called “DC3”).
2.4. Biochemical assays
After days of culture, the glucose and lactate concentration of the culture medium in the dialysis, blocked dialysis, and deep well were measured by bioanalyser (Oji analytical, Japan) to evaluate the changing because of dialysis. Lactic dehydrogenase (LDH) in the medium was measured with a cytotoxicity LDH assay kit-WST (FUJIFILM Wako Pure Chemical Corporation, Japan) following the manufacture protocol. The differentiated hiPSCs aggregates were dissociated and counted their number.
2.5. RT-qPCR analyses
Total RNAs of hiPSCs aggregates sampled before and after differentiation were isolated by using TRIZol reagent (Thermo Fisher Scientific, USA). Their RNAs were purified by adding chloroform (FUJIFILM Wako Pure Chemical Corporation, Japan) and the centrifugation of 15,000 × g for 15 min. The supernatants were collected and purified by adding 2-propanol (FUJIFILM Wako Pure Chemical Corporation, Japan) and the centrifugation of 15,000 × g for 15 min. Following washing with 75% ethanol (FUJIFILM Wako Pure Chemical Corporation, Japan), purified RNA samples were qualitative and quantitative analyzed by Nano drop (Shimadzu, Japan). 100 ng of purified RNA from each sample was reverse-transcribed by PrimeScript™ Reverse Transcriptase (Takara Bio Inc., Japan). The transcribed complementary DNA samples were evaluated their gene expression related to pluripotency (Oct4), definitive endoderm (Sox17, Foxa2) and primitive gut tube (Hnf4a) markers using SYBR Green (TOYOBO CO., LTD.) and detected by StepOne Plus (Thermo Fisher Scientific, USA). The specific primers were used as follows. β-actin: forward, 5′-CCTCATGAAGATCCTCACCGA-3′; reverse, 5′-TTGCCAATGGTGATGACCTGG-3′. Oct4: forward, 5′-AGTGGGTGGAGGAAGCTGACAAC-3′; reverse, 5′-TCGTTGTGCATAGTCGCTGCTTGA-3′. Sox17: forward, 5′-CAGAATCCAGACCTGCACAA-3′; reverse, 5′-TCTGCCTCCTCCACGAAG-3′. Foxa2: forward, 5′-GGTGATTGCTGGTCGTTTGTTGTG-3′; reverse, 5′-GCCGACATGCTCATGTACGTGTT-3′. HNF4a: forward, 5′-CCAAGAGATCCATGGTGTTCAA-3′; reverse, 5′-TTGATGTAGTCCTCCAAGCTCA-3′.
2.6. Statistical analyses
Results were expressed as the mean ± standard deviation of the mean (SD). The Student's t-test was performed to assess the statistical significance of the results. A value of P < 0.05 was considered significant.
3. Results and discussion
3.1. Formation and differentiation of hiPSCs aggregates
The hiPSCs aggregates were formed with rotational suspension culture (Fig. 1a). Whereas the morphology of each condition during differentiation seemed similarly, slight larger number of dead cells was found in DC2 on day 3. Activin A is known as the cytokine which promotes survival and proliferation [16]. The proinflammatory, tumor-promoting, and metastasis-promoting effects of Activin A have been also reported [17], [18], [19]. In DC2, Activin A was added daily without medium change for two days from second day of differentiation, which might cause cellular damages because of the negative effect of Activin A.
