Abstract
Introduction
Venous thromboembolism (VTE) is a life-threatening complication of anti-neutrophil cytoplasmic autoantibody (ANCA) vasculitis whose mechanism remains incompletely elucidated. We tested the hypothesis that elevated microparticle tissue factor activity (MPTFa) or anti-plasminogen antibodies (anti-Plg) may identify patients at risk for VTE.
Methods
In this prospective study, patients were enrolled during active disease and followed longitudinally. Twelve patients who experienced a VTE (VTEpos) were compared with patients without VTE (VTEneg, n = 29) and healthy controls (HC, n = 70). MPTFa, anti-Plg, interleukin-6, high-sensitivity C-reactive protein (hs-CRP), D-dimer, serum creatinine, and serum albumin were assessed. Fisher’s exact tests and Wilcoxon tests compared categorical and continuous variables, respectively. Cox regression for time to VTE or last follow-up was performed.
Results
VTEpos patients had higher MPTFa (peak median = 14.0, interquartile range = 4.3–36.6) than HC (0, 0–3.5) and VTEneg patients (0, 0–1.4). In time-to-event analysis, MPTFa was associated with VTE when measured during both active disease (hazard ratio [HR]; 95% confidence interval [CI]: 1.04; 1.01–1.08) and remission (1.4; 1.11–1.77). Anti-Plg during remission was also associated with VTE (1.17; 1.03–1.33). Each g/dl decrease of serum albumin was associated with a 4-fold increase in VTE risk (4.4; 1.5–12.9). Adjusting for estimated glomerular filtration rate (eGFR), anti-Plg during remission remained significantly associated with VTE.
Conclusion
Elevated MPTFa and increased anti-Plg in remission are strong indicators of VTE independent of renal function. Association of anti-Plg during remission with VTE implies hypercoagulability even during disease quiescence. Hypoalbuminemia strongly portends VTE risk, which is a novel finding in ANCA vasculitis. A thrombotic signature would allow improved management of patients to minimize VTE risk and complications of anticoagulation.
Keywords: autoimmunity, biomarkers, plasminogen, thrombosis, tissue factor, venous thrombosis
VTE is a complication of ANCA vasculitis that affects 8% to 16% of patients and confers significant morbidity and risk of mortality.1, 2, 3, 4 Mechanisms driving the high incidence of VTE in ANCA vasculitis are not fully understood. Moreover, current biomarkers of VTE lack specificity.
Anti-Plg antibodies potentially contribute to VTE by delaying conversion of plasminogen to plasmin, thus prolonging the dissolution time of fibrin clots and inhibiting fibrinolysis.5 Bautz and colleagues5 found that half of proteinase 3 (PR3)–ANCA patients who developed VTE were positive for anti-Plg. However, an association between anti-Plg and VTE in patients with ANCA vasculitis has not been investigated prospectively.
Microparticles (MPs) and tissue factor (TF)–bearing microparticles have been implicated as risk factors for VTE in multiple clinical settings.6, 7, 8, 9, 10, 11, 12, 13 MPs are increased in patients with ANCA vasculitis during active disease.14, 15, 16, 17 Neutrophils treated with ANCA after priming with C5a or tumor necrosis factor-α release TF-bearing MPs that increase thrombin generation in vitro.18, 19 However, the contribution of monocyte-derived TF and association of MPTFa with VTE in patients with ANCA vasculitis has not been explored.
Because VTEs tend to occur during active disease,1 we followed patients with active disease longitudinally, and prospectively measured anti-Plg, D-dimer, and C-reactive protein (CRP). Due to a putative role in thrombosis, MPTFa and interleukin (IL)-620 also were measured. We aimed to determine the role these markers of disease activity and inflammation play in development of VTE in ANCA vasculitis and to elucidate a thrombotic signature that may identify patients at increased risk for VTE.
Methods
Cohort
A prospective longitudinal study was designed to determine if anti-Plg was associated with VTE in ANCA vasculitis. We aimed to enroll 60 patients at high risk for VTE based on historical estimates of willing participants with active disease, anti-Plg positivity, and elevated D-dimer. Fifty-eight patients with active disease (Birmingham Vasculitis Activity Score [BVAS] ≥2) were screened for D-dimer and anti-Plg from 2010 to 2015 at the University of North Carolina at Chapel Hill (UNC) (Figure 1a). All patients had ANCA vasculitis as defined by Chapel Hill Consensus Conference nomenclature21 and a positive enzyme-linked immunosorbent assay (ELISA) for myeloperoxidase (MPO)- or PR3-ANCA at or before study screening. Patients with overlap disease, drug-induced ANCA vasculitis, eosinophilic granulomatosis with polyangiitis, or on dialysis were excluded (n = 22). All study patients (n = 36) were followed longitudinally by serial testing of anti-Plg, D-dimer, and hs-CRP with a primary outcome of VTE. Patients positive for anti-Plg or elevated D-dimer with a Well’s score ≥222 underwent lower extremity compression ultrasonography. Five additional patients diagnosed with VTE during the study period were identified through our Clinical Core and included retrospectively because appropriate samples were available due to the longitudinal nature of our Clinical Core biorepository; samples were obtained when patients were seen in the clinic every 3 to 6 months (Figure 1a). Patients who experienced VTE were defined as VTEpos; patients who did not were designated VTEneg. The sample date associated with the highest BVAS surrounding the time of initial screening (6 months before or after) was used as the active disease time-point for all analytes. The first BVAS = 0 without active disease within 3 months was used as the remission sample time-point. In addition, stored patient samples were tested for IL-6 and MPTFa as potentially relevant biomarkers of thrombosis. HCs were tested for anti-Plg, IL-6, and MPTFa; not all controls were tested for each analyte. The study was approved by the UNC Institutional Review Board. Informed consent was obtained from all patients and HCs.
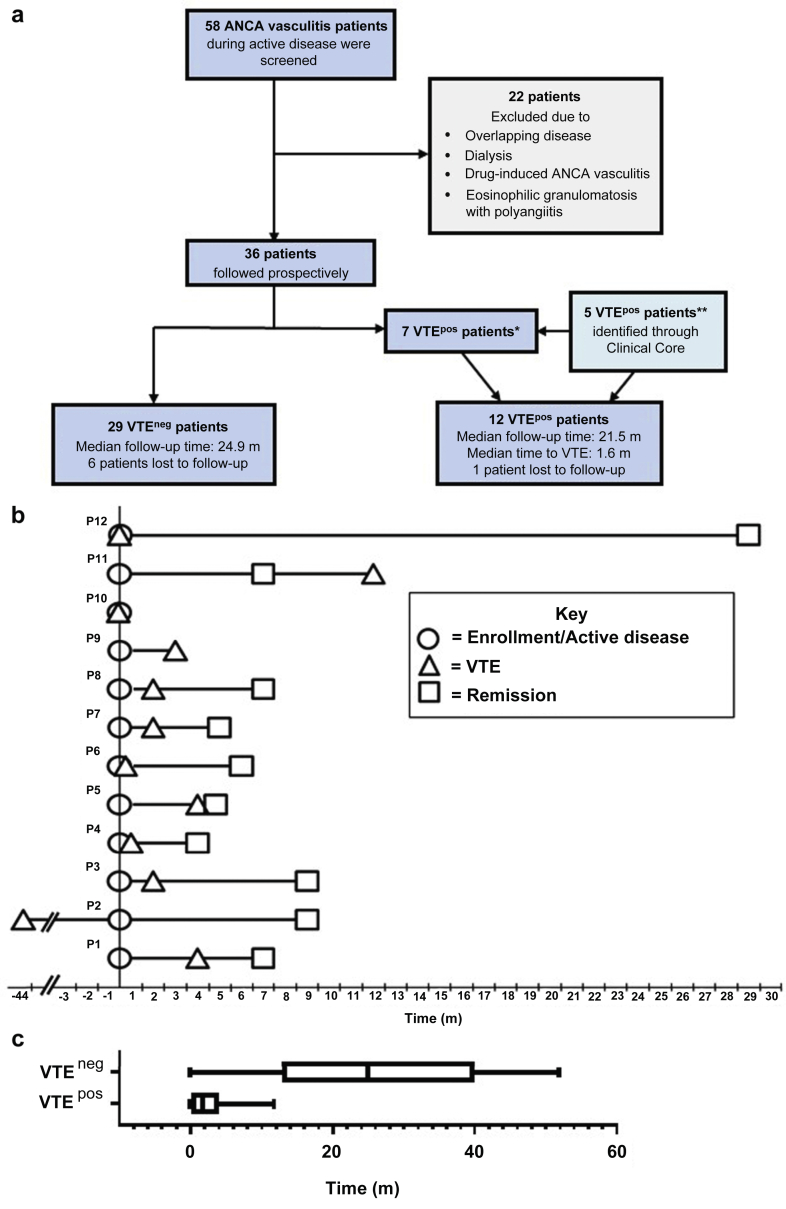
Figure 1.
Patient population and timeline of disease activity and venous thromboembolism (VTE). (a) Flow diagram depicting patient screening, enrollment, and follow-up. VTEneg patients did not experience a VTE during the study, VTEpos patients did experience a VTE during the study. *One VTEpos patient had a remote VTE but was included in the VTEpos group except for time-to-event analyses. **These patients were not screened for enrollment but appropriate samples were available due to the longitudinal nature of our Clinical Core. (b) Diagram depicting timeline of active disease, VTE, and remission dates for VTEpos patients. Time 0 is the enrollment or active disease date. ○ is the enrollment or active disease sample, Δ is the date of the VTE, and □ is the remission sample. Patients 8 through 12 were added retrospectively. (c) Box and whiskers plot showing the follow-up time for VTEneg patients (median 24.9 month [m]) and time to VTE for the VTEpos patients (1.6 m). ANCA, anti-neutrophil cytoplasmic autoantibody; avg, average; neg, negative; pos, positive.
Clinical Information and Research Tests
Clinical characteristics were assessed during active disease. Medications and organ involvement were obtained from electronic medical records. Standard-of-care clinical laboratory tests for serum albumin, serum creatinine, platelet number, hemoglobin, proteinuria, and ANCA were performed by UNC Hospitals McLendon Laboratories. eGFR was calculated using the 4-variable Modification of Diet in Renal Disease equation.23, 24 ANCA titers >20 U/ml (INOVA [San Diego, CA] QUANTA Lite MPO and PR3) were considered positive. D-dimer and hs-CRP were performed by UNC Hospital Laboratories. Positive D-dimer was defined as >229 ng/ml. The lower limit of detection for D-dimer is 150 ng/ml, and for continuous analyses we defined these values as 149 ng/ml. Hs-CRP values >10 mg/l indicate active inflammation. IL-6 levels were determined using stored serum and plasma samples following manufacturer’s protocol in a commercial IL-6 ELISA (R&D Systems, Minneapolis, MN). For IL-6, values >1.57 pg/ml were considered positive based on the mean plus 2 SDs of 20 HCs.
Anti-Plg were detected by ELISA using the native glutamic acid isomer of plasminogen (Haematologic Technologies, Essex Junction, VT) as described previously5 with some modifications. To decrease nonspecific background of the assay, glutamic acid isomer of plasminogen was depleted of contaminating IgG with Protein AG beads (Santa Cruz Biotechnology, Inc., Dallas, TX) before use as the coating protein of the ELISA. Ninety-six well white high-binding ELISA microplates (Greiner Bio-One, Monroe, NC) were coated with glutamic acid isomer of plasminogen at 5 μg/ml in 0.05M carbonate-bicarbonate coating buffer overnight at 4 °C. The next day, the plates were washed with Tris Buffered Saline with 0.1% Tween-20 and blocked with 1% goat serum in Tris Buffered Saline, 0.05% Tween-20, and 0.05% sodium azide (blocking buffer) for 1 hour. The plates were incubated with patient, HC, and 2 historical positive control sera diluted 1:800 in the blocking buffer in duplicate for 3 hours at room temperature or overnight at 4 °C. The plates were then incubated with an alkaline phosphatase conjugated goat anti-human IgG (Invitrogen, Carlsbad, CA) diluted 1:20,000 in blocking buffer. We used a Chemiluminescence detection (Sapphire-II Enhancer; Thermo Fisher Scientific, Waltham, MA; CDP-Star, Roche Applied Science, Indianapolis, IN) method to improve the signal-to-noise ratio and sensitivity of the ELISA. Based on the mean plus 2 SDs of 56 HC samples, the cutoff for positivity was established as 21.7% of the positive control (average of 2 historically positive samples included on each assay).
Microparticle TF Activity Assay
Citrated whole blood from patients and HCs was centrifuged within 1 hour after collection (2500g, 15 minutes, twice) to yield platelet-free plasma, then frozen at −80 °C until use (as recommended by the Scientific Collaborative Workshop of the International Society of Thrombosis and Haemostasis).25 MPs were isolated from platelet-free plasma by centrifugation (20,000g, 30 minutes, 4 °C). MPTFa was determined as described previously.26, 27, 28 In this kinetic assay, TF-dependent Factor Xa generation was determined by subtracting Factor Xa generation in the presence of anti-human TF from Factor Xa generation in the presence of control mouse IgG. TF concentration was determined by comparison with relipidated recombinant human TF (Innovin, Dade Behring; Siemens, Newark, DE). Unstimulated and LPS-stimulated platelet-free plasma from a healthy donor were included on each plate as negative and positive controls; values were expressed as a percentage of this positive control.
Flow Cytometry
To determine baseline TF on leukocytes, whole blood from 10 HCs, 6 VTEneg, and 6 VTEpos patients was stained with CD14-APC (clone 61D3), CD16-FITC (clone eBio6B16), and CD142-PE (clone HTF-1) antibodies (eBioscience, San Diego, CA). CD14 only and CD16 only samples were used to set proper positive/negative gates. Cells stained with all antibodies except CD142, a fluorescence minus 1 control, was used to set the negative gate and determine the threshold for TF positivity. Cells were examined by flow cytometry on a Becton Dickinson (Franklin Lakes, NJ) LSRII flow cytometer and data analyzed using FlowJo 10 (Tree Star, Ashland, OR). The percent TF-positive CD14+ monocytes, CD14+CD16+ inflammatory monocytes, and CD16+ neutrophils were determined.
Statistical Analysis
Continuous variables were expressed as medians with interquartile range; categorical variables were expressed as counts (n) and percentage (%). Wilcoxon 2-sample tests or Kruskal-Wallis tests for continuous variables were compared across 2 or 3 groups, respectively. Fisher exact tests were used to compare groups for categorical measures. A signed-rank test was used for paired measures at active disease and remission within patients. Time-to-event analysis was performed by Cox regression to discern an HR of VTE associated with biological markers. Limited multivariable modeling of several statistically significant measures was done to evaluate independent associations of biomarkers, with only 2 variables in any given model at one time due to small sample size. Pearson correlation analysis measured the relationship of anti-Plg remission values from different time-points. Statistical analyses were conducted by SAS software (Version 9.4; SAS Institute, Cary, NC) and GraphPad Prism (Version 7.04; GraphPad Software, Inc., La Jolla, CA).
Results
Baseline Characteristics
Forty-one patients with ANCA vasculitis (29 VTEneg and 12 VTEpos) and 70 HCs were included (Figure 1a). Eleven VTEpos patients experienced a VTE during the study period. One patient in the study did not experience a VTE during the study period but is classified as VTEpos due to a remote VTE (Figure 1b). Patients and controls did not differ in sex or race. HCs were younger (median = 36 years, interquartile range = 25–56) than patients (56, 45–70; P = 0.0001; Supplementary Table S1). However, the only analyte measured in this study that correlated with age was IL-6 (Supplementary Figure S1).
VTEpos and VTEneg patients were similar in age, sex, race, and distribution of MPO- and PR3-ANCA serotypes (Table 1). Comparable percentages of VTEpos and VTEneg patients were captured at new-onset disease and relapse. At presentation, skin involvement was more common among VTEpos patients (9 of 12 = 75%) compared with VTEneg patients (7 of 29 = 24%, P = 0.004). Patient groups did not differ in other organ involvement, eGFR, proteinuria, platelet count, or hemoglobin concentration. Serum albumin was lower in VTEpos patients (2.9 g/dl, 2.6–3.5) than VTEneg patients (3.8, 3.5–4; P = 0.04). Median time of longitudinal follow-up was similar between the 2 groups. Of the 7 patients lost to follow-up, 1 (8%) was a VTEpos patient and 6 (21%) were VTEneg patients (Figure 1a). More than 90% of the VTEneg patients had longer longitudinal follow-up time than the median time to VTE (1.6 months; Figure 1c).
Table 1.