3.2. Effects of dialysis culture
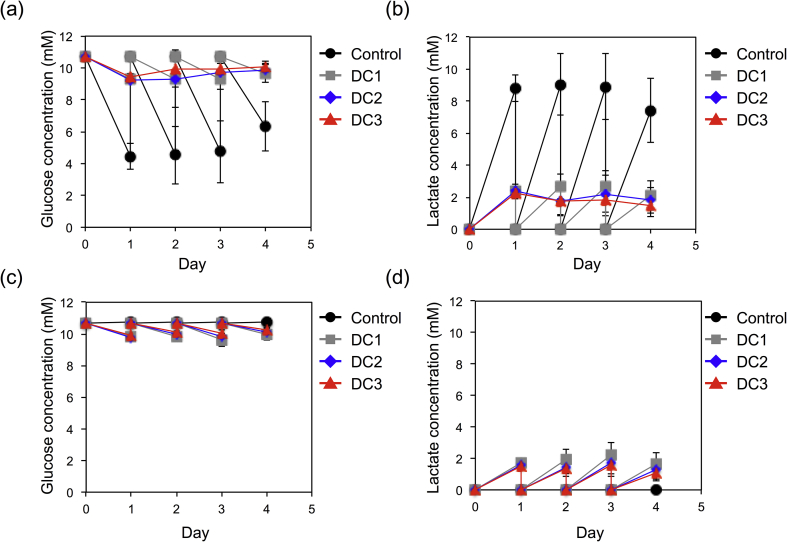
The remaining glucose and produced lactate of the medium in dialysis cup and blocked dialysis cup for every day to evaluate the dialysis effect on glucose supply and lactate removal (Fig. 2). Whereas Control culture consumed all of the glucose in the differentiation medium, DC1, DC2, and DC3 culture with dialysis maintained near the original glucose concentration of the medium (Fig. 2a). The glucose concentration in the deep well were also decreased as the same level of the differentiation medium but still enough glucose was remained in dialysis culture group; DC1, DC2 and DC3. The glucose concentration of the medium in the deep well didn't change in the Control culture, which suggested that dialysis membrane was completely blocked and didn't leak (Fig. 2c). The lactate concentration in the differentiation medium of Control was four times higher than that of in dialysis culture; DC1, DC2, and DC3 (Fig. 2b). The lactate concentration of the medium in the deep well was the same as in the differentiation medium in dialysis culture; DC1, DC2, and DC3, but there was no difference in the control culture because the dialysis membrane was completely sealed (Fig. 2d). Therefore, continuous glucose supply to hiPSCs and lactate removal from differentiation medium though a dialysis membrane were successfully performed by dialysis culture until they were equilibrium between differentiation medium and the medium in the deep well.
Fig. 2.
Glucose and lactate concentration transitions in the culture medium during differentiation. (a) glucose concentration of differentiation medium, (b) lactate concentration of differentiation medium, (c) glucose concentration of medium in deep well, and (d) lactate concentration of medium in deep well. Data represent mean ± SD of n = 5–12 from two to four independent experiments.
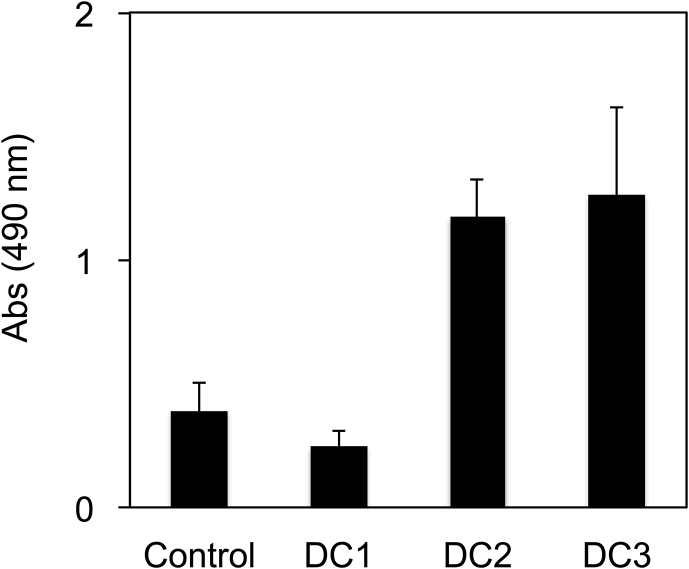
The cellular damages were evaluated by measurement of LDH changes in the differentiation medium (Fig. 3). DC2 and DC3 showed higher value than other two conditions because of the non-medium change. Cellular damage of DC1 might lower than that of Control throughout the whole differentiation days.
Fig. 3.
Lactate dehydrogenase (LDH) cytotoxicity assay on day 4. Data represents mean ± SD of n = 5–6 from two independent experiments.