Patient characteristics at active disease
| Variablea | VTEneg n = 29 |
VTEpos n = 12 |
P valueb |
|---|---|---|---|
| Age, yr, median (interquartile range) | 56 (45, 69) | 62 (51, 77) | 0.28 |
| Sex, male, n (%) | 11 (38) | 8 (67) | 0.17 |
| Race, W, n (%) | 25 (86) | 10 (83) | 1.00 |
| MPO-ANCA serotype, n (%) | 11 (38) | 4 (33) | 1.00 |
| New-onset active sample, n (%) | 11 (38) | 4 (33) | 1.00 |
| Organ involvement, n (%) | – | – | – |
| ENT | 22 (76) | 9 (75) | 1.00 |
| Eye | 12 (41) | 3 (25) | 0.48 |
| Pulmonary | 20 (69) | 9 (75) | 1.00 |
| Renal | 26 (90) | 12 (100) | 0.54 |
| Pulmonary and renal | 17 (59) | 9 (75) | 0.48 |
| Skin | 7 (24) | 9 (75) | 0.004 |
| Neurology | 10 (35) | 2 (17) | 0.45 |
| Musculoskeletal | 17 (59) | 7 (58) | 1.00 |
| Joints | 17 (59) | 7 (58) | 0.73 |
| Gastrointestinal | 3 (10) | 0 (0) | 0.54 |
| Serum albumin (g/dl) | 3.8 (3.5, 4) [n = 16] | 2.9 (2.6, 3.5) [n = 8] | 0.04 |
| UPCR (mg/mg) | 0.5 (0.1, 1.1) [n = 19] | 1 (0.5, 2.8) [n = 7] | 0.27 |
| Serum creatinine (mg/dl) | 1.3 (0.9, 2.3) [n = 26] | 1.8 (1.4, 2.7) | 0.20 |
| Estimated GFR (ml/min per 1.73 m2) | 52 (27, 61) | 35 (24.5, 47) | 0.21 |
| Platelet count (109/l) | 319 (239, 472) [n = 26] | 291 (237, 379) | 0.48 |
| Hemoglobin (g/dl) | 11.8 (10.5, 13.6) [n = 26] | 11.3 (9.7, 12.6) | 0.36 |
ANCA, anti-neutrophil cytoplasmic autoantibodies; ENT, ear, nose and throat; GFR, glomerular filtration rate calculated by Modification of Diet in Renal Disease equation; MPO, myeloperoxidase; UPCR, urine protein to creatinine ratio (mg); VTE, venous thromboembolism; W, white.
Data are presented as median (interquartile range) for continuous variables and n (%) for categorical data. Variables with missing values are noted as [different n].
P values were calculated using a Wilcoxon 2-sample test or Fisher exact test.
The use of anticoagulants (aspirin, unfractionated heparin, warfarin, and low molecular weight heparin) in VTEpos patients before the thrombotic event did not differ from VTEneg patients (Supplementary Table S2). Immunosuppressive therapy was similar in VTEpos and VTEneg patients (Supplementary Table S3). Therapeutic plasma exchange was more prevalent in VTEpos (50%) than VTEneg patients (10%; Supplementary Table S3, P = 0.01); however, disease severity did not vary with VTE status based on BVAS, eGFR, or pulmonary and/or renal involvement (Table 1).
Putative Biomarkers of VTE Correlate With Disease Activity
Putative markers of VTE were first examined during active ANCA vasculitis when VTEs most often occur and compared with a time of disease quiescence. Patients had higher anti-Plg levels (% positive control) in active disease (17.8% positive control, 10.1–22.8) than during remission (10.0, 7.01–13.8; P = 0.0003; Table 2) and compared with HCs (12.4, 10.4–16.4; P = 0.04; Figure 2a). This difference was emphasized in paired active and remission samples (P < 0.0001; Figure 2b). The association of anti-Plg with disease activity was observed in MPO- (P = 0.006) and PR3-ANCA patients (P = 0.0006; Supplementary Figure S2).
Table 2.
Comparison of biomarkers according to disease activity
| Variable | Disease activity |
P value | |
|---|---|---|---|
| Active n = 41 |
Remission n = 30 |
||
| BVAS | 9 (6, 13) | 0 (0, 0) | <0.0001 |
| Anti-Plg (%Pos Co) | 17.8 (10.1, 22.8) | 10 (7, 13.8) | 0.0003 |
| D-dimer (ng/ml) | 493 (183, 1116) [n = 29] | 149 (149, 170) [n = 16] | 0.0004 |
| hs-CRP (mg/l) | 8.6 (3.5, 16) [n = 24] | 6.4 (4, 8.2) [n = 13] | 0.36 |
| IL-6 (pg/ml) | 2.5 (1.6, 6.5) [n = 28] | 1.99 (1.33, 3.03) [n = 22] | 0.27 |
| MPTFa (%Pos Co) | 0 (0, 1.8) [n = 23] | 0 (0, 1.7) [n = 19] | 0.82 |
%Pos Co, % positive control; anti-Plg, anti-plasminogen; BVAS, Birmingham Vasculitis Activity Score; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; MPTFa, microparticle tissue factor activity.
Data are presented as median (interquartile range). P values were calculated using a Wilcoxon 2-sample test. Variables with missing values are noted as [different n].
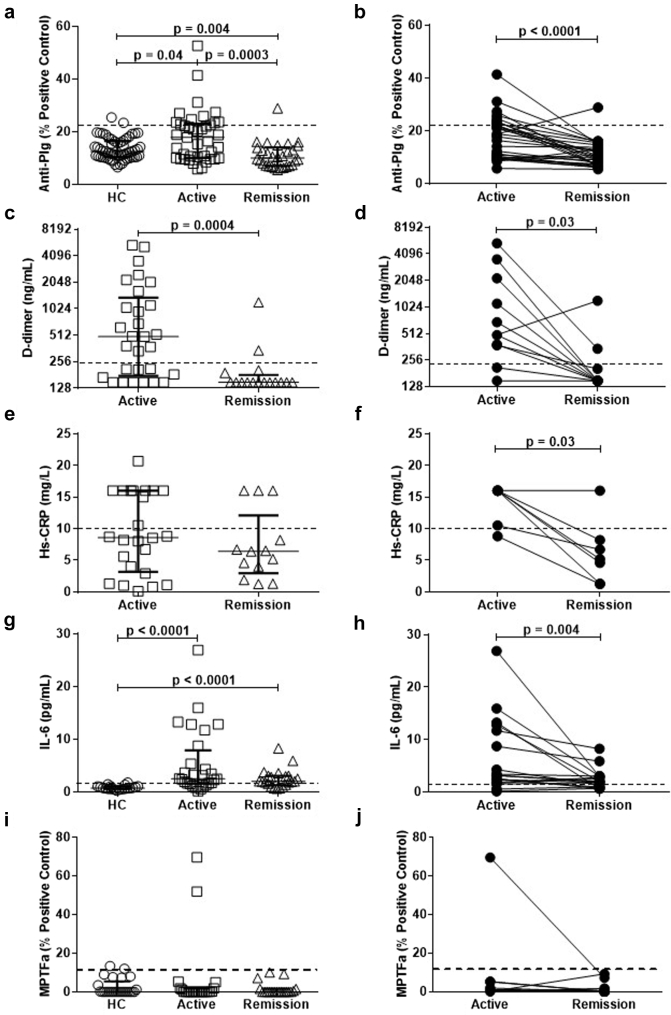
Figure 2.
Putative biomarkers of venous thromboembolism correlate with disease activity. The dashed line represents the threshold for positivity in (a–h), as determined by the mean of healthy control values +2 SDs or values used for reporting clinical laboratory results. (a) Anti-plasminogen (Anti-Plg) were tested in 56 healthy controls (HCs), 41 patients were tested in active disease, and 30 patients were tested in remission. Patients in active disease had higher levels of anti-Plg than HCs and patients in remission. (b) Thirty paired active and remission samples emphasize the decrease in anti-Plg from active disease to remission. (c) Patients in active disease (n = 29) have significantly higher levels of D-dimer than patients in remission (n = 16). (d) Paired active and remission samples confirm that patients in remission have decreased levels of D-dimer (n = 11). (e) High-sensitivity C-reactive protein (hs-CRP) in a group of 24 patients did not differ from 13 patients in remission (P = 0.36). (f) In 6 of 7 patients with active and remission paired samples, patients in remission had lower levels of hs-CRP. (g) Patients in active disease (n = 28) and patients in remission (n = 22) have significantly higher levels of interleukin 6 (IL-6) than HCs (n = 20). (h) In 18 paired samples tested for IL-6, patients in active disease had significantly higher levels of IL-6 than during remission. (i) Microparticle tissue factor activity (MPTFa) tested in 25 HCs, 23 patients tested in active disease, and 19 patients tested in remission showed no difference between HCs and active patients (P = 0.81), active and remission patients (P = 0.82), or HCs and patients in remission (P = 0.67). (j) No significant difference in paired active and remission samples from 15 patients was observed (P = 0.46).
Patients with active disease had elevated D-dimer levels (493 ng/ml, 183–1116) compared with those in remission (149, 149–170; P = 0.0004; Figure 2c); this was confirmed with paired active and remission samples (P = 0.03; Figure 2d). Comparing unpaired samples from patients experiencing active disease (8.6 mg/l, 3.5–16) and patients in remission, no difference in hs-CRP (6.4, 4–8.2; P = 0.36; Table 2; Figure 2e) was detected. However, considering paired samples, a decrease in hs-CRP (P = 0.03; Figure 2f) was observed.
In addition, IL-6 and MPTFa were tested on patient samples due to their contemporary relevance to thrombosis. IL-6 was elevated in both active disease and remission compared with HCs (P < 0.0001; Figure 2g). In paired samples, a marked decrease in IL-6 (P = 0.004; Figure 2h) from active disease to remission was detected. No difference in MPTFa values (Figure 2i) was observed between active patients and HCs or patients in remission. In available paired active and remission samples, no difference in MPTFa levels was discernable (P = 0.46; Figure 2j).
Biomarker Comparison in Patients With and Without VTE at Active Disease
We then investigated whether elevated levels of biomarkers during active disease differed in patients with and without VTE. We detected no differences in anti-Plg, D-dimer, IL-6, or MPTFa between VTEpos and VTEneg patients during active disease (Supplementary Figure S3A, B, D, and E, respectively). Interestingly, hs-CRP was increased in VTEpos (16 mg/ml, 12–16; Supplementary Figure 3C) compared with VTEneg patients (7.4, 2.1–9.6; P = 0.02).
Anti-Plg Prevalence in VTEpos Patients
To further elucidate the correlation between these markers and VTE, we evaluated the highest measured values for each biomarker (peak) to determine if they ever differed between VTEpos and VTEneg patients regardless of disease activity. Peak levels of anti-Plg in VTEpos (21.3% positive control, 13.1–28.9; P = 0.005; Figure 3a) and VTEneg patients (17%, 13.8–23.6; P = 0.0006) were higher than in HCs. VTEpos and VTEneg patients did not differ (P = 0.5, Table 3). Of 12 VTEpos patients, 6 (50%) were positive for anti-Plg compared with 11 of 29 (38%) VTEneg patients (Supplementary Figure S4A). Three VTEpos patients were positive for anti-Plg before VTE (Figure 4a).
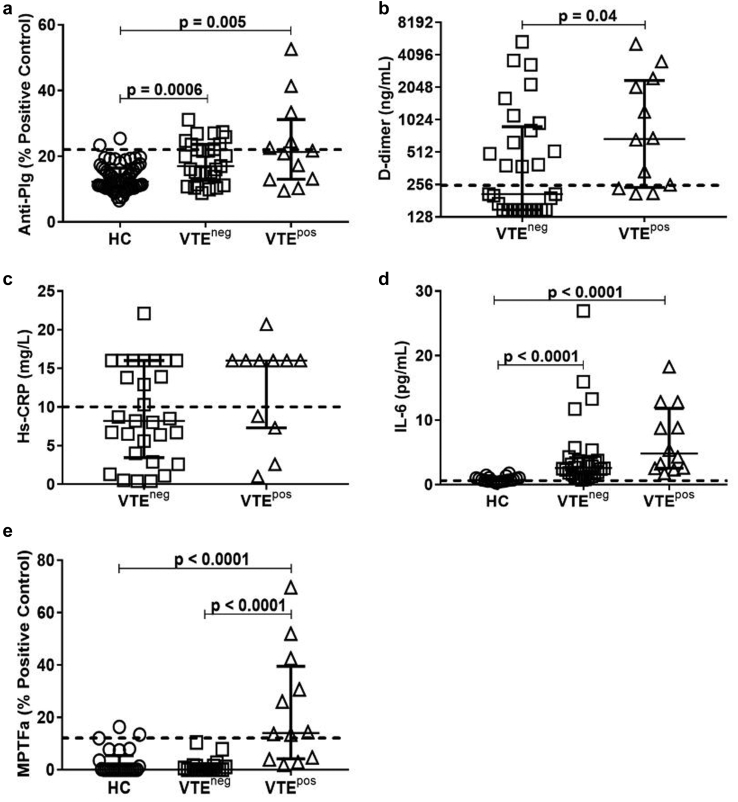
Figure 3.
Comparison of candidate biomarkers in patients with and without venous thromboembolism (VTE). (a) Comparing peak values, VTEpos and VTEneg patients have significantly higher levels of anti-plasminogen (anti-Plg) than healthy controls (HCs). (b) VTEpos patients (n = 12) had significantly higher peak levels of D-dimer than VTEneg patients (n = 29). (c) There was no difference in peak high-sensitivity C-reactive protein (hs-CRP) in VTEpos (n = 11) and VTEneg patients (n = 29, P = 0.1). (d) VTEpos (n = 12) and VTEneg patients (n = 28) had significantly higher peak levels of interleukin 6 (IL-6) than HCs (n = 20). (e) Peak microparticle tissue factor activity (MPTFa) was significantly higher among VTEpos patients (n = 12) than VTEneg patients (n = 21) or HCs (n = 25), whereas peak MPTFa values were similar in VTEneg patients when compared with HCs (P = 0.88). The dashed line represents the threshold for positivity, as determined by the mean of HC values + 2 SDs or values used for reporting clinical laboratory results. neg, negative; pos, positive.
Table 3.
Comparison of biomarkers according to VTE status
| Variable | VTE status |
P value | |
|---|---|---|---|
| VTEneg n = 29 |
VTEpos n = 12 |
||
| BVAS | 12 (8, 13) | 13.5 (7.5, 19.5) | 0.3 |
| Anti-Plg (%Pos Co) | 17 (13.8, 23.6) | 21.3 (13.1, 28.9) | 0.5 |
| D-dimer (ng/ml) | 208 (149, 811) | 677 (245, 2263) | 0.04 |
| hs-CRP (mg/l) | 8.2 (4, 16) | 16 (7.3, 16) [n = 11] | 0.1 |
| IL-6 (pg/ml) | 2.6 (1.6, 4) [n = 28] | 4.8 (2.5, 11) | 0.06 |
| MPTFa (%Pos Co) | 0 (0, 1.4) [n = 21] | 14 (4.3, 36.6) | <0.0001 |
%Pos Co, % positive control; BVAS, Birmingham Vasculitis Activity Score; anti-Plg, anti-plasminogen; hs-CRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; MPTFa, microparticle tissue factor activity; VTE, venous thromboembolism.
Data are presented as median (interquartile range) of highest values measured (peak values). P values were calculated using a Wilcoxon 2-sample test. Variables with missing values are noted as [different n].
Figure 4.
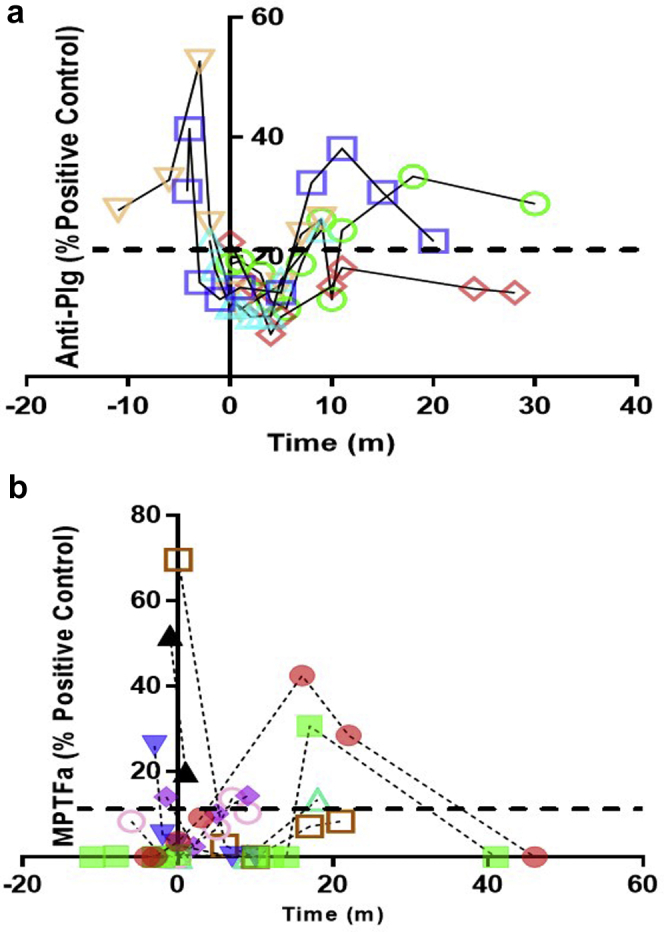
Longitudinal analysis of anti-Plg and MPTFa. (a) Shown are longitudinal samples from venous thromboembolism (VTE)pos patients tested for anti-plasminogen (anti-Plg). Only VTEpos patients who were ever positive (pos) for anti-Plg are shown (n = 5). Anti-Plg was elevated in all 3 patients with samples available before the VTE. One patient was elevated at the time of the VTE and one was elevated after the VTE. (b) Microparticle tissue factor activity (MPTFa) was tested on longitudinal samples from VTEpos patients. Only the 8 of 12 VTEpos patients who were ever pos for MPTFa are shown. In 3 of 6 patients with samples before the VTE, MPTFa was elevated before the diagnosis of the VTE. Five patients had elevated MPTFa after the VTE (3 days–18 months [m]). Elevated MPTFa was defined as greater than 2 SDs above the mean of healthy control (HC) values (11.1%). The dashed line represents the threshold for positivity, as determined by the mean of HC values + 2 SDs. Zero denotes the day of the VTE.