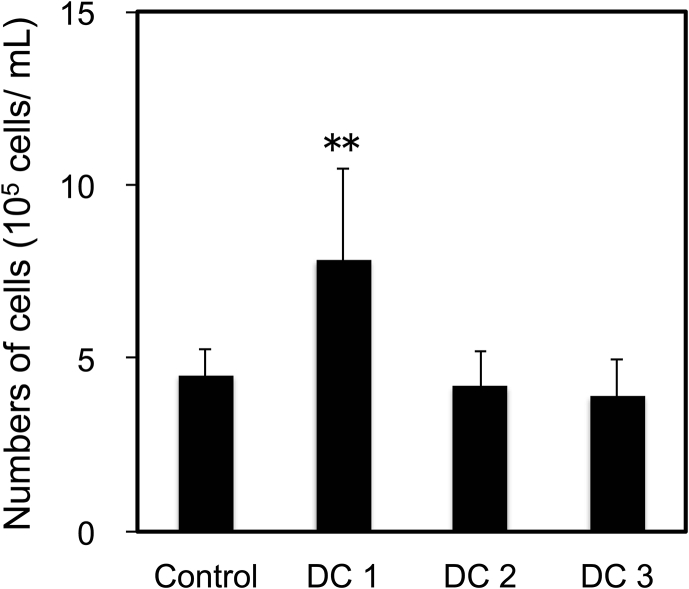
The differentiated cell number seemed to have correlation with the results of LDH assay (Fig. 4). The cell number of DC1 was larger than that of other conditions; Control, DC2, and DC3. The cell number of Control, DC2, and DC3 had no statistical differences. The higher cell damage of DC2 and DC3 between days 3 and 4 suggested that there were toxic macromolecule larger than dialysis MWCO; 3 kDa in the medium. The energy metabolism of hiPSCs shift oxidative phosphorylation from glycolysis during a differentiation [20]. The oxidative phosphorylation shift of hiPSCs has potential to provoke reactive oxygen spices, which reduce hemo oxygenase-1. The lack of hemo oxygenase-1 promotes cellular damage and differentiation of pluripotent stem cells [21]. When the cells are induced apoptosis or autophagy cytotoxic macromolecules are eliminated, e.g. caspase (30–60 kDa) or cytochrome c (12–15 kDa) [22], [23]. To avoid the effect those macromolecules, MWCO of dialysis membrane should be increased or further refinement to remove cytotoxic macromolecule is necessary.
Fig. 4.
Differentiated cell number on day 4. Data represents mean ± SD of n = 5–9 from two to four independent experiments (**P < 0.01).
3.3. Gene expression of differentiated hiPSCs aggregates
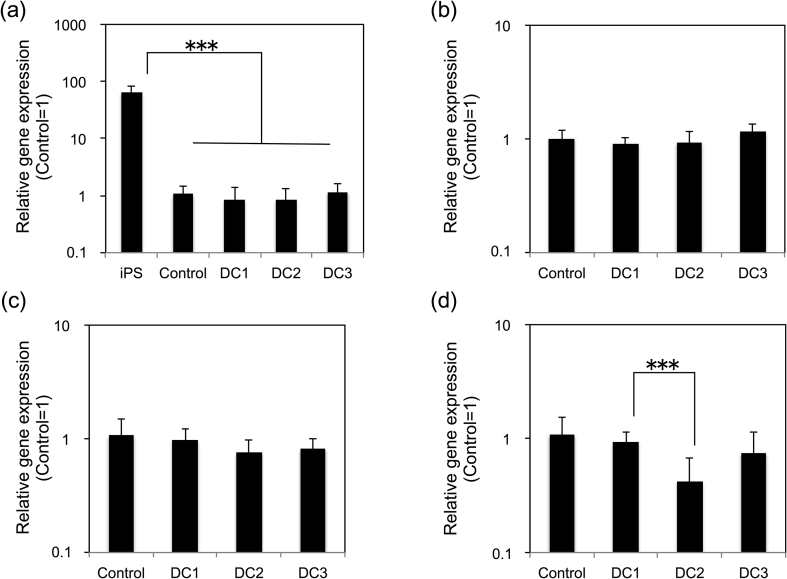
The gene expression of differentiated hiPSCs aggregates was evaluated (Fig. 5). There were no statistical differences between all conditions; Control, DC1, DC2, and DC3 for the gene expression of OCT4, SOX17 and FOXA2. OCT4 expression of differentiated hiPSCs aggregates were statistically lower than hiPSCs sampled right before starting differentiation, which suggests that the hiPSCs aggregates were successfully differentiation, whereas HNF4α expression of DC2 was lower than DC1. The transition of FOXA2 and SOX17 expression is similarly increased at the early stage of endodermal differentiation [24], [25]. The HNF4a expression starts to increase behind the expression of FOXA2 and SOX17 during primitive gut tube development [26]. The decreased expression of HNF4α in DC2 suggested that the excess amount of Activin A inhibited the differentiation. DC3 was similarly differentiated as the Control and DC1 according to the gene expression even if Activin A was not added for two days. Therefore, it was suggested that dialysis culture enabled Activin A usage reduction by recycling of retaining cytokines and autocrine factors.
Fig. 5.
Relative gene expression levels between each culture condition. (a) OCT4, (b) SOX17, (c) FOXA2, and (d) HNF4α. Data represents mean ± SD of n = 5–11 from two to four independent experiments (***P < 0.001).
4. Conclusion
We developed a plate-based simple dialysis culture system for a differentiation of hiPSCs into definitive endodermal lineage. The dialysis culture refined the differentiation medium, which achieved the increase of differentiated cells. We also demonstrated that the reduction of Activin A usage didn't no difference on the gene expression related to definitive endoderm and primitive gut tube markers. The retaining of cytokines and autorcrine factor enabled to promote definitive endodermal differentiation without daily Activin A addition. This study will be expected to contribute to improve the manufacturability of hiPSCs differentiated into endodermal lineage with medium refinement and recycling autocrine factors as well as cytokines.
Author contributions
M.S., H.C., M.I. and Y.S. conceived and designed the study. M.S., H.C. and M.I. conducted the experiments. H.C. and M.S. provided all the figures. M.S., H.C., M.I. and SG.Y acquired the hiPS culture and differentiation technique. M.S., H.C., M.I, SG.Y, H.O, A.M, and Y.S interpreted and analyzed the data. M.S., and H.C. drafted the manuscript. M.S., H.C., M.I, SG.Y, H.O, A.M, and Y.S revised the manuscript.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgment
This work was supported by Japan Agency for Medical Research and Development (AMED, the project of “Center for the development of methods for next-generation pancreatic islet transplantation using iPS cells”).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2019.05.004.
Contributor Information
Marie Shinohara, Email: marie-s@iis.u-tokyo.ac.jp, marie-s@chemsys.t.u-tokyo.ac.jp.
Hyunjin Choi, Email: choihezz@iis.u-tokyo.ac.jp, choihezz@chemsys.t.u-tokyo.ac.jp.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Street C.N., Lakey J.R.T., Shapiro A.M.J., Imes S., Rajotte R.V., Ryan E.A. Implications for predicting long-term clinical outcome. Diabetes. 2004;53:3107–3114. doi: 10.2337/diabetes.53.12.3107. [DOI] [PubMed] [Google Scholar]
- 2.Kwok C.K., Ueda Y., Kadari A., Günther K., Ergün S., Heron A. Scalable stirred suspension culture for the generation of billions of human induced pluripotent stem cells using single-use bioreactors. J Tissue Eng Regen Med. 2018;12:e1076–e1087. doi: 10.1002/term.2435. [DOI] [PubMed] [Google Scholar]
- 3.Massai D., Bolesani E., Diaz D.R., Kropp C., Kempf H., Halloin C. Sensitivity of human pluripotent stem cells to insulin precipitation induced by peristaltic pump-based medium circulation: considerations on process development. Sci Rep. 2017;7:1–15. doi: 10.1038/s41598-017-04158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X., Chen A., Woo T.L., Choo A.B.H., Reuveny S., Oh S.K.W. Investigations into the metabolism of two-dimensional colony and suspended microcarrier cultures of human embryonic stem cells in serum-free media. Stem Cells Dev. 2010;19:1781–1792. doi: 10.1089/scd.2010.0077. [DOI] [PubMed] [Google Scholar]
- 5.Varum S., Rodrigues A.S., Moura M.B., Momcilovic O., Easley I.V.C.A., Ramalho-Santos J. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. 2011;6:e20914. doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moussaieff A., Rouleau M., Kitsberg D., Cohen M., Levy G., Barasch D. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 2015;21:392–402. doi: 10.1016/j.cmet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Meseguer-Ripolles J., Lucendo-Villarin B., Wang Y., Hay D.C. Semi-automated production of hepatocyte like cells from pluripotent stem cells. J Vis Exp. 2018:1–7. doi: 10.3791/57995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamoto R., Takayama K., Akita N., Nagamoto Y., Hosokawa D., Iizuka S. Human iPS cell-based liver-like tissue engineering at extrahepatic sites in mice as a new cell therapy for hemophilia B. Cell Transpl. 2018;27:299–309. doi: 10.1177/0963689717751734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yabe S.G., Fukuda S., Takeda F., Nashiro K., Shimoda M., Okochi H. Efficient generation of functional pancreatic β-cells from human induced pluripotent stem cells. J Diabetes. 2017;9:168–179. doi: 10.1111/1753-0407.12400. [DOI] [PubMed] [Google Scholar]