D-Dimer Higher in VTEpos Patients
Peak values of D-dimer were higher in VTEpos (677 ng/ml, 245–2263; Figure 3b) than VTEneg patients (208, 149–811; P = 0.04; Table 3). Two patients who had elevated D-dimer with a Well’s score ≥2 prompting compression ultrasonography had subclinical VTEs below the knee and one had pulmonary embolism. Peak values of hs-CRP were similar in VTEpos and VTEneg patients (Figure 3c). Although peak IL-6 values were higher in VTEpos (4.8 pg/ml, 2.5–11.0; Figure 3d) and VTEneg patients (2.6, 1.6–4.0) than HCs (P < 0.0001 for both), VTEpos and VTEneg patients did not differ (P = 0.06).
Elevated MPTFa Differentiates Patients With VTE
VTEpos patients had higher levels of peak MPTFa (14.0, 4.3–36.6; Figure 3e) than VTEneg patients (0, 0–1.4; P < 0.0001, Table 3) and HCs (P < 0.0001). Peak MPTFa was similar in VTEneg patients and HC. Eight of 12 VTEpos patients (67%) had at least one elevated MPTFa measurement, whereas 3 of 25 (12%) HCs and no VTEneg patients (0 of 20; P < 0.0001) had increased MPTFa in up to 46 months of follow-up (Supplementary Figure S4B).
To evaluate a temporal relationship, MPTFa was examined longitudinally relative to the time of VTE (Figure 4b). Considering the 8 patients who ever had elevated MPTFa, 3 of 6 patients (50%) with plasma available before VTE had increased MPTFa 1 to 3 months before the VTE. The other 3 patients had elevated MPTFa after VTE (7–17 months). Of 2 patients without plasma samples before VTE, 1 had the highest MPTFa in our cohort measured 3 days after VTE; the other had elevated MPTFa 18 months after VTE.
Increased TF-Bearing Leukocytes in VTEpos Patients
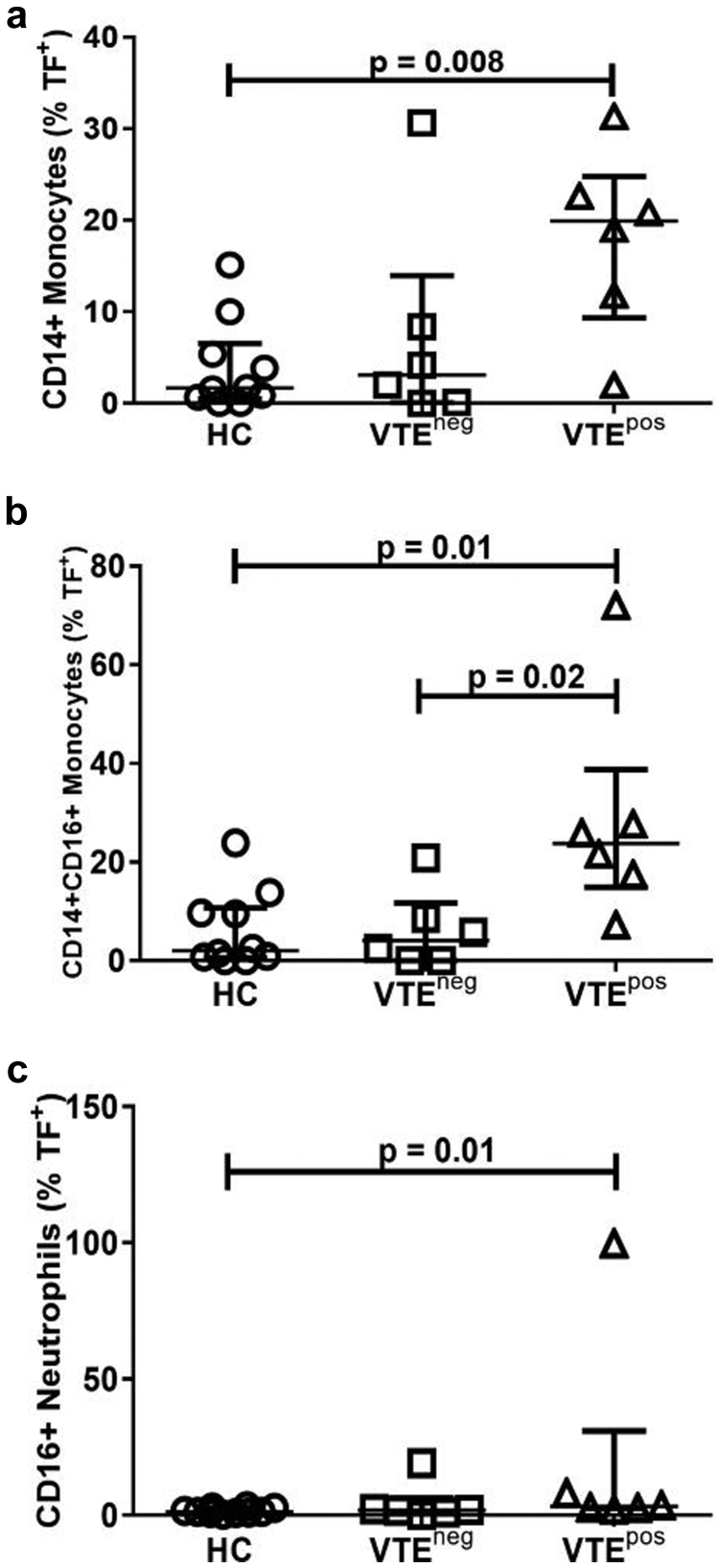
Because MPTFa is elevated in VTEpos patients, we examined whether cells that house ANCA antigens might have increased TF protein. Flow cytometry analysis demonstrated that the percentage of cells positive for TF was increased in monocytes (P = 0.008), inflammatory monocytes (P = 0.01), and neutrophils (P = 0.01) from VTEpos patients compared with HCs (Figure 5a–c). TF was also increased on inflammatory monocytes from VTEpos patients compared with VTEneg patients (Figure 5b, P = 0.02). To confirm that bioactive TF could be released by ANCA, we treated HC whole blood with PR3-ANCA IgG and found that MPs with TFa could be released (data not shown).
Figure 5.
Tissue factor (TF) is increased on cells of venous thromboembolism (VTE)pos patients. Whole blood from healthy controls (HCs), VTEpos, and VTEneg patients were stained with cluster of differentiation 14 (CD14), cluster of differentiation 16 (CD16), and CD142 (anti-TF) to determine the percentage of TF+ leukocytes by flow cytometry. VTEpos patients had a higher surface expression of TF on (a) CD14+ monocytes, (b) CD14+CD16+ inflammatory monocytes, and (c) CD16+ neutrophils than HC. (b) VTEpos patients also had significantly higher surface expression of TF on CD14+CD16+ inflammatory monocytes than VTEneg patients. neg, negative; pos, positive.
Association of Biomarkers With VTE Incidence
Time-to-event analysis was assessed to discern how biological markers relate to incidence of VTE. MPTFa was highly associated with VTE whether using MPTFa at active disease (HR: 1.04; 1.01–1.08; P = 0.01; Table 4) or MPTFa during remission (HR: 1.4; 1.11–1.77; P = 0.005). For every 10% increment of increase in MPTFa during active disease, the risk of VTE increased by 40%. This is a significant unit of measure, as the threshold for positivity was set at 11% of the positive control.
Table 4.