- 10.Musah S., Dimitrakakis N., Camacho D.M., Church G.M., Ingber D.E. Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a Glomerulus chip. Nat Protoc. 2018;13:1662–1685. doi: 10.1038/s41596-018-0007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabeya T., Qiu S., Hibino M., Nagasaki M., Kodama N., Iwao T. Cyclic AMP signaling promotes the differentiation of human induced pluripotent stem cells into intestinal epithelial cells. Drug Metab Dispos. 2018;46:1411–1419. doi: 10.1124/dmd.118.082123. [DOI] [PubMed] [Google Scholar]
- 12.Ramos-Mejia V., Melen G.J., Sanchez L., Gutierrez-Aranda I., Ligero G., Cortes J.L. Nodal/activin signaling predicts human pluripotent stem cell lines prone to differentiate toward the hematopoietic lineage. Mol Ther. 2010;18:2173–2181. doi: 10.1038/mt.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He Z., Jiang J., Kokkinaki M., Dym M. Nodal signaling via an autocrine pathway promotes proliferation of mouse spermatogonial stem/progenitor cells through smad2/3 and oct-4 activation. Stem Cells. 2000;27:2580–2590. doi: 10.1002/stem.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Come J., Nissan X., Aubry L., Toumois J., Girard M., Perrier A.L. Improvement of culture conditions of human embryoid bodies using a controlled perfused and dialyzed bioreactor system. Tissue Eng Part C. 2008;14:289–298. doi: 10.1089/ten.tec.2008.0029. [DOI] [PubMed] [Google Scholar]
- 15.Nath S.C., Nagamori E., Horie M., Kino-oka M. Culture medium refinement by dialysis for the expansion of human induced pluripotent stem cells in suspension culture. Bioprocess Biosyst Eng. 2017;40:123–131. doi: 10.1007/s00449-016-1680-z. [DOI] [PubMed] [Google Scholar]
- 16.Tomizawa M., Shinozaki F., Sugiyama T., Yamamoto S., Sueishi M., Yoshida T. Activin A maintains pluripotency markers and proliferative potential of human induced pluripotent stem cells. Exp Ther Med. 2011;2:405–408. doi: 10.3892/etm.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antsiferova M., Werner S. The bright and the dark sides of activin in wound healing and cancer. J Cell Sci. 2012;125:3929–3937. doi: 10.1242/jcs.094789. [DOI] [PubMed] [Google Scholar]
- 18.Lim R., Mulijadi R., Koulaeva E., Vosdoganes P., Chan S.T., Acharya R. Activin A contributes to the development o hypoxia-induced lung injury in neonatal mice. Pediatr Res. 2015;77:749–756. doi: 10.1038/pr.2015.46. [DOI] [PubMed] [Google Scholar]
- 19.Mayer K., Morty R.E. Activin A: a mediator governing inflammation, immunity, and repair. Am J Respir Crit Care Med. 2012;185:350–352. doi: 10.1164/rccm.201112-2210ED. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J., Nuebel E., Daley G.Q., Koehler C.M., Teitell M.A. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell. 2012;11:589–595. doi: 10.1016/j.stem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin C.-Y., Peng C.-Y., Huang T.-T., We M.-L., Lai Y.-L., Peng D.H. Exacerbation of oxidative stress-induced cell death and differentiation in induced pluripotent stem cells lacking heme oxygenase-1. Stem Cells Dev. 2012;21:1675–1687. doi: 10.1089/scd.2011.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroemer G., Martin S.J. Caspase-independent cell death. Nat Med. 2005;11:725–730. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 23.Vessoni A.T., Muotri A.R., Okamoto O.K. Autophagy in stem cell maintenance and differentiation. Stem Cells Dev. 2012;21:513–520. doi: 10.1089/scd.2011.0526. [DOI] [PubMed] [Google Scholar]
- 24.Tsankov A.M., Gu H., Akopian V., Ziller M.J., Donaghey J., Amit I. Transcription factor binding dynamics during human ES cell differentiation. Nature. 2015;518:344–349. doi: 10.1038/nature14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiyaboonchai A., Cardenas-Diaz F.L., Ying L., Maguire J.A., Sim X., Jobaliya C. GATA6 plays an important role in the induction of human definitive endoderm, development of the pancreas, and functionality of pancreatic β cells. Stem Cell Rep. 2017;8:589–604. doi: 10.1016/j.stemcr.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennarossa G., Maffei S., Campagnol M., Rahman M.M., Brevini T.A.L., Gandolfi F. Reprogramming of pig dermal fibroblast into insulin secreting cells by a brief exposure to 5-aza-cytidine. Stem Cell Rev Rep. 2014;10:31–43. doi: 10.1007/s12015-013-9477-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.