Biomarkers associated with VTE by univariate analysis
| Variable | Univariate analysis |
||
|---|---|---|---|
| Hazard ratio | 95% CI | P value | |
| Anti-Plg (Act) (%Pos Co) | 1.03 | 0.97–1.08 | 0.33 |
| Anti-Plg (Rem) (%Pos Co) | 1.17 | 1.03–1.33 | 0.02 |
| MPTFa (Act) (%Pos Co) | 1.04 | 1.01–1.08 | 0.01 |
| MPTFa (Rem) (%Pos Co) | 1.40 | 1.11–1.77 | 0.005 |
| Serum creatinine (Act) (mg/dl) | 1.29 | 1.00–1.66 | 0.05 |
| Serum albumina (Act) (g/dl) | 4.4 | 1.5–12.9 | 0.008 |
| hs-CRP (Act) (mg/l) | 1.17 | 1.01–1.35 | 0.04 |
| Estimated GFRa (Act) (ml/min per 1.73 m2) | 1.03 | 1.00–1.07 | 0.06 |
%Pos Co, % positive control; Act, active disease; Anti-Plg, anti-plasminogen; CI, confidence interval; GFR, glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; MPTFa, microparticle tissue factor activity; Rem, remission; VTE, venous thromboembolism.
These variables, low serum albumin and low estimated GFR, are inverse associations.
Increased anti-Plg during remission was associated with VTE (HR: 1.17; 1.03–1.33; P = 0.02). Due to the design of the study, some remission time-points were after VTE, but sensitivity analysis of VTEpos patients with multiple remission samples indicated anti-Plg was comparable at all remission time-points (n = 7, Pearson correlation coefficient = 0.93, P = 0.002). Using minimum anti-Plg values during remission still resulted in an HR of 1.23 (1.06–1.43; P = 0.008). Increased hs-CRP (HR: 1.21; 1.02–1.45; P = 0.03) and serum creatinine (HR: 1.29; 1.00–1.66; P = 0.05) during active disease were also associated with VTE. Low baseline serum albumin was associated with an increased risk of VTE (HR: 4.4; 1.5–12.9; P = 0.008).
We performed limited multivariable modeling of statistically significant measures described previously to evaluate independence of associations. The association of anti-Plg at remission with VTE was independent of eGFR (HR: 1.17; 1.03–1.33; P = 0.02) or serum creatinine (HR: 1.19; 1.04–1.36; P = 0.01; Table 5). MPTFa during remission was consistently associated with VTE when adjusted for anti-Plg during remission (HR: 1.44; 1.09–1.9; P = 0.01) or minimum anti-Plg (HR: 1.48; 1.08–2.04; P = 0.01).
Table 5.
Biomarkers associated with VTE by multivariable analysis
| Variable | Multivariable analysis |
||
|---|---|---|---|
| Hazard ratio | 95% CI | P value | |
| MPTFa (Act) (%Pos Co) Adjusted by serum creatinine |
1.04 | 1.01–1.07 | 0.02 |
| MPTFa (Act) (%Pos Co) Adjusted by eGFR |
1.03 | 1.00–1.07 | 0.06 |
| MPTFa (Rem) (%Pos Co) Adjusted by anti-Plg (Rem) |
1.44 | 1.09–1.90 | 0.01 |
| MPTFa (Rem) (%Pos Co) Adjusted by minimum anti-Plg (Rem) |
1.48 | 1.08–2.04 | 0.01 |
| Anti-Plg (Rem) (%Pos Co) Adjusted by serum creatinine |
1.19 | 1.04–1.36 | 0.01 |
| Anti-Plg (Rem) (%Pos Co) Adjusted by eGFR |
1.17 | 1.03–1.33 | 0.02 |
| Serum creatinine (Act) (mg/dl) Adjusted by anti-Plg (Rem) |
1.40 | 1.02–1.92 | 0.04 |
%Pos Co, % positive control; Act, active disease; Anti-Plg, anti-plasminogen; CI, confidence interval; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; MPTFa, microparticle tissue factor activity; Rem, remission; VTE, venous thromboembolism.
Discussion
We explored several biological factors that we hypothesized might contribute to the increased incidence of VTE in patients with ANCA vasculitis. Our group and others identified anti-Plg in a subset of patients.5, 29, 30 These antibodies inhibit fibrinolysis and could contribute to VTE. We corroborate detection of anti-Plg in patients with both MPO- and PR3-ANCA as seen in other cohorts.29, 30
We confirmed that anti-Plg correlate with disease activity in MPO-ANCA as shown previously,30 and extended this finding to patients with PR3-ANCA. Half of both MPO- and PR3-ANCA VTEpos patients were positive for anti-Plg compared with one-third of VTEneg patients. This proportion of VTEpos patients with anti-Plg is similar to a prior observation in PR3-ANCA patients.5 A subsequent study confirmed an anti-Plg–mediated decrease in fibrinolysis via reduced thrombin generation and noted an association of anti-Plg with decreased renal function.29 Correlation of anti-Plg with both systemic and renal disease has been reported.30 Although elevated during active disease, anti-Plg do not discriminate between VTEpos and VTEneg patients at this time-point. Importantly, our HR analysis demonstrated that increased anti-Plg during remission indicated patients more likely to have VTE independent of renal function. This concept of hypercoagulability during remission has been described31 and suggests that some patients with ANCA vasculitis and detectable anti-Plg, even within a normal reference range, may be at risk for VTE during remission.
Because anti-Plg are not universally detectable among VTEpos patients and occur in VTEneg patients, additional factors must contribute to mechanism(s) underlying the increased propensity for VTE in ANCA vasculitis. Multiple studies suggest a higher risk of VTE during active disease1, 2, 3, 4 when inflammation is high.32 We confirmed that D-dimer33 and CRP34 are associated with disease activity in ANCA vasculitis. Unlike Tomasson et al.,20 IL-6 was elevated during active disease in this study, but we did not observe a significant difference in IL-6 between VTEpos and VTEneg patients. These discrepancies may be attributable to fewer patients in our study and our strict definition of remission. While it is possible that increased IL-6 levels in patients compared with HCs may be less robust than shown because of the correlation of IL-6 with age, our analysis of active and remission samples within the same patient demonstrate a strong elevation of IL-6 during active disease. Although D-dimer differed in VTEpos and VTEneg patients, almost half of VTEneg patients were positive for D-dimer, emphasizing its sensitive but nonspecific nature.35 During active disease, hs-CRP distinguished VTEpos from VTEneg patients and was associated with VTE in our HR analysis similar to a prior study.36 Interestingly, 75% of VTEpos patients were positive for both D-dimer and hs-CRP at active disease. It is conceivable that hs-CRP measures may improve the prognostic value of D-dimer. It would be informative to examine this intriguing observation in a larger cohort. Of note, CRP triggers TF protein expression in human monocytes.37
We demonstrate that increased percentages of monocytes, inflammatory monocytes, and neutrophils from VTEpos patients have surface TF than those of healthy individuals. VTEpos patients also have more TF-positive inflammatory monocytes than VTEneg patients. Leukocyte surface expression of TF can be driven by antibody-mediated cell activation.12, 18, 19 ANCA bind both neutrophils and monocytes,38 but ANCA-driven TF expression has not been examined previously in monocytes or inflammatory monocytes. As a proof of concept, we found PR3-ANCA IgG can stimulate release of MPs with TF activity. Because monocytes and inflammatory monocytes from VTEpos patients exhibit elevated TF protein, these cells, in addition to neutrophils, may be sources of increased MPTFa observed in VTEpos patients.
Microparticles and TF are implicated in development of VTE in autoimmune disorders,39, 40, 41 including systemic vasculitis.16, 18, 19, 42 Because TF protein is not equivalent to TF activity,43 a strength of our study is direct measurement of TF activity of MPs. Our study is the first to examine MPTFa in ANCA vasculitis and ascertain an association with VTE. Patients with VTE have strikingly higher levels of MPTFa than patients without VTE. Two-thirds of VTEpos patients had elevated MPTFa during their disease course. In contrast, no VTEneg patients ever had elevated MPTFa, even during active disease when risk for VTE is expected to be highest. Another strength of our study is longitudinal data that allowed discernment of elevations in anti-Plg and MPTFa that might have gone undetected in cross-sectional samples.
A limitation of this small study is the seeming lack of temporal correlation of elevated MPTFa with VTE in some patients, possibly due to the timing of sample acquisition, as therapy around the time of VTE may affect measurement of MPTFa. Although MPTFa did not correlate with disease activity, we tested a limited number of paired samples in this assay. Nonetheless, HR analyses demonstrated a strong association of MPTFa with VTE in active disease and in remission. These data suggest that MPTFa may be a useful biomarker for identifying patients most prone to develop VTE. These findings are intriguing and should be replicated in a larger prospective study. Because a larger percentage of VTEneg patients were lost to follow-up than VTEpos patients, it is conceivable that some potential VTE events were missed in the 6 VTEneg patients lost to follow-up. However, only 2 of the 6 VTEneg patients had shorter follow-up time than the median time to VTE. An interesting finding and possible limitation of the study is that half of the VTEpos patients received therapeutic plasma exchange compared with 10% of VTEneg patients. Plasmapheresis could potentially contribute to the risk of VTE because of the use of a catheter in this procedure. Conversely, plasmapheresis removes clotting factors, anti-Plg, and microparticles that would theoretically decrease the risk of VTE. This is a complicated issue that our study was not designed to address and would be best explored in a large, randomized clinical trial. Additional biological factors not examined in our study may also contribute to thrombosis in ANCA vasculitis. For example, antibodies to anti-tissue plasminogen activator were frequent in patients with anti-Plg; IgG samples containing both antibodies were most effective in delaying fibrinolysis.29
In our cohort, VTEpos patients had 3-fold more skin involvement than VTEneg patients, similar to that reported recently.36 Conversely, we found no overrepresentation of gastrointestinal or eye involvement in VTEpos patients.36 Eye involvement was also similar between VTEpos and VTEneg patients in a UK cohort.44 VTEpos and VTEneg patients had comparable renal involvement, eGFR, and serum creatinine. In time-to-event analysis, serum creatinine was associated with VTE as reported recently36; however, eGFR did not reach significant association with VTE in our study. Increased MPTFa and anti-Plg at remission signify a propensity for VTE in ANCA vasculitis independent of renal function.
Low serum albumin was associated with a 4-fold risk of VTE. The inverse relationship between serum albumin and VTE is not surprising given that hypoalbuminemia is a predictor of VTE in nephrotic syndrome,24 but has not been reported in ANCA vasculitis.24, 45, 46 Although low plasma albumin (hypoalbuminemia) generally correlates with proteinuria, the relationship between these 2 measures may not only reflect the causal glomerular disease of the patient,47 but also the risk of VTE.24 In addition to proteinuria, hypoalbuminemia may be affected by nutritional status, inflammation, and vascular protein permeability.48 Another mechanism that may predispose patients to thrombosis includes the hepatic overproduction of fibrinogen and factors V and VIII as a compensatory response to hypoalbuminemia.49, 50 Decreased plasma levels of antithrombin III, α-1 antitrypsin, and free protein S are known to correlate with serum albumin level.49, 51, 52 In nephrotic patients, low plasma albumin contributes to a compact fibrin clot structure and impaired fibrinolysis; therapeutic albumin replacement improved fibrin clot structure and fibrinolysis.53 Moreover, albumin is a cofactor for the binding of plasminogen to fibrin and their interaction with tissue plasminogen activator54; it is conceivable that anti-Plg may augment the reduced effectiveness of this interaction in the setting of low serum albumin, further impairing fibrinolysis. It will be important to confirm our finding that hypoalbuminemia is associated with an increased risk of VTE in a larger cohort.
Our study identified elevated MPTFa, increased anti-Plg during remission, and hypoalbuminemia as novel biomarkers associated with VTE in patients with ANCA vasculitis. These hypothesis-generating studies contribute to our understanding of the mechanisms of VTE and form a foundation for future studies. Discernment of a thrombotic signature would allow improved management of patients to minimize both risk of VTE and complications of anticoagulation.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank Elizabeth McInnis for IgG purification and Jean Brown for editorial assistance. Nigel Mackman, Nigel Key, Dougald Monroe, Micah Mooberry, Robert Bradford, and Mark Piegore provided expert advice regarding the MPTFa assay. These studies were supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) PO1 DK 058335. EJB received a Vasculitis Foundation Clinical Research Fellowship. VKD was supported by the Nephrotic Syndrome Study Network Consortium (NEPTUNE), NIDDK U-54-DK-083912, and Duke–University of North Carolina Clinical Hematology Research Career Development Program Grant 5K12 HL087097–05 (NIH/National Heart, Lung, and Blood Institute). The UNC Flow Cytometry Core Facility is supported by P30 CA016086 Cancer Center Core Support Grant to the UNC Lineberger Comprehensive Cancer Center.
Author Contributions
DOB, PHN, VKD, RJF, and JCJ designed the study. AF consented patients and acquired data. EJB, CEM, and DOB performed experiments. DOB, CEM, EJB, VKD, MLM, YH, and SLH analyzed data. DOB and CEM prepared figures. DOB, CEM, EJB, and VKD drafted the paper. All authors revised and approved the final version of manuscript.
Footnotes
Table S1. Demographics of study population.
Table S2. Anticoagulation medications in study population.
Table S3. Immunosuppressive therapy in study population.
Figure S1. Correlation of candidate markers with age in healthy controls or patients.
Figure S2. Anti-plasminogen antibodies correlate with disease activity in MPO- and PR3-ANCA vasculitis.
Figure S3. Comparison of candidate biomarkers in patients during active disease.
Figure S4. Some VTEneg patients exceed threshold of positivity for anti-Plg but not MPTFa.
Supplementary Material
References
- 1.Merkel P.A., Lo G.H., Holbrook J.T. Brief communication:high incidence of venous thrombotic events among patients with Wegener granulomatosis:the Wegener's Clinical Occurrence of Thrombosis (WeCLOT) Study. Ann Intern Med. 2005;142:620–626. doi: 10.7326/0003-4819-142-8-200505030-00011. [DOI] [PubMed] [Google Scholar]
- 2.Weidner S., Hafezi-Rachti S., Rupprecht H.D. Thromboembolic events as a complication of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2006;55:146–149. doi: 10.1002/art.21704. [DOI] [PubMed] [Google Scholar]
- 3.Stassen P.M., Derks R.P., Kallenberg C.G. Venous thromboembolism in ANCA-associated vasculitis--incidence and risk factors. Rheumatology (Oxford) 2008;47:530–534. doi: 10.1093/rheumatology/ken035. [DOI] [PubMed] [Google Scholar]
- 4.Allenbach Y., Seror R., Pagnoux C. High frequency of venous thromboembolic events in Churg-Strauss syndrome, Wegener's granulomatosis and microscopic polyangiitis but not polyarteritis nodosa: a systematic retrospective study on 1130 patients. Ann Rheum Dis. 2009;68:564–567. doi: 10.1136/ard.2008.099051. [DOI] [PubMed] [Google Scholar]
- 5.Bautz D.J., Preston G.A., Lionaki S. Antibodies with dual reactivity to plasminogen and complementary PR3 in PR3-ANCA vasculitis. J Am Soc Nephrol. 2008;19:2421–2429. doi: 10.1681/ASN.2008030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park M.S., Owen B.A., Ballinger B.A. Quantification of hypercoagulable state after blunt trauma:microparticle and thrombin generation are increased relative to injury severity, while standard markers are not. Surgery. 2012;151:831–836. doi: 10.1016/j.surg.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liebman H.A., Feinstein D.I. Thrombosis in patients with paroxysmal noctural hemoglobinuria is associated with markedly elevated plasma levels of leukocyte-derived tissue factor. Thromb Res. 2003;111:235–238. doi: 10.1016/j.thromres.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Manly D.A., Wang J., Glover S.L. Increased microparticle tissue factor activity in cancer patients with venous thromboembolism. Thromb Res. 2010;125:511–512. doi: 10.1016/j.thromres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Doormaal F., Kleinjan A., Berckmans R.J. Coagulation activation and microparticle-associated coagulant activity in cancer patients. An exploratory prospective study. Thromb Haemost. 2012;108:160–165. doi: 10.1160/TH12-02-0099. [DOI] [PubMed] [Google Scholar]
- 10.Khorana A.A., Francis C.W., Menzies K.E. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6:1983–1985. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thaler J., Ay C., Mackman N. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–1370. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- 12.Kasthuri R.S., Glover S.L., Jonas W. PF4/heparin-antibody complex induces monocyte tissue factor expression and release of tissue factor positive microparticles by activation of FcgammaRI. Blood. 2012;119:5285–5293. doi: 10.1182/blood-2011-06-359430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ataga K.I., Brittain J.E., Desai P. Association of coagulation activation with clinical complications in sickle cell disease. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daniel L., Fakhouri F., Joly D. Increase of circulating neutrophil and platelet microparticles during acute vasculitis and hemodialysis. Kidney Int. 2006;69:1416–1423. doi: 10.1038/sj.ki.5000306. [DOI] [PubMed] [Google Scholar]
- 15.Erdbruegger U., Grossheim M., Hertel B. Diagnostic role of endothelial microparticles in vasculitis. Rheumatology (Oxford) 2008;47:1820–1825. doi: 10.1093/rheumatology/ken373. [DOI] [PubMed] [Google Scholar]
- 16.Hong Y., Eleftheriou D., Hussain A.A. Anti-neutrophil cytoplasmic antibodies stimulate release of neutrophil microparticles. J Am Soc Nephrol. 2012;23:49–62. doi: 10.1681/ASN.2011030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brogan P.A., Shah V., Brachet C. Endothelial and platelet microparticles in vasculitis of the young. Arthritis Rheum. 2004;50:927–936. doi: 10.1002/art.20199. [DOI] [PubMed] [Google Scholar]
- 18.Kambas K., Chrysanthopoulou A., Vassilopoulos D. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis. 2014;73:1854–1863. doi: 10.1136/annrheumdis-2013-203430. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y.M., Wang H., Wang C. Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol. 2015;67:2780–2790. doi: 10.1002/art.39239. [DOI] [PubMed] [Google Scholar]
- 20.Tomasson G., LaValley M., Tanriverdi K. Relationship between markers of platelet activation and inflammation with disease activity in Wegener's granulomatosis. J Rheumatol. 2011;38:1048–1054. doi: 10.3899/jrheum.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jennette J.C., Falk R.J., Andrassy K. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 22.Mendoza C.E., Brant E.J., McDermott M.L. Microparticle tissue factor activity dominates venou thromboembolism signature in ANCA-vasculitis. J Am Soc Nephrol. 2017;28:118. [abstract] [Google Scholar]
- 23.Levey A.S., Coresh J., Greene T. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 24.Barbour S.J., Greenwald A., Djurdjev O. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 2012;81:190–195. doi: 10.1038/ki.2011.312. [DOI] [PubMed] [Google Scholar]
- 25.Lacroix R., Judicone C., Mooberry M. Standardization of pre-analytical variables in plasma microparticle determination:results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost. 2017;15:1236. doi: 10.1111/jth.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willemze R., Bradford R.L., Mooberry M.J. Plasma microparticle tissue factor activity in patients with antiphospholipid antibodies with and without clinical complications. Thromb Res. 2014;133:187–189. doi: 10.1016/j.thromres.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stravitz R.T., Bowling R., Bradford R.L. Role of procoagulant microparticles in mediating complications and outcome of acute liver injury/acute liver failure. Hepatology. 2013;58:304–313. doi: 10.1002/hep.26307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Key N.S., Mackman N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Semin Thromb Hemost. 2010;36:865–875. doi: 10.1055/s-0030-1267040. [DOI] [PubMed] [Google Scholar]
- 29.Berden A.E., Nolan S.L., Morris H.L. Anti-plasminogen antibodies compromise fibrinolysis and associate with renal histology in ANCA-associated vasculitis. J Am Soc Nephrol. 2010;21:2169–2179. doi: 10.1681/ASN.2010030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao J., Wang C., Gou S.J. The association between anti-plasminogen antibodies and disease activity in ANCA-associated vasculitis. Rheumatology (Oxford) 2014;53:300–306. doi: 10.1093/rheumatology/ket345. [DOI] [PubMed] [Google Scholar]
- 31.Hilhorst M., Winckers K., Wilde B. Patients with antineutrophil cytoplasmic antibodies associated vasculitis in remission are hypercoagulable. J Rheumatol. 2013;40:2042–2046. doi: 10.3899/jrheum.130200. [DOI] [PubMed] [Google Scholar]
- 32.Tichelaar Y.I., Kluin-Nelemans H.J., Meijer K. Infections and inflammatory diseases as risk factors for venous thrombosis. A systematic review. Thromb Haemost. 2012;107:827–837. doi: 10.1160/TH11-09-0611. [DOI] [PubMed] [Google Scholar]
- 33.Ma T.T., Huang Y.M., Wang C. Coagulation and fibrinolysis index profile in patients with ANCA-associated vasculitis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kronbichler A., Kerschbaum J., Grundlinger G. Evaluation and validation of biomarkers in granulomatosis with polyangiitis and microscopic polyangiitis. Nephrol Dial Transplant. 2016;31:930–936. doi: 10.1093/ndt/gfv336. [DOI] [PubMed] [Google Scholar]
- 35.Olson J.D. D-dimer: an overview of hemostasis and fibrinolysis, assays, and clinical applications. Adv Clin Chem. 2015;69:1–46. doi: 10.1016/bs.acc.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Kronbichler A., Leierer J., Leierer G. Clinical associations with venous thromboembolism in anti-neutrophil cytoplasm antibody-associated vasculitides. Rheumatology (Oxford) 2017;56:704–708. doi: 10.1093/rheumatology/kew465. [DOI] [PubMed] [Google Scholar]
- 37.Cermak J., Key N.S., Bach R.R. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513–520. [PubMed] [Google Scholar]
- 38.Jennette J.C., Falk R.J. ANCAs are also antimonocyte cytoplasmic autoantibodies. Clin J Am Soc Nephrol. 2015;10:4–6. doi: 10.2215/CJN.11501114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niccolai E., Emmi G., Squatrito D. Microparticles: bridging the gap between autoimmunity and thrombosis. Semin Thromb Hemost. 2015;41:413–422. doi: 10.1055/s-0035-1549850. [DOI] [PubMed] [Google Scholar]
- 40.Pericleous C., Clarke L.A., Brogan P.A. Endothelial microparticle release is stimulated in vitro by purified IgG from patients with the antiphospholipid syndrome. Thromb Haemost. 2013;109:72–78. doi: 10.1160/TH12-05-0346. [DOI] [PubMed] [Google Scholar]
- 41.Pisetsky D.S., Ullal A.J., Gauley J. Microparticles as mediators and biomarkers of rheumatic disease. Rheumatology (Oxford) 2012;51:1737–1746. doi: 10.1093/rheumatology/kes028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eleftheriou D., Hong Y., Klein N.J. Thromboembolic disease in systemic vasculitis is associated with enhanced microparticle-mediated thrombin generation. J Thromb Haemost. 2011;9:1864–1867. doi: 10.1111/j.1538-7836.2011.04434.x. [DOI] [PubMed] [Google Scholar]
- 43.Johnson G.J., Leis L.A., Bach R.R. Tissue factor activity of blood mononuclear cells is increased after total knee arthroplasty. Thromb Haemost. 2009;102:728–734. doi: 10.1160/TH09-04-0261. [DOI] [PubMed] [Google Scholar]
- 44.Kang A., Antonelou M., Wong N.L. High incidence of arterial and venous thrombosis in antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol. 2019;46:285–293. doi: 10.3899/jrheum.170896. [DOI] [PubMed] [Google Scholar]
- 45.Lionaki S., Derebail V.K., Hogan S.L. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol. 2012;7:43–51. doi: 10.2215/CJN.04250511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee T., Biddle A.K., Lionaki S. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int. 2014;85:1412–1420. doi: 10.1038/ki.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen M., Zhou F.D., Zhao M.H. Normoalbuminaemia is associated with IgA nephropathy in primary glomerulopathy with nephrotic-range proteinuria in Chinese patients. Nephrol Dial Transplant. 2011;26:1247–1252. doi: 10.1093/ndt/gfq553. [DOI] [PubMed] [Google Scholar]
- 48.Rostoker G., Behar A., Lagrue G. Vascular hyperpermeability in nephrotic edema. Nephron. 2000;85:194–200. doi: 10.1159/000045661. [DOI] [PubMed] [Google Scholar]
- 49.Thomson C., Forbes C.D., Prentice C.R. Changes in blood coagulation and fibrinolysis in the nephrotic syndrome. Q J Med. 1974;43:399–407. [PubMed] [Google Scholar]
- 50.Kanfer A. Coagulation factors in nephrotic syndrome. Am J Nephrol. 1990;10(Suppl 1):63–68. doi: 10.1159/000168196. [DOI] [PubMed] [Google Scholar]
- 51.Rydzewski A., Mysliwiec M., Soszka J. Concentration of three thrombin inhibitors in the nephrotic syndrome in adults. Nephron. 1986;42:200–203. doi: 10.1159/000183667. [DOI] [PubMed] [Google Scholar]
- 52.Boneu B., Bouissou F., Abbal M. Comparison of progressive antithrombin activity and the concentration of three thrombin inhibitors in nephrotic syndrome. Thromb Haemost. 1981;46:623–625. [PubMed] [Google Scholar]
- 53.Colle J.P., Mishal Z., Lesty C. Abnormal fibrin clot architecture in nephrotic patients is related to hypofibrinolysis:influence of plasma biochemical modifications:a possible mechanism for the high thrombotic tendency? Thromb Haemost. 1999;82:1482–1489. [PubMed] [Google Scholar]
- 54.Rabelink T.J., Zwaginga J.J., Koomans H.A. Thrombosis and hemostasis in renal disease. Kidney Int. 1994;46:287–296. doi: 10.1038/ki.1994.274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.